Abstract
Cell differentiation, proliferation, and death are vital for immune homeostasis. Wnt signaling plays essential roles in processes across species. The roles of Wnt signaling proteins and Wnt ligands have been studied in the past, but the context-dependent mechanisms and functions of these pathways in immune responses remain unclear. Recent findings regarding the role of Wnt ligands and Wnt signaling in immune cells and their immunomodulatory mechanisms suggest that Wnt ligands and signaling are significant in regulating immune responses. We introduce recent key findings and future perspectives on Wnt ligands and their signaling pathways in immune cells as well as the immunological roles and functions of Wnt antagonists.
The Emerging New Face of Wnt in immunology
Wnt signaling is vital for the development and regeneration of most organs in the body, and Wnt action is broadly conserved across species and cell types. These signaling pathways rely on the interaction between a Wnt protein and a Frizzled (Frz) receptor, but multiple homologs of each of these molecules exist. For example, in mammals, 19 Wnt ligands, 10 Frz receptors, and several coreceptors (e.g., low-density lipoprotein receptor-related proteins LRP5 and LRP6) have been identified (see ‘The Wnt homepage’ at www.stanford.edu/group/nusselab/cgi-bin/wnt/). Depending on the nature of the ligands and downstream events, Wnt signaling pathways have been broadly divided into two types – the canonical and non-canonical Wnt pathways.
With remarkable progress in the stem cell field, Wnt signaling pathways are now garnering increased attention as emerging putative targets for molecular therapeutics in cancer [1]. The Wnt system is an essential regulator of tissue homeostasis [2]. A variety of inflammatory diseases (e.g., infection, allergy, cancer, and autoimmune diseases) can be viewed as host responses for tissue injury and repair [3].
It is logical to consider that Wnt ligands and Wnt signaling pathway proteins play key roles in both acute and chronic inflammation. Consistent with this view, dysregulation of Wnt signaling pathways and aberrant expression of Wnt antagonists have been reported recently in cancer, asthma, and autoimmunity in mice and humans [4–7]. One of the most important aspects to consider is that recent findings in immunology implicate multiple layers of immune regulation via Wnt ligands and Wnt signaling proteins. Owing to the context-dependent roles of Wnt ligands and Wnt signaling proteins, it is expected that future experimental or translational findings regarding Wnt in Immunology may have fundamental and broad importance in biomedical science. This brings an exciting opportunity to discover new layers of immune-modulation mechanisms and to revisit the role of Wnt proteins in multiple diseases. In this review, we attempt to provide novel aspects of Wnt biology, integrating the roles of Wnt ligands and their signaling pathways with immune modulation in tissue injury and repair. First, we briefly describe canonical and non-canonical Wnt signaling pathways. Next, we discuss recent findings regarding Wnt ligands and Wnt pathway proteins in myeloid lineage cells (e.g., macrophages and dendritic cells, DCs) and lymphoid lineage cells (e.g., CD8+, CD4+, and regulatory T cells, Tregs; see Glossary). Lastly, we introduce the role of Wnt antagonists and place them in context of recent findings in the field of immunology.
Molecular Players in Canonical and Non-Canonical Wnt Signaling
In the canonical Wnt signaling pathway, the Wnt ligand binds to the Frz receptor and its coreceptor, LRP 5/6 (Figure 1A). The Wnt1 class ligands (Wnt2, Wnt3, Wnt3a, and Wnt8a) function via the canonical Wnt/β-catenin signaling pathway. When Wnt signaling is ‘off’, β-catenin levels are maintained at low levels by the β-catenin destruction complex [1]. The complex consists of protein kinases such as casein kinase (CK)-1 and glycogen synthase kinase (GSK)-3β, the tumor-suppressor adenomatous polyposis coli (APC) protein, and the scaffolding protein Axin. Activation of the canonical Wnt pathway induces cell proliferation, differentiation, maturation, body-axis specification, and morphogenetic signaling [8].
Figure 1. Canonical and Non-Canonical Wnt Pathways in Mammals (A) Degradation of phosphorylated (P) β-catenin is mediated by the ubiquitin pathway that involves interactions between β-transducin repeat-containing protein (β-TrCP) and the β-catenin destruction complex.
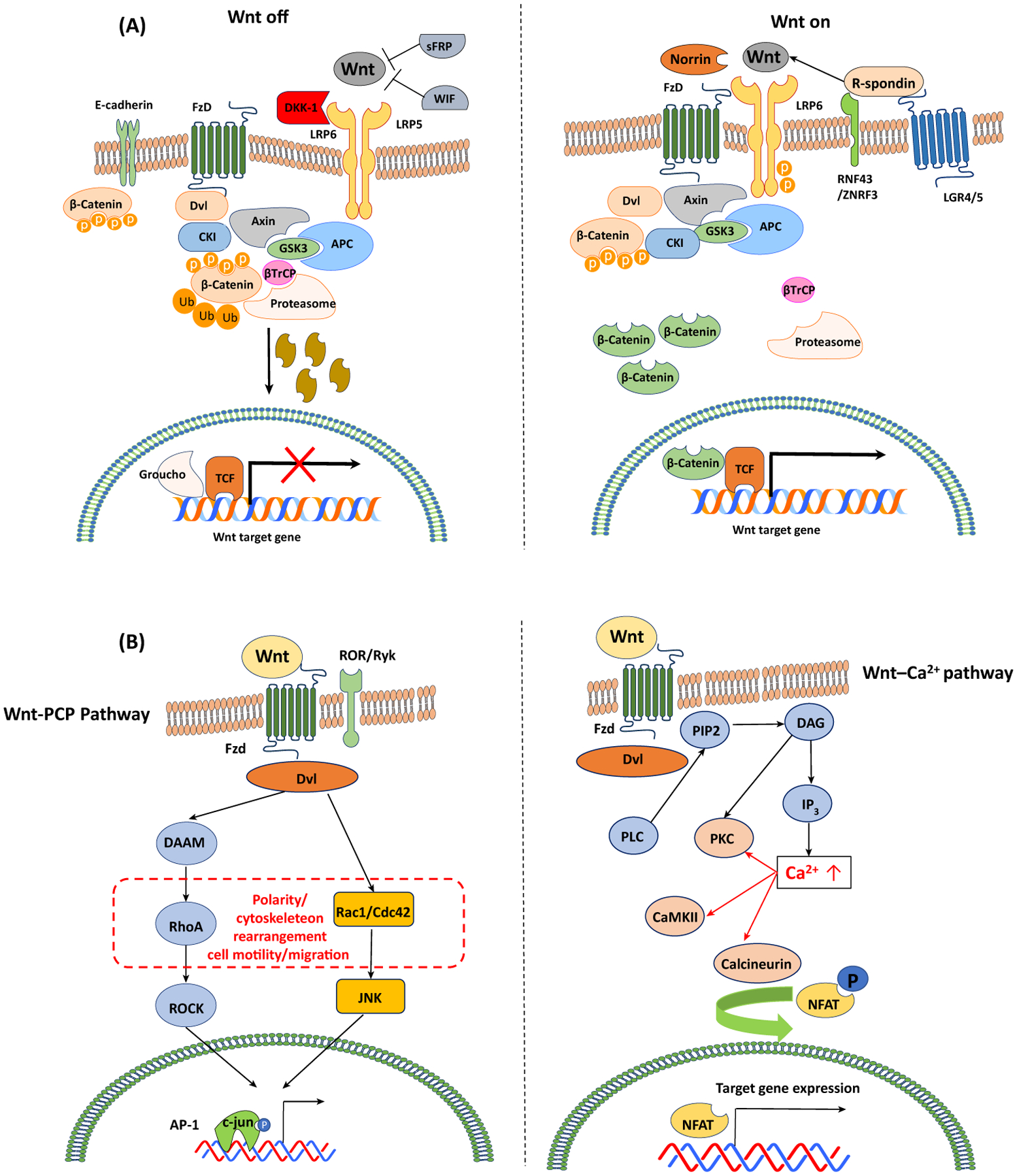
The elimination of cellular β-catenin depletes its pool to translocate to the nucleus; thus, Wnt target gene expression is repressed (Wnt-OFF). Upon Wnt ligand binding to Frz and LRP5/6, the activation of the adaptor protein disheveled (Dvl) and recruitment of Axin complex to the LRP coreceptor are initiated. The decreased proteasomal degradation of phosphorylated β-catenin leads to the accumulation and nuclear translocation of β-catenin, subsequently forming molecular complexes with TCF-1/LEF-1. This interaction eliminates transcriptional repressors (e.g., Groucho), initiating the expression of Wnt target genes (Wnt-ON). Wnt signaling is enhanced by R-spondin binding to its receptor LGR-5 and RNF43. (B) In the Wnt/Ca2+ pathway, Wnt ligands induce the release of intracellular calcium. The Wnt/Ca2+ pathway involves activation of phospholipase C (PLC) and turnover of phospholipid membranes in the endoplasmic reticulum (ER), and the release of intracellular Ca2+. The increased Ca2+ levels result in the activation of Ca2+-dependent signaling molecules such as protein kinase C (PKC), Ca2+/calmodulin-dependent protein kinase (CamKII), and the calcineurin-dependent transcriptional nuclear factor of activated T cells (NFAT). In the planar cell polarity (PCP) pathway, Wnt ligands stimulate activation of the small GTPases Rho and Rac, leading to cytoskeletal rearrangements and cell motility. Ca2+–NFAT signaling plays crucial roles in lymphocyte activation. Signaling crosstalk between the Wnt/Ca2+ pathway and the canonical Wnt pathway to regulate NFAT translocation via GSK-3β has been proposed.
By contrast, non-canonical Wnt signaling is initiated by the Wnt5a type (Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11). The Wnt5a type ligands generate the non-canonical Wnt/planar cell polarity (PCP) or Wnt/Ca2+ pathways and regulate cellular polarization and migration (Figure 1B). Wnt ligands are recognized by Frz/retinoic acid-related orphan receptor (ROR)/receptor tyrosine kinase (RYK) receptor complexes. Both canonical and non-canonical Wnt pathways regulate crucial cell functions across species (e.g., survival, apoptosis, proliferation, cell fate decision, cell motility, and cytoskeletal rearrangements) [8,9]. In fact, a recent report suggested that RYK could regulate natural killer (NK) cell development in a temporal fashion, and diminished self-renewal of hematopoietic stem cells was observed in RYK-deficient mice relative to littermate controls, indicating a novel role of RYK in hematopoiesis [9].
