Abstract
Background
Follow-up is integral for hematopoietic cell transplant (HCT) care to ensure surveillance and intervention for complications. We characterized the incidence of, and predictors for, being lost to follow-up.
Methods
Two-year survivors of first allogeneic (10,367 adults and 3,865 children) or autologous (7,291 adults and 467 children) HCT for malignant/non-malignant disorders from 2002–2013 reported to the Center for International Blood and Marrow Transplant Research were selected. The cumulative incidence of being lost to follow-up (defined as having missed 2 consecutive follow-up reporting periods) was calculated. Marginal Cox models (adjusted for center effect) were fit to evaluate predictors.
Results
The 10-year cumulative incidence of being lost to follow-up among adult allogeneic and autologous HCT survivors was 13% (95% CI, 12–14) and 15% (95% CI, 14–16), respectively. Among pediatric HCT survivors, estimates were 25% (95% CI, 24–27) and 24% (95% CI, 20–29), respectively. In adult allogeneic HCT survivors, younger age, non-malignant disease, public/no insurance (reference: private), living farther from the HCT center, and being unmarried were associated with being lost to follow-up. For adult autologous HCT survivors, older age and testicular/germ cell tumor (reference: non-Hodgkin lymphoma) were associated with greater risk of being lost to follow-up. Among pediatric allogeneic HCT survivors, older age, public/no insurance (reference: private), and non-malignant disease were associated with being lost to follow-up. Among pediatric autologous HCT survivors, older age was associated with greater risk of being lost to follow-up.
Conclusion
Follow-up focusing on minimizing attrition in high-risk groups is needed to ensure surveillance for late effects.
Keywords: Bone marrow transplantation, stem cell transplantation, survivors, lost to follow-up
Introduction
By 2030, it is estimated that there will be 0.5 million hematopoietic cell transplant (HCT) survivors in the United States (US) [1]. Despite the increasing survival rates of HCT recipients, there is ongoing risk for early morbidity related to acute graft-versus-host disease (GVHD) and late morbidity related to late effects including cardiovascular, gastrointestinal, endocrinologic dysfunction, psychosocial distress, and risk of subsequent malignant neoplasms [2–4]. Follow-up is an integral part of HCT care which ensures surveillance and intervention for early and late complications [5]. Among populations with chronic health conditions [6–9] as well as non-HCT cancer survivors [10], studies have characterized the proportion of survivors that are lost to follow-up and risk factors for being lost to follow-up.
An overall assessment of the number of HCT survivors that are lost to follow-up is needed. Information is also needed to identify HCT survivors at greatest risk for being lost to follow-up. The Center for International Blood and Marrow Transplant Research (CIBMTR) Registry, the largest sample of HCT recipients, has the potential to be a vital resource in the characterization of lost to follow-up patterns among HCT survivors. These analyses provide the first characterization of, and predictors for being lost to follow-up among HCT survivors reported to the CIBMTR. The primary objective of this study was to estimate the cumulative incidence of being lost to follow-up among HCT survivors. A secondary aim was to identify patient, disease, and HCT-related factors that predict being lost to follow-up.
Methods
Study Design, Setting, and Data Source
CIBMTR represents a voluntary working group of more than 500 HCT centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT procedures to a Statistical Center at the Medical College of Wisconsin and the NMDP Coordinating Center. Centers that participate in CIBMTR report all transplants consecutively. Participants are followed longitudinally. Data quality is ensured by the completion of computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of CIBMTR research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
The CIBMTR collects data which includes disease type, age, sex, pre-transplant disease stage and chemotherapy-responsiveness, diagnosis date, graft type, conditioning regimen, post-HCT disease progression and survival, development of a subsequent malignancy, and cause of death. More detailed disease and pre- and post-HCT clinical information is collected on a subset of CIBMTR patients sampled using a weighted randomization scheme. Data are collected at specific intervals including pre-HCT, 100 days post-HCT, six months post-HCT, and annually for the first 6 years post-HCT and biennially thereafter or until death.
Study Population
Two-year survivors were selected if they had received a first HCT for malignant and non-malignant disorders performed in the US from 2002–2013 and reported to the CIBMTR. Adult (≥18 years of age at the time of HCT) and pediatric (<18 years of age at the time of HCT) recipients of autologous and allogeneic HCT were included in these analyses. Overall, 14,232 (10,367 adults and 3,865 pediatric) allogeneic HCT recipients and 7,758 (7,291 adult and 467 pediatric) autologous HCT recipients met the study eligibility criteria.
