Abstract
Background
Current guidelines emphasize an active lifestyle in the management of knee osteoarthritis (OA), but up to 90% of patients with OA are inactive. In a previous study, we demonstrated that an 8-week physiotherapist (PT)-led counseling intervention, with the use of a Fitbit, improved step count and quality of life in patients with knee OA, compared with a control.
Objective
This study aimed to examine the effect of a 12-week, multifaceted wearable-based program on physical activity and patient outcomes in patients with knee OA.
Methods
This was a randomized controlled trial with a delay-control design. The immediate group (IG) received group education, a Fitbit, access to FitViz (a Fitbit-compatible app), and 4 biweekly phone calls from a PT over 8 weeks. Participants then continued using Fitbit and FitViz independently up to week 12. The delay group (DG) received a monthly electronic newsletter in weeks 1 to 12 and started the same intervention in week 14. Participants were assessed in weeks 13, 26, and 39. The primary outcome was time spent in daily moderate-to-vigorous physical activity (MVPA; in bouts ≥10 min) measured with a SenseWear Mini. Secondary outcomes included daily steps, time spent in purposeful activity and sedentary behavior, Knee Injury and OA Outcome Score, Patient Health Questionnaire-9, Partners in Health Scale, Theory of Planned Behavior Questionnaire, and Self-Reported Habit Index.
Results
We enrolled 51 participants (IG: n=26 and DG: n=25). Compared with the IG, the DG accumulated significantly more MVPA time at baseline. The adjusted mean difference in MVPA was 13.1 min per day (95% CI 1.6 to 24.5). A significant effect was also found in the adjusted mean difference in perceived sitting habit at work (0.7; 95% CI 0.2 to 1.2) and during leisure activities (0.7; 95% CI 0.2 to 1.2). No significant effect was found in the remaining secondary outcomes.
Conclusions
A 12-week multifaceted program with the use of a wearable device, an app, and PT counseling improved physical activity in people with knee OA.
Trial Registration
ClinicalTrials.gov NCT02585323; https://clinicaltrials.gov/ct2/show/NCT02585323
Keywords: physical activity, counseling, knee osteoarthritis, physiotherapy, wearables
Introduction
Background
Arthritis is the most common cause of severe chronic pain and disability worldwide [1,2]. Analysis by the Arthritis Alliance of Canada estimates a new diagnosis of osteoarthritis (OA) every 60 seconds [3]. Current evidence supports the use of physical activity to manage OA because of its benefits on pain, mobility, and quality of life [4-6]. Guidelines by the OA Research Society International recommend the use of physical activity and therapeutic exercise as first-line treatment for knee OA [7]. Canadian physical activity guidelines recommend ≥150 min a week of moderate-to-vigorous physical activity (MVPA), performed in bouts of ≥10 min [8]. A study using accelerometers, however, found that over 90% of people with knee OA did not meet the physical activity guidelines [9]. This concurs with a systematic review that only 13% of people with OA accumulated ≥150 min per week of MVPA in bouts of ≥10 min [10].
Several modifiable factors are associated with low physical activity participation in patients with arthritis. These include lack of motivation [11], doubts about the effectiveness of exercise [12], and lack of health professional advice [13]. To promote an active lifestyle, the use of activity tracking devices has been explored based on the assumption that providing direct feedback on the amount of physical activity encourages people to meet specific targets. A systematic review of 14 randomized controlled trials (RCTs) of accelerometer-based interventions reported a small effect on physical activity participation (standardized mean difference 0.26; 95% CI 0.04 to 0.49). Notably, consumer-grade accelerometers promote physical activity behaviors through the use of behavioral change techniques [14], such as goal setting, self-monitoring, feedback, and rewards [15]. However, effective techniques such as action planning and problem-solving are absent from the use of these devices alone [15]. These techniques require contact with a health professional with counseling experience.
Our previous study [16] and Lyons et al [17] have demonstrated the feasibility of a physical activity counseling program with the use of a Fitbit (Fitbit Inc), a consumer-grade wearable device. Our subsequent study showed that an 8-week physiotherapist (PT)-led counseling program improved step count and quality of life in people with knee OA, compared with a control [18].