There are Wnt antagonistic ligands that inhibit Wnt pathways, including the secreted frizzled-related proteins (sFRP1–5), Wnt inhibitory factor (Wif1), Cerberus, Wise/SOST, and the Dickkopf family proteins across species. On the one hand, sFRPs and WIF1 bind to Wnt agonistic ligands, sequestering Wnt agonists in the extracellular space [10]. On the other hand, Dickkopf family proteins and Wise/SOST competitively prevent binding of Wnt agonists to their receptor LRP5/6 [10].
Wnt ligands in tissue injury and repair play important roles in inflammation and some immune diseases. These pathways are well-reviewed elsewhere [1,2,8,11,12]. In addition to the ligands and receptors mentioned above, Wnt signaling is also regulated by specific modulators and coreceptors. For example, R-spondin can enhance Wnt signaling, an effect that depends on R-spondin binding to one of its receptors, the leucine-rich repeat-containing G protein-coupled receptor (LGR) proteins 4, 5, and 6 in mouse [10,13]. Binding of R-spondin to LGR proteins prevents Frz ubiquitination and subsequent lysosomal degradation [14]. Collectively, Wnt ligands and their signaling pathways are regulated by multiple ligands and pathways, implicating context-dependent roles in a variety of immune responses in tissue injury and repair processes.
Wnt Signaling in Myeloid Cells
Dendritic Cells
DCs are an important subset of immune cells that drive a selective adaptive immune response. Wnt and Notch signaling can promote DC differentiation in mice and humans both in vitro and in vivo [15]. It has been reported that specific ablation of β-catenin expressed in DCs promoted type 1/17T helper cell (Th1/Th17) responses while it decreased Treg responses, suggesting that β-catenin plays a tolerogenic role in gut DCs to control colitis in the murine DSS (dextran sodium sulfate) colitis model [16]. In separate studies, β-catenin-mediated immunological tolerance in DCs was achieved by expressing IL-10 and vitamin A-metabolizing enzymes, operating in cooperation with other signaling molecules such as PI3K/Akt, TLRs, MAPKs, Fas, and PLCγ2 in mice [17–20] (Figure 2A). Another mechanism of β-catenin-mediated immunological tolerance induction in DCs is β-catenin activation by disruption of homophilic E-cadherin interaction [21]. The disruption caused human DC maturation but failed to produce proinflammatory cytokines, resulting in IL-10-producing CD4+ T cell differentiation. Because phosphorylated E-cadherin binds to cellular β-catenin, thereby serving as a reservoir of cellular β-catenin, disruption of human E-cadherin phosphorylation by point mutations of three serine residues in the protein releases β-catenin into the cytosol in a Wnt-independent manner in human embryonic kidney (HEK) 293T and Madin-Darby canine kidney (MDCK) cells [22]. Wnt ligands such as Wnt3a and Wnt5a contribute to the induction of tolerogenic murine DCs by reprogramming DC responses to lipopolysaccharide (LPS), although they do not alter DC maturation in vitro [23]. Recently, however, it has been shown that melanomas induce DC-mediated tolerance to impede effective immunotherapy via a Wnt5a-mediated mechanism in mice [24]. In this study, melanoma-derived Wnt5a triggered fatty acid oxidation (FAO) in DCs via β-catenin and PPAR-γ to induce tolerogenic DCs promoting Treg generation. Inhibition of this pathway using DC-specific ablation of β-catenin improved anti-PD-1 immunotherapy outcomes in a syngeneic tumor model using the BrafV600EPten−/− melanoma-derived cell line, suggesting a promising perspective of targeting Wnt ligands in cancer immunology.
Figure 2. Wnt Proteins in T cells and Dendritic Cells (DCs) in Mice and Humans.
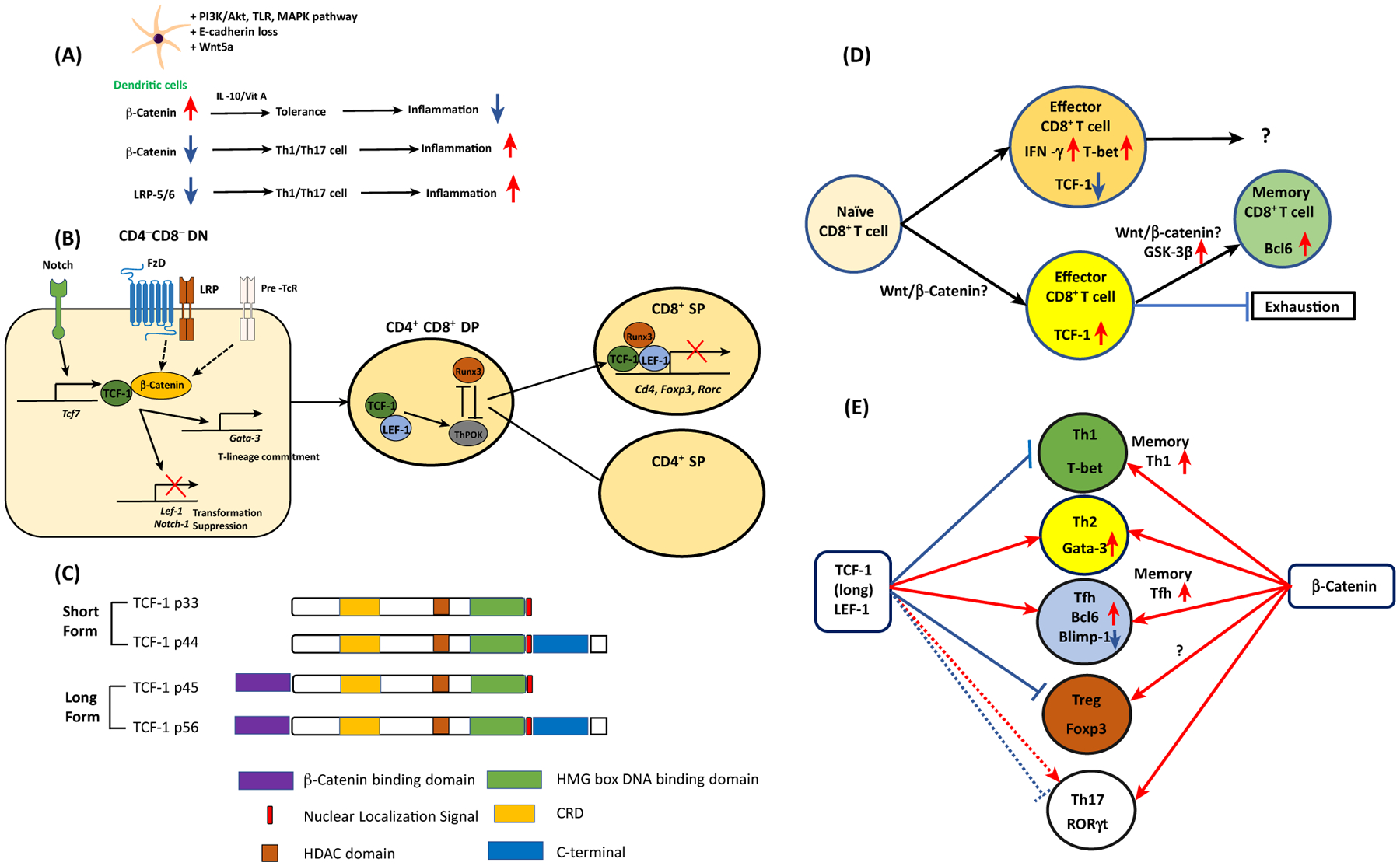
(A) β-Catenin accumulation by various signaling pathways and Wnt5a induces tolerogenic DCs to suppress inflammation. When DCs lack β-catenin or Wnt coreceptors LRP5/6, CD4+ T cells are preferentially differentiated into type 1/17 T helper (Th1/Th17) cells to promote inflammation. (B) In the CD4CD8 DN stage, thymocytes receive Notch signals and induce TCF-1. Together with β-catenin, TCF-1 inhibits thymocyte transformation while directing T-lineage commitment via Gata-3 expression. In the CD4CD8 DP stage, TCF-1 interacts with RUNX3 and ThPOK. The interaction between TCF-1/LEF-1 and RUNX3 directs the development of CD8+ T cells. This transcriptional factor complex represses Cd4, Foxp3, and Rorc gene expression. (C) TCF-1 short and long isoforms and their domains. (D) Naïve CD8 T cells are resistant to become CD8 effector T cells if they are TCF-1hi cells, while TCF-1lo cells become active IFN-γ+ T-bet+ effector T cells. TCF-1hi CD8+ effector T cells become more stem-cell-like memory CD8+T cells with elevated expression of Bcl6. Whether the canonical Wnt pathway is involved in this process is controversial. (E) In CD4+ T cells, naïve CD4+ T cells can be differentiated into five different types of helper T cells according to their lineage-specification transcriptional factors. TCF-1 expression favors Th2 and follicular helper T (Tfh) cell formation while it inhibits Th1 differentiation and Treg suppressor function. The role of TCF-1 in Th17 cells is dependent on the microenvironment. In memory CD4+ T cells, TCF-1 enhances memory Th1 and Tfh cell formation. Unlike TCF-1, expression of β-catenin enhances all types of T cell differentiation and function. Abbreviations: DN, double negative; DP, double positive.
The role of Wnt signaling in neuronal inflammation has been proposed previously [11]. For instance, loss of the Wnt receptor LRP5/6 in DCs has been shown to decrease the production of IL-10 and TGF-β expression but increased IL-12, TNF-α, and IL-6 protein expression in bone marrow-derived DCs, exacerbating Th1/Th17 cell-mediated central nervous system (CNS) inflammation in the experimental autoimmune encephalomyelitis (EAE) model, as evidenced by elevated concentrations of IFN-γ and IL-17 secretion in CNS-derived CD4+ T cells, and increased clinical pathology score [25]. Consistent with this finding, loss of LRP5/6 in DCs using DC-specific LRP-5/6 deficient mice induced enhanced antitumor immunity by directing less Treg cell expansion and increasing effector CD8+ T cell functions (e.g., increased granzyme B, TNF-α, and IFN-γ expression) relative to wild-type tumor-bearing mice, thus resulting in reduced tumor growth in murine thymoma (EL4), melanoma (B16F10), and Lewis lung carcinoma (LLC) mouse models [26]. DCs are crucial subsets in antitumor immunotherapy and are able to modulate T cell responses; the potential of therapeutically targeting Wnt pathways in DCs is reviewed elsewhere [27].
