Abstract
Treatment for relapse of chronic myeloid leukemia (CML) following hematopoietic cell transplantation (HCT) includes tyrosine kinase inhibitors (TKI) with or without donor lymphocyte infusions (DLI), however the most effective treatment strategy is unknown. This study was performed through the Center for International Blood and Marrow Transplant Research (CIBMTR) database. We retrospectively reviewed all patients reported to the CIBMTR registry from 2002–2014 who underwent HCT for CML and were alive 30 days post relapse. A total of 215 HCT recipients relapsed and were analyzed in the following groups 1) TKI alone n=128, 2) TKI with DLI n=48, and 3) DLI without TKI n=39. In multivariate analysis, disease status prior to HCT had a significant effect on overall survival (OS). Patients that received a DLI alone compared to a TKI with DLI had inferior survival HR 2.28 (95% CI 1.23–4.24; p = 0.009). Those who received TKI alone had similar survival compared to those who received TKI with DLI (p=0.81). These data supports that despite use of TKI pre-transplantation, TKI salvage therapy continues to provide significant survival following relapse in patients with CML following HCT. These data does not suggest that adding a DLI to TKI adds an improvement in OS.
Introduction
The optimal approach to patients with chronic myeloid leukemia (CML) that relapse following allogeneic hematopoietic cell transplantation (HCT) in the tyrosine kinase inhibitors (TKIs) era is unknown. Imatinib was introduced in 2001 being followed by the introduction of second generation TKIs such as dasatinib or nilotinib, in subsequent years.1–2 In later years, the spectrum of TKIs has further broadened, given the introduction of bosutinib and ponatinib.3–4 Nevertheless, allogeneic HCT remains an important rescue strategy for some CML patients at adverse risk. Currently HCT is indicated in patients with advanced phase CML at disease presentation or disease progression to blast phase.5 Also HCT is indicated in patients with a lack of response from multiple TKIs or in patients who cannot tolerate TKIs.5–6 Allogeneic HCT also may be discussed in the case of ABL1 mutations conferring a high resistance level such as T315i mutations.7 In contrast, prior to the TKI era, patients were evaluated for HCT early during their disease course, especially in younger patients with low comorbidity.
Strategies to treat relapse following HCT have historically relied on the use of donor lymphocyte infusions (DLIs). The graft-versus-leukemia effect is well-described in CML and DLIs have been shown to induce durable remissions in CML.8–9 Since the approval of imatinib, the tyrosine kinase inhibitors have been used as maintenance therapy following HCT and at disease relapse.5,9–16 Several small studies have demonstrated benefit in combining DLIs with TKIs.17–19 In this analysis, we examine outcomes following relapse after HCT in patients that received TKI in combination with DLI (Combination arm), TKI without DLI (Primary TKI) and DLI without TKI (Primary DLI).
Materials and Methods
Data Source
This is a registry-based retrospective study using the Center for International Blood & Marrow Transplant Research (CIBMTR) database. This includes a collaboration of more than 420 transplant centers in the United States and worldwide. Registration and research data are collected pre-transplantation, at 100 days and 6 months post-transplantation, and annually thereafter until death or last follow-up.
Patients
This was a multi-institutional, retrospective study using data from patients with CML who had received TKI, and subsequently underwent HCT from an allogeneic donor, and relapsed following transplantation. The median time to DLI post HCT relapse was 4 weeks, therefore, one-month post HCT relapse was used as a landmark to avoid selection bias against DLI due to early death post HCT relapse. Data were retrieved from CIBMTR from 134 centers during the years of 2002–2014. Treatment at relapse was determined and grouped into three arms 1) TKI in combination with DLI (Combination arm), 2) TKI without DLI (Primary TKI) and 3) DLI without TKI (Primary DLI). Further patient related variables included age, gender, and Karnofsky Performance Status (KPS). Disease related variables included disease status prior to HCT, time from diagnosis to HCT, time from HCT to relapse, time from diagnosis to relapse. Transplant related variables assessed included donor type, HLA match, year of transplant, graft source, cytomegalovirus (CMV) status, conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and presence or absence of planned TKI maintenance. Patients were also assessed for presence of GVHD (acute II-IV/chronic) prior to the landmark analysis. Patients were excluded if they did not receive a TKI prior to HCT. Umbilical cord blood were excluded due to patients not able to undergo DLI.