Study Aim
In this study, we aimed to assess the effect of a 12-week multifaceted intervention on improving activity participation in people with knee OA. Our primary hypothesis was that, compared with controls, those who received the program would increase mean daily MVPA time as determined by an objective measure. In addition, we explored the effect of the program on OA disease status, depressive symptoms, perceived habitual behaviors, and psychological constructs of being active.
Methods
Study Design
The Supporting Physical activity & Reducing sedentary behaviour in Arthritis (SuPRA) project was a proof-of-concept RCT with a delay-control design. Participants were randomly assigned to start the intervention either immediately (immediate group [IG]) or 14 weeks (delay group [DG]) after completing the baseline assessment. All participants were reassessed in weeks 13 (primary end point), 26, and 39 (Figure 1).
Figure 1.
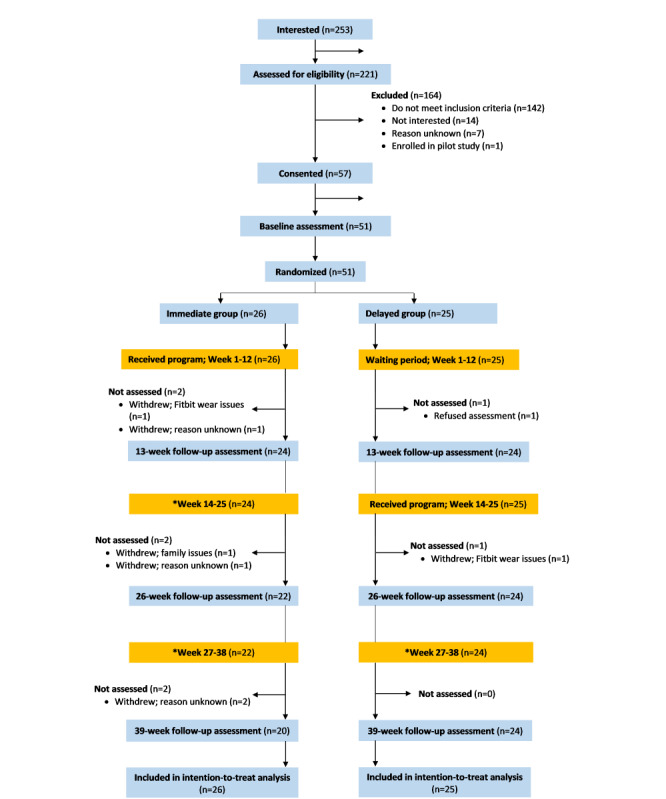
Consolidated Standards of Reporting Trials flowchart.
Participants
Participants were recruited from the Mary Pack Arthritis Program (Vancouver Coastal Health Authority) and the Fraser Health Authority in British Columbia, Canada. We also posted study information on Facebook, Twitter, Kajiji, and Craigslist. Individuals were eligible if they had a diagnosis of knee OA or met 2 criteria for early OA [19]. People with other chronic musculoskeletal conditions or contraindications to be physically active without medical supervision were excluded (Textboxes 1 and 2).
Inclusion criteria.
Inclusion criteria
Patients who had a physician-confirmed diagnosis of knee osteoarthritis or were aged ≥50 years and had felt pain or discomfort in or around the knee during the previous year lasting >28 separate or consecutive days [19]
Patients who had no previous diagnosis of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, polymyalgia rheumatica, connective tissue diseases, fibromyalgia, or gout
Patients who had no history of using disease-modifying antirheumatic drugs or gout medications
Patients who had no prior knee arthroplasty and not on a waiting list for total knee or hip replacement surgery
Patients who did not have surgery in the back, hip, knee, foot, or ankle joints in the past 12 months
Patients who had no history of acute injury to the knee in the past 6 months
Patients who had an email address and access to the internet daily
Patients who were able to attend a 1.5-hour group education session
Exclusion criteria.