DC-derived Wnt ligands can modulate B cell and T cell-mediated immune responses. For example, follicular dendritic cells (FDCs) from human tonsils can express Wnt5a. Specifically, FDC-derived Wnt5a expression protected germinal center (GC) B cell death in the absence of serum in vitro, via Ca2+ activation, also inducing CD40 and interferon regulatory factor (IRF4) mRNA expression, indicating that rapid activation of GC B cells was achieved [28]. DC-derived Wnt5a was also able to induce IFN-γ-expressing CD4+ T cells in human samples. In this study, knockdown ofWnt5a using siRNA in human monocyte-derived DCs impaired IL-12 secretion – an important process in the differentiation of CD4+ T cells into IFN-γ expressing CD4+ T cells [29].
Collectively, recent studies on Wnt pathway proteins in DCs suggest that the role of the Wnt–β-catenin pathway may be involved in generating tolerogenic DCs. However, the involvement of Wnt protein crosstalk with other pathways and context-dependent DC functions suggests the existence of Wnt-independent mechanisms as well as prompting caution in concluding that Wnt pathways play a primary role in DC functions.
Macrophages
Because macrophages establish a tissue repair program after tissue injury in many organs, the expression of Wnt ligands or Wnt signaling in macrophages is considered to be crucial for tissue regeneration and fibrosis [30]. A recent study showed that β-catenin is activated in alveolar macrophages (AMs) in uninjured lungs of mice [31]. A previous study reported early expression of WNT5A and matrix metalloproteinase (MMP)-7 for collagen synthesis in the human lung epithelial cell line (BEAS-2B), as well as in lung biopsy samples from human sepsis patients and in a murine cecal ligation and perforation sepsis model [32]. Because wound healing is mediated by collagen synthesis, the study implicated WNT5A in tissue repair [32]. Another study showed that tick-borne bacteria Francisella tularensis infection in mouse peritoneal macrophages induced GSK-3β activation in vitro and in vivo. Specifically, inhibition of GSK-3β resulted in a marked reduction of anti-inflammatory cytokine production (e.g., IL-6, TNF-α, IL-12p40) in the mouse tularemia model [33]. Two recent studies showed that attenuation of the Wnt–β-catenin pathway using Lrp5-deficient mice reduced lung fibrosis, and conditional genetic ablation of β-catenin in macrophages improved resolution of organ fibrosis in a mouse model bleomycin-induced lung injury [31,34].
In the heart, cardiac repair after myocardial infarction (MI) can be mediated, at least in part, by macrophages in mice [35]. A recent study demonstrated that macrophage-specific deficiency of Wntless, a protein required for Wnt ligand secretion, improved cardiac repair and heart function after ischemic injury in a mouse isofluorane myocardial infarction (MI) model [35]. Of note, this study showed that the loss of Wnt ligand secretion using Wntless-deficient myeloid cells preferentially generated M2-like macrophages with anti-inflammatory (decreased IL-1α protein), reparative, and angiogenic properties (increased VEGF protein expression). This finding suggested that targeting natural Wnt inhibitors might be a potentially effective strategy to improve cardiac repair after MI, but this remains to be determined.
In the intestine, genetic macrophage-specific deletion of porcupine, a protein required for Wnt ligand palmitoylation, failed to rescue intestinal stem cells from radiation-induced cell death and resulted in increased susceptibility to radiation-induced injury relative to controls, suggesting that macrophage-derived Wnt ligands might be important for tissue repair in mice [36]. In a separate study, Stat6 deficiency reduced the mRNA expression of Wnt ligands Wnt2b, Wnt7b, and Wnt10a in the mucosa and in cells of the lamina propria in a TNBS (2,4,6-trinitrobenzene sulfonic acid)-induced colitis mouse model. In vitro differentiation of M2a macrophages from wild-type peritoneal macrophages (so-called wound-healing macrophages) overexpressed Wnt ligands Wnt2b, Wnt7b, and Wnt10a mRNA relative to Stat6-deficient M2a macrophages. Adoptive transfer of M2a macrophages or administration of a Wnt agonist accelerated wound healing in mice with TNBS-induced colitis relative to controls [6]. A crucial reparative function of Wnt7bfrom macrophages has been further shown. Somatic deletion of the third exon of Wnt7b in macrophages prevented normal tissue repair and regeneration of kidney in a post-ischemia reperfusion injury model in mice [37]. Moreover, by engulfing hepatocyte debris during adult biliary regeneration in mice, macrophages have been reported as a source of Wnt ligands (including Wnt3a) in liver (macrophages were depleted via liposomal clodronate to verify the source of Wnt ligands) [38]. This process, in turn, prompted the specification of mouse hepatic progenitor cells (HPCs) into hepatocytes via this β-catenin-specific pathway in vivo in mice [38]. Another example is that of Wnt7B, whose protein expression in myeloid cells, including tumor-associated macrophages (TAMs), can serve as a molecular switch for angiogenesis and macrophage-mediated lung metastasis in mice and humans [39]. In this study, WNT7B was highly expressed in human mammary tumors and was associated with the presence of tumor-associated macrophages (TAMs). Moreover, in a MMTV-PyMT mouse model, genetic ablation of the Wnt7b gene in myeloid cells led to reduced mammary gland tumor mass and volume relative to wild-type mice [39].
Other reports indicate that aberrant expression of non-canonical Wnt ligands such as Wnt5a in macrophages can be associated with metabolic inflammation in sepsis, cancer, obesity, and atherosclerosis in humans and mice [40]. A molecular clue from the presumed proinflammatory role of Wnt5a in macrophages has been reported: specifically, upon the recognition and internalization of E. coli or Chandipura virus (CHPV) infection by the CD14 receptor, the Wnt5a–Frz5–Rac1–p65 signaling axis led to subsequent Toll-like receptor (TLR) signaling in the murine RAW 264.7 macrophage cell line and in bone marrow-derived macrophages (BMDMs) [41]. This enabled macrophages to perform their immune functions (IFN production) and ensure cell survival (increased BCL-2 expression) upon activation of this signaling axis [41]. Wnt5a may not always harbor a proinflammatory role in organ injury/repair and infection; for instance, a report indicated that Wnt5a could induce macrophages of the immunosuppressive type in mice and humans [42]. Wnt5a inhibited human M1 type macrophage differentiation via inhibition of the NF-κB pathway while inducing the production of immunosuppressive cytokines (e.g., IL-10 and TGF-b), thus exhibiting an M2-like macrophage phenotype [42]. In human breast cancer patient tissue microarrays and immunohistochemistry assays, Wnt5a expression in breast tumors was associated with the presence of immunosuppressive CD163+ TAMs [42]. The opposing roles of macrophage-derived Wnt5a in a variety of inflammatory diseases and biological functions are reviewed elsewhere [40,43]. In sum, studies indicate that, when induced, Wnt ligands in macrophages can play reparative roles in tissue injury and repair, although not universally. Indeed, not all Wnt ligands show similar biological functions and activities. Thus, more extensive studies will be necessary to assess the mechanisms of action of each Wnt ligand and Wnt signaling component to obtain a comprehensive understanding of their functional roles in macrophages and whether these roles are species-specific or not.
Wnt Signaling in T Lymphocytes
The individual roles of Wnt signaling proteins have been studied in T cell development and function (Figure 2A and Box 1). In the past, by using conditional deletion of Wnt signaling proteins in mice or by using small molecules to inhibit Wnt pathways in T cell subsets, studies have proposed that the canonical Wnt pathway can play a vital role in T cell biology [12]. However, cumulative evidence has revealed that elimination of each Wnt signaling component does not necessarily phenocopy elimination of other components. A closer look at the recent developments in assessing the role of individual Wnt signaling proteins in T cells may help to better elucidate their biological role in the immune response.
Box 1. Wnt Signaling in T Cell Development.
Wnt signaling has been characterized in thymopoiesis. Wnt transcription factors (TFs)TCF-1 and LEF-1 are essential in early-stage mouse thymocyte development [100]. It has been shown that TCF-1 is a downstream target gene of the Notch signaling pathway, and it directly restrains LEF1 to prevent thymocyte transformation in TCF-1-deficient mice while it promotes maturation of T-lineage commitment [101]. After positive selection, both TFs interact with β-catenin to dictate differentiation of CD4CD8 double-positive (DP) cells into CD4+ T cells upstream of Th-POK, while CD8+ T cell development is mediated by the interaction between Runx3 and TCF-1 to silence Cd4 gene expression [102]. A further study revealed that TCF-1 and LEF-1 are essential to establish CD8 T cell identity with their intrinsic HDAC activity, repressing Cd4, Foxp3, and Rorc in mice [103]. This suggests a novel function of TCF/LEF in addition to their TFs to turn on Wnt target gene expression (see Figure 2B in main text). TCF-1 is expressed in multiple isoforms with intrinsic HDAC and DNA-binding activity. Among them, the long isoforms possess an N-terminal β-catenin-interacting domain. The importance of the interaction between β-catenin and long TCF-1 isoforms via its N-terminal domain was demonstrated in the long TCF-1 isoform-deficient (p45−/−) mice, revealing that the interaction is crucial for thymocyte survival but not thymic maturation [49]. The role of β-catenin in thymopoiesis has been studied, demonstrating that it upregulates IL-7Rα expression in thymocytes during positive selection [49,104–106]. In thymus-derived Treg (tTreg) generation, it has been shown using a proteomics approach that TCF-1 limits Treg generation in a gene-dosage-dependent manner [107].
Role of Wnt Proteins in CD8+ T Cell Responses
The dichotomy of CD4+ and CD8+ T cell-mediated immune responses is central to our understanding of adaptive immunity. In acute viral infections, the activation of naive T cells leads to effector T cell generation, eliminating the pathogen and forming pools of memory T cells to provide heightened protection.
Indeed, the differentiation and persistence of memory CD8+ T cells appears to be regulated by Wnt pathway transcriptional factors such as T cell factor (TCF)-1 and lymphoid enhancer factor (LEF)-1 in Listeria monocytogenes-expressing OVA (LM-OVA) infected mice, respectively [44,45]. Deficiency of TCF-1 and LEF-1 in CD8+ T cells markedly decreased the killer cell lectin-like receptor G1 (KLRG1)loIL-7Rα+ memory T cell precursor population relative to wild-type mice, resulting in a poor recall response to infection (IFN-γ secretion), as evidenced from insufficient and functionally inferior memory T cell populations relative to TCF-1- and LEF-1-expressing CD8+ T cells which were able to mediate a secondary response [44,45]. These studies showed the essential function of TCF-1 and LEF-1 in the maturation, longevity, and secondary expansion of mouse memory CD8+ T cells. A prior report using the acute lymphocytic choriomenignitis virus (LCMV) or L. monocytogenes infection mouse infection models was consistent with these findings because TCF-1 was deemed to be a transcriptional repressor and not a transcriptional activator of an effector CD8+ T cell phenotype CD8+ T cells (based on differences in IFN-γ and T-bet expression) [46].
