Abstract
Nutrients from dehydrated sewage sludge play an essential role in the development of many plants such as Terminalia argentea, in the recovery of degraded areas. The aims were to assess the abundance, diversity and species richness of phytophagous, pollinators and predators arthropods, as well as the percentage of defoliation of T. argentea trees, fertilized (or not) with dehydrated sewage sludge in a degraded area. The abundance, diversity and species richness of phytophagous Coleoptera and total predators (predator insects + protocooperating ants + spiders); abundance and species richness of Diptera, pollinator insects, spiders, and predators (predator insects + spiders) were higher on trees fertilized with dehydrated sewage sludge. The abundance of phytophagous Coleoptera declined with the presence of phytophagous Hemiptera and protocooperating ants; population of phytophagous Orthoptera declined in response to phytophagous Coleoptera and total predators; the numbers of the leafminer Lyriomyza sp. directly increased with the numbers of spiders. The ecological indices of phytophagous, pollinators, and predator arthopods increased on Terminalia argentea trees fertilized with dehydrated sewage sludge; such a better ecological indices in fertilized than in unfertilized trees, show it more suitable for the recovery of degraded areas. We discuss the competition between phytophagous insects groups as well as herbivory reduction by predators.
Subject terms: Ecology, Environmental sciences, Entomology
Introduction
Sewage sludge, a residual and semi-solid material, produced as a by-product during domestic and industrial waste water treatment, is rich in organic matter, shows potential for fertilization and production of seedling substrates1,2. Sewage sludge can be used safely in agriculture and forests plantations as fertilizer and in the recovery of degraded areas, with a low-cost alternative to reduce the environmental impacts and to avoid contamination of the human food chain3–5. Furthermore, dehydrated sewage sludge (DSS) does not affect the heavy metal contents in grains of maize, Zea mays L. (Poales: Poaceae) and cowpea, Vigna unguiculata (L.) Walp. (Fabales: Fabaceae)6.
Terminalia argentea Mart. & Zucc (Combretaceae), a secondary native tree from the Southeastern and Central-western Brazil, is used for landscaping, wood and coal production, civil construction and the recovery of degraded areas7. Continuous release of exudates by T. argentea in the trunk is typical due to pathogens attack, affecting the constant visitation by Trigona branneri (Crockere) and Mesembrinella bicolor (Fabricius) (Hymenoptera: Apidae)8.
Insect diversity may be used to assess the recovery of degraded area, as these organisms easily respond to environmental changes9. Different orders of insects, with a large number of families and species, including Coleoptera, are widely used as a bioindicator5,10. Nutritional indices and chemical plant defenses are associated with factors such as fertilization and plant development (i.e. age), affecting phytophagous insects and therefore, the natural enemies’ diversity5,11–13. Sewage sludge increases the humus content in the soil and it is rich in macro (e.g. N, P and K) and micronutrients (e.g. Cu and Zn)14, favoring plants and, consequently, insect development.
The aims here were to assess for 24 months the ecological indices (abundance, diversity and species richness) and ecological processes (herbivory and predation) of phytophagous, pollinators and predators arthropods on T. argentea trees, fertilized (or not) with DSS in a degraded area. We hypothesize that (i) T. argentea trees resemble living islands, and that the fertilization with DSS may increase the canopy size (canopy islands), and thus accommodate larger numbers of phytophagous, pollinators and predators arthropods (> the equilibrium theory of island biogeography—ETIB)5,15–17; (ii) there is competition between groups of phytophagous insects, such as hemipterans, coleopterans and orthopterans18,19; and (iii) arthropod predators, such as insects and spiders, reduce the number of phytophagous insects and thus herbivory on T. argentea trees19–21.
Results
Terminalia argentea trees and arthropods
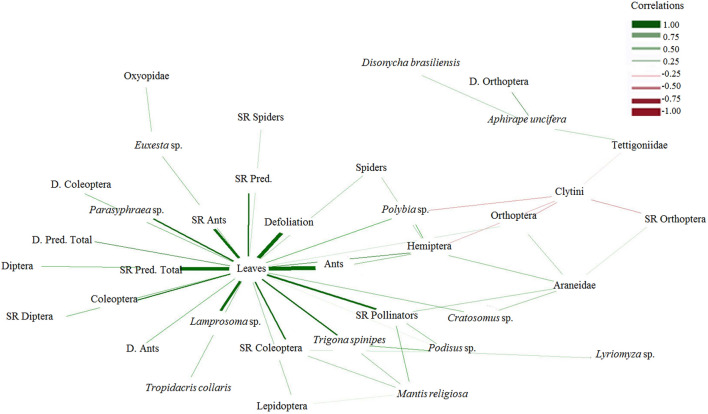
The phytophagous Coleoptera and the abundance, diversity and species richness of total predators and Diptera, pollinators, spiders, predator abundance and species richness were higher (P < 0.05) on T. argentea trees fertilized with DSS (Table 1). Percentage of defoliation and phytophagous Coleoptera Psiloptera sp. (Buprestidae), Cerambycidae, Cerotoma sp., Lamprosoma sp., Parasyphraea sp. (Chrysomelidae) and Cratosomus sp. (Curculionidae); Euxesta sp. (Diptera: Otitidae), Lepidoptera caterpillars and Tropidacris collaris Stoll (Orthoptera: Romaleidae); pollinators Trigona spinipes Fabricius (Hymenoptera: Apidae); and predators Araneidae and Salticidae (Araneae), Podisus sp. (Hemiptera: Pentatomidae), Polybia sp. (Hymenoptera: Vespidae), protocooperating ants (Hymenoptera: Formicidae) and Mantis religiosa L. (Mantodea: Mantidae) were higher (P < 0.05) on T. argentea trees fertilized with DSS (Tables 2 and 3). The abundance of Coleoptera, Diptera, Hemiptera and Orthoptera, spiders and protocooperating ants; the diversity of Coleoptera, protocooperating ants and total predator; the species richness of Coleoptera, Diptera, pollinators, protocooperating ants, spiders, predators and total predator; the percentage of defoliation; the numbers of phytophagous insects Cratosomus sp., Euxesta sp., Lamprosoma sp., Lepidoptera, Parasyphraea sp. e T. collaris, pollinators T. spinipes, and predators Pentatomidae and Polybia sp. increased with the total numbers of T. argentea leaves (Fig. 1).
Table 1.