Exclusion criteria
Patients who had previously used a physical activity wearable tracker
Patients who received a steroid injection in a knee in the last 6 months
Patients who received a hyaluronate injection in a knee in the last 6 months
Patients who used medication that may impair activity tolerance (eg, beta blockers)
Patients who faced a level of risk by exercising as identified by the Physical Activity Readiness Questionnaire [20]. If a participant did not pass the Physical Activity Readiness Questionnaire, a physician’s note was requested to determine the eligibility
Randomization and Blinding
After completing the baseline measures, participants were randomly assigned to the IG or the DG in a 1:1 allocation ratio. Randomization was performed by a research staff not involved in the study using numbers generated by SAS version 9.4 (SAS Institute Inc) in variable block sizes to ensure allocation concealment. Participants were not blinded as they knew whether they received the program during the intervention period. Daily MVPA time (the primary outcome) was measured with a wearable multisensor device couriered to the participant to wear for 7 days. All research personnel processing the objectively measured physical activity data were blinded to the participants’ group assignment. Self-reported measures were completed by the participant via a web-based questionnaire.
Intervention
The intervention has 3 components: (1) an in-person session with 20 min of group education and 30 min of individual counseling with a PT, (2) the use of a Fitbit Flex-2 wristband, and (3) PT counseling by phone to review physical activity goals (20-30 min). The in-person session was held in a meeting room at either the Mary Park Arthritis Centre, Fraser Health Authority, or Arthritis Research Canada. Fitbit is a consumer-grade wearable device that tracks and displays steps walked, gross level of physical exertion, and time spent being active. It is easy for users to put on and remove for charging. Fitbit Flex-2 is splash proof but cannot be used during water-based activities (eg, shower, swimming). Participants may record activities not captured by the device on the Fitbit website. Participants could view their physical activity goal attainment on FitViz, a new Fitbit-compatible web-based app developed for this study [21].
All 8 study PTs completed a 2-day basic training in motivational interviewing at the University of British Columbia [22]. In addition, they attended an orientation session, received a counseling guide, and shadowed at least one education and counseling session before they were paired with a participant.
Participants in the IG were scheduled to attend a PT-led group education session that focused on physical activity in OA management and strategies to manage joint symptoms. Next, each participant was paired with the next available study PT. During the individual counseling, PTs used the Brief Action Planning approach [23] to guide participants to set specific, measurable, attainable, relevant, and time-bound (SMART) physical activity goals. An example of a SMART goal is “attending a pool exercise class in a community center every Tuesday and Saturday for the next three months.”
Study PTs then set the parameters on their assigned participants’ FitViz accounts based on the participant’s goals. These parameters included the following: (1) the upper and lower limits of intensity and duration of MVPA (ie, to promote physical activity based on the participant’s goal), (2) the duration when a sedentary behavior should be interrupted (ie, to promote less sitting), and (3) the rest time in between sessions of MVPA (ie, to promote pacing).
During weeks 1 to 8, the study PT remotely reviewed participants’ progress on FitViz and counseled them to modify their physical activity goals via 4 biweekly phone calls. A counseling guide was provided, and the discussion was documented by the PT. During weeks 9 to 12, participants continued using their Fitbit and FitViz but had no counseling calls with their PTs. However, participants could email their PTs if there were questions related to being physically active. At the end of the program, participants could keep their Fitbit and FitViz account.
The DG received the same intervention in week 14. During the waiting period, they received monthly emails of arthritis news that were unrelated to physical activity.
Outcome Measures
The primary outcome measure was mean daily MVPA time measured with SenseWear Mini (BodyMedia Inc). SenseWear integrates triaxial accelerometer data, physiological sensor data, and personal demographic information to provide estimates of steps and energy expenditure. A strong relationship has been found between SenseWear and indirect calorimetry measures of energy expenditure for activities of daily living (Pearson r=0.85) [24]. The device can be worn 24 hours a day and can capture a full picture of physical activity and off-body time throughout the day [25]. An important feature of SenseWear is its ability to differentiate between sedentary and light physical activities [26]; hence, it is an ideal instrument to assess both active and sedentary behaviors.