A collection of recent studies assessed the mechanistic role of TCF-1 in CD8+ T cells, mainly implicating this transcription factor in (i) effector CD8 T cell and memory T cell pool formation, (ii) suppression of cytotoxic CD8+ T cells and follicular helper T (Tfh) cell-associated gene upregulation, (iii) differential regulation of CD8+ T cells by long and short TCF-1 isoforms, and (iv) mouse models of cancer and chronic LCMV infection.
First, the role of TCF-1 in CD8+ memory T cell generation and effector function was assessed in acute infections of L. monocytogenes or LCMV (Armstrong strain) in mice [47]. The first three CD8+ T cell divisions after the infection produced daughter cells contributing to the memory cell pool [47]. The daughter cells that showed more robust proliferation with effector-prone cells became TCF-1-negative cells, while TCF-1-positive daughter cells showed a stem cell-like phenotype with self-renewal activity after resolution of acute infection (Figure 2D) [47].
Second, TCF-1 has been implicated in the regulation of cytotoxic CD8+ T cells and in Tfh cell-associated gene expression [48]. For instance, Runx3 deficiency in CD8+ T cells resulted in aberrantly increased gene expression of Bcl6 and Tcf7 (encoding TCF-1), thus inhibiting the upregulation of cytotoxic molecules (e.g., granzyme B and perforin) in effector CD8+ T cells in mice infected with LCMV or L. monocytogenes [48]. In addition, ablation of Tcf7 in Runx3-deficient CD8+ effector T cells blocked Tfh-associated gene upregulation of Bcl6 and Il21. This indicated that Runx3-mediated Tcf7 gene repression might upregulate the expression of cytotoxic proteins, enforcing acquisition of cytotoxic functions. Although there was no direct test, the plausible underlying mechanism presumably occurred via protection of cytotoxic lineage integrity by preventing differentiation towards the Tfh lineage, and implicating TCF-1 in driving cellular differentiation and cytotoxicity [48].
Third, differential promoter usage and alternative splicing have been shown to generate multiple TCF-1 isoforms in mouse thymocytes [49]. Although the TCF-1 short isoforms (which lack the β-catenin binding domain) were initially thought to be dominant negatives or non-functional, a later study using TCF-1 long isoform (p45−/−) deficient mice revealed that TCF-1 short isoforms were adequate to support thymocyte development and regulate the majority of TCF-1-mediated gene regulation, despite some moderate reduction of thymic output [49,50] (Figure 2C). It was noted that the TCF-1 long isoform contributed to central memory CD8+ T cell maturation and secondary expansion during a recall response in the acute LCMV infection model using TCF-1 long isoform-deficient mice [51]. This study suggested that TCF-1 isoforms might have distinct functions and represent an evolutionarily conserved means to ensure proper programming of T cell responses, as in the case of viral infections [51].
Lastly, two recent reports identified a population of TCF-1hi antigen-specific CXCR5+CD8+ T cells that could proliferate following PD-1 inhibitory pathway blockade in two murine models, namely chronic LCMV (clone 13 strain) infection and the 3-methylcholanthrene (MCA)-induced fibrosarcoma model [52,53]. These studies showed that antigen-specific TCF-1hi CD8+ T cells, related to inhibitory immune checkpoints, exhibited less exhaustion and harbored a more stem cell-like phenotype upon Bcl-6 expression than did TCF-1loCD8+ T cells; this seemed to counteract type I IFN-mediated CD8+ T cell exhaustion given that type I IFNAR-deficient mice, or neutralizing type IIFN production via antibodies, led to increased numbers of TCF-1hiCD8+T cells [52,53]. These studies were significant because they suggest that high expression of TCF-1 and the activation of the TCF-1-Bcl6 pathway could be important in the generation of antigen-specific effector responses in vivo.
Additional reports have suggested that, during unresolved viral infections (e.g., HIV-1 or chronic LCMV infection), human and mouse infected CD4+ Tfh cells can establish persistent reservoirs within lymph nodes [54,55]. Specifically, TCF-1 and BCL-6 were shown to be required for the development of CXCR5+ ‘follicular cytotoxic T cells’ (TFCs) to eradicate virus-infected Tfh cells in mice. Moreover, CD4+ T cell specific ablation of TCF-1 and Bcl6 in mice showed decreased TFC development upon LCMV infection, resulting in more virus-infected Tfh cells in lymph nodes [55]. Another study showed that Wnt ligands from human progenitor-derived astrocytes (Wnt1, Wnt2b, and Wnt3) could induce anti-HIV-1 CD4dimCD8bright T cells from CD8+ T cells in vitro [56]. Furthermore, depletion of various Wnt ligands in the culture supernatant of human progenitor-derived astrocytes resulted in decreased CD4dimCD8bright T cell conversion from CD8+ T cells. In vivo, a humanized HIV-1 infection mouse model was used to compare the role of human CD4dimCD8bright T cells to CD8+ single-positive T cells in controlling infection in the mouse brain; CD4dimCD8bright T cells from human PBMC-reconstituted NOD/SCID/IL-2rcγ−/− infected mice presented better survival and HIV-1-infected target cell cytolysis ex vivo than CD8+ T cells. In addition increased numbers of CD4dimCD8-bright infiltrating cells correlated with reduced HIVGAG mRNA transcripts in mouse brains and with better control of HIV-1 infection than single-positive cells [56]. These effects likely occurred in a Wnt signaling-dependent manner, although further studies to directly demonstrate this dependency are needed.
With regard to β-catenin, its role may be to endow CD8+ T with more stem cell-like properties, similar to results regarding the function of TCF-1, whereby TCF-1 expression led to a stem cell-like and undifferentiated phenotype, as previously discussed [47]. Accordingly, β-catenin is continuously degraded in T cells, and the stabilized expression of β-catenin in mice using adenoviral transduction of a β-catenin mutant was shown to negatively regulate proximal T cell activation events in vitro [57]. A further two independent studies reported that β-catenin-mediated activation of CD8+ T cells could limit effector cell differentiation while allowing the maintenance of a stem cell-like phenotype in mouse models of syngeneic tumors and xenograft mesotheliomas (NSG mice), respectively [58,59]. These studies suggested that β-catenin-mediated activation of CD8+T cells might preserve more memory T cell-like phenotypes. However, it should be noted that the Wnt–β-catenin pathway was dispensable for memory CD8+ T cell generation, suggesting that different experimental systems may need to be interpreted with caution to determine the precise roles of β-catenin in CD8+ T cell differentiation and function [60].
Collectively, these results suggest a regulatory role of Wnt signaling proteins in modulating CD8+T cell effector function and memory T cell pool generation in different contexts. Primarily, the expression of TCF-1/β-catenin can lead to a stem cell-like phenotype to form memory CD8+ T cells or differentiation towards a Tfh cell-like gene expression profile in mice. This switch might be potentially important in achieving a balance between effector CD8+ T cells (expressing low TCF-1) and memory CD8+ T cells, and may contribute to determining the outcome of optimal primary and secondary immune responses during viral infections and/or in other contexts. However, functional regulation of β-catenin-mediated CD8+ T cell immune responses remains controversial [46,58–60] owing to the use of different experimental systems. This indicates that results achieved under varying experimental conditions need to be interpreted with caution (e.g., by using pharmacologic inhibition, genetic ablation, and/or overexpression of Wnt–β-catenin proteins). Thus, extensive studies are warranted to precisely determine whether various canonical Wnt ligands such as Wnt3a are upregulated upon viral infection (e.g., LCMV) and can effectively influence CD8+ T cell function.
Role of Wnt Proteins in CD4+ T Cells
CD4+ T cells differentiate into five helper T cell types, including Tfh and Treg cells (Figure 2E). Follicular helper T cells are important to mount humoral immunity [61], while regulatory T cells are required for maintaining immunological tolerance in humans and mice [62].
In CD4+ T cells, the generation of memory Th1 and memory Tfh cells has been found to be dependent on TCF-1 long isoforms in the LCMV chronic infection mouse model [51]. Moreover, TCF-1 can induce the Gata-3–1b isoform directly upon type 2 T helper (Th2) cell differentiation, and TCF-1-deficiency can protect mice from ovalbumin (OVA)-induced asthma by impairing Th2 cell development [63]. In naïve CD4+ T cells from human cord blood, Wnt3a was reported to promote Th2 cell differentiation via the chromatin organizer SATB1 (special AT-rich sequence binding protein-1) in addition to β-catenin [64]. A recent report also showed that Wnt10b deficiency exacerbated house dust mite (HDM)-induced asthma in mice, suggesting that Wnt10b might potentially regulate type 2 inflammation and Th2 cell activation [65].
Further investigations showed that selective ablation of TCF-1 or LEF-1 resulted in Tfh defects in the LCMV acute infection model via two plausible mechanisms [66]. Specifically, both TCF-1 and LEF-1 were reported to set the responsiveness of naïve CD4+ T cells into becoming Tfh cells at an early stage of Tfh cell differentiation, sustaining the expression of IL-6Rα and gp130. This in turn facilitated the expression of the Tfh cell transcriptional factor Bcl6 [66]. A separate study also suggested that TCF-1 modulated virus-specific Tfh cell responses at very early stages of acute viral infection with LCMV before Tfh cell marker CXCR5 expression [67]. Although the study suggested a possible role of Wnt signaling in Tfh cell differentiation at the transcriptional level, there was no experimental evidence that Wnt ligands elicited canonical Wnt signaling.
In an EAE mouse model using MOG (myelin oligodendrocyte glycoprotein) peptide, TCF-1 deficiency led to an exacerbated average clinical score, and the mechanism of this outcome was reported to be mediated by IL-17-producing CD4+ T cells (Th17 cells). Specifically, Th17 cell differentiation was shown to be driven by direct binding of TCF-1 to the regulatory region of the II17 gene in EAE [68]. However, a different outcome was observed in a recent report showing that β-catenin overexpression in CD4+ T cells induced a4p1 integrin expression during CNS infiltration and promoted Th1 cell-mediated progressive lethal neurologic manifestations in the mice [69]. Wnt pathway activation was found in Th17 cells in a separate study that used an antigen-specific tyrosine-related protein (TRP) mouse tumor model where tumor rejection was achieved by adoptively transferring TRP-specific Th17 cells into recipient mice [70]. These Th17 cells efficiently eradicated tumors and were long-lived, harboring a stem cell-like molecular signature that resembled early memory CD8+ T cells, expressing high levels of Wnt proteins (e.g., β-catenin, TCF-7) [70].