The abundance (Abun.), diversity (D), and species richness (RS) of phytophagous insects, pollinators, spiders, predators (predators + spiders) (Pred.), total predators (predators + spiders + protocooperating ants) (Tot. Pred.) on Terminalia argentea Mart & Zucc (Combretaceae) trees (mean ± SE) fertilized or non-fertilized with dehydrated sewage sludge in degraded area.
| Ecological indices | Sewage sludge | Wilcoxon test | ||
|---|---|---|---|---|
| Fertilized | Non-fertilized | VT* | P | |
| Abund. Coleoptera | 9.79 ± 1.32 | 2.54 ± 0.79 | 4.3 | 0.00 |
| D Coleoptera | 7.63 ± 1.08 | 2.68 ± 0.50 | 3.1 | 0.00 |
| SR Coleoptera | 4.21 ± 0.35 | 1.54 ± 0.28 | 4.5 | 0.00 |
| Abund. Diptera | 1.21 ± 0.55 | 0.00 ± 0.00 | 3.3 | 0.00 |
| D Diptera | 0.00 ± 0.00 | 0.00 ± 0.00 | –- | –- |
| SR Diptera | 0.38 ± 0.10 | 0.00 ± 0.00 | 3.3 | 0.00 |
| Abund. Orthoptera | 1.00 ± 0.28 | 0.38 ± 0.11 | 1.6 | 0.06 |
| D Orthoptera | 0.30 ± 0.12 | 0.30 ± 0.12 | –- | –- |
| SR Orthoptera | 0.50 ± 0.10 | 0.33 ± 0.09 | 1.2 | 0.12 |
| Abund. pollinators | 10.33 ± 3.01 | 0.38 ± 0.26 | 4.3 | 0.00 |
| D pollinators | 0.00 ± 0.00 | 0.00 ± 0.00 | –- | –- |
| SR pollinators | 0.83 ± 0.13 | 0.08 ± 0.05 | 4.4 | 0.00 |
| Abund. spiders | 4.38 ± 1.38 | 1.50 ± 0.36 | 2.3 | 0.01 |
| D spiders | 2.25 ± 0.65 | 2.35 ± 0.71 | 0.0 | 0.48 |
| SR spiders | 1.75 ± 0.21 | 1.25 ± 0.27 | 1.8 | 0.04 |
| Abund. Pred | 6.21 ± 1.46 | 1.75 ± 0.40 | 4.1 | 0.00 |
| D Pred | 4.42 ± 0.94 | 3.06 ± 0.81 | 0.9 | 0.18 |
| SR Pred | 3.04 ± 0.22 | 1.42 ± 0.31 | 3.8 | 0.00 |
| Abund. Tot. Pred | 37.83 ± 3.99 | 7.17 ± 1.05 | 5.6 | 0.00 |
| D Tot. Pred | 14.18 ± 1.36 | 8.89 ± 1.30 | 2.8 | 0.00 |
| SR Tot. Pred | 8.33 ± 0.40 | 3.83 ± 0.43 | 5.2 | 0.00 |
n = 24 per treatment. VT* = value of the test. –- = it was not possible to generate due to zero in the treatments.
Table 2.
The abundance of phytophagous insects on Terminalia argentea Mart & Zucc (Combretaceae) and defoliation (%) of trees (mean ± SE) fertilized or non-fertilized with dehydrated sewage sludge in a degraded area.
| Order: family | Species | Sewage sludge | Wilcoxon test | ||
|---|---|---|---|---|---|
| Fertilized | Non-fertilized | VT* | P | ||
| Coleoptera | |||||
| Buprestidae | Psiloptera sp. | 0.21 ± 0.13 | 0.00 ± 0.00 | 1.8 | 0.04 |
| Cerambycidae | Non-identified | 0.25 ± 0.10 | 0.00 ± 0.00 | 2.3 | 0.01 |
| Chrysomelidae | Alagoasa sp. | 0.00 ± 0.00 | 0.42 ± 0.41 | 1.0 | 0.16 |
| Clytrini | 0.25 ± 0.09 | 0.54 ± 0.14 | 1.4 | 0.08 | |
| Cerotoma sp. | 0.46 ± 0.19 | 0.08 ± 0.05 | 1.6 | 0.05 | |
| Diabrotica speciose Germar | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.0 | 0.50 | |
| Disonycha brasiliensis Costa Lima | 0.13 ± 0.06 | 0.08 ± 0.05 | 0.5 | 0.32 | |
| Eumolpus sp. | 0.04 ± 0.04 | 0.00 ± 0.00 | 1.0 | 0.16 | |
| Gynandrobrotica sp. | 0.04 ± 0.04 | 0.00 ± 0.00 | 1.0 | 0.16 | |
| Lamprosoma sp. | 1.63 ± 0.25 | 0.13 ± 0.09 | 5.2 | 0.00 | |
| Parasyphraea sp. | 1.92 ± 0.58 | 0.04 ± 0.04 | 3.8 | 0.00 | |
| Walterianella sp. | 0.00 ± 0.00 | 0.13 ± 0.06 | 1.8 | 0.04 | |
| Wanderbiltiana sp. | 0.08 ± 0.05 | 0.04 ± 0.04 | 0.6 | 0.28 | |
| Curculionidae | Non-identified | 0.04 ± 0.04 | 0.00 ± 0.00 | 1.0 | 0.16 |
| Cratosomus sp. | 1.13 ± 0.42 | 0.00 ± 0.00 | 3.1 | 0.00 | |
| Diorymerus sp. | 1.17 ± 0.48 | 0.38 ± 0.29 | 1.5 | 0.07 | |
| Lordops sp. | 0.00 ± 0.00 | 0.04 ± 0.04 | 1.0 | 0.16 | |
| Tenebrionidae | Epitragus sp. | 0.29 ± 0.12 | 0.04 ± 0.04 | 1.8 | 0.04 |
| Diptera | |||||
| Agromyzidae | Lyriomyza sp. | 0.79 ± 0.55 | 0.00 ± 0.00 | 1.4 | 0.08 |
| Otitidae | Euxesta sp. | 0.42 ± 0.17 | 0.00 ± 0.00 | 2.8 | 0.00 |
| Hemiptera | – | 21.04 ± 8.46 | 2.79 ± 1.17 | 3.1 | 0.00 |
| Blattodea | |||||
| Termitidae | Nasutitermes sp.§ | 4.17 ± 2.88 | 0.00 ± 0.00 | 1.4 | 0.08 |
| Lepidoptera | Non-identified | 0.29 ± 0.09 | 0.04 ± 0.04 | 2.3 | 0.01 |
| Orthoptera | |||||
| Gryllidae | Non-identified | 0.04 ± 0.04 | 0.00 ± 0.00 | 1.0 | 0.16 |
| Proscopiidae | Cephalocoema sp. | 0.17 ± 0.16 | 0.00 ± 0.00 | 1.0 | 0.16 |
| Romaleidae | Tropidacris collaris Stoll | 2.08 ± 0.48 | 0.54 ± 0.19 | 3.1 | 0.00 |
| Tettigoniidae | Non-identified | 0.79 ± 0.25 | 0.38 ± 0.11 | 1.0 | 0.17 |
| % defoliation | – | 7.88 ± 0.28 | 3.70 ± 0.21 | 5.7 | 0.00 |
n = 24 per treatment. VT* = value of the test. §Observed on T. argentea trunk.
Table 3.