Definitions
HLA ‘well matched’ is defined as no identified HLA mismatch and informative data at 4 loci or allele matching at HLA-A, -B and –DRB1; ‘partially matched’ is defined as single-locus mismatch and/or missing HLA data.20 Myeloablative conditioning (MAC) is defined as irreversible cytopenia and stem cell support is mandatory.21 Nonmyeloablative (NMA) is defined as causing minimal cytopenia and can be given also without stem cell support. 21 Reduced-intensity conditioning (RIC) is defined as not fitting criteria for either MAC or NMA. 21 Criteria used to identify acute GVHD and chronic GVHD are as defined by Glucksberg et al. and Shulman et al.22–23 Variables were reported and collected from the CIBMTR forms and defined in the CIBMTR manual (e.g. relapse).
Statistical Analysis
Descriptive statistics tables of patients including demographics, disease-related factors, transplant-related factors and cause of death were prepared. Medians and ranges were listed for continuous variables. The total numbers of patients and the percentages of each subgroup were calculated for categorical variables. Baseline characteristics of patients were compared between three relapse treatment groups, “TKI + DLI” vs. “TKI without DLI” vs. “DLI without TKI”, using chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables.
The Cox proportional hazards model was used for multi-variate analysis to compare the differences in overall survival of the three treatment groups. The clock started at 30 days after relapse. A stepwise variable selection procedure was used for choosing adjusting variables. The final model was selected with adjustments for disease status prior to HCT, post-HCT maintenance TKI therapy, and year of HCT. Other variables considered but not selected in the final model included age, gender, country, KPS, anti-thymocyte globulin (ATG)/alemtuzumab, time from HCT to relapse, TBI usage, conditioning intensity, recipient CMV status, donor type, graft source, GVHD prophylaxis, and GVHD prior to the landmark analysis. Proportionality of all covariates were checked by using the time dependent covariates. Possible interactions between the main variable and other adjusted covariates were also examined. The effect of the main variable was tested at 0.05 significance level. Results were expressed as hazard ratios with 95% confidence intervals and p-values. Direct adjusted survival probabilities based on stratified Cox proportional hazards model were estimated at 1 year, 2 years and 3 years, with their point- wise 95% confidence intervals and p-values. Survival plots were generated from the final Cox model stratified on treatment of conditioning regime and weighted averages of covariate values using the pooled sample proportion as the weight functions. These adjusted probabilities estimate likelihood of outcomes in populations with similar prognostic factors. The analyses were conducted using SAS 9.4 TS1M4.
Study Endpoints
The primary endpoint was effect of salvage treatment starting at 30 days post HCT relapse on overall survival (OS) in the three treatment groups. Secondary endpoints examined included evaluation of cause of death, impact of disease status on overall survival, impact of maintenance on overall survival, and impact of GVHD on treatment effect.
Results
Patient-, Disease- and Transplantation-Related Variables
We identified 215 patients transplanted for CML between the years 2002 and 2014 who had received TKI prior to HCT and who had relapsed following their first allogeneic HCT. Table 1 displays patient-related, disease-related, and HCT-related baseline characteristics for the three groups, Combination arm, n = 48, Primary TKI, n = 128, and Primary DLI, n = 39. The completeness of follow-up, since 30 days post-HCT relapse in our defined population was 94% at 3 years. The median follow-up of survivors, 30 days post-HCT relapse was 58 months (6–132 months) for the Combination arm, 64 months (1–160months) for the Primary TKI arm and 46 month (3–91 months) for the Primary DLI. Other patient related factors such as age, gender, KPS status were well balanced between the groups. The HCT-CI was available for the years 2007–2014 (not available on CIBMTR form prior to 2007). Chronic phase disease status was more prevalent in those that received Primary DLI therapy compared to other groups, p =0.03.
Table 1.
