Abstract
Brain endocannabinoids serve as retrograde neurotransmitters, being synthesized in postsynaptic neurons “on demand” and released to bind presynaptic cannabinoid receptors and suppress glutamatergic or GABAergic transmission. The most abundant brain endocannabinoid, 2 arachidonoyl glycerol (2-AG), is primarily synthesized by diacylglycerol lipase-α (DGLα), which is activated by poorly understood mechanisms in response to calcium influx following postsynaptic depolarization and/or the activation of Gq-coupled group 1 metabotropic glutamate receptors. However, the impact of other neurotransmitters and their downstream signaling pathways on synaptic 2-AG signaling has not been intensively studied. Here, we found that DGLα activity in membrane fractions from transfected HEK293T cells was significantly increased by in vitro phosphorylation using cyclic AMP-dependent protein kinase (PKA). Moreover, PKA directly phosphorylated DGLα at Ser798 in vitro. Elevation of cAMP levels in HEK293 cells expressing DGLα increased Ser798 phosphorylation, as detected using a phospho-Ser798 specific antibody, and enhanced DGLα activity; this in situ enhancement of DGLα activity was prevented by mutation of Ser798 to Ala. We investigated the impact of PKA on synaptic 2-AG mobilization in mouse striatal slices by manipulating D1-dopamine receptor (D1R) signaling and assessing depolarization-induced suppression of excitation (DSE), a DGLα- and 2-AG-dependent form of short-term synaptic depression. The magnitude of DSE in direct pathway medium spiny neurons was increased by preincubation with a D1R agonist, and this enhancement was blocked by postsynaptic inhibition of PKA. Taken together, these findings provide new molecular insights into the complex mechanisms regulating synaptic endocannabinoid signaling.
Graphical Abstract
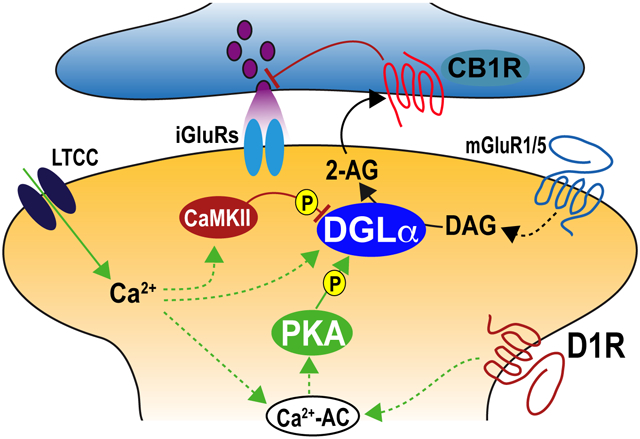
Postsynaptic synthesis of a major brain endocannabinoid, 2-arachidonoyl glycerol (2-AG), from diacylglycerol (DAG) by diacylglycerol lipase-α (DGLα) is stimulated by L-type voltage-gated calcium channels (LTCC) and/or metabotropic glutamate receptors (mGluR1/5). Shonesy et al show that cyclic AMP-dependent protein kinase (PKA) phosphorylates Ser798 in DGLα to increase activity. Their data indicate that D1-dopamine receptors (D1R) stimulate adenylyl cyclase (Ca2+-AC) and PKA to enhance synaptic 2-AG production by DGLα and short-term depression of glutamatergic transmission, which depends on presynaptic endocannabinoid 1 receptors (CB1R). Ca2+/calmodulin-dependent protein kinase II (CaMKII) was previously shown to phosphorylate distinct sites in DGLα to restrain synaptic 2-AG synthesis.
INTRODUCTION
Levels of brain endocannabinoids (eCBs) are dynamically modulated by regulating the rates of synthesis and degradation (reviewed in: (Fowler et al. 2017; Murataeva et al. 2014)). Many of the known central effects of eCBs are mediated by binding to the type 1 cannabinoid receptor (CB1R), which is concentrated at glutamatergic and GABAergic synaptic terminals. CB1Rs signal via Gi/Go heterotrimeric G proteins to suppress the release of neurotransmitters, resulting in either short- or long-term synaptic depression (reviewed in: (Kano et al. 2009)). Emerging findings illustrate that eCB signaling modulates a variety of behaviors and that myriad neuropsychiatric conditions may be associated with disruptions of eCB signaling and/or respond to eCB modulation (reviewed in: (Augustin & Lovinger 2018; Cristino et al. 2019; Hill et al. 2018; Wei et al. 2017; Gunaydin & Kreitzer 2016)). Postsynaptic mobilization of the most abundant brain eCB, 2-arachidonoylglycerol (2-AG), can be triggered by a variable combination of Ca2+ influx(Puente et al. 2011) and activation of group 1 metabotropic glutamate receptors (mGluR1/5) (Maejima et al. 2005; Hashimotodani et al. 2007; Lerner & Kreitzer 2012), but the underlying molecular mechanisms are poorly understood. However, 2-AG mobilization is critically gated by its on-demand synthesis by postsynaptic diacylglycerol lipase-α (DGLα) (Gao et al. 2010; Tanimura et al. 2010; Yoshino et al. 2011), and the specific mechanisms regulating DGLα are key to understanding synaptic regulation in diverse pathophysiological states. While it has been shown that direct phosphorylation of DGLα by calcium/calmodulin-dependent protein kinase II (CaMKII) can restrain synaptic 2-AG synthesis (Shonesy et al. 2013), the impact of other neurotransmitter receptor signaling pathways on DGLα activity and synaptic 2-AG signaling has not been intensively studied.
Medium spiny neurons (MSNs) in the dorsal striatum integrate excitatory synaptic inputs from the cortex and thalamus to modulate action selection and motor coordination (reviewed in: (Mathur & Lovinger 2012; Zhai et al. 2018)). MSNs in the direct and indirect output pathways (dMSNs and iMSNs, respectively) express either D1- or D2-dopamine receptors (D1Rs or D2Rs), respectively (Shonesy et al. 2014; Shonesy et al. 2018; Wu et al. 2015; Xu et al. 2018), and are critically modulated by dopamine innervation from the substantia nigra (reviewed in: (Burguiere et al. 2015; Gunaydin & Kreitzer 2016; Zhai et al. 2018; Gerfen & Surmeier 2011)). Moreover, glutamatergic inputs to both dMSNs and iMSNs can be modulated by cannabinoid receptor-dependent short- and long-term depression (Shonesy et al. 2018; Shonesy et al. 2013; Lerner & Kreitzer 2012; Song et al. 2018). Thus, the intersecting striatal actions of D1Rs and D2Rs, canonically acting to increase or decrease cAMP signaling in d/iMSNs, respectively, with eCBs are critical for the dynamic regulation of striatal output to the basal ganglia, exerting complex behavioral effects. However, the impact of dopamine signaling on synaptic 2-AG mobilization at striatal synapses is poorly understood.
Here we began to test the hypotheses that cyclic AMP-dependent protein kinase (PKA), a canonical downstream target of dopamine signaling, directly modulates DGLα activity and plays a novel role in the dopamine-dependent modulation of 2-AG signaling at excitatory synaptic inputs to dMSNs. Using a combination of in vitro and heterologous cell experiments, as well as electrophysiological experiments in mouse striatal slices, we identify a mechanism underlying a novel role of D1Rs in the regulation of 2-AG mobilization and eCB-dependent synaptic plasticity in dMSNs, with potentially important implications for understanding the flow of information through the striatum.