The abundance of predators, protocooperating ants, and pollinators on Terminalia argentea Mart & Zucc (Combretaceae) trees (mean ± SE) fertilized or non-fertilized with dehydrated sewage sludge in a degraded area.
| Order: family | Species | Sewage sludge | Wilcoxon test | ||
|---|---|---|---|---|---|
| Fertilized | Non-fertilized | VT* | P | ||
| Araneae | |||||
| Araneidae | Non-identified | 2.96 ± 1.39 | 0.46 ± 0.14 | 1.8 | 0.04 |
| Anyphaenidae | Teudis sp. | 0.00 ± 0.00 | 0.04 ± 0.04 | 1.0 | 0.16 |
| Salticidae | Non-identified | 0.54 ± 0.14 | 0.25 ± 0.12 | 1.8 | 0.04 |
| Aphirape uncifera Tullgren | 0.04 ± 0.04 | 0.21 ± 0.10 | 1.4 | 0.08 | |
| Uspachus sp. | 0.13 ± 0.09 | 0.04 ± 0.04 | 0.6 | 0.27 | |
| Sparassidae | Quemedice sp. | 0.04 ± 0.04 | 0.13 ± 0.06 | 1.0 | 0.16 |
| Oxyopidae | Non-identified | 0.42 ± 0.14 | 0.17 ± 0.07 | 1.4 | 0.09 |
| Oxyopes salticus Hentz | 0.04 ± 0.04 | 0.04 ± 0.04 | 0.0 | 0.50 | |
| Tetragnathidae | Leucauge sp. | 0.08 ± 0.05 | 0.04 ± 0.04 | 0.6 | 0.28 |
| Thomisidae | Aphantochilus rogersi O.P.Camb | 0.08 ± 0.05 | 0.08 ± 0.05 | 0.0 | 0.50 |
| Tmarus sp. | 0.04 ± 0.04 | 0.04 ± 0.04 | 0.0 | 0.50 | |
| Hemiptera | |||||
| Pentatomidae | Podisus sp. | 0.29 ± 0.17 | 0.00 ± 0.00 | 2.1 | 0.02 |
| Hymenopera | |||||
| Apidae | Apis mellifera L | 0.08 ± 0.05 | 0.21 ± 0.20 | 0.5 | 0.29 |
| Tetragonisca angustula Latreille | 0.08 ± 0.05 | 0.00 ± 0.00 | 1.4 | 0.08 | |
| Trigona spinipes Fabricius | 10.17 ± 3.00 | 0.17 ± 0.16 | 4.4 | 0.00 | |
| Formicidae | Protocooperating | 28.67 ± 3.98 | 5.08 ± 0.18 | 5.2 | 0.00 |
| Vespidae | Polybia sp. | 0.75 ± 0.16 | 0.21 ± 0.12 | 3.0 | 0.00 |
| Mantodea | |||||
| Mantidae | Mantis religiosa L | 0.38 ± 0.14 | 0.04 ± 0.04 | 2.1 | 0.02 |
n = 24 per treatment. VT* = value of the test.
Figure 1.
Estimated network structures based on the Spearman correlation (P < 0.05) generated for total leaves per tree, defoliation (%), and the abundances of Aphirape uncifera, Araneidae, spiders, phytophagous Coleoptera, Clytini, Cratosomus sp., Diptera, Disonycha brasiliensis, Euxesta sp., protocooperating ants, phytophagous Hemiptera, Lamprosoma sp., Lepidoptera, Mantis religiosa, Lyriomyza sp. mines, phytophagous Orthoptera, Oxyopidae, Parasyphraea sp., Podisus sp., Polybia sp., Tettigoniidae, Trigona spinipes, and Tropidacris collaris; the diversity (D.) of protocooperating ants, phytophagous Coleoptera, total predators (predators + spiders + protocooperating ants), and phytophagous Orthoptera; and species richness (SR) of spiders, phytophagous Coleoptera, Diptera, protocooperating ants, Orthoptera, pollinators, predators, and total predadors on Terminalia argentea trees. n = 48.
Competition between phytophagous insects
The abundance of phytophagous Hemiptera and pollinators reduced (P < 0.05) the abundance of phytophagous Coleoptera, phytophagous Orthoptera and T. spinipes; the abundance of phytophagous Hemiptera and phytophagous Orthoptera reduced Clytini (Coleoptera: Chrysomelidae); the species richness of pollinators reduced (P < 0.05) phytophagous Coleoptera; and the species richness of phytophagous Hemiptera reduced pollinators (Table 4, Fig. 1).
Table 4.
Simple regression equation analysis of the variables of phytophagous Coleoptera (Ab.Col.) with phytophagous Hemiptera (Ab.Hem.), pollinator insects (Ab.Pol.), protocooperating ants (Ab.Ant.), and predators (predators + spiders) (Ab.Pred.); phytophagous Orthoptera (Ab.Orth.) with Ab.Col. and total predators (predators + spiders + protocooperating ants) (Ab.Tot.Pred..); Euxesta sp. (Ab.Eux.) with Ab.Ant.; Lyriomyza sp. (Ab.Lyr.) with Araneidae (Ab.Aranei), spiders (Ab.Spid.) with Ab.Tot.Pred.; Ab.Pol. with Ab.Ant. and Ab.Tot.Pred.; T. spinipes (Ab.Ts.) with Ab.Ant., Ab.Tot.Pred. and Ab.Col.; Ab.Spid. with Ab.Pol. and Ab.Ts.; Ab.Aranei with Ab.Ts.; Ab.Tot.Pred. with Ab.Col.; species richness of phytophagous Coleoptera (SR.Col.) with species richness of pollinators (SR.Pol.) and predators (SR.Pred.); SR.Pol.) with phytophagous Hemiptera (SR.Hem.); diversity of phytophagous Orthoptera (D.Orth.) with diversities of spiders (D.Spid.) and total predators (D.Tot.Pred.); protocooperating ants (D.Ant.) with phytophagous Hemiptera (D.Hem.) and D.Ara. on Terminalia argentea Mart & Zucc (Combretaceae) trees in a degraded area.