Study Endpoint
The primary outcome was lost to follow-up status which was defined as a patient having missed 2 consecutive follow-up reporting periods (using the date of last contact).
Independent Variables
Sociodemographic variables considered included: patient’s age at the time of HCT, sex, Karnofsky/Lansky performance status pre-HCT, race, median income of the ZIP code where the HCT recipient resides (obtained from US census data) [11], health insurance status, marital status, and distance from HCT survivor’s home to their HCT center (estimated as the geodetic distance in miles between the two ZIP code locations – that of the patient and the transplant center). The centroid of each ZIP code is used in the calculation [12]. Disease and HCT-related variables included disease type as well as year of transplant.
Statistical Analyses
Descriptive statistics were calculated for sociodemographic, disease, and HCT-related variables. Cumulative incidence of being lost to follow-up for HCT recipients was estimated treating death as a competing event. Cumulative incidence estimates accompanied by 95% confidence intervals are provided. For the multivariable analyses, marginal Cox models were constructed to adjust for center effect. Individual models were constructed for four distinct populations: 1) adult allogeneic HCT survivors, 2) adult autologous HCT survivors, 3) pediatric allogeneic HCT survivors, and 4) pediatric autologous HCT survivors. Backward model selection procedures with an α <0.05 was used to identify significant independent variables to be included in the final models. SAS version 9.4 (Cary, NC) was used for all analyses.
Results
Patient, disease, and transplant characteristics
Patient, disease, and HCT-related characteristics of the study population are summarized in Table 1A, B. Among 2-year adult HCT survivors, the median age at the time of HCT was 49 years (range, 18–81) and 58 years (range, 18–82) for allogeneic and autologous patients, respectively. Corresponding median follow-up was 80 months (range, 24–174) and 85 months (range, 24–175). For adults, the majority of allogeneic HCT procedures were completed for acute myelogenous leukemia (35%). For adult survivors the majority of autologous HCT procedures were completed for multiple myeloma (65%). The majority of adult allogenic and autologous HCT survivors were covered by private health insurance (64% and 57%, respectively).
Table 1A:
Sociodemographic characteristics of the study population
| Adult | Pediatric | ||||
|---|---|---|---|---|---|
| Allogeneic (10,367) | Autologous (n=7,291) | Allogeneic (n=3,865) | Autologous (n=468) | ||
| % | % | % | % | ||
| Age at HCT (years) | |||||
| Median (range) | 49 (18–81) | 58 (18–82) | 7 (<1–18) | 5 (<1–18) | |
| Sex | |||||
| Male | 57 | 60 | 60 | 59 | |
| Female | 43 | 40 | 40 | 41 | |
| Race | |||||
| Caucasian | 89 | 83 | 76 | 74 | |
| African American | 5 | 14 | 11 | 15 | |
| Other | 4 | 2 | 8 | 4 | |
| Missing | 2 | 1 | 5 | 7 | |
| Marital Status | |||||
| Married | 64 | 68 | NA | NA | |
| Single/Separated/Divorced/Widowed | 29 | 24 | NA | NA | |
| Missing | 7 | 8 | NA | NA | |
| Health Insurance Status | |||||
| None | <1 | <1 | 1 | <1 | |
| Public | 23 | 28 | 40 | 48 | |
| Private | 64 | 57 | 43 | 38 | |
| Other/Unspecified | 4 | 9 | 5 | 4 | |
| Missing | 8 | 6 | 10 | 8 | |
| Income | |||||