Baseline Characteristics for Patients ≥ 18 years who Relapsed after HCT for CML, 2002–2014
| Variable | TKI + DLI | TKI but no DLI | DLI but no TKI | P-value |
|---|---|---|---|---|
| Number of patients | 48 | 128 | 39 | |
| Number of centers | 40 | 63 | 31 | |
| Patient related | ||||
| Age, median (range), yrs | 41 (22–66) | 41 (18–73) | 47 (19–61) | 0.34 |
| Gender | 0.76 | |||
| Female | 18 (38) | 51 (40) | 13 (33) | |
| Karnofsky score at time of HCT | 0.83 | |||
| 90–100 | 33 (69) | 87 (68) | 27 (69) | |
| HCT-CI | 0.002 | |||
| 0 | 18 (38) | 31 (24) | 1 (3) | |
| 1–2 | 6 (12) | 19 (15) | 1 (3) | |
| 3+ | 7 (15) | 17 (13) | 5 (13) | |
| Missing/NA | 17 (35) | 61 (48) | 32 (82) | |
| Pre-transplant disease related | ||||
| Disease status prior to HCT | 0.03 | |||
| CP1 | 14 (29) | 39 (30) | 16 (41) | |
| AP | 7 (15) | 17 (13) | 11 (28) | |
| CP2+ | 15 (31) | 33 (26) | 4 (10) | |
| BP | 7 (15) | 12 (9) | 6 (15) | |
| Hematologic CR | 5 (10) | 27 (21) | 2 (5) | |
| Time from diagnosis to HCT (CP1), median (range), ms | 14 (3–46) | 14 (5–181) | 24 (5–52) | 0.66 |
| Time from diagnosis to relapse, median (range), ms | 31 (6–175) | 38 (4–215) | 38 (6–345) | 0.70 |
| Time from HCT to relapse, median (range), ms | 6 (<1–99) | 8 (<1–107) | 4 (<1–70) | 0.04 |
| Transplant related | ||||
| Year of transplant | < 0.01 | |||
| 2002–2005 | 9 (19) | 31 (25) | 25 (64) | |
| 2006–2009 | 28 (59) | 63 (49) | 11 (28) | |
| 2010–2014 | 11 (22) | 34 (26) | 3(8) | |
| Graft source | 0.01 | |||
| Bone marrow | 7 (15) | 48 (38) | 13 (33) | |
| Peripheral blood | 41 (85) | 80 (63) | 26 (67) | |
| Recipient CMV status | 0.85 | |||
| Negative | 18 (38) | 52 (41) | 18 (46) | |
| Positive | 30 (63) | 75 (59) | 21 (54) | |
| Missing | 0 | 1 (<1) | 0 | |
| Donor type | 0.22 | |||
| HLA-identical sibling | 25 (52) | 48 (38) | 16 (41) | |
| Other related | 2 (4) | 8 (6) | 0 | |
| Well-matched unrelated | 19 (40) | 53 (41) | 19 (49) | |
| Partially-matched unrelated | 2 (4) | 19 (15) | 4 (10) | |
| Conditioning intensity | 0.85 | |||
| MAC - Chemo | 18 (38) | 56 (44) | 17 (44) | |
| MAC - TBI | 17 (35) | 37 (29) | 9 (23) | |
| RIC/NMA | 13 (27) | 34 (27) | 13 (33) | |
| Missing | 0 | 1 (<1) | 0 | |
| ATG/Alemtuzumab | 0.006 | |||
| ATG alone | 12 (25) | 28 (22) | 16 (41) | |
| Alemtuzumab alone | 4 (8) | 2 (2) | 4 (10) | |
| No ATG or Alemtuzumab | 32 (67) | 98 (77) | 19 (49) | |
| GVHD prophylaxis | 0.56 | |||
| TAC based | 27 (56) | 77 (60) | 17 (44) | |
| CSA based | 16 (33) | 39 (30) | 18 (46) | |
| Post-HCT CY | 2 (4) | 7 (5) | 2 (5) | |
| Other | 3 (6) | 3 (2) | 2 (5) | |
| Missing | 0 | 2 (2) | 0 | |
| Post-transplant disease related | ||||
| Post-HCT maintenance TKI therapy | 0.03 | |||
| No | 35 (73) | 87 (68) | 35 (90) | |
| Yes | 13 (27) | 41 (32) | 4 (10) | |
| Choice of treatment TKI therapy in response to relapse | ||||
| No TKI given | 0 | 0 | 39 | |
| IM + DA + NI | 5 (10) | 1 (<1) | 0 | |
| IM + DA | 6 (13) | 11 (9) | 0 | |
| IM + NI | 3 (6) | 3 (2) | 0 | |
| DA + NI | 7 (15) | 14 (11) | 0 | |
| IM | 9 (19) | 24 (19) | 0 | |
| DA | 10 (21) | 47 (37) | 0 | |
| NI | 6 (13) | 20 (16) | 0 | |
| Other | 2 (4) | 8 (6) | 0 | |
| Relapse assessment method | < 0.01 | |||
| Hematologic/clinical | 38 (79) | 81 (63) | 15 (38) | |
| FISH | 3 (6) | 2 (2) | 1 (3) | |
| Conventional cytogenetics | 2 (4) | 9 (7) | 9 (23) | |
| Molecular | 4 (8) | 22 (17) | 2 (5) | |
| Assessment method unknown | 1 (2) | 14 (11) | 12 (31) | |
| Distribution of GVHD prior to the Landmark Analysis | 0.09 | |||
| Patients that developed acute and chronic GVHD | 3 (6) | 19 (15) | 1 (3) | |