Participants wore a SenseWear Mini over the triceps for 7 days at each assessment. Almeida et al [27] determined that a minimum of 4 days of wear was required to reliably assess energy expenditure from different levels of physical activity in people with rheumatoid arthritis (intraclass correlation coefficient >0.80). We calculated the average MVPA accumulated in bouts (min per day). A bout was defined as ≥10 min at the level of ≥3 metabolic equivalent of the task (MET), with an allowance for interruption of up to 2 min below the threshold [28].
The secondary outcomes included the following:
Average daily time in purposeful activity performed in ≥4 MET in bouts of ≥10 min, with allowance for interruption of up to 2 min below the threshold (eg, brisk walking) [29].
Average daily step count.
Average daily time in sedentary behavior performed in ≤1.5 MET in bouts of ≥20 min during waking hours [30-33].
Partners in Health Scale (PIHS) [36].
Patient Health Questionnaire-9 (PHQ-9) [39].
We used SenseWear to measure purposeful activity time, steps, and sedentary behavior time. The KOOS consists of 5 subscales: pain, symptoms, activities of daily living, sports/recreation, and knee-related quality of life. It was originally developed for people recovering from injuries such as the anterior cruciate ligament and meniscus injury and was validated in patients with OA [34,35]. The PIHS is a 12-item measure designed to assess self-efficacy, knowledge of health conditions and treatment, and self-management behavior, such as adopting a healthy lifestyle (Cronbach α=.82) [36]. Motivation for engaging in physical activity was measured using the Rhodes 7-point Likert-type Theory of Planned Behavior questionnaire [37,38]. The questionnaire consists of 16 items measuring all components of the theory. Previous studies using this measure showed good predictive validity and internal consistency in adult populations [37,38].
The PHQ-9 consists of 9 questions that correspond to the diagnostic criteria for major depressive disorder. A score greater than 11 indicates a major depressive disorder [39]. The Self-Reported Habit Index is a 12-item scale, rated on a 7-point Likert scale, which measures characteristics of habitual behavior (reliability minimum α=.81) [40,41]. We asked participants to rate their strength of habit for 3 specific activity-related behaviors: sitting during leisure time at home, sitting during usual occupational activities, and walking outside for 10 min. A higher score indicates a stronger habit or behavior that is done frequently and automatically. Demographic variables and comorbid conditions were collected at baseline.
Adverse Event and Intervention Fidelity Monitoring
We tracked adverse events (falls as well as cardiovascular and musculoskeletal events) related to their physical activity [42] in the follow-up questionnaire at weeks 13, 26, and 39. Participants were deemed adhering to the 12-week intervention protocol if they (1) attended the education session, (2) used their Fitbit ≥5 days per week in ≥11 weeks, and (3) participated in ≥3 of 4 counseling calls. We monitored participants’ Fitbit wear using FitViz, which wirelessly synchronized physical activity data recorded by a Fitbit 150 times per hour [43]. We calculated the percentage of participants meeting each criterion and all 3 criteria.
Data Analysis
Descriptive analysis was used to summarize participant characteristics and comorbid conditions. We performed an intention-to-treat analysis using SAS version 9.4. The analysis of covariance (ANCOVA) was used for the main analysis to estimate an adjusted mean difference comparing time in MVPA (primary outcome) at 13 weeks between groups, adjusting for baseline MVPA and blocking. In a secondary analysis, we used a longitudinal mixed effects model that allowed us to additionally examine the intervention effects at 26 and 39 weeks after intervention initiation. The mixed effects models included the following variables as fixed effects: (1) the randomization group indicator for baseline difference, (2) a set of indicator variables for follow-up assessment time points (weeks 13, 26, and 39) to account for secular trend, and (3) a set of indicator variables for the lengths of time since intervention initiation (weeks 12, 25, or 38) to estimate intervention effects after these amounts of time postintervention initiation. The models additionally included participants as random effects to account for the repeated measures nature of the data. We used the sandwich estimators for linear mixed models [44] to compute empirical standard errors that were robust to model specifications. We examined the secondary outcomes at 13 weeks using ANCOVA and over weeks 26 and 39 using the mixed effects models mentioned earlier.