| $0–39,000 | 10 | 9 | 14 | 6 | |
| $40–59,000 | 30 | 23 | 31 | 20 | |
| $60–79,000 | 21 | 14 | 18 | 12 | |
| $80–99,000 | 11 | 6 | 9 | 6 | |
| $100,000+ | 8 | 4 | 6 | 2 | |
| Missing | 19 | 44 | 21 | 53 | |
| Distance to Center (miles) | |||||
| 0–100 miles | 58 | 44 | 53 | 39 | |
| 100+ miles | 24 | 13 | 27 | 9 | |
| Missing | 18 | 43 | 20 | 52 | |
Table 1B:
Disease and transplant-related characteristics of the study population
| Adult | Pediatric | ||||
|---|---|---|---|---|---|
| Allogeneic (10,367) | Autologous (n=7,291) | Allogeneic (n=3,865) | Autologous (n=468) | ||
| % | % | % | % | ||
| Karnofsky Score at HCT | |||||
| <90 | 27 | 34 | 12 | 17 | |
| ≥90 | 67 | 60 | 83 | 78 | |
| Missing | 6 | 6 | 6 | 6 | |
| Disease | |||||
| AML | 35 | 0 | 18 | 0 | |
| CLL | 5 | 0 | <1 | ||
| NHL | 13 | 26 | 2 | 2 | |
| HL | 3 | 8 | <1 | 8 | |
| Mutiple Myeloma | 4 | 65 | <1 | <1 | |
| Testicular/Germ Cell Tumor | 0 | 1 | 0 | 2 | |
| CNS Tumor | 0 | <1 | 0 | 78 | |
| Other Solid Tumor | 0 | <1 | 0 | 10 | |
| Other Malignant | 36 | 0 | 34 | 0 | |
| Other Non-Malignant | 4 | 0 | 46 | 0 | |
| Year of HCT | |||||
| 2002–2005 | 30 | 29 | 33 | 31 | |
| 2006–2009 | 42 | 47 | 44 | 53 | |
| 2010–2013 | 28 | 25 | 23 | 17 | |
Abbreviation: AML=acute myelogenous leukemia; CLL=chronic lymphocytic leukemia; HL=Hodgkin lymphoma; NHL=Non-Hodgkin lymphoma
For 2-year pediatric HCT survivors, the median age at the time of HCT was 7 years (range, <1–18) and 5 years (range, <1–18) for the allogeneic and autologous survivors, respectively. Corresponding median follow-up was 77 months (range, 24–175) and 76 months (range, 24–173). For pediatric HCT survivors the majority of allogeneic HCT procedures were completed for acute lymphoblastic leukemia (22%). For pediatric survivors the majority of autologous HCT procedures were completed for central nervous system tumors (78%) which included neuroblastoma. Slightly less than half (43%) of pediatric allogeneic HCT survivors were covered by private health insurance while nearly half of the pediatric autologous HCT survivors had public insurance (48%).
The Cumulative Incidence of Lost to Follow-up
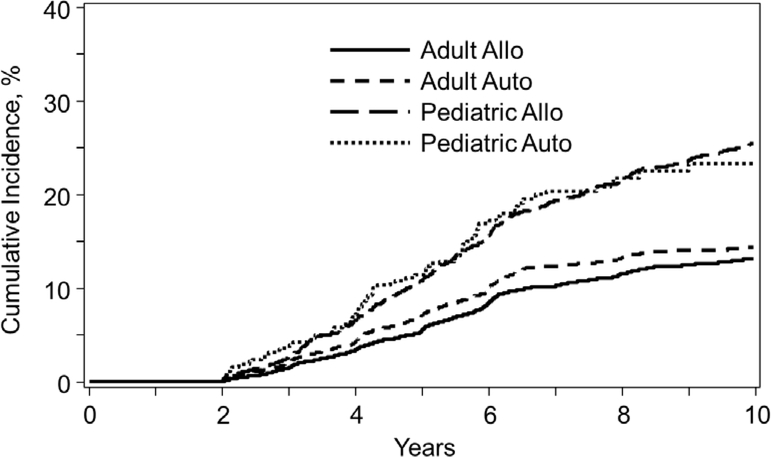
Figure 1 depicts the cumulative incidence of being lost to follow-up since HCT by age at the time of HCT (adult versus pediatric) and by type of HCT (allogeneic versus autologous) among 2-year survivors.
Figure 1:
Among 2-year survivors, the cumulative incidence of becoming lost to follow-up by age at the time of transplantation (adult versus pediatric) and by type of transplant (allogeneic versus autologous).
The 3-year, 5-year, and 10-year cumulative incidence of being lost to follow-up post-HCT among adult allogeneic HCT survivors was 2% (95% confidence interval [CI], 1–2), 5% (95% CI, 5–6), and 13% (95% CI, 12–14). Among adult autologous HCT survivors, the 3-year, 5-year, and 10-year cumulative incidence of being lost to follow-up post-HCT was 2% (95% CI, 2–3), 7% (95% CI, 7–8), and 15% (95% CI, 14–16).
For pediatric allogeneic HCT survivors the 3-year, 5-year, and 10-year cumulative incidence of being lost to follow-up post-HCT was 3% (95% CI, 2–3), 11% (95% CI, 10–12), and 25% (95% CI, 24–27). For pediatric autologous HCT survivors the 3-year, 5-year, and 10-year cumulative incidence of being lost to follow-up post-HCT was 4% (95% CI, 2–6), 12% (95% CI, 9–15), and 24% (95% CI, 20–29).