| Patients that developed only acute GVHD | 12 (25) | 30 (23) | 7 (18) | |
| Patients that developed only chronic GVHD | 6 (13) | 22 (17) | 4 (10) | |
| Patients that did not develop GVHD | 27 (56) | 57 (45) | 27 (69) | |
| Median follow-up of survivors (range) since 30-d post relapse, months | 58 (6–132) | 64 (1–160) | 46 (3–91) |
The median time from HCT to relapse was slightly longer in the Primary TKI arm at 8 months (<1–107 months) compared to 6 months (<1–99 months) in the Combination arm, and 4 months (<1–70 months) in the Primary DLI arm, p = 0.04. The year of transplant differed, p < 0.01, as more patients that received primary DLI were treated prior to 2007. The majority of patients received peripheral blood as the graft source, this was greatest in the Combination arm at 85%, p = 0.01 (Table 1). A higher proportion in the Primary DLI arm received in vivo T cell depletion, compared to the other two groups, p = 0.006. It is noted that fewer patients that received maintenance therapy received a DLI as part of the salvage treatment, p=0.03, prompting planned inclusion of this variable in multivariate analysis below. Most common reported cause of death in all arms was relapse (Appendix 1).
In multivariate analysis (Table 3), as expected, the biggest impact on OS was disease status prior to HCT. As compared to those in chronic phase 1 (CP1), recipients that went to transplant in blast phase (BP) had a hazard ratio (HR) of 5.51 (95% confidence intervals [CI] 2.93–10.37; p<0.001) and patients in CP2 or higher had a HR 2.64 (95% CI 1.46–4.79; p = 0.001). Patients that received Primary DLI compared to Primary TKI had inferior survival HR 2.15 (95% CI 1.27–3.65; p = 0.004). Those who received Primary TKI had similar OS compared to those who received Combination therapy (p=0.81). In a sensitivity multivariate analysis, after controlling for how relapse was detected [clinical/hematologic vs. subclinical [molecular or cytogenetic]), TKI with or without DLI remained significantly associated with superior OS compared to DLI primary (p=0.003). Recipients who did not receive post-HCT maintenance had inferior OS with HR 2.00 (95% CI 1.21–3.31), p = 0.007. The presence of acute GVHD prior to the landmark analysis was not significantly associated with post-relapse OS (p= 0.77).
Table 3.
A Cox regression model was fitted for all eligible patients to evaluate the impact of main effect on overall survival since 30-d post relapse, while controlling for other risk factors.
| Cox Model Estimates (N=215) | ||||
|---|---|---|---|---|
| Parameter | N (N Event) | HR (%95 CI) | P-value | Overall P-value |
| Disease status prior to HCT | ||||
| CP1 (reference) | 69 (22) | 1.00 | <.001 | |
| AP | 35 (16) | 1.60 (0.83, 3.09) | 0.157 | |
| BP | 25 (21) | 5.51 (2.93, 10.37) | <.001 | |
| CP2+ | 52 (31) | 2.64 (1.46, 4.79) | 0.001 | |
| Hematologic CR | 34 (18) | 1.90 (0.94, 3.86) | 0.075 | |
| Main effect | ||||
| TKI + DLI (reference) | 48 (24) | 1.00 | 0.011 | |
| DLI but no TKI | 39 (24) | 2.28 (1.23, 4.24) | 0.009 | |
| TKI but no DLI | 128 (60) | 1.06 (0.65, 1.72) | 0.813 | |
| Post-HCT maintenance TKI Therapy | ||||
| Yes (reference) | 58 (20) | 1.00 | 0.007 | |
| No | 157 (88) | 2.00 (1.21, 3.31) | 0.007 | |
| Year of Transplant | ||||
| 2002–2007 (reference) | 110 (49) | 1.00 | 0.026 | |
| 2008–2014 | 105 (59) | 1.68 (1.06, 2.64) | 0.026 |
In addition, the other variables we considered but were not selected are age group, gender, country, KPS, ATG/Alemtuzumab, time from transplant to relapse, TBI given, conditioning intensity, recipient CMV status, donor type, graft source, GVHD Prophylaxis and GVHD prior to the landmark analysis.