MATERIALS AND METHODS
DNA constructs and DGLα protein expression
The pcDNA3.1D vector (Invitrogen) with a CMV promoter was used to express human DGLα (WT or S798A mutant) and DGLβ with a C-terminal V5 tag in HEK293T cells by transfection using a 1:3 (w/w) ratio of DNA to polyethylenimine (linear MW 25,000 Da) (Polysciences Cat: # 23966). HEK293T cells are not listed in the Register of Misidentified Cells Lines (version 9: https://iclac.org/databases/cross-contaminations/) and were obtained from the American Type Culture Collection. The cells and were not authenticated for these studies and were used after ≤20 passages.
GST fusion proteins containing the indicated DGLα fragments were expressed from a pGEX6P-1 vector backbone (GE Healthcare Life Sciences) and purified as described previously(Shonesy et al. 2013).
The sequences of all WT and mutant DNA constructs were confirmed (Genewiz). Custom made materials will be shared upon reasonable request.
DGL Activity Assay
Transfected HEK293T cells were Dounce-homogenized in lysis buffer containing 20 mM HEPES (pH 7.5), 2 mM DTT, 250 mM sucrose, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM PMSF and 1 μM microcystin-LR. Following homogenization, membranes were pelleted by centrifugation at 10,000 X g for 25 min at 4°C. Membranes were resuspended using a Dounce homogenizer in 20 mM HEPES (pH 7.5), 2 mM DTT, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 1 mM PMSF and 1 μM microcystin-LR.
Resuspended membranes (~5 μg total protein, 50 μl total volume) containing equal levels of V5-tagged DGLα by immunoblot, were pre-incubated at 30°C for 15 min with 10 mM MgCl2, 0.4 mM ATP and variable amounts purified bovine heart PKA catalytic subunit (generous gift from Dr. J.D. Corbin, Vanderbilt University). For Figure 1A, membrane extracts were pre-incubated with 0, 100, 300, or 1000 nM PKA. For Figure 1B, membranes were preincubated with parallel aliquots of PKA (1 μM) with or without heat-inactivation by incubation for 1 hour at 70°C. For Figure 1C, PKA (10 nM) was incubated at 4°C with or without PKA inhibitor peptide PKI (10 μM) for 10 minutes prior to the addition to membranes. DGLα assays were then initiated by direct addition of 1-steroyl-2-arachidonoylglycerol (SAG; Cayman Chemical) in 100% methanol (final concentration: 5%). After an additional incubation at 37°C for 15 min, assays were terminated by adding 200 μl of 100% methanol containing 125 pmol 2-AG-d8 and 50 pmol arachidonic acid-d8. Insoluble material was removed by centrifugation (2000 X g, 10 min, 4°C), and 20 μl of supernatant, containing extracted lipids, was analyzed by LC/MS. Activity measured in membrane fractions from mock-transfected cells that do not express exogenous V5-DGLα was subtracted as a blank from activities in membrane fractions from transfected cells.
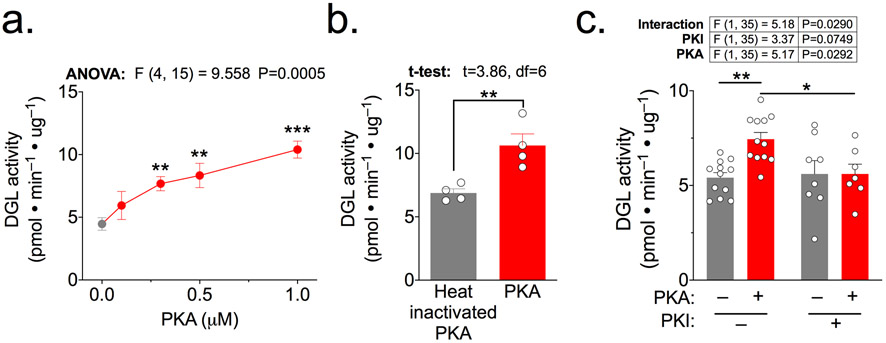
Figure 1. DGLα in membrane fractions from transfected HEK293T cells is activated by PKA.
DGLα activity in membrane fractions isolated from HEK293T cells expressing DGLα-WT and preincubated as indicated below for 15 min at 30°C, was measured as described in Methods.
a. DGLα activities after preincubation of the membrane fraction with MgATP in the presence of increasing concentrations of purified PKA. Results of a one-way ANOVA are shown above, with Holm-Sidak’s multiple comparisons test used to compare each data point to the 0 μM PKA control.
b. DGL activities following preincubation of the membrane fraction with MgATP and either PKA or heat-inactivated PKA (1 μM each). Results from an unpaired t-test comparing the two groups are shown above.
c. DGLα activities after preincubation of the membrane fraction with MgATP with and without purified PKA (10 nM) and the PKA inhibitor peptide, PKI (10 μM), as indicated. Results of a two-way ANOVA are shown above with Holm-Sidak’s multiple comparisons test used to compare between conditions.
LC/MS detection of lipids
The lipid samples were analyzed on a SCIEX 6500 QTRAP mass spectrometer equipped with an IonDrive Turbo-V ion source in tandem with a Shimadzu Nexera X2 UHPLC. The sample (20 μl) was injected onto a Waters Acquity BEH C18 column (50 mm × 2.1 mm, 1.7 μm) under the following gradient (mobile phase B is indicated): 65% from 0 to 0.4 min, increased to 99% from 0.4 to 4.5 min and held for 1.7 min, and decreased to 35% from 5.7 to 6.0 min. Mobile phase A was composed of H2O, 0.1% formic acid and mobile phase B was composed of Acetonitrile, 0.1% formic acid. Analytes were detected via multiple reaction monitoring (MRM) as [M+H] in positive ion mode using the following MRM transitions (the mass in parentheses represents the mass of the deuterated internal standard): AA (m/z 519(527) → 409(417)); 2-AG (m/z 485(493) → 411(419)). Quantification was achieved via stable isotope dilution for AA and 2-AG. Levels of analytes are given in pmol per total μg protein for DGL activity assays.
In Vitro Phosphorylation Assay
V5-tagged DGLα (or DGLβ) was isolated from transfected HEK293T cell lysates using a V5 antibody (Bethyl Laboratories, A190-120A) and protein-G magnetic beads (Dynabeads, Invitrogen), as previously described(Shonesy et al. 2013), and then incubated (30°C, 20 min) in assay buffer (50 mM HEPES, pH 7.5, 10 mM MgCl2, 1 μM dithiothreitol, 400 μM [γ−32P]ATP (700–1000 cpm/pmol)) in the presence or absence of PKA catalytic subunit (as indicated in figure legends). Reactions were stopped by adding LDS sample buffer (Invitrogen)and heating (70°C, 10 min), and the eluted proteins were resolved by SDS-polyacrylamide gel electrophoresis (8 or 10% acrylamide) and transferred to nitrocellulose membranes (see below), which were then exposed overnight to X-ray film. To verify V5-DGL expression and the specificity of the immunoprecipitation, membranes were then immunoblotted with a mouse anti-V5 antibody.
Purified GST-fusion proteins were phosphorylated using essentially the same conditions. Phosphorylation stoichiometries were assessed by spotting 15 μl of the reaction on P81 Whatman paper at the indicated times. Papers were then washed and 32P incorporation was determined using a scintillation counter. Alternatively, aliquots of the reactions were quenched with sample buffer and resolved by SDS-polyacrylamide gel electrophoresis (12% acrylamide) to visualize phosphorylated products by autoradiography.
Proteomics detection of phosphorylation sites
The V5-tagged DGLα was isolated from transfected HEK293T cells and phosphorylated by PKA (see above). The V5-DGLα band was excised from gels and incubated with 100 mM ammonium bicarbonate, pH 8, reduced with 4 mM DTT or TCEP, alkylated with 8 mM iodoacetamide and finally digested overnight with trypsin (10 ng/μl; 37°C). Extracted peptides were reconstituted in 0.1% formic acid.