Risks factors for Lost to Follow-up
Risk factors for lost to follow-up among adult allogeneic and autologous HCT survivors as well as pediatric allogeneic and autologous HCT survivors are depicted in Tables 2–5.
Table 2:
Multivariate analysis results of predictors for being lost to follow-up among adult 2-year survivors of allogeneic hematopoietic cell transplantation (n=10,367)
| Characteristics | N | HR (95% CI) | P-Value | |
|---|---|---|---|---|
| Age at transplant (years) | <0.0001 | |||
| 51+ | 4665 | Reference | ||
| 41–50 | 2307 | 1.38 (1.14–1.67) | 0.0009 | |
| 18–40 | 3395 | 2.19 (1.80–2.66) | <0.0001 | |
| Insurance | <0.0001 | |||
| Private | 6584 | Reference | ||
| None | 70 | 2.33 (1.30–4.17) | 0.004 | |
| Public | 2394 | 1.29 (1.13–1.48) | 0.0002 | |
| Other/Unspecified | 449 | 1.41 (1.04–1.92) | 0.03 | |
| Missing | 870 | 1.22 (0.85–1.74) | 0.29 | |
| Distance to center (miles) | 0.0008 | |||
| 0–99 | 6056 | Reference | ||
| 100+ | 2438 | 1.39 (1.11–1.72) | 0.004 | |
| Unknown | 1873 | 1.23 (1.00–1.53) | 0.05 | |
| Marital status | 0.008 | |||
| Married | 6644 | Reference | ||
| Single/Divorced/Widowed | 3034 | 1.26 (1.09–1.46) | 0.002 | |
| Missing | 689 | 0.99 (0.75–1.31) | 0.93 | |
| Disease | 0.0008 | |||
| Malignant/except CLL | 9389 | Reference | ||
| CLL | 525 | 0.61 (0.43–0.88) | 0.007 | |
| Non-malignant | 453 | 1.52 (1.18–1.94) | 0.001 | |
Abbreviations: CLL=chronic lymphocytic leukemia
Table 5:
Multivariate analysis results of predictors for being lost to follow-up among pediatric 2-year survivors of autologous hematopoietic cell transplantation (n=467)
| Characteristics | N | HR (95% CI) | P-Value | |
|---|---|---|---|---|
| Age at transplant (year) | 0.04 | |||
| <11 | 370 | Reference | ||
| 11–17 | 97 | 1.72 (1.01–2.93) | 0.04 | |
| Gender | 0.05 | |||
| Female | 192 | Reference | ||
| Male | 275 | 1.57 (0.99–2.49) | 0.05 | |
| Disease | 0.01 | |||
| Lymphoma (HL/NHL) | 43 | Reference | ||
| Testicular/Germ cell | 8 | 1.43 (0.33–5.26) | 0.59 | |
| CNS tumor | 366 | 0.55 (0.34–0.90) | 0.02 | |
| Other solid tumor | 45 | 0.50 (0.24–1.05) | 0.07 | |
Abbreviations: CNS=central nervous system: HL=Hodgkin lymphoma; NHL=Non-Hodgkin lymphoma
For adult allogeneic HCT survivors, younger age at the time of HCT was significantly associated with greater risk of being lost to follow-up (41–50 years versus 51+ years: HR 1.38; 95% CI 1.14–1.67, and 18–40 years versus 51+ years: HR 2.19; 95% CI 1.80–2.66). Diagnosis of non-malignant disease was associated with greater risk of lost to follow-up when compared to malignant diseases excluding chronic lymphocytic leukemia (HR 1.52; 95% CI 1.18–1.94). Compared to adult allogeneic HCT survivors with private health insurance, public (HR 1.29; 95% CI 1.13–1.48) or no insurance (HR 2.33; 95% CI 1.30–4.17) was significantly associated with higher risk of lost to follow-up. Living from the HCT center 100+ miles away (HR 1.39; 95% CI 1.11–1.72) compared to living 0–99 miles away from the HCT center was associated with greater risk of lost to follow-up. Unmarried (single/divorced/widowed) HCT survivors were significantly more likely to be lost to follow-up (HR 1.26; 95% CI 1.09–1.46).
For adult autologous HCT survivors, older age at the time of HCT was significantly associated with risk of being lost to follow-up. Specifically, patients 21–30 years of age had significantly greater risk of being lost to follow-up as compared to those transplanted at 18–20 years of age (Hazard Ratio [HR] 2.26; 95% CI 1.17–4.34). As compared to NHL patients, a diagnosis of a testicular or germ cell tumor was associated with greater risk of lost to follow-up (HR 1.95; 95% CI 1.32–2.87) whereas a diagnosis of multiple myeloma (reference: NHL) was associated with decreased risk of lost to follow-up (HR 0.72; 95% CI 0.56–0.92).