Wnt5a has also been thought to affect T cell migration [71]. Specifically, chemokine CXCL12 has been shown to induce Wnt5a expression in human CD4 T+ cells, and to be necessary to achieve T cell migration in a chemotaxis assay in vitro [71]. Based on this evidence, Wnt ligands and Wnt proteins are able to regulate T cell differentiation, effector function, and, in some instances, migration. Of note, Wnt pathway proteins such as β-catenin and GSK3-β can mediate Wnt-independent signaling pathways via growth factors such as FGF or EGF [72,73]. This may in turn suggest that non-Wnt ligands might also signal via canonical Wnt signaling pathways in CD4+ T cells, but this remains to be determined.
From another angle, Tregs are an important subset of CD4+ T cells and can be generated both in the periphery (pTreg) and in the thymus (tTreg) [62]. Recent work has identified a subset of Tregs that can regulate B cells and Tfh cell-mediated responses in the GC, suggesting functional roles in the control of humoral immune responses in autoimmunity and infectious diseases [74]. A recent report showed that Bcl6-mediated follicular regulatory T (Tfr) cell generation was triggered by mTORC1-mediated Stat3 phosphorylation [75]. Stat3 bound to the 5’-regulatory region of the Tcf7 gene, inducing TCF-1 to further induce Bcl6 in infection with acute LCMV infection or OVA protein immunization in mice [75]. Similar to its negative regulatory role of TCF-1 in thymus for the tTreg generation, a previous report showed that Treg cell function could be inhibited by Wnt agonists (Wnt3a) during inflammatory conditions in a murine model of T cell-mediated colitis and in blood samples from patients with rheumatoid arthritis [76]. However, other reports suggested that activation of the Wnt–β-catenin pathway via pharmacologic GSK-3β inhibition or retroviral β-catenin overexpression in Tregs enhanced Treg functions in allograft islet transplantation and inflammatory bowel disease (IBD) murine models [77,78]. APC, a negative regulator of the Wnt–β-catenin pathway, has been studied in mouse Tregs. Treg-specific ablation of APC resulted in an autoimmune phenotype with splenomegaly, and the Apc/Min+ mutation in Tregs altered their suppressor function, promoting intestinal tumorigenesis in mice, and implicating APC in Treg development and function [79]. Further studies revealed that APC could regulate NFAT-driven T cell signal transduction in mice, where Apc/Min+ mouse Tregs exhibited reduced differentiation and IL-10 production relative to wild-type Tregs, and would be essential to suppress intestinal inflammation in the Apc/Min+ colorectal cancer mouse model [79–81]. From these findings it can be surmised that the roles of TCF-1 and β-catenin in CD4+ T cell and Treg differentiation and function are not necessarily redundant because functional studies regarding TCF-1 and β-catenin have not shown a high level of consistency in phenotypes.
The roles of Wnt pathway signaling proteins have been dissected individually and in isolation, while the integrative and putative role of Wnt–β-catenin signaling in T cell function, differentiation, and Treg cell development remains to be addressed. The presence of adequate Wnt agonists and antagonists in a given microenvironment needs to be tested, and potential crosstalk between Wnt pathways and other signaling pathways also needs to be assessed. Furthermore, more careful consideration should be given to various types of extracellular stimulation that can utilize Wnt proteins to identify whether Wnt proteins can mediate signaling via other inflammatory microenvironment signals.
Wnt Antagonists and Inflammatory Diseases
DKK Family Members and Immunity
The canonical Wnt pathway has been shown to play a crucial role in hematopoiesis. DKK family proteins DKK-1, 2, and 4 are known to inhibit canonical Wnt signaling while the mode of action for DKK-3 is less clear [10]. A previous study using human cord blood cells showed that Wnt3a together with the Notch ligand Delta 1 favored T cell differentiation, while inhibition of the Wnt pathway favored NK cell differentiation [82]. The lack of Wnt agonist Wnt3a negatively regulated hematopoietic stem cell (HSC) renewal and subsequently impaired progenitor cell differentiation [83]. Thus, research on the importance of Wnt ligands in immunological diseases has been considered most relevant in recent years [84]. One report showed that DKK-1 promoted HSC regeneration following total body irradiation in mice, raising the possibility that DKK-1 might harbor additional roles in hematopoiesis via Wnt-dependent and independent pathways [85].
The best-characterized Wnt antagonist is Dickkopf-1 (DKK-1). DKK-1 has recently emerged as a proinflammatory immunomodulator in multiple immune diseases. Deficiency for DKK-1 has been found to protect mice from HDM-induced asthma and cutaneous leishmaniasis, independently of canonical Wnt pathway inhibition in CD4+ T cells [5]. A reduced asthma phenotype via DKK-1 was predicted in this study because previous reports had shown that deficiency of canonical Wnt proteins or inhibition of the canonical Wnt pathway in mouse T cells could block Th2 cell differentiation [63,64]. This could suggest that DKK-1 potentially elicits Wnt-independent immune modulation activity. Alternatively, different cell types and model systems may explain the opposing outcomes. A proinflammatory role of DKK-1 in acute lung inflammation was demonstrated in mice. DKK-1 inhibited the canonical Wnt pathway in alveolar epithelial cells (AECs), resulting in the promotion of macrophage/neutrophil adhesion to AECs [86]. DKK-1 has also been implicated in cancer, inhibiting the canonical Wnt pathway and seemingly mediating escape from immunosurveillance and promoting myeloid-derived suppressor cell (MDSC) accumulation in the tumor microenvironment by [4,87]. Indeed, DKK-1 has been reported to promote the recruitment of macrophages and neutrophils in a mouse model of cancer metastasis [7]. In this study, DKK-1 modulated non-canonical Wnt/PCP–Rac-JNK1 signaling in immune cells while it inhibited the canonical Wnt pathway in osteoblasts to promote metastasis. A new DKK-1 receptor, cytoskeleton-associated protein (CKAP)-4, was identified in tumor cells but not in normal cells, suggesting that inhibition of DKK-1–CKAP-4 interactions might be considered as a potential therapeutic approach for specific cancers (e.g., pancreatic cancer) [88]. Recently, DKK-2, another Dickkopf family member protein, was found to dysregulate antitumor immunity by inhibiting the cytotoxic activity of immune cells in a β-catenin and LRP6-independent manner in a mouse model of melanoma [89]. Such studies on DKK-1 and DKK-2 suggested that these two antagonists might potentially have additional roles that are independent of canonical Wnt pathway inhibition, although this remains to be addressed. Another study demonstrated that stress-induced, tubular epithelial cell (TEC)-derived DKK-3 was a driver of fibrosis in the mouse chronic kidney disease (CKD) model. Genetic ablation of DKK3 or anti-DKK-3 antibody treatment of mice yielded increased Th1-skewed immune responses, diminished tubular damage and interstitial fibrosis, and reduced Wnt–β-catenin pathway activity [90]. DKK-3 was also expressed in CD8+ T cells and contributed to CD8+ T cell-mediated tolerance [90]. In another study, abrogation of DKK-3 function enhanced antitumor immunity and promoted skin allograft rejection by CD8+ T cells in a mouse P815.Kb.B7 tumor model [91]. Whether DKK-3 could modulate Wnt signaling was not addressed in this study. For DKK-1, the expression of this protein in regulatory T cells was demonstrated in a murine IBD model with a lymphopenic environment, suggesting that DKK-1 harbors an immunosuppressive role in Tregs, and prevents CD4+ T cell proliferation [92]. The study also showed that DKK-1 expression was independent of Wnt pathway activation. Collectively, these studies suggest that DKK family proteins are able to function not only as Wnt pathway inhibitors but also as Wnt-independent immunomodulatory ligands. However, their role in the microenvironment may vary according to the inflammatory milieu (Box 2). Taken together, studies on Wnt antagonists suggest that Wnt ligands may have dual functions as Wnt inhibitors or immunomodulators. Nevertheless, further studies evaluating different immune response contexts are warranted to define the presumed functional role of these proteins in pathological inflammation.
Box 2. Other Wnt Ligands in Immune Responses.
Owing to the importance of Frz receptors to initiate the canonical and non-canonical Wnt signaling transduction, five mammalian sFRP proteins (sFRP1–5) were deemed to be crucial in lymphopoiesis and immune responses. sFRPs harbor an N-terminal cysteine-rich domain (CRD) that serves as a binding site for Wnt proteins because of its homology to the CRD of Frz receptors [108]. sFRPs contains a cysteine-rich domain (CRD) in the N-terminal region, which shares homology with the CRD of Frz receptors and serves as a binding surface for Wnt proteins [6,8,11]. Wnt proteins play vital roles in various biological activities, including development and immune system function [12,13]. Because Frz receptors initiate Wnt signal transduction in both canonical and noncanonical pathways, sFRPs that modulate the association between Wnt and Frz receptors are presumed to have biological importance.
In lymphopoiesis, a recent study showed that estrogen-induced sFRP5 expression decreases B cell progenitors, indicating that sFRP5 is a negative regulator of maternal lymphopoiesis [109]. Although it is known that Wnt signaling promotes lymphopoiesis, this study did not address whether sFRP5 modulates the Wnt pathway, and this warrants further studies. In immune responses, adipocyte-derived sFRP5 plays an anti-inflammatory role in obesity-related inflammation in mice by regulating the accumulation of activated macrophages and modulating metabolic dysfunction (e.g., glucose intolerance and hepatic steatosis) [110]. In this study, the anti-inflammatory activity of sFRP5 was achieved by canceling Wnt5a-mediated JNK activation in macrophages independently of the canonical Wnt pathway. Similarly, the anti-inflammatory role of sFRP5 in antagonizing Wnt5a-JNK-mediated macrophage activation was shown in an ischemia/reperfusion (I/R) injury model [111]. By contrast, sFRP1 was elevated in synovial fluids of human rheumatoid arthritis (RA) patients, and it promoted Th17 cell differentiation in both human naïve and memory CD4 T cells [112]. The function ofsFRP1 was to enhance TGF-β-mediated Th17 cell differentiation by increasing Smad-2/3 activity. This study is in line with the TCF-1-mediated Th17 cell repression by the canonical Wnt pathway activation.