Peptide mixtures were fractionated on a C18 reverse phase column (360 μm O.D. x 100 μm I.D. Jupiter, 3 μm beads, 300 Å, Phenomenex) equipped with a laser-pulled emitter tip (flow rate: 500 nl/min). Mobile phase solvents consisted of 0.1% formic acid, 99.9% water (solvent A) and 0.1% formic acid, 99.9% acetonitrile (solvent B). A 90–minute gradient was used: 0–10 min, 2% B; 10–50 min, 2–35% B; 50–60 min, 35–90% B; 60–65 min, 90% B, 65–70 min, 90–2% B, 70–90 min, 2% B. The eluted peptides were analyzed on an LTQ Orbitrap Velos mass spectrometer (Thermo Scientific), operated using a data-dependent method with dynamic exclusion enabled. Full scan (m/z 300–2000) spectra were acquired with the Orbitrap as the mass analyzer (resolution 60,000), and the five most abundant (LTQ Orbitrap XL) or twelve most abundant ions (LTQ Orbitrap Velos) in each MS scan were selected for fragmentation via collision-induced dissociation (CID) in the LTQ. For analyses performed on the LTQ Orbitrap XL, the data-dependent method included neutral loss triggered MS3 scan events, where MS3 scans were performed when neutral losses of 97.98, 48.99, or 32.66 (neutral losses specific for phosphorylation) were detected in the preceding MS2 spectra. All tandem mass spectra were converted into DTA files using Scansifter, and searched against a human subset of the UniProtKB protein database (uniprot.org), also containing reversed (decoy) protein sequences. Database searches were performed using a custom version of SEQUEST (Eng et al. 1994) on the Vanderbilt ACCRE Linux cluster, and results were assembled in Scaffold 4 (Proteome Software) with minimum filtering criteria of 95% peptide probability. All searches were configured to use variable modifications of carbamidomethylation on cysteine, oxidation of methionine, and phosphorylation of serine, threonine, and tyrosine. Sites of modification were validated by manual interpretation of the raw tandem mass spectra using QualBrowser software (Xcalibur 2.1.0, Thermo Scientific). To determine the relative abundance of phosphorylated peptides in digests of in vitro phosphorylated DGLα, accurate mass measurements acquired in the Orbitrap were used to generate extracted ion chromatograms in Skyline software (MacLean et al. 2010) for analysis. A window of 10 ppm around the theoretical monoisotopic m/z values of the observed precursor ions of the unmodified and phosphorylated peptide pairs was used. Using Skyline, the integrated area under each peak was determined, and the percent relative abundance of each phosphorylated peptide was calculated as a percentage of the total area under the curve (AUC) obtained for both the phosphorylated and unmodified forms for each DGLα peptide.
Generation and validation of phospho-Ser798 specific DGLα antibody
Polyclonal rabbit anti-serum to a Ser798-phosphorylated (pS798) DGLα was generated by Cocalico Biologicals. Rabbits were immunized with a phosphorylated peptide spanning residues 793-803 of human DGLα (CDSRRSpSGFRSI) following conjugation to Keyhole limpet, and boosted twice after 14 and 21 days. A test bleed (day 35) was screened by immunoblotting lysates of HEK293T cells expressing human V5-DGLα that had been incubated with FSK+IBMX (see Fig. 3). Serum was then collected for affinity purification of antibodies using Sulfo-Link (Thermo) resin coupled to either the non-phosphorylated or pS798 peptides. Serum was first incubated (4°C, 4 h) with non-phosphorylated peptide resin. The unbound material was collected and then incubated with pS798-peptide resin (4°C overnight). After washing the pS798-peptide resin with TBS-Tween (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 0.1% (v/v) Tween-20), antibodies were eluted using 0.2 M glycine pH 2.5, neutralizing the eluate as it was collected in tubes containing Tris-HCl, pH 9.0. Pooled fractions were concentrated using a Centricon-10 device (Millipore-Sigma) and then dialyzed against TBS/50% (v/v) glycerol. Available samples of the pS798-DGLα antibody will be shared upon reasonable request.
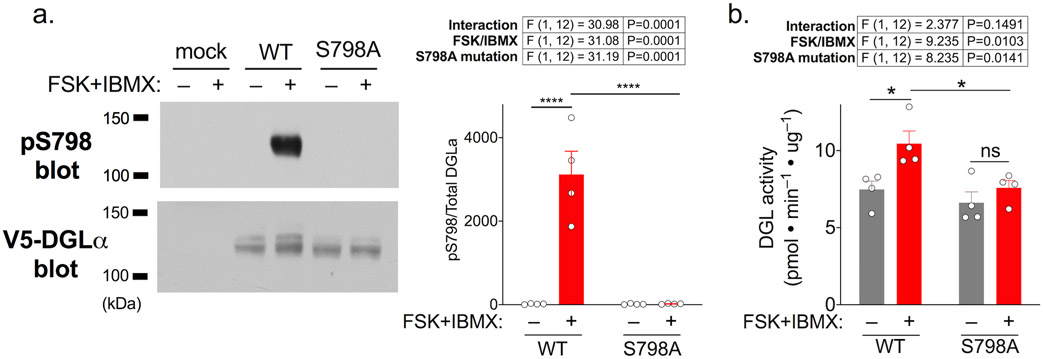
Figure 3. DGLα is phosphorylated at Ser798 and activated by stimulating cAMP signaling in situ.
HEK293T cells expressing DGLα (WT or S798A) or mock transfected cells were incubated for 30 min with forskolin and IBMX (25/200 μM, respectively) and then membrane fractions were prepared in the presence of phosphatase inhibitors
a. Left: representative immunoblots of membrane fractions using antibodies to the V5 tag (total DGLα) and phospho-Ser798. Right: Bar graph with superimposed data points reporting the ratio of phospho-Ser798 to total V5 signals from four independent replicates immunoblotted in parallel. Data are representative of >3 other experiments in which cells were incubated similarly.
b. Bar graph with superimposed data points reporting DGL activities in membrane fractions prepared independently from HEK293T cells expressing DGLα-WT or -S798A.
Data in all panels are presented as mean ± s.e.m., with results of a two-way ANOVA shown above with results from post-hoc Holm-Sidak’s multiple comparisons test indicated with *p<0.05, ****p<0.0001.
Immunoblotting
Immunoblotting of HEK293T cell lysates was performed as described by Shonesy et al., 2013 (Shonesy et al. 2013) using rabbit anti-V5 (Bethyl Laboratories, A190-120A, 1:10,000) or affinity-purified pS798 antibodies (see above: 1:4000). SDS-polyacrylamide gels (8-12% acrylamide) were transferred at 4°C to nylon-backed nitrocellulose membranes in 10 mM CAPS, 10% (v:v) methanol, pH 10.5 at 1 Amp for 1-2.5 hr. Membrane blocking, antibody incubations and membrane washing were conducted in 50 mM Tris-HCl, pH7.5, 0.15 M NaCl containing 5% (w/v) dried milk.
Animals
All animal procedures were pre-approved by the Vanderbilt University Institutional Animal Care and Use Committee (Protocol Number: M/15/065), in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Tg(Drd2-EGFP)S118Gsat (D2-eGFP) (MGI:3843608) and Tg(Drd1-tdTomato)5Calak (D1-tdTomato) (MGI:4360387) BAC transgenic mice on a C57Bl6 background were housed on a 12 h light-dark cycle with food and water ad libitum. The animal studies were not pre-registered and used a total of 48 male mice at P27-P42 days of age to generate data for Figure 4 and Supplementary Figures S1 and S2, with no randomization. Numbers of animals in each slice treatment group are shown in each figure. As described below, pain and suffering was minimized by anesthetizing mice with isofluorane.