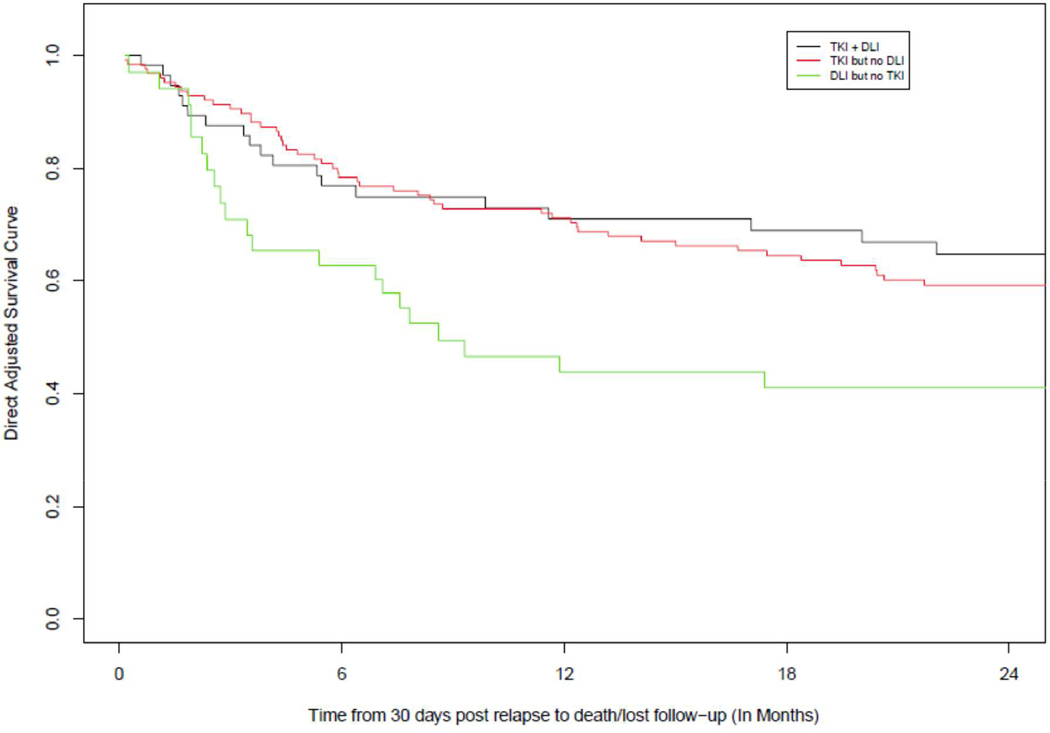
Adjusted survival shows OS advantage of TKI use at 2 years, with OS of 65%, (95% CI: 0.53–0.77) in the Combination arm, 59%, (95% CI: 0.51–0.67) in the Primary TKI arm, and 41%, (95% CI: 0.27–0.55) in the Primary DLI arm (p=0.013) (Fig 1, Table 2).
Figure 1.
Adjusted OS curves
Table 2.