| Equations of the simple regression | R2 | ANOVA | |
|---|---|---|---|
| F | P | ||
| Ab.Col. = 3.59 + 20.36 × √¯Ab.Hem. − 0.14 × Ab.Hem | 0.16 | 4.4 | 0.02 |
| Ab.Col. = 4.59 + 0.56 × Ab.Pol. − 0.01 × Ab.Pol.2 | 0.18 | 4.9 | 0.01 |
| Ab.Col. = 0.89 + 0.45 × Ab.Ant. − 0.004 × Ab.Ant.2 | 0.43 | 17.2 | 0.00 |
| Ab.Orth. = 0.11 + 0.19 × Ab.Col. − 0.01 × Ab.Col.2 | 0.16 | 4.2 | 0.02 |
| Ab.Orth. = 0.10 + 0.21 × Ab.Tot.Pred. − 0.01Ab.Tot.Pred.2 | 0.19 | 5.3 | 0.01 |
| Ab.Eux. = − 0.10 + 0.04 × Ab.Ant. − 0.01 × Ab.Ant.2 | 0.13 | 3.3 | 0.04 |
| Ab.Lyr. = 7.62 + 0.19 × Ab.Aranei | 0.23 | 13.8 | 0.00 |
| Ab.Lyr. = − 0.12 + 0.17 × Ab.Ara | 0.21 | 12.3 | 0.00 |
| Ab.Lyr. = − 0.23 + 0.16 × Ab.Tot.Pred | 0.21 | 12.2 | 0.00 |
| Ab.Pol. = − 0.76 + 0.74 × Ab.Ant. − 0.01 × Ab.Ant.2 | 0.17 | 4.7 | 0.01 |
| Ab.Pol. = − 3.30 + 0.71 × Ab.Tot.Pred. − 0.01 × Ab.Tot.Pred.2 | 0.22 | 6.3 | 0.00 |
| Ab.Ts. = − 0.97 + 0.74 × Ab.Ant. − 0.01 × Ab.Ant.2 | 0.17 | 4.7 | 0.01 |
| Ab.Ts. = − 3.42 + 0.70 × Ab.Tot.Pred. − 0.01 × Ab.Tot.Pred.2 | 0.22 | 6.2 | 0.00 |
| Ab.Ts. = − 1.41 + 2.01 × Ab.Col. −0.08 × Ab.Col.2 | 0.19 | 5.1 | 0.01 |
| Ab.Spid. = 1.54 + 0.26 × Ab.Pol | 0.35 | 24.3 | 0.00 |
| Ab.Spid. = 1.62 + 0.26 × Ab.Ts | 0.33 | 22.4 | 0.00 |
| Ab.Aranei = 0.28 + 0.28 × Ab.Ts | 0.41 | 31.4 | 0.00 |
| Ab.Tot.Pred. = 10.15 + 2.00 × Ab.Col | 0.38 | 27.9 | 0.00 |
| SR.Col = 2.00 + 0.40 × SR.Pol. − 1.71 × SR.Pol.2 | 0.30 | 9.6 | 0.00 |
| SR.Col. = 0.93 + 1.15 × SR.Pred. − 0.18 × SR.Pred.2 | 0.27 | 8.4 | 0.00 |
| SR.Pol. = 0.16 + 0.51 × SR.Hem. − 0.10 × SR.Hem.2 | 0.17 | 4.6 | 0.02 |
| D.Orth. = 0.04 + 0.24 × D.Spid. − 0.02 × D.Spid.2 | 0.24 | 7.3 | 0.00 |
| D.Orth. =—0.01 + 0.18 × D.Tot.Pred. − 0.01 × D.Tot.Pred.2 | 0.22 | 6.4 | 0.00 |
| D.Ant. = 4.54 + 0.53 × D.Hem | 0.10 | 5.3 | 0.03 |
| D.Ant. = 3.72 + 1.21 × D.Spid. – 0.08 × D.Spid.2 | 0.23 | 6.7 | 0.00 |
ANOVA. n = 48, degrees of freedom: treatment = 1, replicates = 23, and of residue = 23.
Predators and phytophagous insects
The abundance of Araneidae and spiders reduced (P < 0.05) the number of the leafminer Lyriomyza sp.; the abundance of protocooperating ants reduced phytophagous Coleoptera, Euxesta sp., pollinators, and T. spinipes; the abundance of total predators reduced phytophagous Orthoptera, pollinators and T. spinipes; and the abundance of Polybia sp. reduced Clytini. The diversity and species richness of total predators reduced (P < 0.05) the numbers of phytophagous Coleoptera and Orthoptera, respectively. On the other hand, the abundance of pollinators and T. spinipes increased (P < 0.05) spiders; the abundance of T. spinipes increased Araneidae; the abundance of phytophagous Coleoptera increased total predators and the leafminer Lyriomyza sp.; the abundance of Lepidoptera caterpillars and T. spinipes increased M. religiosa; the abundance of the leafminer of Lyriomyza sp. and T. spinipes increased Podisus sp.; the abundance of Polybia sp., spiders and protocooperating ants increased phytophagous Hemiptera; the abundance of Oxyopidae (Araneae) increased Euxesta sp.; the abundance of Cratosomus sp., phytophagous Hemiptera and Orthoptera increased Araneidae; the abundance of Disonycha brasiliensis Lima (Coleoptera: Chrysomelidae) and Tettigoniidae (Orthoptera) increased Aphirape uncifera Tullgren (Araneae: Salticidae). The diversity of Orthoptera increased (P < 0.05) the abundance of A. uncifera; the diversity of phytophagous Hemiptera increased protocooperating ants; the species richness of phytophagous Coleoptera and pollinators increased the abundance of M. religiosa and Podisus sp.; and the species richness of phytophagous Orthoptera and pollinators increased the abundance of Araneidae (Table 4, Fig. 1).
Discussion
The highest ecological indices (abundance, diversity and species richness) of phytophagous, pollinator and predators arthropods on T. argentea, fertilized with dehydrated sewage sludge (DSS), are related to a higher nitrogen levels6 and consequently a better development of these plants (e.g. > leaves/tree = > ETIB)5,15. The apparent competition between Coleoptera and Hemiptera for space and food, and the negative effect between protocooperating ants and phytophagous Coleoptera, are in accordance to findings on Caryocar brasiliense Camb. (Malpighiales: Caryocaraceae) trees19,21.
The highest ecological indices of phytophagous Cerambycidae, Cerotoma sp., Cratosomus sp., Euxesta sp., Lamprosoma sp., Lepidoptera caterpillars, Parasyphraea sp., Psiloptera sp., T. collaris and Hemiptera, ; pollinator T. spinipes; predators Araneidae, Salticidae, Pentatomidae and Polybia sp.; protocooperating ants and ecological processes (herbivory) on T. argentea trees fertilized with DSS, may be due to the highest numbers of leaves of this plant (> ETIB). Leaves are food resource with a better quality for these phytophagous insects, which in turn may attract a higher number of predators. Such an observation confirms the first hypothesis (i.e. ETIB), that the diversity and abundance of phytophagous insects, pollinators and their predators are usually higher on larger trees with higher leaf mass5,15–17. Thus, trees such as T. argentea, may seem as islands (as proposed by ETIB), and those with lower leaf mass present a higher chance to get extinct the endangered species5,17,22,23. In addition, the number of free amino acids and proteins in leaves, pollen and/or nectar production and quality (more protein and amino acids) in flowers, are superior in plants with higher nitrogen fertilization levels, e.g. T. argentea trees fertilized with DSS, increasing the attractiveness to phytophagous and pollinator insects6,24–26. Dehydrated sewage sludge used as a biofertilizer improved the macrofauna recovery, including scarab beetles’ larvae and adults in degraded soils of the Cerrado (Brazilian Savanna) biome27.