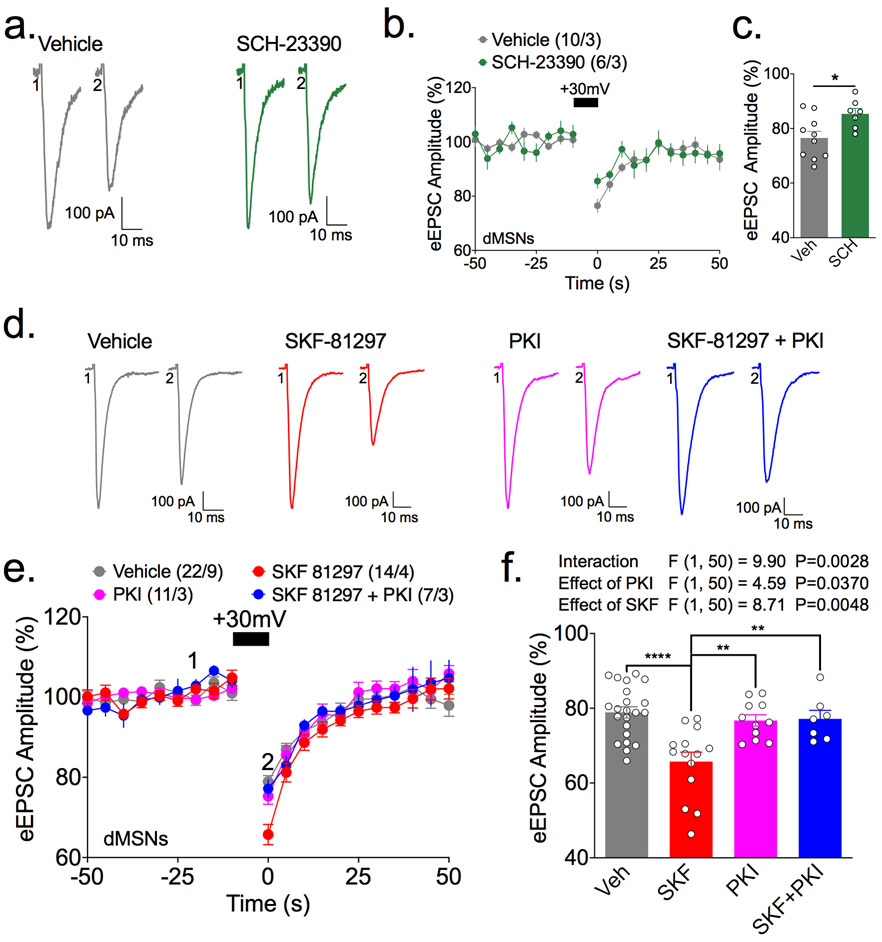
Figure 4. D1 dopamine receptor signaling via PKA enhances depolarization-induced suppression of excitation (DSE) in direct pathway striatal medium spiny neurons (dMSNs).
Evoked excitatory postsynaptic currents (eEPSCs) were recorded every 5 s from dMSNs in striatal slices preincubated with pharmacological reagents as described. Panels a and d show representative eEPSC traces from single cells immediately before and immediately after the induction of DSE with a 10 s depolarization to +30 mV. Graphs in panels b and e plot the eEPSCs over time after normalization to the baseline (mean ± s.e.m.), with the number of cells and animals analyzed in each data set indicated. Bar graphs in panels c and f summarize the mean ± s.e.m. eEPSC amplitudes at the peak of DSE as a percentage of the baseline eEPSC, with individual data points from each cell superimposed.
a-c. Preincubation of striatal slices with the D1R antagonist, SCH23390 (2 μM), reduces DSE in dMSNs (* , p<0.05 by Students t-test).
d-f. Preincubation of striatal slices with the D1R agonist, SKF-81297 (10 μM) enhances DSE in dMSNs, and this enhancement is blocked by including the PKA inhibitor, PKI (10 μM), in the recording pipet solution. Results from a two-way ANOVA (factors: SKF-81297 and PKI) are tabulated above the bar graph, with results of post-hoc Holm-Sidak multiple comparison test also indicated: **, p<0.01. ****, p<0.001.
Brain Slice Preparation and electrophysiology
Acute brain slice preparation and whole cell patch clamp recordings were performed essentially as previously described (Shonesy et al. 2014; Shonesy et al. 2018; Shonesy et al. 2013). D1-tdTomato or D2-eGFP mice were anesthetized with isoflurane and transcardially perfused with cold, oxygenated (95% v/v O2, 5% v/v CO2) N-methyl-D-glucamine (NMDG)-containing artificial cerebrospinal fluid (ACSF), comprised of (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 25 glucose, 5 ascorbate, 20 HEPES, 3 Na-pyruvate, 0.5 CaCl2·4H2O, 10 MgSO4·7H2O. NMDG-ACSF was titrated to pH 7.3-7.4 with HCl. Following decapitation, the brain was removed and a 3 mm coronal block containing the striatum was cut using a cold brain matrix. A Leica VT1000S vibratome (Leica Microsystems, Bannockburn, IL) was used to cut 200-300 μm coronal slices in cold, oxygenated NMDG-ACSF. Slices were transferred to a recovery chamber containing NMDG-ACSF kept at 34°C for 10 minutes after which they were held at 24°C in HEPES-ACSF pH 7.3-7.4 containing (in mM): 92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 ascorbate, 3 Na-pyruvate, 2 CaCl2·4H2O, 2 MgSO4·7H2O. Slices were typically prepared between 8 am and noon.
Depolarization induced suppression of excitation (DSE) experiments were carried out in striatal medium spiny neurons (MSNs) identified as D1-tdTomato positive (referred to as direct pathway or dMSNs throughout) or D2-eGFP positive (indirect pathway or iMSNs throughout). Slices were continuously perfused at a flow rate of 2-3 ml/min with oxygenated recording-ACSF (30-32°C), containing (in mM): 113 NaCl, 2.5 KCl, 1 NaH2PO4, 26 NaHCO3, 20 glucose, 1 ascorbate, 3 Na-pyruvate, 2.5 CaCl2·2H2O, 1.2 MgSO4·7H2O, 0.05 picrotoxin. Electrophysiological recordings were performed up to 6 hours after slicing.
Patch electrodes were pulled on a Flaming/Brown microelectrode puller (Sutter Instruments) to a resistance of 2.0-4.0 MΩ, and filled with a solution containing (in mM): 130 K-gluconate, 4 NaCl, 10 HEPES, 4 Mg-ATP, 0.3 Na-GTP, and 10 Na-phosphocreatine (pH 7.3, adjusted with KOH). After establishing access to the cell, a 15 min equilibration period was observed prior to starting recordings to allow the pipette solution to equilibrate with the cell cytoplasm. Baseline evoked excitatory postsynaptic currents (eEPSCs) were elicited by stimulation through a glass pipette filled with recording ACSF and placed ~100 μm dorsal of the patched cell. Stimulation pulses were delivered every 5 sec prior to and immediately following application of a postsynaptic depolarization (+30 mV; 3 or 10 sec, as indicated). This paradigm was repeated two times on each cell, and normalized eEPSC amplitudes were averaged for each cell. Responses were normalized to an average of the baseline responses. Average normalized response amplitudes during the baseline were compared to the first time point following depolarization and statistical significance between groups was determined by an unpaired Student’s t-test. Sample sizes were not precalculated and genotype blinding of the experimenter was not possible. For all experiments, access resistance was monitored online and cells that demonstrated a >20% change were excluded from analysis.