Among pediatric allogeneic HCT survivors, age 10–17 years at the time of HCT (reference: <10 years) was significantly associated with higher risk of being lost to follow-up (HR 1.97; 95% CI 1.65–2.36). Compared to those pediatric allogeneic HCT survivors with private health insurance, publicly insured (HR 1.34; 95% CI 1.09–1.63) or uninsured (HR 3.53; 95% CI 2.11–5.92) were significantly more likely to be lost to follow-up. Non-malignant disease was associated with greater risk of becoming lost to follow-up compared to patients diagnosed with maligant diseases (HR 1.25; 95% CI 1.05–1.49).
Among pediatric autologous HCT survivors age 11–17 years at the time of HCT (reference: < 11 years) was associated with greater risk of lost to follow-up (HR 1.72; 95% CI 1.01–2.93). Male gender was associated with greater risk of being lost to follow-up (HR 1.57; 95% CI 0.99–2.49) although this achieved only marginal statistical significance. A diagnosis of a central nervous system tumor (reference: lymphoma) was associated with decreased risk of being lost to follow-up (HR 0.55; 95% CI 0.34–0.90).
Discussion
We demonstrate that the cumulative incidence of lost to follow-up in the US is high at 10-years (e.g., ~25% among pediatric HCT survivors). Among pediatric HCT survivors follow-up may be even more important given the longer life expectancy. Adolescent and young adult age appeared to be associated with lost to follow-up among pediatric and adult autologous and allogeneic HCT survivors. Public health insurance or a lack of health insurance was associated with increased risk of lost to follow-up among both pediatric and adult allogeneic HCT survivors, whereas being unmarried and living at a greater distance from the HCT center was associated with greater lost to follow-up risk among only adult allogeneic HCT survivors. Non-malignant disorders were associated with greater risk of lost to follow-up among pediatric and adult allogeneic HCT survivors.
Over time survivors may perceive that their susceptibility to late effects is diminished [13]. Many late effects have a long latency period prior to manifestation [3–4] underscoring the importance of regular surveillance for late effects for an extended period following HCT [5,14]. Becoming lost to follow-up can diminish opportunities to prevent late effects or to limit the progression of late effects. Efforts are needed to ensure the retention of all HCT survivors in follow-up HCT care. Examples include the use of dedicated resources (e.g., specialized staff) to identify at-risk survivors or outreach efforts focused on those that are lost to follow-up or their referring providers [15]. Efforts to educate survivors about the importance of surveillance for late effects may also be beneficial [16]. Few HCT centers have long-term follow-up programs; however, these may also provide an important structure to minimize lost to follow-up [14,17–18].
Our findings suggest that adolescent and young adult-aged HCT survivors may be at risk for lost to follow-up. Data document similar findings across other chronic disease populations [19–20] including non-HCT cancer survivors [21–23]. Among non-HCT cancer survivors, barriers to the provision of follow-up care for adolescent and young adult-aged survivors are numerous such as cost and a lack of health insurance, transition of care away from a parental locus of control, competing responsibilities, diminished perceived vulnerability to late effects, and lack of providers that are comfortable providing care to aging pediatric HCT survivors. For adolescent and young adult-aged HCT survivors there is a need to develop age appropriate approaches to HCT survivor care [24–26]. Strategies aimed at engaging survivors through the delivery of text messages, emails, and app notifications are examples [27–28]. Transitioning programs may also provide an important structure to minimize the lost to follow-up among this population [29].
Those children and adults with public or a lack of health insurance who were survivors of allogeneic HCT experienced greater risk for lost to follow-up. In general, children and adults with public insurance experience notable delays in obtaining necessary care [30–31]. Pediatric and adult allogeneic HCT survivors with public or a lack of health insurance may continue to face financial hardships or other challenges related to a lack of socioeconomic resources when compared to children and adult HCT survivors with private insurance [32]. Further research is needed to evaluate potential strategies that can be used to improve access to care and ensure health care delivery to these underserved populations of HCT survivors [33].