The abundance of phytophagous Hemiptera and pollinators (e.g. T. spinipes) reduced the number of phytophagous Coleoptera; whilst this insect order reduced the numbers of Orthoptera and T. spinipes, as well as those of phytophagous Hemiptera and phytophagous Orthoptera reduced Clytini. These correlations confirm the second hypothesis that there was competition between those insect groups for space and feeding. Moreover, protocooperating ants, associated with phytophagous Hemiptera, for instance, may have attacked beetles. However, further studies are needed to elucidate this hypothesis. Competition between defoliators (e.g. Coleoptera), sucking and galling insect species for space and feeding was observed on C. brasiliense trees17,19.
Trigona spinipes, by flying in flocks with aggressive behavior, chases other pollinators, such as Apis mellifera L. and Tetragonisca angustula Latreille (Hymenoptera: Apidae)28, and also likely other insects (e.g. beetles); beyond damages shoot and plant growth tissues to remove fibers for nests construction5,29,30. Food web studies are intricate due to interactions among host plants, phytophagous, predators and parasitoids insects, soil and climatic conditions31. Only a few studies have examined food webs in complex ecosystems, such as in the Cerrado18,31,32.
Spiders, the dominant predators group (excluding the protocooperating ants), correlated negatively with some phytophagous insects (e.g. Lyriomyza sp. and Orthoptera), confirming the third hypothesis on the negative correlation between phytophagous insects and predators. On the other hand, T. spinipes is perhaps the major prey to spiders on T. argentea trees. Spiders are important in the biological control of phytophagous (r = − 0.73; P = 0.00) and leafminer insects (r = − 0.62; P = 0.01) on C. brasiliense trees19,21. Spiders are important in pest control in agroforestry systems, especially in tropical regions21,33–35 since a wide range of pest insects can get caught in their webs, resulting in deaths36. The importance of these arthropods for biological control was confirmed by population reduction of Epiphyas postvittana (Walker) (Lepidoptera: Tortricidae) on Malus domestica Bork (Rosaceae) and Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) on Citrus sinensis (L.) Osbeck (Rutaceae)37,38. In addition to spiders, the protocooperating ants were very abundant on T. argentea trees fertilized with DSS, probably due to the highest numbers of phytophagous insects—protocooperation39–41. The increased abundance of protocooperating ants reduced the numbers of phytophagous Coleoptera and T. spinipes on T. argentea trees, as observed in C. brasiliense, where the highest number of these ants reduced defoliation by beetles19,21. In addition, ants are bioindicators in the recovery of degraded area because they respond quickly to environmental complexity and by interacting mutually with other insects42–45. The abundance of the predatory wasp Polybia sp. was higher on fertilized plants probably due to a higher numbers of caterpillars (Lepidoptera) and the leafminer Lyriomyza sp.. Predatory wasps (Vespidae) are important natural enemies in agricultural systems such as Brassica campestris L. and kale B. oleracea L. var. acephala DC (Brassicales: Brassicaceae); Arabian coffee Coffea arabica L. (Gentianales: Rubiaceae) and tomato Solanum lycopersicon L. (Solanales: Solanaceae), preying mainly on caterpillars and leafminers (Lepidoptera)46–49.
In general, arthropod predators on T. argentea trees reduced herbivory by insects. However, in a few cases the presence of arthropod predators increased the numbers of phytophagous such as the leafminer Lyriomyza sp., likely by reducing competition with other more dominant groups (e.g. phytophagous Coleoptera). It shows how complex are interactions in food webs in natural and agroforestry systems18,19,21,31,32,36. Predators are often generalist in their feeding habits, and the greatest complexity of canopy architecture increases niches options for phytophagous insects and consequently for the natural enemy diversity50. For example, sewage sludge increases the richness of the ground beetle Carabidae (Coleoptera) in the area of Oxford, USA51.
The largest T. argentea tree canopy size (> ETIB) fertilized with DSS may explain the largest abundance of phytophagous insects (> defoliation), pollinators and predators, showing that this plant is adequate to recovery degraded areas. There was competition between groups of phytophagous insects and predator arthropods in high populations and consequent herbivory reduction.
Material and methods
Study
The study was conducted in a degraded area at the “Instituto de Ciências Agrárias (ICA)” of the “Universidade Federal de Minas Gerais (UFMG)”, Montes Claros, Minas Gerais, Brazil (S 16º51′38″ W 44º55′00″ 943 m) from March 2015 to February 2017 (24 months; arthropod collection period). The area presents soil loss and changes in soil chemistry and hydrology due to degradation52,53. Köppen’s climate classification54 defines this area as tropical dry climate; annual rainfall, 1,000–1,300 mm, with dry winter; annual mean temperature, ≥ 18 °C. The type of soil is litolic neosoil55 and chemical and physical details were described5.
Study design
Seeds were collected from five-years old Terminalia argentea trees at ICA/UFMG campus before sowing. Terminalia argentea seedlings were produced in March 2014 by sowing one seed per plastic polybag (8 × 12 cm), and these kept in a nursery covered with black shed net. The mixed substrate contained 30% organic materials (i.e. two parts of debris gardening pruning < 5 cm in length, and one of brown bovine manure), 30% clay soil, 30% sand, and 10% of mineral fertilizer (i.e. 160 g reactive natural phosphate per seedling)5. The soil pH in the pits (40 × 40 × 40 cm) was corrected with dolomitic limestone with anhydrous carbonate mineral composed of calcium magnesium carbonate (90% relative total neutralization power) (187 g per pit), increasing base saturation to 50%56. Natural phosphate (80 g per pit), fritted trace elements (FTE) (10 g/pit), and marble roch dust (1 kg per pit) were added when needed. Thirty-centimeters tall T. argentea seedlings were planted in pits in a two-meters spacing , in six parallel lines on flat terrain with two-meters spacing lines, with four trees per treatment (fertilized or not with dehydrated sewage sludge—DSS) per line. The seedlings in experimental area were supplied with water until the beginning of the rainy season. The seedlings with five-cm long branches were pruned with a sterilized razor, eliminating the additional shoots (i.e. others different from the leader shoot) and branches up to 1/3 of crown height. The experimental design was in random blocks with two levels of fertilization (i.e. a single dose of 20 L of DSS per pit or none fertilization) and 24 replications with one plant each5.
DSS (with 5% mean moisture content) was obtained from a sewage treatment plant (STP) in Juramento, Minas Gerais, Brazil. The STP is operated by the Minas Gerais Sanitation Company – “Companhia de Saneamento de Minas Gerais S.A. (COPASA)”. The STP is highly efficient, removing more than 90% of the organic material from the domestic waste water. The sewage sludge is dumped off into coarse sand tanks, staying there for three months to reduce the amount of thermotolerant coliforms (and other pathogenic microrganisms) and reach the ideal levels for agricultural use that is < 103 of the most likely number per g of total solids (as recommended by the National Council for the Environment—“Conselho Nacional do Meio Ambiente—CONAMA”). The chemical and biological characteristics of the DSS were described5,6.