Pharmacological reagents used
The highly D1R- and D2R-selective antagonists, SCH-23390 and RS-sulpiride, and the highly selective D1R agonist, SKF-81297 (Tocris Biosciences) were resuspended as 10 mM stocks in DMSO. On the day of the recording, the stocks were diluted into ACSF at final concentrations of 10 μM, 10 μM and 2 μM for SCH-23390, RS-sulpiride and SKF-81297 respectively. The highly specific PKA inhibitor, PKI(5-24) (Tocris Biosciences) was resuspended as a 1 mM stock in H2O and was diluted into the pipette solution to a final concentration of 1 μM on the day of recording.
Statistical Analyses
Data were analyzed using Student’s t test (unpaired, 2-tail), 1-way ANOVA or 2-way ANOVA according to the numbers of sample groups and variable factors in each experiment, as indicated in the figure legends. No outlier tests were performed and no data were excluded from the analyses. The normality of the data distribution was confirmed using the Shapiro-Wilk test where allowed by the sample size. All testing was performed using GraphPad Prism (versions 6 or 8).
RESULTS
PKA activates DGLα
We previously showed that CaMKII suppresses DGLα activity by phosphorylating Ser782 and Ser808 (Shonesy et al. 2013). Since dopamine signaling has complex interactions with eCB signaling in the striatum (see Introduction), we hypothesized that PKA, a canonical dopamine-regulated protein kinase, may modulate 2-AG production by phosphorylating DGLα. As an initial test for PKA modulation of DGLα, membrane fractions from HEK293T cells expressing wild type V5-tagged DGLα were pre-incubated in the presence of Mg2+ and ATP for 15 min with increasing concentrations of purified PKA (0-1 μM), before assaying DGLα activity. PKA increased DGLα activity ~2-fold in a concentration-dependent manner (Fig. 1a). Notably, the PKA-dependent increase of DGLα activity was prevented by prior heat-inactivation of PKA (Fig 1b), or by inclusion of the PKA inhibitor PKI (Fig. 1c), confirming that PKA activity is required.
PKA phosphorylates DGLα at Ser798
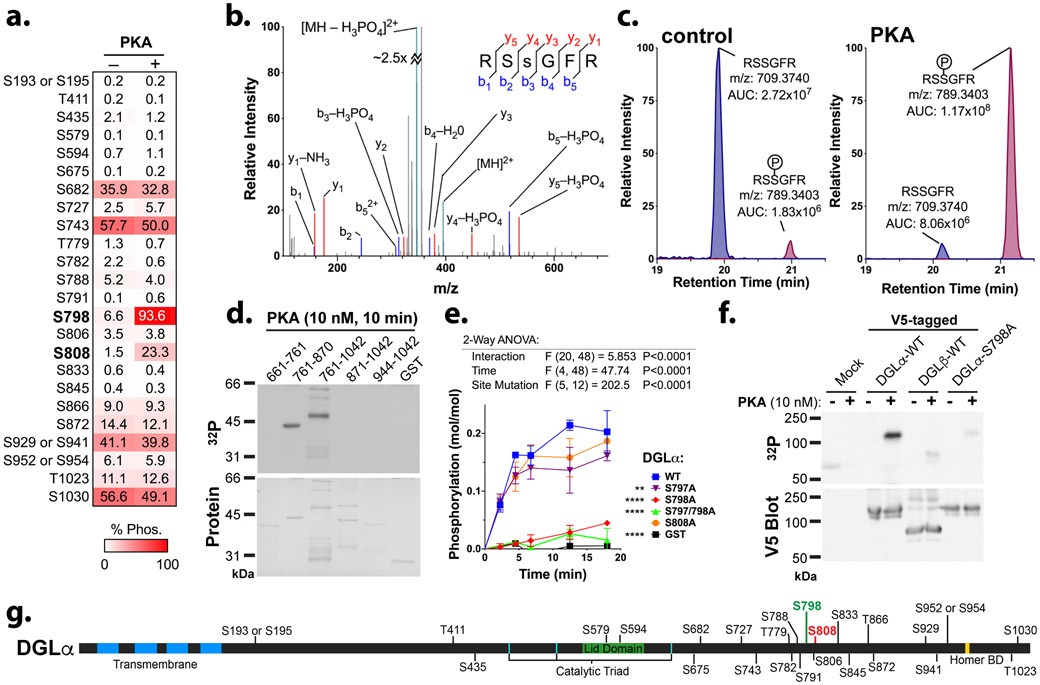
To test the hypothesis that PKA directly phosphorylates DGLα, we incubated full-length V5-tagged DGLα (immunoprecipitated from HEK293T cells) with MgATP in the absence or presence of PKA (100 nM; 1 hour), and detected phosphorylated peptides/residues by LC-MS/MS analysis. Approximate levels of phosphorylation at each site were estimated from extracted ion chromatograms (see Methods). A total of 24 phosphorylated residues were detected in V5-DGLα that had not been incubated with PKA, but only 6 sites were ≥10% phosphorylated (S682, S743, S872, S929/S941, T1023 and S1030) (Fig. 2a). The pre-incubation with PKA increased levels of phosphorylation at only two sites by ≥2 fold; Ser798 (6.6% to 93.6% phosphorylated) and Ser808 (1.5% to 23.3% phosphorylated) (Fig. 2a). MS/MS spectral data identifying the Ser798 phosphorylation site and chromatograms comparing levels of Ser798 phosphorylation with and without PKA are shown in Figs. 2b, c. These data suggest that while DGLα can be phosphorylated at many sites, presumably by intrinsic HEK293 cell kinases, PKA selectively phosphorylates only two residues in the C-terminal domain (CTD), even after prolonged incubation with relatively high concentrations of PKA.
Figure 2. PKA preferentially phosphorylates Ser798 in DGLα.
a. Immunoprecipitated V5-tagged DGLα was incubated at 30°C for 1 hour in the presence of MgATP, with or without the addition of purified PKA catalytic subunit (100 nM). Table summarizing the estimated percentage phosphorylation at identified serine or threonine residues without or with preincubation with PKA, as determined by LC-MS/MS analysis overlaid on a heat map coding the extent of phosphorylation.
b. Annotated MS/MS spectrum identifying the Ser798 phosphorylation site.
c. Extracted ion chromatograms showing the relative abundance of phosphorylated and non-phosphorylated peptides containing Ser798 with calculated areas under the curve (AUC) without or with PKA addition.
d. A family of purified GST fusion proteins containing the indicated regions of the DGLα CTD, which together include 19 of the phosphorylation sites detected in panel a, were incubated at 30°C for 10 min with Mg[γ−32P]ATP and the PKA catalytic subunit (10 nM) and then analyzed by SDS-polyacrylamide gel electrophoresis. An autoradiograph (top) of the Coomassie blue stained gel (bottom) is shown. Data are representative of >3 similar experiments.
e. GST-DGLα(761-870) proteins (WT or with the indicated serine residues mutated to alanine) were incubated at 30°C with Mg[γ−32P]ATP and the PKA catalytic subunit (10 nM). Phosphorylation stoichiometries were assessed at the indicated times as described in methods. Results of a two-way ANOVA (factors: time, mutation) are tabulated above.
f. Immunoprecipitated V5-tagged full-length DGLα (WT or S798A mutant) was incubated at 30°C for 10 min in the presence of Mg.[γ−32P]ATP, with or without the addition of purified PKA catalytic subunit (10 nM), as indicated, and then analyzed by SDS-polyacrylamide gel electrophoresis and transfer to nitrocellulose membrane. An autoradiograph (top) of a membrane that was immunoblotted using an antibody to the V5 tag (bottom) is shown.
g. Diagram summarizing the location of phosphorylation sites within the DGLα domain structure, demonstrating the strong enrichment of phosphorylation sites in the unique C-terminal domain. The preferred PKA site identified herein is highlighted in green, and the previously identified preferred CaMKII phosphorylation site (inhibitory) is highlighted in red.