Given the complexity of the HCT process many patients that require post-HCT care are unable to receive care locally and may have to travel to specialized centers to receive care. HCT survivors face a variety of physical, psychological, and social barriers (e.g., getting time off work, lack of transportation) that influence the ability to travel a long distance to seek care at their respective HCT center [34]. Improving clinic efficiency to engage survivors [35], the provision of affordable transportation alternatives for HCT survivors, greater use of telemedicine, or the establishment of satellite clinics [36] may be valuable approaches to mitigate the burden of distance and its impact on lost to follow-up. Facilitating long-term follow-up care at a center near the HCT survivor may also be a valuable approach using survivorship care plans and the education of non-HCT providers [37–38].
Across many chronic disease populations, it has been documented that married or partnered persons tend to experience better physical, mental, and social health outcomes [39]. For example, married individuals with chronic health conditions may experience greater social support. A recent study from the CIBMTR found that marital status was not associated with overall survival and graft-versus-host disease risk among HCT survivors [40]. Despite these findings, given the resource intensive nature of the allogeneic HCT process, caregivers such as spouses or partners remain critical to supporting the retention of survivors in long-term follow-up.
Pediatric and adult allogeneic HCT survivors with non-malignant disease may perceive that they are cured and that their susceptibility to late effects is minimal. Moreover, adult autologous HCT recipients with a diagnosis of testicular or germ cell tumors are often exposed to limited outpatient therapy and may perceive that they are cured without susceptibility to late effects. On the other hand, pediatric patients with central nervous system tumors and adult patients with indolent diseases such as chronic lymphocytic leukemia and multiple myeloma may not be lost to follow-up due to the nature of their diseases (e.g., need for ongoing maintenance therapy and likelihood of relapse). Greater efforts are needed to educate survivors and their families about the importance of adequate surveillance for complications which may occur following HCT.
Limitations
We were unable to evaluate potentially important factors (e.g., educational attainment, current health status, and the presence of a long-term follow-up program) that may be associated with being lost to follow-up. The possibility of under-reporting or over-reporting of lost to follow-up remains. There may be a failure to update contact information, HCT survivors may be deceased or may be receiving follow-up care elsewhere. Alternatively, if a patient had missed two follow-up reporting periods, but later resumed their care at their respective HCT center they would still be classified as lost to follow-up. In the latter instance, the proportion in which this was felt to be the case was very small and unlikely to have impacted the results of our analyses. Some of these results (e.g., the association between insurance status and lost to follow-up status) may lack generalizability when considering other systems of health care delivery or health insurance coverage.
Conclusions
A national, comprehensive, risk-based approach to long-term follow-up focusing on minimizing the attrition in high-risk groups is needed. Patient reported outcomes may help describe reasons for lack of long-term follow-up. Collection of accurate epidemiologic and clinical data from all survivors can help refine strategies to improve the retention of HCT survivors which will support surveillance and intervention for potential complications.
Table 3:
Multivariate analysis results of predictors for being lost to follow-up among adult 2-year survivors of autologous hematopoietic cell transplantation (n=7,291)
| Characteristics | N | HR (95% CI) | P-Value | |
|---|---|---|---|---|
| Age at transplant (years) | 0.0002 | |||
| 18–20 | 71 | Reference | ||
| 21–30 | 268 | 2.26 (1.17–4.34) | 0.01 | |
| 31–40 | 473 | 1.82 (0.95–3.46) | 0.07 | |
| 41+ | 6479 | 1.21 (0.63–2.34) | 0.57 | |
| Disease | 0.0002 | |||
| NHL | 1877 | Reference | ||
| HL | 552 | 1.14 (0.85–1.54) | 0.38 | |
| Multiple myeloma | 4738 | 0.72 (0.56–0.92) | 0.008 | |
| Testicular/Germ cell | 91 | 1.95 (1.32–2.87) | 0.0008 | |
| CNS tumor | 16 | 1.03 (0.17–6.27) | 0.98 | |
| Other solid tumor | 17 | 2.14 (0.79–5.81) | 0.13 | |
| Year of transplant | 0.04 | |||
| 2010–2013 | 1802 | Reference | ||