Adjusted Survival Estimates for OS Since 30-d Post Relapse
| Time | TKI + DLI | 95% CI | TKI no DLI | 95% CI | DLI no TKI | 95% CI | P-value TKI + DLI vs. TKI only | P-value TKI+DL I vs. DLI only | P-value TKI only vs. DLI only |
|---|---|---|---|---|---|---|---|---|---|
| 1 year | 0.71 | (0.60–0.82) | 0.71 | (0.64–0.79) | 0.44 | (0.29–0.59) | 0.977 | 0.004 | 0.001 |
| 2 years | 0.65 | (0.53–0.77) | 0.59 | (0.51–0.67) | 0.41 | (0.27–0.55) | 0.441 | 0.013 | 0.031 |
| 3 years | 0.63 | (0.51–0.75) | 0.54 | (0.46–0.62) | 0.41 | (0.27–0.55) | 0.254 | 0.023 | 0.117 |
Discussion
CIBMTR analyses show that patients with CML who relapse after HCT have the highest survival if treated with a TKI with or without immunotherapy with DLI. This study confirms that the major determining impact on survival after post-HCT relapse in CML is institution of TKI therapy. It further identifies DLI without TKI has inferior survival to any TKI containing therapy. This study did not aim to identify superiority of any particular TKI, however the most commonly used TKI at relapse was dasatinib.
The decision of which treatment modality to use at disease relapse can be difficult for clinicians as there are no large, prospective studies directing therapy. Each therapy comes with risks and benefits that must be weighed and the patient scenario at relapse may dictate which treatment strategy is used. Complications of DLI include GVHD, which can lead to morbidity and mortality, including increased use of immunosuppression followed by infection. A publication identified pre-DLI factors associated with prolonged survival in remission without secondary GVHD. The probability of survival in remission without secondary GVHD was highest (>50% at 5 years) when DLI were given beyond 1-year form HCT for molecular and/or cytogenetics CML relapse.24 In the current study pre-relapse GVHD did not affect survival, however clinicians would be hesitant to use DLI in patients that had severe acute GVHD or ongoing chronic GVHD. DLI appears to be most effective in patients with CP, in our study there were relatively more patients with CP1 in the DLI arm.25–26
Imatinib has been studied in patients relapsing following HCT.10 In one study of 128 patients, 50 patients had failed treatment with a DLI prior to starting imatinib therapy. Imatinib showed activity with complete cytogenetic response in 58% of patients in chronic phase (CP), 48% patients in accelerated phase (AP) and 22% of patients in blast crisis (BC). The 2-year survival in CP was 100%, 86% for AP and 12% for BC. Other studies have shown similar results.11–16 Adverse effects of imatinib include neutropenia, thrombocytopenia, and gastrointestinal (GI) intolerance. Imatinib is most effective when patients are in CP.10–13
Imatinib combined with DLI has also been studied in 37 patients relapsing following HCT, where 13 patients received DLI only, 9 patients received imatinib only and 11 patients received DLI and imatinib combined; 4 patients received a DLI and imatinib, but not concurrently.17 Ten of the 11 patients that received the combination achieved a molecular remission in 3 months, however only 2/22 patients that received either DLI only and imatinib only achieved a molecular remission at 3 months. The study concluded that imatinib and DLI are synergistic in the treatment of relapsed CML. A more recent study included 71 patients at a single institution with CML that had undergone HCT comparing DLI, TKI or DLI + TKI for the treatment of relapsed CML post HCT. Out of the 71 patients, 45 patients relapsed following HCT and 40 patients went on to receive one of the 3 treatments following relapse. There was no statistically significant finding; however, the TKI-only group had the highest cumulative incidence of complete molecular remission and lowest cumulative incidence of death compared to DLI and TKI+DLI.18 Shanavas et al analyzed 46 patients retrospectively who either received a DLI or TKI following relapse of HCT. This study found that TKI had improved OS with a HR of 37.4; 95% CI, 2.2–625.4, p = 0.01.19
We observed that patients had the highest OS when receiving a TKI with or without a DLI. There appears to be no added benefit to including DLI as part of the salvage therapy. Our study showed that disease status prior to HCT continues to confer poor prognosis. Interestingly, the presence of GVHD prior to the landmark analysis shows no impact on OS in this population of patients that had relapsed following HCT. Although the study is not designed to assess maintenance, as a covariate, maintenance TKI post-HCT was associated with superior post-relapse OS and this was independent of time from HCT to relapse. Post relapse overall OS was highest in HCT recipients who received a salvage TKI containing regimen compared to DLI primary salvage therapy. This data supports that despite use of TKI pre-transplantation and in maintenance therapy, TKI salvage therapy continues to provide significant OS following relapse in patients with CML following HCT. This data contradicts previously held thoughts that dosing of maintenance therapy might confer resistance at relapse and does not suggest that adding a DLI to TKI adds an improvement in OS, although the sample size is small.27