Concluding Remarks
The importance of Wnt signaling in inflammatory and fibrotic diseases [3,93] is consistent with the known roles of Wnt proteins in tissue injury and repair. Experimental findings in recent years suggest that Wnt signaling plays important roles in lymphomyelopoiesis and immune responses. The canonical Wnt signaling proteins can exert their roles in T cell differentiation and effector function in various inflammatory diseases including cancer, as well as in autoimmunity and viral infections. Enhancing stem cell-like properties via Wnt signaling activation may be potentially beneficial in vaccine development efforts against viral infections and possibly cancer, but this remains to be determined. Immunologic and pharmacological interventions to stimulate Wnt signaling or deplete Wnt antagonists might be a potential promising approach, depending on the context. DCs and macrophages are also regulated by Wnt ligands and Wnt signaling components, modulating innate immune and adaptive responses in various types of inflammatory diseases. The link between these Wnt signaling proteins and available Wnt ligands in given immune microenvironments need to be assessed in the future for each case. The expression of various types of Wnt ligands in an inflammatory microenvironment represents a complex but promising aspect of being able to control immune responses in tissue injury and repair processes. Furthermore, because Wnt signaling is governed by gene-dosage effects [83,94], it needs to be closely examined along with the spatiotemporal dynamics of Wnt pathways (e.g., APC). Indeed, these may be important considerations in better understanding Wnt signaling in immune modulation, and further research is warranted.
The response to Wnt ligands in immune cells should be analyzed with caution because they may have both pro- and anti-inflammatory effects in a given microenvironment [95]. The use of conditional deletion mice to ablate specific Wnt signaling components, Wnt ligands receptors (e.g., LRP5/6), and Wnt ligands is encouraged, but whether the Wnt–β-catenin pathway represents a primary immunomodulation pathway under specific contexts warrants thorough investigation.
In addition, the expression of receptors for canonical and non-canonical Wnt receptors may not always be clearly defined, which is a limitation in dissecting their roles. For example, Ryk can bind to both Wnt3a and Wnt5a, and, in addition, different experimental conditions, tissue/cellular contexts, and spatiotemporal changes may affect outcomes [9]. Simultaneously, potential crosstalk between canonical and non-canonical Wnt pathways in immune cells may also be an important consideration. In addition, in host-pathogen interactions, previous studies have shown that infectious pathogens employ hijacking mechanisms to alter host Wnt signaling pathways to favor their survival in the host [96–99]. Thus, investigating the cellular sources of Wnt ligands may facilitate the identification of early targets for immunologic intervention in infectious diseases. Evidently, in translational studies, proper comparisons with human specimens will be required to validate any findings from mouse studies.
With many recent developments and the quest for developing putative therapeutic agents, more studies are warranted to understand the role of Wnt signaling in infections, immune responses, and in tissue injury and repair process (see Outstanding Questions, Figure 3, and Table 1). Owing to the complexity and context-dependence of Wnt immunology, the development of new immunotherapeutics will need to be carefully designed, and site-specific delivery should be considered to minimize off-target effects.
Outstanding Questions.
How do Wnt ligands direct innate and adaptive immune responses in different microenvironments and in the context of acute versus chronic inflammation? Are there new signaling pathways? The exact spatiotemporal expression of Wnt ligands and Wnt receptors in immune cell subsets may contribute to determining the outcomes of immune responses.
Are host Wnt pathways altered to favor pathogen survival by pathogen-derived factors? If so, how might these be intercepted and cause persistent inflammation? Pathogen-derived factors may hijack or change the host Wnt pathway such that tissue repair processes may be delayed. Alternatively, these pathogen-derived factors may compromise immune cells such that the host fails to eliminate pathogens.
Can Wnt-induced signaling pathways and Wnt ligands in cancer be targeted to develop new putative cancer immunotherapies? Excessive Wnt pathway activation can lead to the development of tumorigenesis with uncontrolled cell proliferation. Simultaneously, Wnt ligands can modulate immune cells to favor tumorigenesis or tumoricidal activity.
Can Wnt ligands be used as biomarkers for chronic inflammatory diseases? Many proinflammatory diseases have elevated systemic or local levels of Wnt antagonist (e.g., DKK-1). Addressing this may potentially lead to a faster and more accurate diagnosis of different types of inflammatory diseases.
Figure 3. Wnt Signaling Pathways, Inflammation, and Tissue Repair in Mice and Humans.
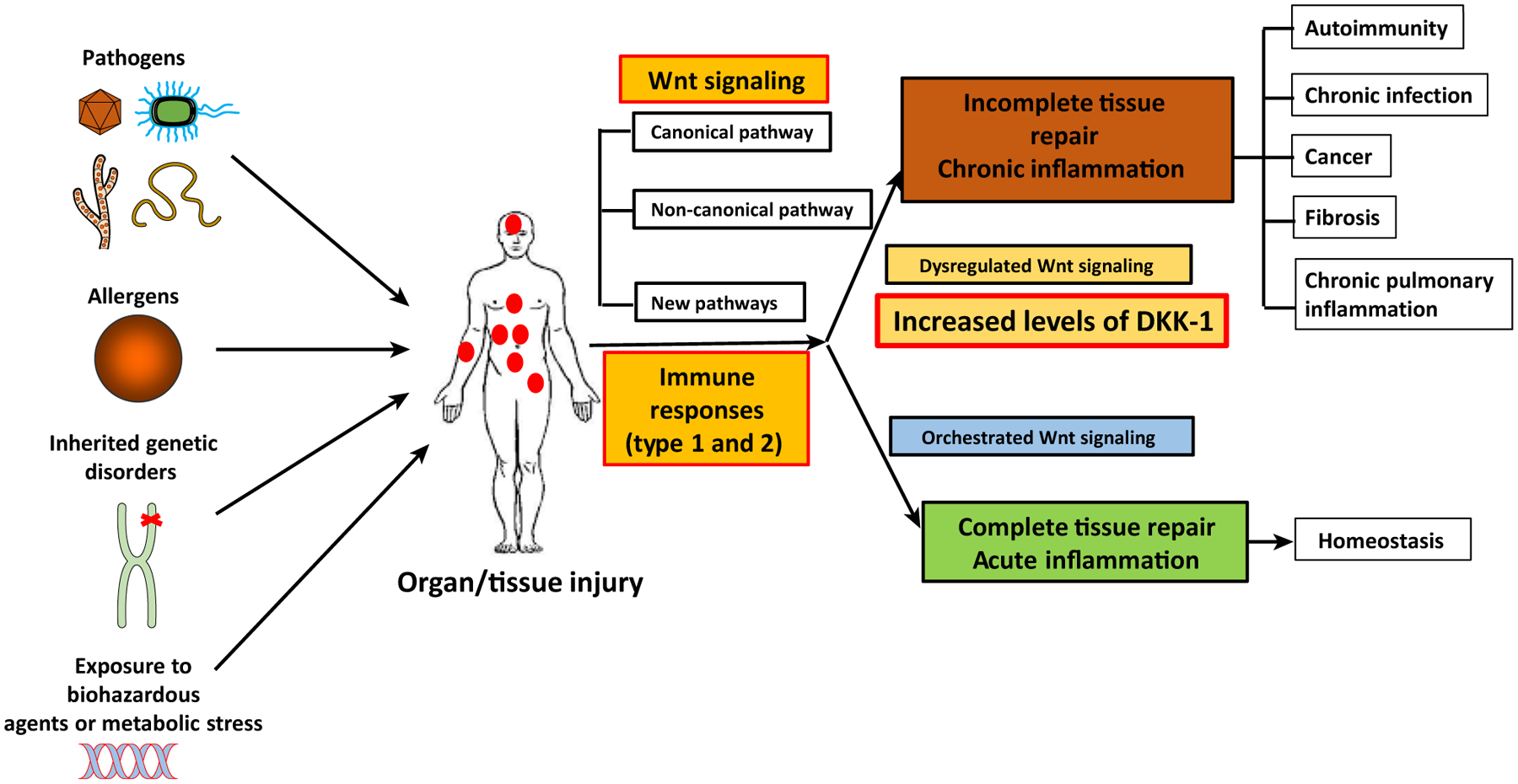
A plethora of challenges (e.g., viruses, helminths, fungus, bacteria, allergens, carcinogens, and inherited genetic mutations) cause injuries in various organs and tissues in the body. Upon injury, the host initiates tissue/organ repair by activating Wnt signaling pathways. The canonical Wnt pathway is a central axis of the Wnt immunology. However, recent studies reveal that non-canonical Wnt pathways and non-Wnt pathways that are induced by Wnt ligands are important for type 1 and type 2 core immune responses in tissue injury/repair processes. If tissue repair and immune responses are well coordinated, the host will achieve tissue/organ homeostasis. When these two processes are dysregulated, the host develops multiple chronic inflammatory diseases such as chronic infections, cancer, autoimmune diseases, pulmonary diseases (asthma), and organ fibrosis. Thus, new roles of Wnt ligands and signal transduction pathways are important targets of immunotherapy in many immunological diseases.
Table 1.