Sample Size
We estimated that our collaboration with health authorities and patient groups allowed the study to recruit 60 eligible participants within 12 months. Our previous study of a similar physical activity counseling program resulted in an estimated MVPA time of 75.5 min per day (SD 54.3) in the intervention group and 50.0 min per day (SD 46.8) in the controls [18]. Assuming approximately 15% attrition, we anticipated that 50 of the 60 participants would complete the study. With a sample size of 50, we would have 74% power at an α level of 0.1 (via a one-sided test).
Ethics
The research protocol was approved by the University of British Columbia Behavioural Research Ethics Board (application number: H15-02038) and published in ClinicalTials.gov (NCT02585323).
Results
Baseline Characteristics
In the years 2017 to 2019, 253 people indicated an interest to participate, and 221 met the eligibility criteria (Figure 1). Of these, we recruited 51 participants (IG: 23/26, 88% were women; DG: 19/25, 76% were women). Both groups were similar in age (IG: mean 65.0, SD 8.3 years; DG: mean 64.8, SD 9.0 years) and BMI (IG: mean 29.8, SD 9.0 kg/m2; DG: mean 28.9, SD 6.2 kg/m2). Approximately 55% (28/51) of the participants did not meet the Canadian physical activity guidelines at baseline (Table 1).
Table 1.
Baseline characteristics of participants.
| Variables | All (N=51) | Immediate group (n=26) | Delay group (n=25) | |
| Women, n (%) | 42 (82) | 23 (89) | 19 (76) | |
| Age (years), mean (SD) | 64.9 (8.5) | 65.0 (8) | 64.8 (9) | |
| Marital status, n (%) | ||||
|
|
Married/common law | 30 (59) | 18 (69) | 12 (48) |
|
|
Separated/divorced | 10 (20) | 5 (19) | 5 (20) |
|
|
Widowed/never married/other | 11 (22) | 3 (12) | 8 (32) |
| University degree or trades certificate, n (%) | 25 (49) | 14 (54) | 11 (44) | |
| Gross annual household income (US $), n (%) | ||||
|
|
≤24,000 | 2 (4) | 0 (0) | 2 (8) |
|
|
24,001-40,000 | 6 (12) | 2 (8) | 4 (16) |
|
|
40,001-60,000 | 9 (18) | 4 (15) | 5 (20) |
|
|
60,001-80,000 | 8 (16) | 4 (15) | 4 (16) |
|
|
80,001-100,000 | 7 (14) | 5 (19) | 2 (8) |
|
|
>100,000 | 7 (14) | 3 (12) | 4 (16) |
|
|
No answer | 12 (24) | 8 (31) | 4 (16) |
| Diagnosed with OAa, n (%) | ||||
|
|
Yes | 37 (73) | 20 (77) | 17 (68) |
|
|
No, but met the likely OA criteria | 14 (28) | 6 (23) | 8 (32) |
| In general, would you say your health is, n (%) | ||||
|
|
Excellent | 6 (12) | 1 (4) | 5 (20) |
|
|
Very good | 14 (27) | 10 (39) | 4 (16) |
|
|
Good | 22 (43) | 11 (42) | 11 (44) |
|
|
Fair | 8 (16) | 3 (12) | 5 (20) |
|
|
Poor | 1 (2) | 1 (4) | 0 (0) |
| Compared with 1 year ago, how would you rate your health in general now? n (%) | ||||
|
|
Much better | 3 (6) | 1 (4) | 2 (8) |
|
|
Somewhat better | 8 (16) | 4 (15) | 4 (16) |
|
|
About the same | 24 (47) | 11 (42) | 13 (52) |
|
|
Somewhat worse | 16 (31) | 10 (39) | 6 (24) |
|
|
Much worse | 0 (0) | 0 (0) | 0 (0) |
| Number of comorbid conditions, median (25th, 75th percentile) | 3.0 (2.0, 5.0) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) | |
| BMI (kg/m2), mean (SD) | 29.4 (7.7) | 29.8 (9.0) | 28.9 (6.2) | |
| Participants did not meet the Canadian physical activity guideline (≥150 min of MVPAb in bouts of ≥10 min per week), n (%) | 28 (55) | 15 (58) | 13 (52) | |
aOA: osteoarthritis.
bMVPA: moderate-to-vigorous physical activity.