There are limitations to this study. While this is the largest study of this population to date, the decision made by the clinician to use one strategy vs. another was not captured, and even though multivariate regression analysis was performed to adjust for any captured baseline characteristics imbalances, it can only adjust for variables that are measured and captured. Furthermore, the registry did not collect dose quantification of DLI allowing better identification of immunotherapy thresholds/GVHD risk thresholds. There has been evidence linking increasing efficacy with increasing CD3+ cell dose, especially in patients with a molecular or cytogenetic relapse compared to a hematologic relapse, which could help better define dose requirements.28 Our study included relapsed patients post HCT, given this design and selection procedure, some questions are not possible to address, such as why time to relapse was shorter in the Primary DLI arm; while we have adjusted for this factor, we are not able to answer the question since we did not include all patients (relapsed or not relapsed). Unfortunately, we did not have exact disease status (chronic, accelerated or blast phase) at the time of relapse to further define relapse severity. The registry does not collect data on responses to therapies given post HCT relapse, or the details of such therapies. Although these limitations are present, this study nevertheless represents a comprehensive picture of the current therapeutic landscape for CML therapy at relapse with current immunotherapy and TKI treatment options. While registration data is not all encompassing, it is exceedingly unlikely a clinical trial will be conducted to address this question for patients with CML.
In conclusion, our data suggest that TKI is the most effective treatment strategy at disease relapse following HCT for CML. Combination therapy of TKI plus DLI did not confer additional benefit, and DLI alone showed inferior OS when compared to TKI with or without a DLI.
Supplementary Material
Highlights.
In the setting of CML relapse after HCT, TKI is an effective treatment strategy.
DLI added to TKI does not appear to add benefit.
DLI alone when compared to TKI alone has inferior survival in CML patients relapsing after HCT.
Acknowledgements
The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement U24CA076518 with the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement U24HL138660 with NHLBI and NCI; grant U24CA233032 from the NCI; grants OT3HL147741, R21HL140314 and U01HL128568 from the NHLBI; contract HHSH250201700006C with Health Resources and Services Administration (HRSA); grants N00014–18-1–2888 and N00014–17-1–2850 from the Office of Naval Research; subaward from prime contract award SC1MC31881–01-00 with HRSA; subawards from prime grant awards R01HL131731 and R01HL126589 from NHLBI; subawards from prime grant awards 5P01CA111412, 5R01HL129472, R01CA152108, 1R01HL131731, 1U01AI126612 and 1R01CA231141 from the NIH; and commercial funds from Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Allovir, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Anthem, Inc.; Astellas Pharma US; Atara Biotherapeutics, Inc.; BARDA; Be the Match Foundation; bluebird bio, Inc.; Boston Children’s Hospital; Bristol Myers Squibb Co.; Celgene Corp.; Children’s Hospital of Los Angeles; Chimerix, Inc.; City of Hope Medical Center; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Dana Farber Cancer Institute; Enterprise Science and Computing, Inc.; Fred Hutchinson Cancer Research Center; Gamida-Cell, Ltd.; Genzyme; Gilead Sciences, Inc.; GlaxoSmithKline (GSK); HistoGenetics, Inc.; Immucor; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen Research & Development, LLC; Janssen Scientific Affairs, LLC; Japan Hematopoietic Cell Transplantation Data Center; Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Mayo Clinic and Foundation Rochester; Medac GmbH; Mediware; Memorial Sloan Kettering Cancer Center; Merck & Company, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; OptumHealth; Orca Biosystems, Inc.; PCORI; Pfizer, Inc.; Phamacyclics, LLC; PIRCHE AG; Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; Sanofi Genzyme; Seattle Genetics; Shire; Sobi, Inc.; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; The Medical College of Wisconsin; University of Minnesota; University of Pittsburgh; University of Texas-MD Anderson; University of Wisconsin - Madison; Viracor Eurofins and Xenikos BV. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Acknowledgments of research support for the study
Footnotes
Author Disclosures:
Olsson, RF – AstraZeneca
Gergis, U – Incyte, Merck, Astellas, Jazz Pharmaceutical
Orti, G – Novartis, Bristol-Myers Squibb, Incyte, Pfizer
Lazarus, H – Novartis, Bristol-Myers Squibb
Cerny, J – Pfizer
For all other authors, nothing COI to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251–2259. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260–2270. [DOI] [PubMed] [Google Scholar]
- 3.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011;118:4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013;369:1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCCN Guidelines. Chronic Myelogenous Leukemia V 1.2019. https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf, accessed on 9/10/2018.