Arthropods
Insects and spiders were visually counted, every two weeks, on the adaxial and abaxial surfaces of the leaves between 7:00 and 11:00 AM at the apical, middle and basal canopy in the northerly, southerly, easterly and westerly directions, in 12 leaves per plant (i.e. 27,648 leaves from 48 T. argentea trees) during 24 months. Only insects and spiders collected for identification were removed from trees during the assessment. At least three specimens per insect or spider species were collected using aspirator, stored in glass flasks with 70% ethanol or mounted, separated into morphospecies, and sent for identification. Insect defoliation was assessed visually as the leaf area loss on a 0–100% scales with 5% increments for removed leaf area57,58.
Ecological indices
To avoid pseudoreplication, mean numbers of data per tree were ever used. Ecological indices (abundance, diversity, and species richness) were calculated for each species per tree in the treatments (fertilization or not with DSS) using the software BioDiversity Professional, Version 259. The arthropod diversity was calculated using the Hill’s formula60,61 and the species richness with the Simpson indices62,63. The predator (i.e. insects and spiders) and prey ratio on T. argentea was calculated per tree. Predators were classified as spiders (most important group), predators (predators + spiders) and total predators (spiders + predators + protocooperating ants).
Statistical analyses
Data on defoliation percentage, abundance, diversity, and species richness of phytophagous insects, pollinators and predators were submitted to non-parametric statistical hypothesis, the Wilcoxon signed-rank test (P < 0.05)64, using the statistical program “Sistema para Análises Estatísticas e Genéticas” (SAEG), version 9.165. Simple regression analyzes and parameters (P < 0.05) were performed with SAEG to test the interactions between groups of phytophagous, pollinators and predators, and foliar mass (see41).
The Spearman correlation matrix, among the most significant characteristics, was calculated. The matrices were submitted to correlation networks66. Edge thickness was controlled by application of a cut value of 0.28 (from which the Spearman correlation becomes significant, meaning that only edges with |rij|≥ 0.28 are highlighted). These analyses were performed in R version 3.4.167. The correlation network procedure was performed using the package qgraph66.
Ethics
No specific permits are required to Terminalia argentea tree in Brazil. The laboratory and field studies did not involve endangered or protected species.
Acknowledgements
We would like to thank to Dr. Antônio Domingos Brescovit (Instituto Butantan, São Paulo, Brasil—Aracnidae) and Dr. Ayr de Moura Bello (Fundação Oswaldo Cruz, Rio de Janeiro, Brasil—Coleoptera) for arthropod species identifications. The voucher number for spiders is IBSP 36921–36924 (Instituto Butantan, São Paulo, Brasil) and that of insects is 1595/02 and 1597/02 (Centro de Estudos Faunísticos e Ambientais, Universidade Federal do Paraná, Curitiba, Paraná, Brasil). The study was financially supported by the following Brazilian agencies “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-Finance Code 001)”, “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)”, and “Programa Cooperativo sobre Proteção Florestal (PROTEF)” of the “Instituto de Pesquisas e Estudos Florestais (IPEF)”.
Author contributions
The study was conceived and designed by G.L.D.L. and M.A.S., data were collected by F.W.S.S. and J.C.N.C. with support from G.L.D.L., data analysis was performed by G.L.D.L. and A.M.A., figures, tables and manuscript preparation was done by G.L.D.L., F.W.S.S., G.L.T., A.M.A., M.A.S., J.C.Z. and J.C.L. All authors contributed to revisions and approve the final manuscript.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Frišták V, Pipíška M, Soja G. Pyrolysis treatment of sewage sludge: a promising way to produce phosphorus fertilizer. J. Clean. Prod. 2018;172:1772–1778. doi: 10.1016/j.jclepro.2017.12.015. [DOI] [Google Scholar]
- 2.Antonkiewicz J, Baran A, Pełka R, Wisła-Świder A, Nowak E, Konieczka P. A mixture of cellulose production waste with municipal sewage as new material for an ecological management of wastes. Ecotoxicol. Environ. Saf. 2019;169:607–614. doi: 10.1016/j.ecoenv.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 3.Kimberley MO, Wang H, Wilks PJ, Fisher CR, Magesan GN. Economic analysis of growth response from a pine plantation forest applied with biosolids. Forest Ecol. Manag. 2004;189:345–351. doi: 10.1016/j.foreco.2003.09.003. [DOI] [Google Scholar]
- 4.Caldeira MVW, Favalessa M, Gonçalves EO, Demaelarmelina WMF, Santos EV, Vieira M. Lodo de esgoto como componente de substrato para produção de mudas de Acacia mangium Wild. Comun. Sci. 2014;5:34–43. [Google Scholar]
- 5.Silva JL, Leite GLD, Tavares WS, Silva FWS, Sampaio RA, Azevedo AM, Serrão JE, Zanuncio JC. Diversity of arthropods on Acacia mangium (Fabaceae) and production of this plant with dehydrated sewage sludge in degraded area. R. Soc. Open Sci. 2020;7:2. doi: 10.1098/rsos.191196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira TAR, Sampaio RA, Fonseca IM, Ferreira CS, Santos SE, Ferreira LC, Gomes E, Fernandes LA. Metais pesados e patógenos em milho e feijão caupi consorciados, adubados com lodo de esgoto. Rev. Bras. Eng. Agríc. Ambient. 2007;11:331–338. doi: 10.1590/S1415-43662007000300014. [DOI] [Google Scholar]
- 7.Lorenzi H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. Nova: Instituto Plantarum; 2002. [Google Scholar]
- 8.Boff S, Graciolli G, Boaretto AG, Marques MR. Visiting insects of exudated gums by Terminalia argentea Mart. & Zucc (Combretaceae) Rev. Bras. Entomol. 2008;52:477–479. doi: 10.1590/S0085-56262008000300025. [DOI] [Google Scholar]
- 9.Santos MS, Louzada JNC, Dias N, Zanetti R, Delabie JHC, Nascimento IC. Riqueza de formigas (Hymenoptera, Formicidade) da serapilheira em fragmentos de floresta semidecídua da Mata Atlântica na região do Alto do Rio Grande, MG, Brasil. Iheringi. Sér. Zool. 2006;96:95–101. doi: 10.1590/S0073-47212006000100017. [DOI] [Google Scholar]
- 10.Davis AJ, Holloway JD, Huijbregts H, Krikken J, Kirk-Spriggs AH, Sutton SL. Dung beetles as indicators of change in the forests of northern Borneo. J. Appl. Ecol. 2001;38:593–616. doi: 10.1046/j.1365-2664.2001.00619.x. [DOI] [Google Scholar]
- 11.Bowers MD, Stamp NE. Effects of plant-age, genotype, and herbivory on Plantago performance and chemistry. Ecology. 1993;74:1778–1791. doi: 10.2307/1939936. [DOI] [Google Scholar]
- 12.Coley, P. D. & Barone, J. A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 27, 305–335, 10.1146/annurev.ecolsys.27.1.305 (1996.)