To confirm the specificity of PKA phosphorylation in DGLα, we tested a family of GST-fusion proteins containing different fragments of the DGLα-CTD (Shonesy et al. 2013) as phosphorylation substrates using lower PKA concentrations (10 nM, 10 min incubation) and [γ−32P]ATP. GST-fusion protein fragments containing residues 661-761 or 871-1042 were not significantly phosphorylated, but PKA efficiently phosphorylated fragments containing residues 761-870 and 761-1042 (Fig. 2d), which both contain the Ser798 and Ser808 sites detected by LC-MS/MS analysis of PKA-phosphorylated full length protein. The site-specificity of this phosphorylation was further tested by monitoring the time courses for PKA phosphorylation of GST-DGLα(761-870) with various serine-to-alanine mutations. A S798A single mutation or a S797A/S798A double mutation substantially reduced phosphorylation at every time point relative to WT, such that there was no significant phosphorylation above a GST control (Fig. 2e). In contrast, the S808A mutation had no significant effect on phosphorylation at any time point. Moreover, mutation of the Ser797 to Ala, adjacent to the Ser798 residue identified by LC-MS/MS, also had a minimal effect on the rate of phosphorylation. Taken together, these data indicate that under these conditions PKA preferentially phosphorylates Ser798 in the GST-DGLα(761-870) fusion protein, consistent with our LC-MS/MS analyses of the phosphorylated full length protein.
To verify that Ser798 is the primary PKA phosphorylation site in full-length DGLα, we investigated the impact of the S798A mutation on PKA phosphorylation of full-length V5-tagged DGLα. Immunoprecipitated V5-DGLα (WT or S798A) was phosphorylated using [γ−32P]ATP and 10 nM PKA for 10 min and then analyzed by SDS-PAGE and autoradiography. An ~116 kDa 32P-labeled protein co-migrated with the V5-DGLα wild-type (WT) protein detected by immunoblotting with an antibody to the V5 tag (Fig. 2f). Neither the 32P-labeled protein nor the V5-tagged protein were detected in samples prepared in parallel from control (mock transfected) cells. Notably, 32P-labeling of the ~116 kDa protein was markedly reduced by S798A mutation, even though similar levels of V5-DGLα-S798A were immunoprecipitated. Moreover, V5-DGLβ was phosphorylated to a much lower extent than the V5-DGLα (Fig. 2f). Taken together, these data indicate that PKA preferentially phosphorylates DGLα compared to DGLβ in vitro, and identify Ser798 as the primary phosphorylation site in the full length protein. The locations of Ser798 and other known phosphorylation sites relative to the transmembrane and catalytic domains of DGLα are summarized in Fig. 2g.
DGLα is phosphorylated at Ser798 and activated in situ
To determine the impact of stimulating PKA on DGLα in situ, intact HEK293T cells expressing V5-DGLα-WT or V5-DGLα-S798A were incubated with vehicle control or with forskolin plus IBMX (25 μM and 100μM, respectively) for 30 min to increase intracellular cAMP concentrations (forskolin is a direct adenylyl cyclase activator and IBMX is a broad-spectrum inhibitor of cAMP phosphodiesterases). Membrane fractions isolated in the presence of phosphatase inhibitors were then immunoblotted using V5 and phospho-Ser798-DGLα antibodies (see Methods), or assayed for DGLα activity. Ser798 phosphorylation was barely detectable in membrane fractions from vehicle-treated cells expressing DGLα-WT, but activation of cAMP signaling increased Ser798 phosphorylation by >100-fold (Fig. 3a). Importantly, there was no detectable signal for phospho-Ser798 in membrane fractions from either mock-transfected cells or cells expressing V5-DGLα-S798A after the activation of cAMP signaling, demonstrating the specificity of the pS798-DGLα antibody. Moreover, parallel studies showed that DGLα activity in membrane fractions was significantly increased following stimulation of cAMP signaling in cells expressing V5-DGLα-WT but not V5-DGLα-S798A (Fig. 3b). Taken together, these data indicate that stimulation of cAMP signaling can increase Ser798 phosphorylation to activate DGLα in intact cells.
Synaptic 2-AG mobilization is enhanced by DA and PKA signaling
Depolarization-induced suppression of excitation (DSE) in both subtypes of striatal MSNs is a well-established functional read out of retrograde eCB signaling that is dependent on DGLα-mediated synthesis of 2-AG (Alger 2002; Tanimura et al. 2010; Shonesy et al. 2014). Stimulation of D1Rs in dMSNs canonically activates PKA, but the potential modulatory role of dopamine in eCB signaling has not been systematically investigated. Therefore, we investigated the impact of D1Rs on DSE in striatal dMSNs identified in slices from transgenic mice expressing tdTomato driven by the D1R promoter.
Pre-incubation of striatal slices with the D1R antagonist SCH-23390 (10 μM) for ≥30 min reduced the extent of DSE in dMSNs (Fig. 4a-c), suggesting that tonic D1R signaling facilitates striatal 2-AG release under these conditions. However, SCH-23390 has no effect when DHPG (10 μM) is included during DSE induction (Supplementary Figure 1). DHPG stimulates group 1 metabotropic glutamate receptors, which activate phospholipase C, and is thought to enhance 2-AG release and DSE induction by increasing the supply of diacylglycerol substrates for DGLα (Hashimotodani et al. 2008; Maejima et al. 2001; Zhang et al. 2011), perhaps overcoming the modest effect of SCH-23390 on DSE. In contrast, pre-incubation with the D1R agonist SKF-81297 (2 μM) for ≥30 min, resulted in a significant enhancement of DSE whether or not DHPG is present (Fig. 4d-f; Supplementary Figure 1). Consistent with the hypothesis that canonical D1R signaling via Gαs and PKA is required for this effect, pre-loading dMSNs with the highly-selective PKA peptide inhibitor PKI (1 μM) prevented the effect of SKF-81297 on DSE (Fig. 4g, h, i). Taken together, these data indicate that D1R activation enhances postsynaptic eCB mobilization via PKA activation.
DISCUSSION
Long- and short-term eCB-dependent synaptic plasticity has been intensively studied in striatal dMSNs and iMSNs (Gunaydin & Kreitzer 2016; Mathur & Lovinger 2012). While eCB-dependent forms of LTD appear to be more easily induced in iMSNs, with only a few reports of eCB-LTD in dMSNs, the lack of specific genetic tools until quite recently made it difficult to unequivocally identify the specific eCB involved (2-AG or anandamide), obscuring underlying molecular induction mechanisms. For example, one form of eCB-LTD in iMSNs is regulated by D2Rs via a mechanism that requires cAMP/PKA signaling and RGS4 (Lerner & Kreitzer 2012) and was blocked by a DGLα inhibitor, but DGLα knockout mice were not available to confirm this interpretation. However, DGLα knockout mice were recently used to show that DGLα is required for the on-demand synthesis and mobilization of 2-AG to induce DSE, a well-defined form of short term striatal synaptic plasticity, in both dMSNs and iMSNs (Shonesy et al. 2014; Shonesy et al. 2018). In general, dMSNs and iMSNs express either D1Rs or D2Rs, which can simulate or inhibit adenylyl cyclase, respectively (Beaulieu & Gainetdinov 2011). Moreover, the specific knockout of DGLα in either dMSNs or iMSNs has additional unique effects on striatal function (see below). Taken together, these prior studies suggest that dopamine receptor/cAMP signaling has potentially complex interactions with DGLα/2-AG signaling in the striatum.