| 2006–2009 | 3395 | 1.22 (0.75–1.98) | 0.43 | |
| 2002–2005 | 2094 | 1.70 (0.95–3.02) | 0.07 | |
Abbreviations: CNS=central nervous system; HL=Hodgkin lymphoma; NHL=Non-Hodgkin lymphoma
Table 4:
Multivariate analysis results of predictors for being lost to follow-up among pediatric 2-year survivors of allogeneic hematopoietic cell transplantation (n=3,865)
| Characteristics | N | HR (95% CI) | P-Value | |
|---|---|---|---|---|
| Age at transplant (year) | <0.0001 | |||
| <10 | 2738 | Reference | ||
| 10–17 | 1127 | 1.97 (1.65–2.36) | <0.0001 | |
| Race | 0.0009 | |||
| Caucasian | 2938 | Reference | ||
| African American | 439 | 1.18 (0.86–1.63) | 0.31 | |
| Other | 304 | 1.48 (1.20–1.84) | 0.0003 | |
| Unknown | 184 | 1.42 (0.85–2.36) | 0.18 | |
| Insurance | <0.0001 | |||
| Private | 1676 | Reference | ||
| None | 54 | 3.53 (2.11–5.92) | <0.0001 | |
| Public | 1543 | 1.34 (1.09–1.63) | 0.005 | |
| Other/Unspecified | 197 | 1.23 (0.87–1.75) | 0.24 | |
| Missing | 395 | 1.24 (0.85–1.83) | 0.26 | |
| Disease | 0.01 | |||
| Malignant | 2076 | Reference | ||
| Non-malignant | 1789 | 1.25 (1.05–1.49) | 0.01 | |
| Year of transplant | 0.004 | |||
| 2010–2013 | 901 | Reference | ||
| 2006–2009 | 1694 | 1.46 (1.13–1.89) | 0.0037 | |
| 2002–2005 | 1270 | 1.75 (1.24–2.45) | 0.0012 | |
Footnotes
Conflict of Interest Disclosure
Dr. Ganguly receives support from Seattle Genetics, Kite Phrama, Janssen Pharmaceuticals, and Daiichi Sankyo.
References
- 1.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquini MC. Impact of graft-versus-host disease on survival. Best Pract Res Clin Haematol. 2008;21(2):193–204. [DOI] [PubMed] [Google Scholar]
- 3.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SJ, Inamoto Y. Late Effects of Blood And Marrow Transplantation. Haematologica 2017; 102: 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18(3):348–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tien Y, Chiu Y, Liu M. Frequency of Lost to Follow-Up and Associated Factors for Patients with Rheumatic Diseases. PLoS One. 2016; 11(3): e0150816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawatsu L, Uchimura K, Ohkado A, Kato S. A combination of quantitative and qualitative methods in investigating risk factors for lost to follow-up for tuberculosis treatment in Japan – Are physicians and nurses at a particular risk? PLoS One. 2018; 13(6): e0198075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: age really matters. J Int AIDS Soc. 2017; 20(1): 21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hicks CW, Zarkowsky DS, Bostock IC, et al. Endovascular aneurysm repair patients who are lost to follow-up have worse outcomes. J Vasc Surg. 2017. June; 65(6): 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill A, Gosain R, Bhandari S, et al. “Lost to Follow-up” Among Adult Cancer Survivors. Am J Clin Oncol. 2017. October 12 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau; Census 2017, using American FactFinder; <http://factfinder2.census.gov>.; (20 December 2018).
- 12.Khera N, Gooley T, Flowers MED, et al. Association of Distance from Transplantation Center and Place of Residence on Outcomes after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(7):1319–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staba Hogan MJ, Ross WL, Balsamo L, Mitchell HR, Kadan-Lottick NS. Parental perception of child vulnerability. Pediatr Blood Cancer. 2018: e27364. [DOI] [PubMed] [Google Scholar]
- 14.Hashmi S, Carpenter P, Khera N, et al. Lost in Transition: The Essential Need for Long-Term Follow-Up Clinic for Blood and Marrow Transplantation Survivors. Biol Blood Marrow Transplant 2015; 21(2): 225–232. [DOI] [PubMed] [Google Scholar]
- 15.Nekhlyudov L, O’Malley DM, Hudson SV. Integrating Primary Care Providers in the Care of Cancer Survivors: Gaps in Evidence and Future Opportunities. Lancet Oncol. 2017. January; 18(1): e30–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera N, Martin P, Edsall K, et al. Patient centered care coordination in hematopoietic cell transplantation. Blood Adv. 2017;1(19):1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battiwalla M, Tichelli A, Majhail NS. Long-Term Survivorship after Hematopoietic Cell Transplantation: Roadmap for Research and Care. Biol Blood Marrow Transplant. 2017; 23(2): 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syrjala KL, Stover AC, Yi JC, et al. Development and implementation of an Internet-based survivorship care program for cancer survivors treated with hematopoietic stem cell transplantation. J Cancer Surviv. 2011;5(3):292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackie AS, Rempel GR, Kovacs AH, et al. Transition Intervention for Adolescents With Congenital Heart Disease. J Am Coll Cardiol. 2018;71(16):1768–1777. [DOI] [PubMed] [Google Scholar]