- 6.Craddock CE. We do still transplant CML, don’t we? Hematology Am Soc Hematol Educ Program 2018;1:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolini FE, Basak GW, Kim DW, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer 2017;123:2875–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb HJ, Schattenberg A, Boldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995;86:2041–2050. [PubMed] [Google Scholar]
- 9.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000;96:2712–2716. [PubMed] [Google Scholar]
- 10.Olavarria E, Ottmann OG, Deininger M, et al. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia 2003;17:1707–1712. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, O’Brien S, Cortes JE, et al. Imatinib mesylate therapy for relapse after allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood 2002;100:1590–1595. [PubMed] [Google Scholar]
- 12.DeAngelo DJ, Hochberg EP, Alyea EP, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chornic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res 2004;10:5065–5071. [DOI] [PubMed] [Google Scholar]
- 13.Hess G, Bunjes D, Siegert W, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for choric myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol 2005;23:7583–7593. [DOI] [PubMed] [Google Scholar]
- 14.Palandri F, Amabile M, Rosti G, et al. Imatinib therapy for chronic myeloid leukemia patients who relapse after allogeneic stem cell transplantation: a molecular analysis. Bone Marrow Transplant 2007;39:189–191. [DOI] [PubMed] [Google Scholar]
- 15.Conchon M, Sanabani SS, Bendit I, et al. The use of imatinib mesylate as a lifesaving treatment of chronic myeloid leukemia relapse after bone marrow transplantation. J Transplant 2009: 357093–357093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright MP, Shepherd JD, Barnett MJ, et al. Response to tyrosine kinase inhibitor therapy in patients with chronic myelogenous leukemia relapsing in chronic and advanced phase following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16:639–646. [DOI] [PubMed] [Google Scholar]
- 17.Svani BN, Montero A, Kurlander R, et al. Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant 2005;36:1009–1015. [DOI] [PubMed] [Google Scholar]
- 18.Zeidner JF, Zahurak M, Rosner GL, et al. The evolution of treatment strategies for patients with chronic myeloid leukemia relapsing after allogeneic bone marrow transplantation: Can tyrosine kinase inhibitors replace donor lymphocyte infusions? Leuk Lymphoma 2015; 56:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shanavas M, Messner HA, Kamel-Reid S, et al. A comparison of long-term outcomes of donor lymphocyte infusions and tyrosine kinase inhibitors in patients with relapsed CML after allogeneic hematopoietic cell transplantation. Clin Lymphoma Myeloma Leuk 2014;14:87–92. [DOI] [PubMed] [Google Scholar]
- 20.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008;14:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacigalupo A, Ballen K, Rizzo D, et al. : Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, et al. : Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974;18:295–304. [DOI] [PubMed] [Google Scholar]
- 23.Shulman HM, Sullivan KM, Weiden PL, et al. : Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 seattle patients. The American Journal of Medicine 1980;69:204–217. [DOI] [PubMed] [Google Scholar]
- 24.Radujkovic A, Guglielmi C, Bergantini S, et al. Donor lymphocyte infusions for chronic myeloid leukemia relapsing after allogeneic stem cell transplantation: May we predict graft-versus-leukemia without graft-versus-host disease? Biol Blood Marrow Transplant 2015;21:1230–1236. [DOI] [PubMed] [Google Scholar]
- 25.Luznik L and Fuchs EJ. Donor lymphocyte infusions to treat hematologic malignancies in relapse after allogeneic blood or marrow transplantation. Cancer Control 2002;9:123–137. [DOI] [PubMed] [Google Scholar]
- 26.Collins RH Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997;15:33–444, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Eide CA and O’Hare T. Chronic myeloid leukemia: Advances in understanding disease biology and mechanisms of resistance to tyrosine kinase inhibitors. Curr Hematol Malig Rep 10:158–66, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simula MP, Marktel S, Fozza C, et al. Response to donor lymphocyte infusions for chronic myeloid leukemia is dose-dependent: the importance of escalating the cell dose to maximize therapeutic efficacy. Leukemia 21:943–948, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.