The specific mechanisms that induce synaptic 2-AG signaling are poorly understood. Postsynaptic calcium influx is sufficient to induce 2-AG mobilization (Shonesy et al. 2015), but the molecular basis of this response is unclear because calcium does not directly modulate DGLα activity when assayed in vitro(Farooqui et al. 1989; Rosenberger et al. 2007) (data not shown). In fact, under some conditions, calcium can exert negative feedback to prevent excess 2-AG synthesis by recruiting CaMKII to bind to and phosphorylate DGLα, inhibiting its activity (Shonesy et al. 2013). Synaptic 2-AG signaling also can be augmented by stimulating group 1 metabotropic glutamate receptors (mGlu1 or mGlu5); in some cases, this effect has been attributed to phospholipase C activation, enhancing the supply of diacylglycerol substrate to DGLα (Hashimotodani et al. 2005). Appropriate subcellular targeting of DGLα is likely important for on-demand synaptic 2-AG synthesis, and there is evidence that PKC inhibitors, but not PKA inhibitors, modulate DGLα trafficking between plasma membrane and intracellular membrane compartments in heterologous cells (Zhou et al. 2016). However, mechanism(s) underlying this trafficking effect in heterologous cells and the role of PKC in modulating 2-AG release at synapses have not been defined. Rosenberger et al. (2007) previously reported that PKA activates diacylglycerol lipases in bovine brain microsomal or plasma membrane fractions (Rosenberger et al. 2007). These bovine enzymes were previously reported to be purified as ~27 kDa and ~52 kDa proteins, respectively (Farooqui et al. 1989), but their potential direct phosphorylation by PKA was not investigated and neither protein appears large enough to be a proteolytic DGLα fragment containing the entire catalytic and C-terminal domains. Thus, our current data provide substantial new insights by showing that PKA directly phosphorylates intact DGLα (~110 kDa) at Ser798 to enhance 2-AG synthesis in vitro and in heterologous cells (Figs. 1-3).
We detected ten phosphorylated serine and threonine residues in DGLα using phospho-proteomic methods in a previous study (Shonesy et al. 2013). All but one of these sites is included in the total of twenty four phosphorylated residues detected using the improved methodologies of the current studies. Most of the phosphorylation sites are located in the C-terminal domain (CTD) of DGLα (Fig. 2g) that is not conserved in DGLβ. Like all mammalian cells, HEK293 cells express numerous endogenous protein kinases that may contribute to the substantial (10-60%) phosphorylation of several sites (S682, S743, S872, S929/S941, T1023, S1030). However, we found that exogenous PKA is highly selective in targeting Ser798 and to a lesser extent Ser808 (Fig. 2a). Interestingly, CaMKII targets the same region in DGLα, but preferentially phosphorylates Ser808 and to a lesser extent Ser782(Shonesy et al. 2013). Thus, this region of the CTD appears to play an important role in modulating DGLα, even though the baseline intrinsic activity of DGLα is enhanced by PKA phosphorylation but suppressed by CaMKII phosphorylation. However, wild type DGLα is active even when Ser798 phosphorylation cannot be reliably detected (in membrane fractions from vehicle-treated cells), and DGLα-S798A has similar basal activity to the WT enzyme (Fig. 3), showing that PKA phosphorylation is not absolutely required for DGLα activity. Thus, it appears that phosphorylation of unique sites within the CTD of DGLα by PKA (Ser798) or CaMKII (Ser808) exerts opposing modulatory actions to enhance and suppress 2-AG synthesis, respectively. In some ways, DGLα regulation may resemble the modulation of hormone-sensitive lipase and adipocyte lipolysis by several kinases, which phosphorylate multiple clustered sites in HSL (reviewed in: (Lampidonis et al. 2011)).
Our electrophysiological studies show that DSE in dMSNs is enhanced by a D1R agonist via a mechanism requiring PKA activity (Fig. 4), strongly supporting the physiological relevance of our biochemical data showing that PKA enhances DGLα activity by phosphorylating Ser798 (Fig. 1-3). Interestingly, even though DGLα expression is required for DSE in both dMSNs and iMSNs (Shonesy et al. 2014), the specific deletion of DGLα from dMSNs significantly reduces baseline total striatal levels of 2-AG, whereas deletion of DGLα from iMSNs had no significant impact on baseline 2-AG levels (Shonesy et al. 2018). A potential explanation of these findings is that while activity-dependent synaptic 2-AG mobilization and DSE can be similarly induced in both cells types, tonic DGLα activity is higher in dMSNs than in iMSNs under basal, unstimulated conditions. This higher basal DGLα activity in dMSNs may be driven in part by D1R signaling via PKA because SCH-23390 reduces the amount of DSE (Fig. 4), although SCH-23390 has no effect when DHPG is included during DSE induction (Supplementary Figure 1). However, our current data do not preclude roles for other kinases in regulating Ser798 phosphorylation and DGLα activity, and protein phosphatases must also contribute to this dynamic regulation. Indeed, we previously reported that total striatal DGLα activity, baseline striatal levels of 2-AG, and DSE in dMSNs are enhanced in mice with a global knock-in Thr286 to Ala (T286A) mutation of CaMKIIα (which reduces autonomous CaMKII activity, presumably to relieve the aforementioned suppression of DGLα activity by Ser808/Ser782 phosphorylation), whereas DSE in iMSNs is unaffected in these mice (Shonesy et al. 2013). The selectivity of the effect of the T286A mutation on DSE in dMSNs may be related to higher levels of DGLα activity due to D1R/PKA signaling in these neurons. Thus, the opposing actions of PKA and CaMKII on DGLα activity may be more important in setting the level of tonic 2-AG signaling in dMSNs. Notably, selective deletion of DGLα from dMSNs increases baseline glutamatergic drive of the direct striatal output pathway, presumably contributing to deficits in social interactions, excessive grooming, and decreased exploration of a novel environment that are observed in these mice (Shonesy et al. 2018). In contrast, mice with a selective loss of DGLα from iMSNs exhibit none of these synaptic or behavioral deficits(Shonesy et al. 2018).
The present findings add to a body of work showing that both D1R and eCB signaling play a vital role in controlling dMSN output to modulate numerous behaviors. Although D1R signaling via PKA more typically acts to increase direct pathway output (e.g., by enhancing the activities of glutamate receptors and L-type calcium channels (Gerfen & Surmeier 2011)), the enhancement of DSE defined here acts to reduce excitatory drive to dMSNs. Spatio-temporal differences in how D1Rs signal to diverse downstream targets may allow for precise fine-tuning of dMSN output in different contexts. We also speculate that eCB signaling may be modulated by other neurotransmitter receptors that are positively or negatively coupled to cAMP and PKA in different neuronal contexts. For example, striatal D2Rs, which inhibit adenylyl cyclase and decrease PKA activity, are expressed in iMSNs, excitatory synaptic terminals that drive both MSN subtypes and in some striatal interneurons, and modulate various forms of long-term synaptic plasticity in both dMSNs and iMSNs (Lerner & Kreitzer 2012; Wu et al. 2015; Xu et al. 2018; Shen et al. 2008). Although we found that DSE induction in iMSNs and dMSNs is unaffected by preincubation with sulpiride, a D2R-antagonist (Supplementary Fig. 2), the role of striatal D2Rs in regulating synaptic 2-AG signaling deserves further study. Nevertheless, the present findings indicate that 2-AG signaling can be differentially regulated in various neuronal subtypes, perhaps reflecting their diverse neurochemical environments that act in part via multiple signaling pathways to modulate DGLα.