- 13.Leite GLD, Picanço M, Zanuncio JC, Moreira MD, Jham GN. Hosting capacity of horticultural plants for insect pests in Brazil. Chil. J. Agric. Res. 2011;71:383–389. doi: 10.4067/S0718-58392011000300006. [DOI] [Google Scholar]
- 14.Mass, K. D. B. Biossólido Como Substrato na Produção de Mudas de Timburi. Graduate theses and dissertations (2010).
- 15.MacArthur RH, Wilson EO. The Theory of Island Biogeography. Princeton: Princeton University Press; 1967. [Google Scholar]
- 16.Espírito Santo MM, Neves FS, Andrade Neto FR, Fernandes GW. Plant architecture and meristem dynamics as the mechanisms determining the diversity of gall-inducing insects. Oecologia. 2007;153:353–364. doi: 10.1007/s00442-007-0737-8. [DOI] [PubMed] [Google Scholar]
- 17.Leite GLD, Veloso RVS, Zanuncio JC, Azevedo AM, Silva JL, Wilcken CF, Soares MA. Architectural diversity and galling insects on Caryocar brasiliense trees. Sci. Rep. 2017;7:16677. doi: 10.1038/s41598-017-16954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris RJ, Lewis OT, Godfray HCJ. Experimental evidence for apparent competition in a tropical forest food web. Nature. 2004;428:310–313. doi: 10.1038/nature02394. [DOI] [PubMed] [Google Scholar]
- 19.Leite GLD, Veloso RVS, Zanuncio JC, Almeida CIM, Ferreira PSF, Fernandes GW, Soares MA. Habitat complexity and Caryocar brasiliense herbivores (Insecta; Arachnida; Araneae) Fla. Entomol. 2012;95:819–830. doi: 10.1653/024.095.0402. [DOI] [Google Scholar]
- 20.Auslander M, Nevo E, Inbar M. The effects of slope orientation on plant growth, developmental instability and susceptibility to herbivores. J. Arid Environ. 2003;55:405–416. doi: 10.1016/S0140-1963(02)00281-1. [DOI] [Google Scholar]
- 21.Leite GLD, Veloso RVS, Zanuncio JC, Almeida CIM, Ferreira PSF, Serrão JE, Ramalho FS. Seasonal damage caused by herbivorous insects on Caryocar brasiliense (Caryocaraceae) trees in the Brazilian savanna. Rev. Colombiana Entomol. 2012;38:108–113. [Google Scholar]
- 22.Kitahara M, Fujii K. An island biogeographical approach to the analysis of butterfy community patterns in newly designed parks. Res. Popul. Ecol. 1997;91:23–35. doi: 10.1007/BF02765247. [DOI] [Google Scholar]
- 23.Burns, K. C. Native–exotic richness relationships: a biogeographic approach using turnover in island plant populations. Ecology 97, 2932–2938, 10.1002/ecy.1579 (2016) [DOI] [PubMed]
- 24.Taiz, L., Zeiger, E., Moller, I. M. & Murphy, A. Fisiologia e desenvolvimento vegetal. (Artmed, 2017).
- 25.Tiedge K, Lohaus G. Nectar sugars and amino acids in day- and night-flowering Nicotiana species are more strongly shaped by pollinators' preferences than organic acids and inorganic ions. PLoS ONE. 2017;12:1–25. doi: 10.1371/journal.pone.0176865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stabler D, Power EF, Borland AM, Barnes JD, Wright GA. A method for analysing small samples of floral pollen for free and protein-bound amino acids. Methods Ecol. Evol. 2018;9:430–438. doi: 10.1111/2041-210X.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura AE, Alves MC, Sanches S, Akihiro LG, Antonio PG. Recuperação de um solo degradado com a aplicação de adubos verdes e lodo de esgoto. Rev. Bras. Cienc. Solo. 2008;32:405–416. doi: 10.1590/S0100-06832008000100038. [DOI] [Google Scholar]
- 28.Serra BDV, Campos LA. Polinização entomófila de abobrinha, Cucurbita moschata (Cucurbitaceae) Neotrop. Entomol. 2010;39:153–159. doi: 10.1590/S1519-566X2010000200002. [DOI] [PubMed] [Google Scholar]
- 29.Silva FWS, Leite GLD, Guanabens REM, Sampaio RA, Gusmão CAG, Zanuncio JC. Spatial distribution of arthropods on Acacia mangium (Fabales: Fabaceae) trees as windbreaks in the Cerrado. Fla. Entomol. 2014;97:631–638. doi: 10.1653/024.097.0240. [DOI] [Google Scholar]
- 30.Damascena JG, Leite GLD, Silva FWS, Soares MA, Guanabens REM, Sampaio RA, Zanuncio JC. Spatial distribution of phytophagous insects, natural enemies, and pollinators on Leucaena leucocephala (Fabaceae) trees in the Cerrado. Fla. Entomol. 2017;100:558–565. doi: 10.1653/024.100.0311. [DOI] [Google Scholar]
- 31.Gratton C, Denno RF. Seasonal shift from bottom-up to top-down impact in phytophagous insect populations. Oecology. 2003;134:487–495. doi: 10.1007/s00442-002-1137-8. [DOI] [PubMed] [Google Scholar]
- 32.Marquis RJ, Diniz IR, Morais HC. Patterns and correlates of the interspecific variation in foliar insect herbivory and pathogen attack in Brazilian Cerrado. J. Trop. Ecol. 2001;17:127–148. doi: 10.1017/S0266467401001080. [DOI] [Google Scholar]
- 33.Landis D, Wratten SD, Gurr GM. Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann. Rev. Entomol. 2000;45:175–201. doi: 10.1146/annurev.ento.45.1.175. [DOI] [PubMed] [Google Scholar]
- 34.Langellotto, G. A. Aggregation of Invertebrate Predators in Complex-Structured Habitats: Role of Altered Cannibalism, Intraguild Predation, Prey Availability, and Microclimate. Graduate theses and dissertations (2003).
- 35.Halaj J, Halpern CB, Yi H. Responses of litter-dwelling spiders and carabid beetles to varying levels and patterns of green-tree retention. Forest Ecol. Manag. 2008;255:887–900. doi: 10.1016/j.foreco.2007.09.083. [DOI] [Google Scholar]
- 36.Sunderland K, Samu F. Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: a review. Entomol. Exp. Appl. 2000;95:1–13. doi: 10.1046/j.1570-7458.2000.00635.x. [DOI] [Google Scholar]
- 37.Amalin DM, Reiskind J, Peña JE, Mcsorley R. Predatory behavior of three species of sac spiders attacking citrus leafminer. J. Arachnol. 2001;29:72–81. doi: 10.1636/0161-8202(2001)029[0072:PBOTSO]2.0.CO;2. [DOI] [Google Scholar]
- 38.Hogg BN, Mills NJ, Daane KM. Temporal patterns in the abundance and species composition of spiders on host plants of the invasive moth Epiphyas postvittana (Lepidoptera: Tortricidae) Environ. Entomol. 2017;146:502–510. doi: 10.1093/ee/nvx065. [DOI] [PubMed] [Google Scholar]
- 39.Del-Claro K, Oliveira PS. Conditional outcomes in a neotropical treehopper-ant association: temporal and species-specific variation in ant protection and homopteran fecundity. Oecologia. 2000;124:156–165. doi: 10.1007/s004420050002. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz OJ. Indirect Effects in Communities and Ecosystems: The Role of Trophic and Nontrophic Interactions. Princeton: Princeton University Press; 2006. [Google Scholar]
- 41.Costa, S. S. D. Insects and growth of Terminalia argentea Mart & Zucc (Combretaceae) fertilized with dehydrated sewage sludge. Graduate theses and dissertations (2019).