- 20.Treadwell M, Telfair J, Gibson RW, et al. Transition from pediatric to adult care in sickle cell disease: Establishing evidence-based practice and directions for research. Am J Hematol. 2011;86(1):116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaupin LM, Boldt A, Amato K. Come back: Identifying targets to engage young adult survivors who have been lost to follow-up. ASCO. Presented Friday, February 16, 2018. [Google Scholar]
- 22.Smith AW, Parsons HM, Kent EE, et al. Unmet Support Service Needs and Health-Related Quality of Life among Adolescents and Young Adults with Cancer: The AYA HOPE Study. Front Oncol. 2013; 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett M, McDonnell G, DeRosa A, et al. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): a systematic review. J Cancer Surviv. 2016;10(5):814–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke L, Chung C, Grant M. Psychosocial Care for Adolescent and Young Adult Hematopoietic Cell Transplant Patients. J Psychosoc Oncol. 2011; 29(4): 394–414. [PMC free article] [PubMed] [Google Scholar]
- 25.Ortiz JM, Rosipal JM, Woodworth G, et al. Addressing the Unmet Social Needs Of the Aya Stem Cell Transplant Patient. Biol Blood Marrow Transplant. 2009;15(2): s148. [Google Scholar]
- 26.Miyamura K, Yamashita T, Atsuta Y, et al. High probability of follow-up termination among AYA survivors after allogeneic hematopoietic cell transplantation. Blood Adv. 2019. February 12; 3(3): 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson MM, Tyc VL, Srivastava DK, et al. Multi-component behavioral intervention to promote health protective behaviors in childhood cancer survivors: the protect study. Med Pediatr Oncol. 2002;39(1):2–1; discussion 2. [DOI] [PubMed] [Google Scholar]
- 28.Wesley KM, Fizur PJ. A review of mobile applications to help adolescent and young adult cancer patients. Adolesc Health Med Ther. 2015; 6:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cupit MC, Duncan C, Savani BN, Hashmi SK. Childhood to adult transition and long-term follow-up after blood and marrow transplantation. Bone Marrow Transplant. 2016;51(2):176–81. [DOI] [PubMed] [Google Scholar]
- 30.Focusing on Children’s Health: Community Approaches to Addressing Health Disparities: Workshop Summary. Institute of Medicine (US) and National Research Council (US) Roundtable on Health Disparities. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 31.Miller S, Wherry LR. Health and Access to Care during the First 2 Years of the ACA Medicaid Expansions. N Engl J Med. 2017;376(10):947–956. [DOI] [PubMed] [Google Scholar]
- 32.Anand A, Theodore R, Mertens A, Lane PA, Krishnamurti L. Health Disparity in Hematopoietic Cell Transplantation for Sickle Cell Disease: Analyzing the Association of Insurance and Socioeconomic Status Among Children Undergoing Hematopoietic Cell Transplantation. Blood 2017. 130:4636. [Google Scholar]
- 33.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2010; 16(8):1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Nassar KE, Kim HT, Blossom J, et al. The impact of geographic proximity to transplant center on outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(5):708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ragon BK, Clifton C, Savani BN, et al. Follow-up in Long Term Transplant Clinic Overcomes the Barrier of Geographic Distance From Transplant Center. Blood 2012. 120:2070. [Google Scholar]
- 36.Dyer G, Gilroy N, Brown L, et al. What They Want: Inclusion of Blood and Marrow Transplantation Survivor Preference in the Development of Models of Care for Long-Term Health in Sydney, Australia. Biol Blood Marrow Transplant. 2016; 22(4):731–743. [DOI] [PubMed] [Google Scholar]
- 37.Majhail NS, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gajewski JL, McClellan MB, Majhail NS, et al. Payment and Care for Hematopoietic Cell Transplantation Patients: Toward a Specialized Medical Home for Complex Care Patients. Biol Blood Marrow Transplant. 2018;24(1):4–12. [DOI] [PubMed] [Google Scholar]
- 39.Beattie S, Lebel S, Tay J. The influence of social support on hematopoietic stem cell transplantation survival: a systematic review of literature. PLoS One. 2013;8(4): e61586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tay J, Brazauskas R, He N, et al. The Impact of Marital Status on Hematopoietic Stem Cell Transplant (HCT) Recipient Outcomes: A Surrogate for Consistent Caregiver. a CIBMTR Registry Study. Blood. 2018; 132:4788. [Google Scholar]