In summary, our present and previous data indicate that multiple protein kinases can differentially modulate DGLα activity by targeting distinct residues within the C-terminal domain. It will be interesting to determine which kinases phosphorylate other residues in DGLα that were detected here (Fig. 1a) and whether they also contribute to the overall modulation of 2-AG synthesis by regulating DGLα activity, or perhaps the subcellular localization (Zhou et al. 2016). Such mechanisms may allow multiple neurotransmitters to fine-tune the rate of 2-AG synthesis to establish an eCB “set point” that is uniquely poised to modulate synaptic tone under basal conditions. In the case of PKA actions in striatal dMSNs, this presumably contributes to the ability of dopamine to alter the balance of striatal output via the direct and indirect pathways to modulate striatal-based behaviors.
Supplementary Material
Acknowledgements
We are grateful for outstanding technical assistance from Walker Parrish and the generous gift of purified PKA catalytic subunit from Dr. Jackie D. Corbin (Vanderbilt University). Thanks also for critical input into a draft of this manuscript from Drs. Danny Winder and Sachin Patel (both Vanderbilt University).
Funding:
This work was supported by the National Institutes of Health (K01 MH107765 to B.C.S., T32-MH065215 to J.R.S., F31-MH109196 and T32-DK07563 to C.R.M., R01-NS078291 to R.J.C.), the American Heart Association (15PRE25110020 to C.R.M), and Vanderbilt University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funding agencies. Endocannabinoid measurements were conducted at the Vanderbilt Mass Spectrometry Research Center Facility with an instrument purchased with support from the National Institutes of Health (Grant No. S10 OD017997). The authors report no significant biomedical financial interests or potential conflicts of interest.
Abbreviations used:
- 2-AG
2-arachidonoyl glycerol
- ACSF
artificial cerebrospinal fluid
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CB1R
type 1 cannabinoid receptor
- D1R
D1-dopamine receptor
- D2R
D2-dopamine receptor
- DGLα
diacylglycerol lipase-α
- d/iMSN
striatal medium spiny neurons projecting via the direct/indirect output pathways, respectively
- DSE
depolarization-enhanced suppression of excitation
- eCB
endocannabinoid
- eEPSC
evoked excitatory postsynaptic current
- PKA
cyclic AMP-dependent protein kinase
REFERENCES CITED
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68, 247–286. [DOI] [PubMed] [Google Scholar]
- Augustin SM and Lovinger DM (2018) Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chem Neurosci 9, 2146–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM and Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63, 182–217. [DOI] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Mallet L, Feng G and Graybiel AM (2015) Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol 30, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Bisogno T and Di Marzo V (2019) Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat Rev Neurol. [DOI] [PubMed] [Google Scholar]
- Eng JK, Mccormack AL and Yates JR (1994) An Approach to Correlate Tandem Mass-Spectral Data of Peptides with Amino-Acid-Sequences in a Protein Database. J Am Soc Mass Spectr 5, 976–989. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Rammohan KW and Horrocks LA (1989) Isolation, characterization, and regulation of diacylglycerol lipases from the bovine brain. Ann N Y Acad Sci 559, 25–36. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Doherty P and Alexander SPH (2017) Endocannabinoid Turnover. Adv Pharmacol 80, 31–66. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB et al. (2010) Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci 30, 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR and Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA and Kreitzer AC (2016) Cortico-Basal Ganglia Circuit Function in Psychiatric Disease. Annu Rev Physiol 78, 327–350. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T and Kano M (2007) Ca(2+)-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol 17, 360–365. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K and Kano M (2008) Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology 54, 58–67. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS and Kano M (2005) Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron 45, 257–268. [DOI] [PubMed] [Google Scholar]
- Hill MN, Campolongo P, Yehuda R and Patel S (2018) Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 43, 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M and Watanabe M (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Lampidonis AD, Rogdakis E, Voutsinas GE and Stravopodis DJ (2011) The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene 477, 1–11. [DOI] [PubMed] [Google Scholar]
- Lerner TN and Kreitzer AC (2012) RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron 73, 347–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean B, Tomazela DM, Shulman N et al. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A and Kano M (2001) Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31, 463–475. [DOI] [PubMed] [Google Scholar]
- Maejima T, Oka S, Hashimotodani Y, Ohno-Shosaku T, Aiba A, Wu D, Waku K, Sugiura T and Kano M (2005) Synaptically driven endocannabinoid release requires Ca2+-assisted metabotropic glutamate receptor subtype 1 to phospholipase Cbeta4 signaling cascade in the cerebellum. J Neurosci 25, 6826–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur BN and Lovinger DM (2012) Endocannabinoid-dopamine interactions in striatal synaptic plasticity. Front Pharmacol 3, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murataeva N, Straiker A and Mackie K (2014) Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol 171, 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P and Manzoni OJ (2011) Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci 14, 1542–1547. [DOI] [PubMed] [Google Scholar]
- Rosenberger TA, Farooqui AA and Horrocks LA (2007) Bovine brain diacylglycerol lipase: substrate specificity and activation by cyclic AMP-dependent protein kinase. Lipids 42, 187–195. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P and Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321, 848–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Bluett RJ, Ramikie TS et al. (2014) Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep 9, 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Parrish WP, Haddad HK et al. (2018) Role of Striatal Direct Pathway 2-Arachidonoylglycerol Signaling in Sociability and Repetitive Behavior. Biol Psychiatry 84, 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Wang X, Rose KL et al. (2013) CaMKII regulates diacylglycerol lipase-alpha and striatal endocannabinoid signaling. Nat Neurosci 16, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonesy BC, Winder DG, Patel S and Colbran RJ (2015) The initiation of synaptic 2-AG mobilization requires both an increased supply of diacylglycerol precursor and increased postsynaptic calcium. Neuropharmacology 91, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Anderson GR, Sutton LP, Dao M and Martemyanov KA (2018) Selective Role of RGS9-2 in Regulating Retrograde Synaptic Signaling of Indirect Pathway Medium Spiny Neurons in Dorsal Striatum. J Neurosci 38, 7120–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y et al. (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327. [DOI] [PubMed] [Google Scholar]
- Wei D, Allsop S, Tye K and Piomelli D (2017) Endocannabinoid Signaling in the Control of Social Behavior. Trends Neurosci 40, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Kim JI, Tawfik VL, Lalchandani RR, Scherrer G and Ding JB (2015) Input- and cell-type-specific endocannabinoid-dependent LTD in the striatum. Cell Rep 10, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Perez S, Cornil A et al. (2018) Dopamine-endocannabinoid interactions mediate spike-timing-dependent potentiation in the striatum. Nat Commun 9, 4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G et al. (2011) Postsynaptic diacylglycerol lipase alpha mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Tanimura A, Graves SM, Shen W and Surmeier DJ (2018) Striatal synapses, circuits, and Parkinson’s disease. Curr Opin Neurobiol 48, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang M, Bisogno T, Di Marzo V and Alger BE (2011) Endocannabinoids generated by Ca2+ or by metabotropic glutamate receptors appear to arise from different pools of diacylglycerol lipase. PLoS One 6, e16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Howell FV, Glebov OO, Albrecht D, Williams G and Doherty P (2016) Regulated endosomal trafficking of Diacylglycerol lipase alpha (DAGLalpha) generates distinct cellular pools; implications for endocannabinoid signaling. Mol Cell Neurosci 76, 76–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.