Summary of Wnt Ligands and Their Functionsa
| Wnt ligand | Cellular sources | Target cells | Function |
|---|---|---|---|
| Wnt3a | Unknown | Tregs Cord blood cells CD8 T cells |
Inhibition of suppressor function [76] (m,h) Enhancing human Th2 cell differentiation [64] (h) Inhibits proliferation/effector differentiation [58] (m) |
| Stromal cells | HSC cells | Promotes HSC self-renewal/progenitor cell differentiation [83] (m) | |
| Macrophages | HPCs Thymocytes |
Normal T cell development [9] (m) Transition from HPCs to hepatocytes [38] (m,h) |
|
| Wnt5a | Melanoma cells | DCs (m) | Fatty acid oxidation to induce tolerogenic DCs [24] (m) |
| Macrophages | Kidney cells | Wound healing [37] (m) | |
| DCs | CD4 T cells T cells |
Enhances IL-2 and IFN-γ secretion [29] (h) | |
| Thymic stromal epithelium | Thymocytes | Sensitizes T cells to CXCL12-mediated migration (h,m) [71] | |
| Follicular DCs | GC B cells | Inhibits apoptosis of αβ TcR+ thymocytes [9] (m) Protects GC B cells from apoptosis [28] (h) |
|
| Wnt 2b | Macrophages | Unknown | Wound healing in the TNBS colitis model [6] (m) |
| Wnt 7b | Myeloid cells | Unknown | Angiogenic switch/supports macrophage-mediated lung metastasis [39] (m,h) |
| Macrophages | Unknown | Metabolic sepsis, cancer, obesity, atherosclerosis | |
| Macrophages | Unknown | Induce immunosuppressive macrophages in sepsis and breast cancer [40] (m,h) Required for kidney injury repair [37] (m) |
|
| Wnt 10a | Macrophages | Unknown | Required for kidney injury repair [37] (m) |
| Wnt 10b | Unknown | CD4 T cells | Inhibiting Th2-mediated asthma [65] (m) |
| DKK-1 | Platelets | T cells | Th2 cell differentiation [5] (h,m) |
| Cancer cells | NK cells | Inducing senescence of cancer cells to evade NK cell-mediated cytolysis [4] (m) | |
| Myeloid cells | Unknown | Promoting cancer inflammation [87] (m) | |
| Osterix-1+ cells | HSCs | Promoting HSC compartment repopulation [85] (m) | |
| Tregs | Effector CD4 T cells | Preventing T cell-mediated colitis [92] (m) | |
| DKK-2 | Unknown | CD8 T cells/NK cells | Inhibition of cytotoxic activity [89] (m) |
| DKK-3 | Tubular epithelial cells | Unknown | Promoting kidney disease with more Th1 cell differentiation in Dkk3-deficient condition [90] (m) |
| CD8 T cells | Unknown | CD8 T cell-mediated tolerance induction [91] (m) | |
| sFRP5 | Adipocytes | Unknown | Anti-inflammatory role in obesity-related inflammation [110] (m) |
| Unknown | B cells | Decreases B cell progenitor development [106] (m) | |
| sFRP1 | Synovial fluid | CD4+ T cells | Promotes Th17 cells [112] (h) |
h, human; m, mouse.
Highlights.
Wnt ligands are important in inducing crucial functions in cell proliferation, differentiation, apoptosis, motility, and survival. Wnt ligands and their signaling pathways are therefore important for tissue repair and immune responses.
T cell development and peripheral immune responses of T cells are highly dependent on Wnt pathway components TCF-1 and β-catenin. The importance of canonical Wnt signaling must be interpreted with caution depending on different microenvironments.
Both canonical and non-canonical Wnt pathways appear to be important for macrophage-mediated tissue injury and repair in major organs (e.g., heart).
Wnt antagonists such as DKK-1 can harbor immunomodulatory functions that are often β-catenin-independent.
Acknowledgments
This work is supported by National Institutes of Health grants R01CA168670, R21AI23858, R56AI27794, and R21AI107957, and National Cancer Institute grant P30CA016359.
Glossary
- Follicular helper T (Tfh) cells
CD4+ T cells that are present in the periphery within B cell follicles of secondary lymphoid organs such as lymph nodes and spleen. They are identified by the expression of CXCR5. Tfh cells are important to form and to maintain germinal centers (GCs) via the secretion of IL-21 and IL-4, and the expression of CD40L.
- Follicular regulatory T (Tfr) cells
CD4+ regulatory T cells (Treg cells) that are found in the GC after immunization with protein antigens. They share phenotypic characteristics with Tfh and Treg cells but are distinct from the two subsets. Tfr cells originate from thymic (t)Treg precursors, not naïve or Tfh cells. They limit Tfh cell and germinal B cell numbers in vivo.
- Leishmaniasis
a disease caused by infection with Leishmania parasites. It is categorized as a neglected tropical disease and is spread by the bite of phlebotomine sand flies. Typically, there are two forms of leishmaniasis depending on the site of infection. One is cutaneous leishmaniasis in skin and the other is visceral leishmaniasis in internal organs such as spleen, liver and bone marrow.
- M2-like macrophages
by convention, macrophages are divided into three groups: M2a, M2b, and M2c. M2a macrophages are connected with Th2 cell-mediated immune responses and they produce IL-4. M2b macrophages are stimulated by immune complexes and TLRs. They produce IL-10 and their antigen presentation is upregulated. M2c macrophages secrete IL-10 and TGF-β. They contribute to extracellular matrix components production and tissue remodeling.
- MMTV-PyMT mouse model
a mouse breast cancer metastasis model in which the long terminal repeat (LTR) of mouse mammary tumor virus (MMTR) drives mammary gland-specific expression of polyoma virus middle-T antigen.
- Myeloid-derived suppressor cells (MDSCs)
immune cells from the bone marrow-derived myeloid lineage. MDSCs are heterogeneous, immunosuppressive cell type that expand in pathological inflammation such as cancer via an altered hematopoiesis.
- PD-1 inhibitory pathway
is a signaling pathway to regulate T cells. Programmed cell death protein (PD)-1 is a coinhibitory ‘checkpoint’ molecule, and is induced upon T cell activation. PD-1 plays an essential role in balancing immune homeostasis and tolerance, but its expression can limit protective immunity and immunosurveillance against tumors.
- Regulatory T cells (Tregs)
a subset of the CD4+ T cell population that suppress inflammation. Tregs express a lineage-specification transcriptional factor Foxp3, and play a key role to maintain immunological homeostasis in autoimmunity.
- SATB1 (special AT-rich sequence binding protein-1)
a transcriptional factor and chromatin organizer that integrates higher-order chromatin architecture for gene expression regulation.
- TCF-1 isoforms
both long and short isoforms of TCF-1 possess a high mobility group (HMG) DNA-binding domain and a HDAC domain. Only the TCF-1 long isoforms (p45 and p42) contain an N-terminal β-catenin-binding domain to interact with the protein, whereas the TCF-1 short isoforms (p33 and p30) lack this domain.
- Toll-like receptor (TLR) signaling
a pathway mediated by TLRs which play key roles in innate immune responses. TLRs recognize structurally conserved molecules such as PAMP (pathogen-associated molecular patterns) from microbes.
- Type 1/17T helper (Th1/17) cell responses
these are mediated by Th1 cells (expressing type 1 cytokine IFN-γ) and Th17 cells (expressing cytokine IL-17). Th1/Th17 responses are involved in autoimmunity, pathogen infection and cancer.
- Type 2T helper (Th2) cells
a subset of differentiated naïve CD4+ T cells that express type 2 cytokines such as IL-4, IL-5, and IL-13, and which play a major role in type 2 inflammation. Th2 cell-mediated responses are important for helminth infections, allergic inflammation, and tissue injury and repair.
References
- 1.Nusse R and Clevers H (2017) Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 [DOI] [PubMed] [Google Scholar]
- 2.Karin M and Clevers H (2016) Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gieseck RL 3rd et al. (2018) Type 2 immunity in tissue repair and fibrosis. Nat. Rev. Immunol 18, 62–76 [DOI] [PubMed] [Google Scholar]
- 4.Malladi S et al. (2016) Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chae WJ et al. (2016) The Wnt antagonist Dickkopf-1 promotes pathological type 2 cell-mediated inflammation. Immunity 44, 246–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosin-Roger J et al. (2016) The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 9, 986–998 [DOI] [PubMed] [Google Scholar]
- 7.Zhuang X et al. (2017) Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol 19, 1274–1285 [DOI] [PubMed] [Google Scholar]
- 8.Acebron SP and Niehrs C (2016) Beta-catenin-independent roles of Wnt/LRP6 signaling. Trends Cell Biol. 26, 956–967 [DOI] [PubMed] [Google Scholar]
- 9.Famili F et al. (2016) The non-canonical Wnt receptor Ryk regulates hematopoietic stem cell repopulation in part by controlling proliferation and apoptosis. Cell Death Dis. 7, e2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driehuis E and Clevers H (2017) WNT signalling events near the cell membrane and their pharmacological targeting for the treatment of cancer. Br. J. Pharmacol 174, 4547–4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchetti B and Pluchino S (2013) Wnt your brain be inflamed? Yes, it Wnt! Trends Mol. Med 19, 144–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staal FJ and Arens R (2016) Wnt signaling as master regulator of T-lymphocyte responses: implications for transplant therapy. Transplantation 100, 2584–2592 [DOI] [PubMed] [Google Scholar]
- 13.Carmon KS et al. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U. S. A 108, 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao HX et al. (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 [DOI] [PubMed] [Google Scholar]
- 15.Zhou J et al. (2009) Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity 30, 845–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manicassamy S et al. (2010) Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoharan I et al. (2014) TLR2-dependent activation of beta-catenin pathway in dendritic cells induces regulatory responses and attenuates autoimmune inflammation. J. Immunol 193, 4203–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manicassamy S et al. (2009) Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat. Med 15, 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian C et al. (2013) Fas signal promotes the immunosuppressive function of regulatory dendritic cells via the ERK/beta-catenin pathway. J. Biol. Chem 288, 27825–27835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capietto AH et al. (2013) Down-regulation of PLCgamma2-beta-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J. Exp. Med 210, 2257–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang A et al. (2007) Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity 27, 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen AE et al. (2014) E-cadherin phosphorylation occurs during its biosynthesis to promote its cell surface stability and adhesion. Mol. Biol. Cell 25, 2365–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oderup C et al. (2013) Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J. Immunol 190, 6126–6134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao F et al. (2018) Paracrine Wnt5a-beta-catenin signaling triggers a metabolic program that drives dendritic cell tolerization. Immunity 48, 147–160 e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suryawanshi A et al. (2015) Canonical wnt signaling indendritic cells regulates Th1/Th17 responses and suppresses autoimmune neuroinflammation. J. Immunol 194, 3295–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong Y et al. (2016) Deletion of LRP5 and LRP6 in dendritic cells enhances antitumor immunity. Oncoimmunology 5, e1115941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suryawanshi A et al. (2016) Modulation of inflammatory responses by Wnt/beta-catenin signaling in dendritic cells: a novel immunotherapy target for autoimmunity and cancer. Front. Immunol 7, 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J et al. (2012) Wnt5a is secreted by follicular dendritic cells to protect germinal center B cells via Wnt/Ca2+/NFAT/NF-kappaB-B cell lymphoma 6 signaling. J. Immunol 188, 182–189 [DOI] [PubMed] [Google Scholar]
- 29.Valencia J et al. (2014) Wnt5asignaling increases IL-12 secretion by human dendritic cells and enhances IFN-gamma production by CD4+ T cells. Immunol. Lett 162, 188–199 [DOI] [PubMed] [Google Scholar]
- 30.Vannella KM and Wynn TA (2017) Mechanisms of organ injury and repair by macrophages. Annu. Rev. Physiol 79, 593–617 [DOI] [PubMed] [Google Scholar]