- 42.Economo EP, Klimov P, Sanart EM, Guénard B, Weiser MD, Lecroq B, Nowles LL. Global phylogenetic structure of the hyperdiverse ant genus Pheidole reveals the repeated evolution of macroecological patterns. Proc. R. Soc. B. 2014;282:1–10. doi: 10.1098/rspb.2014.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez-Lachaud G, Lachaud JP. Arboreal ant colonies as ‘hot-points’ of cryptic diversity for myrmecophiles: the weaver ant Camponotus sp. aff. textor and its interaction network with its associates. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomicki G, Ward PS, Renner SS. Macroevolutionary assembly of ant/plant symbioses: Pseudomyrmex ants and their ant-housing plants in the Neotropics. Proc. R. Soc. B. 2015;282:1–9. doi: 10.1098/rspb.2015.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanches A. Fidelity and promiscuity in an ant-plant mutualism: a case study of triplaris and Pseudomyrmex. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0143535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranda MMM, Picanço M, Leite GLD, Zanuncio JC, Clercq P. Sampling and non-action levels for predators and parasitoids of virus vectors and leaf miners of tomato in Brazil. Med. Facul. Landb. Univ. Gent. 1998;63:519–523. [Google Scholar]
- 47.Leite GLD, Oliveira IR, Guedes RNC, Picanço M. Comportamento de predação de Protonectarina sylveirae (Saussure) (Hymenoptera: Vespidae) em mostarda. Agro-Ciencia (Chillán) 2001;17:93–96. [Google Scholar]
- 48.Picanço M, Ribeiro LJ, Leite GLD, Gusmão MR. Seletividade de inseticidas a Polybia ignobilis (Haliday) (Hymenoptera: Vespidae) predador de Ascia monuste orseis (Godart) (Lepidoptera: Pieridae) An. Soc. Entomol. Bras. 1998;27:85–90. doi: 10.1590/S0301-80591998000100011. [DOI] [Google Scholar]
- 49.Picanço MC, Oliveira IR, Fernandes FL, Martinez HEP, Bacci L, Silva EM. Ecology of Vespidae (Hymenoptera) predators in Coffea arabica plantations. Sociobiology. 2012;59:1269–1280. [Google Scholar]
- 50.Condon MA, Scheffer SJ, Lewis ML, Whaeton R, Adams DC, Forbes AA. Lethal interactions between parasites and prey increase niche diversity in a tropical community. Science. 2014;343:1240–1244. doi: 10.1126/science.1245007. [DOI] [PubMed] [Google Scholar]
- 51.Larsen KJ, Purrington FF, Brewer SR, Taylor DH. Influence of sewage sludge and fertilizer on the ground geetle (Coleoptera: Carabidae) fauna of an old-field community. Environ. Entomol. 1996;25:452–459. doi: 10.1093/ee/25.2.452. [DOI] [Google Scholar]
- 52.Milton SJ, Dean WRJ, du Plessis MA, Siegfried WR. A conceptual model of arid rangeland degradation. Bioscience. 1994;44:70–76. doi: 10.2307/1312204. [DOI] [Google Scholar]
- 53.Whisenant SG. Repairing damaged wildlands: a process-oriented, landscape-scale approach. Restor. Ecol. 1999;9:249–249. doi: 10.1046/j.1526-100x.2001.009002249.x. [DOI] [Google Scholar]
- 54.Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. Köppen's climate classification map for Brazil. Meteorol. Z. 2013;22:711–728. doi: 10.1127/0941-2948/2013/0507. [DOI] [Google Scholar]
- 55.Santana PHL, Frazão LA, Santos LDF, Fernandes LA, Sampaio RA. Soil attributes and production of Eucalyptus in monoculture and silvopastoral systems in the north of Minas Gerais, Brazil. J. Agric. Sci. Technol. 2016;6:361–370. doi: 10.17265/2161-6264/2016.06.001. [DOI] [Google Scholar]
- 56.Kopittke PM, Menzies NW. A review of the use of the basic cation saturation ratio and the “ideal” soil. Soil Sci. Soc. Am. J. 2007;71:259–265. doi: 10.2136/sssaj2006.0186. [DOI] [Google Scholar]
- 57.Sastawa BM, Lawan M, Maina YT. Management of insect pests of soybean: effects of sowing date and intercropping on damage and grain yield in the Nigerian Sudan savanna. Crop Prot. 2004;23:155–161. doi: 10.1016/j.cropro.2003.07.007. [DOI] [Google Scholar]
- 58.Mizumachi E, Mori A, Osawa N, Akiyama R, Tokuchi N. Shoot development and extension of Quercus serrata saplings in response to insect damage and nutrient conditions. Ann. Bot. 2006;98:219–226. doi: 10.1093/aob/mcl091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krebs, C. J. Bray-Curtis cluster analysis. 1. Biodiversity Pro Versão 2. https://biodiversity-pro.software.informer.com (1989).
- 60.Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54:427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- 61.Jost L. Entropy and diversity. Oikos. 2006;113:363–375. doi: 10.1111/j.2006.0030-1299.14714.x. [DOI] [Google Scholar]
- 62.Begon, M., Townsend, C. R., Harper, J. L. Ecologia: de indivíduos a ecossistemas. (Artmed, 2007).
- 63.Lazo JA, Valdés NV, Sampaio RA, Leite GLD. Diversidad zoológica asociada a um silvopastoreo leucaena-guineacon diferentes edades de establecimiento. Pesq. Agropec. Bras. 2007;42:1667–1674. doi: 10.1590/S0100-204X2007001200001. [DOI] [Google Scholar]
- 64.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bull. 1945;1:80–83. doi: 10.2307/3001968. [DOI] [Google Scholar]
- 65.SAEG—Sistema para Análises Estatísticas. https://arquivo.ufv.br/saeg/. (accessed on 30 june 2018) (2007).
- 66.Epskamp S, Cramer AOJ, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: Network visualizations of relationships in psychometric data. J. Stat. Softw. 2012;48:1–18. doi: 10.18637/jss.v048.i04. [DOI] [Google Scholar]
- 67.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/ (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.