- 31.Sennello JA et al. (2017) Lrp5/beta-catenin aignaling controls lung macrophage differentiation and inhibits resolution of fibrosis. Am. J. Respir. Cell Mol. Biol 56, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villar J et al. (2014) Early activation of pro-fibrotic WNT5A in sepsis-induced acute lung injury. Crit Care 18, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang P et al. (2009) Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol. Immunol 46, 677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam AP et al. (2014) Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 190, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palevski D et al. (2017) Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice. J. Am. Heart Assoc 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha S et al. (2016) Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun 7, 13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SL et al. (2010) Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. U. S. A 107, 4194–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulter L et al. (2012) Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med 18, 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeo EJ et al. (2014) Myeloid WNT7b mediates the angiogenic switch and metastasis in breast cancer. Cancer Res. 74, 2962–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao Y et al. (2016) Biological functions of macrophage-derived Wnt5a, and its roles in human diseases. Oncotarget 7, 67674–67684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naskar D et al. (2014) Wnt5a-Rac1-NF-kappaB homeostatic circuitry sustains innate immune functions in macrophages. J. Immunol 192, 4386–4397 [DOI] [PubMed] [Google Scholar]
- 42.Bergenfelz C et al. (2012) Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J. Immunol 188, 5448–5458 [DOI] [PubMed] [Google Scholar]
- 43.Brandenburg J and Reiling N (2016) The Wnt blows: on the functional role of Wnt signaling in Mycobacterium tuberculosis infection and beyond. Front. Immunol 7, 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X and Xue HH (2012) Cutting edge: generation of memory precursors and functional memory CD8+ T cells depends on T cell factor-1 and lymphoid enhancer-binding factor-1. J. Immunol 189, 2722–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X et al. (2010) Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity 33, 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiemessen MM et al. (2014) T cell factor 1 represses CD8+ effector T cell formation and function. J. Immunol 193, 5480–5487 [DOI] [PubMed] [Google Scholar]
- 47.Lin WW et al. (2016) CD8+ T lymphocyte self-renewal durinc effector cell determination. Cell Rep. 17, 1773–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shan Q et al. (2017) The transcription factor Runx3 guards cytotoxic CD8+ effector T cells against deviation towards follicular helper T cell lineage. Nat. Immunol 18, 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z et al. (2017) Beta-catenin-interacting Tcf1 isoforms are essential for thymocyte survival but dispensable for thymic maturation transitions. J. Immunol 198, 3404–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van de Wetering M et al. (1996) Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol. Cell. Biol 16, 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gullicksrud JA et al. (2017) Differential eequirements for Tcf1 long isoforms in CD8+ and CD4+ T cell responses to acute viral infection. J. Immunol 199, 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Im SJ et al. (2016) Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu T et al. (2016) The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci. Immunol 1, eaai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lindqvist M et al. (2012) Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest 122, 3271–3280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leong YA et al. (2016) CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol 17, 1187–1196 [DOI] [PubMed] [Google Scholar]
- 56.Richards MH et al. (2016) Migration ofCD8+T cells into the central nervous system gives rise to highly potent anti-HIV CD4dimCD8bright T cells in a Wnt signaling-dependent manner. J. Immunol 196, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Driessens G et al. (2011) Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells – phospholipase C-gamma1 phosphorylation. J. Immunol 186, 784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gattinoni L et al. (2009) Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med 15, 808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gattinoni L et al. (2011) A human memory T cell subset with stem cell-like properties. Nat. Med 17, 1290–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Driessens G et al. (2010) Beta-catenin does not regulate memory T cell phenotype. Nat. Med 16, 513–514 author reply 514–5 [DOI] [PubMed] [Google Scholar]
- 61.Qi H (2016) T follicular helper cells in space-time. Nat. Rev. Immunol 16, 612–625 [DOI] [PubMed] [Google Scholar]
- 62.Josefowicz SZ et al. (2012) Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol 30, 531–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu Q et al. (2009)T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat. Immunol 10, 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Notani D et al. (2010) Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol. 8, e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trischler J et al. (2016) Immune modulation of the T cell response in asthma through Wnt10b. Am. J. Respir. Cell Mol. Biol 54, 584–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi YS et al. (2015) LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat. Immunol 16, 980–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu T et al. (2015) TCF1 is required for the T follicular helper cell response to viral infection. Cell Rep. 12, 2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu Q et al. (2011) T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. J. Immunol 186, 3946–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorcini D et al. (2017) Wnt/beta-catenin signaling induces integrin alpha4beta1 in T cells and promotes a progressive neuroinflammatory disease in mice. J. Immunol 199, 3031–3041 [DOI] [PubMed] [Google Scholar]
- 70.Muranski P et al. (2011) Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh MC et al. (2009) Activation of Wnt5A signaling is required for CXC chemokine ligand 12-mediated T-cell migration. Blood 114, 1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang S et al. (2016) Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3beta/Foxp3 axis. J. Biol. Chem 291, 21085–21095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao H et al. (2016) FGF23 promotes myocardial fibrosis in mice through activation of beta-catenin. Oncotarget 7, 64649–64664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sage PT and Sharpe AH (2015) T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 36, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu L et al. (2017) The kinase mTORC1 promotes the generation and suppressive function of follicular regulatory T cells. Immunity 47, 538–551 e5 [DOI] [PubMed] [Google Scholar]
- 76.van Loosdregt J et al. (2013) Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 39, 298–310 [DOI] [PubMed] [Google Scholar]
- 77.Graham JA et al. (2010) Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3beta inhibition. J. Biol. Chem 285, 32852–32859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding Y et al. (2008) Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med 14, 162–169 [DOI] [PubMed] [Google Scholar]
- 79.Chae WJ and Bothwell AL (2015) Spontaneous intestinal tumorigenesis in ApcMin+ mice requires altered T cell development with IL-17A. J. Immunol. Res 2015, 860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aguera-Gonzalez S et al. (2017) Adenomatous polyposis coli defines Treg differentiation and anti-inflammatory function through microtubule-mediated NFAT localization. Cell Rep. 21, 181–194 [DOI] [PubMed] [Google Scholar]
- 81.Dennis KL et al. (2015) T-cell Expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol. Res 3, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aoyama K et al. (2007) The interaction of the Wnt and Notch pathways modulates natural killer versus T cell differentiation. Stem Cells 25, 2488–2497 [DOI] [PubMed] [Google Scholar]
- 83.Luis TC et al. (2011) Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9, 345–356 [DOI] [PubMed] [Google Scholar]
- 84.Staal FJ et al. (2008) WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol 8, 581–593 [DOI] [PubMed] [Google Scholar]
- 85.Himburg HA et al. (2017) Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat. Med 23, 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo Y et al. (2015) Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 126, 2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D’Amico L et al. (2016) Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med 213, 827–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kimura H et al. (2016) CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J. Clin. Invest 126, 2689–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiao Q et al. (2018) DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med 24, 262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Federico G et al. (2016) Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight 1, e84916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papatriantafyllou M et al. (2012) Dickkopf-3, an immune modulator in peripheral CD8 T-cell tolerance. Proc. Natl. Acad. Sci. U. S. A 109, 1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chae WJ et al. (2017) Membrane-bound Dickkopf-1 in Foxp3+ regulatory T cells suppresses T-cell-mediated autoimmune colitis. Immunology 152, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whyte JL et al. (2012) Wnt signaling and injury repair. Cold Spring Harb. Perspect. Biol 4, a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacDonald BT et al. (2004) Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development 131, 2543–2552 [DOI] [PubMed] [Google Scholar]
- 95.Staal FJ et al. (2016) Caught in a Wnt storm: complexities of Wnt signaling in hematopoiesis. Exp. Hematol 44, 451–457 [DOI] [PubMed] [Google Scholar]
- 96.Luo T et al. (2015) Ehrlichia chaffeensis exploits canonical and noncanonical host wnt signaling pathways to stimulate phagocytosis and promote intracellular survival. Infect. Immun 84, 686–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kessler M et al. (2012) Chlamydia trachomatis disturbs epithelial tissue homeostasis in fallopian tubes via paracrine Wnt signaling. Am. J. Pathol 180, 186–198 [DOI] [PubMed] [Google Scholar]
- 98.Fujimuro M et al. (2003) A novel viral mechanism for dysregulation of beta-catenin in Kaposi’s sarcoma-associated herpes-virus latency. Nat. Med 9, 300–306 [DOI] [PubMed] [Google Scholar]
- 99.vanZuylen WJ et al. (2016) The Wnt pathway: akeynetworkin cell signalling dysregulated by viruses. Rev. Med. Virol 26, 340–355 [DOI] [PubMed] [Google Scholar]
- 100.Steinke FC and Xue HH (2014) From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunol. Res 59, 45–55 [DOI] [PubMed] [Google Scholar]
- 101.Tiemessen MM et al. (2012) The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. PLoS Biol. 10, e1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steinke FC et al. (2014) TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4+ T cell fate and interact with Runx3 to silence Cd4 in CD8+ T cells. Nat. Immunol 15, 646–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xing S et al. (2016) Tcf1 and Lef1 transcription factors establish CD8+ T cell identity through intrinsic HDAC activity. Nat. Immunol 17, 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Y et al. (2003) Deletion of beta-catenin impairs T cell development. Nat. Immunol 4, 1177–1182 [DOI] [PubMed] [Google Scholar]
- 105.Yu Q and Sen JM (2007) Beta-catenin regulates positive selection of thymocytes but not lineage commitment. J. Immunol 178, 5028–5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Q and et al. (2007) Beta-catenin expression enhances IL-7 receptor signaling in thymocytes during positive selection. J. mmunol 179, 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barra MM et al. (2015) Transcription factor 7 limits regulatory T cell generation in the thymus. J. Immunol 195, 3058–3070 [DOI] [PubMed] [Google Scholar]
- 108.Surana R et al. (2014) Secreted frizzled related proteins: implications in cancers. Biochim. Biophys. Acta 1845, 53–65 [DOI] [PubMed] [Google Scholar]
- 109.Yokota T et al. (2015) Estrogen-inducible sFRP5 inhibits early B-lymphopoiesis in vivo, but not during pregnancy. Eur. J. Immunol 45, 1390–1401 [DOI] [PubMed] [Google Scholar]
- 110.Ouchi N et al. (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329, 454–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakamura K et al. (2016) Secreted Frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J. Biol. Chem 291, 2566–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee YS et al. (2012) The Wnt inhibitor secreted Frizzled-related protein 1 (sFRP1) promotes human Th17 differentiation. Eur. J. Immunol 42, 2564–2573 [DOI] [PubMed] [Google Scholar]