Comparison of the Immediate Group With Delay Group
Table 2 and Figure 2 present the results of the primary outcome from 4 time points. At baseline, the mean MVPA time was 31.0 min per day (SD 37.3) for the IG and 71.3 min per day (SD 99.8) for the DG. The DG accumulated significantly more MVPA time—2 outliners accumulated a mean of >300 min per day (Figure 3). At 13 weeks (the primary end point), the IG accumulated a mean MVPA of 37.7 min per day (SD 30.5), whereas the DG had 49.4 min per day (SD 63.6). The adjusted mean difference in time spent in MVPA between groups following the intervention at 13 weeks was 13.1 min per day (95% CI 1.6 to 24.5), favoring the IG. Analyses adjusted for blocking yielded nearly identical results; thus, it was removed from subsequent analyses. A secondary analysis using a mixed effects model revealed smaller intervention effects at 12 weeks (9.4 min per day; 95% CI −3.0 to 21.7), 25 weeks (−3.0 min per day; 95% CI −34.9 to 29.0), and 38 weeks (0.2 min per day; 95% CI −44.0 to 44.4) postprogram initiation (Table 3).
Table 2.
Participant outcomes and results of the primary analysis.
| Measures | Immediate group, mean (SD) | Delay group, mean (SD) | Adjusted difference in mean change at T0-T1 | ||||||||
| Baseline (T0; n=26) | 13 weeks (T1; n=24) | 26 weeks (T2; n=22) | 39 weeks (T3; n=20) | Baseline (T0; n=25) | 13 weeks (T1; n=24) | 26 weeks (T2; n=22) | 39 weeks (T3; n=23) | Mean difference (95% CI) | P value | ||
| Time in MVPAa,b (min) | 31.0 (37.3) | 37.7 (30.5) | 37.0 (32.3) | 34.0 (25.2) | 71.3 (99.8) | 49.4 (63.6) | 74.6 (102.1) | 54.8 (66.2) | 13.1 (1.6 to 24.5) | .03 | |
| Time in purposeful activityc (min) | 11.1 (19.5) | 13.3 (20.0) | 13.7 (18.8) | 12.8 (16.8) | 42.1 (80.2) | 23.1 (37.1) | 36.5 (62.6) | 22.0 (42.3) | 1.6 (−3.0 to 6.1) | .50 | |
| Daily steps | 6294.0 (3418.0) | 7133.3 (3603.3) | 6381.6 (3492.0) | 5845.3 (2575.5) | 7030.1 (3921.6) | 6232.7 (3086.1) | 8162.3 (6642.3) | 7445.1 (4713.2) | 1106.5 (−19.9 to 2232.9) | .05 | |
| Sedentary timed (min) | 567.5 (183.1) | 531.4 (173.5) | 492.5 (156.6) | 502.8 (135.3) | 551.1 (234.9) | 558.3 (224.9) | 499.5 (248.9) | 483.1 (225.4) | −29.5 (−75.8 to 16.7) | .21 | |
| Knee Injury and Osteoarthritis Outcome Score (0-100; higher=better) | |||||||||||
| Symptoms | 68.5 (10.7) | 69.3 (12.7) | 65.4 (45.8) | 68.4 (16.3)e | 65.7 (12.3) | 66.9 (14.9)f | 72.2 (16.8)g | 72.5 (13.3)g | −1.4 (−7.8 to 4.9) | .66 | |
| Pain | 72.6 (13.5) | 73.1 (15.3) | 72.5 (18.3) | 72.1 (19.8)e | 65.1 (13.7) | 65.9 (15.6)f | 74.8 (15.4)g | 72.8 (13.2)g | 2.5 (−4.2 to 9.5) | .49 | |
| Activity of daily living | 75.5 (14.7) | 75.0 (13.1) | 77.7 (18.9) | 74.8 (20.7)e | 72.2 (15.8) | 70.3 (16.9)f | 80.3 (13.1)g | 80.0 (14.0)g | 2.8 (−3.3 to 8.8) | .37 | |
| Sports and recreation | 47.9 (23.7) | 47.1 (22.4) | 50.5 (30.1) | 51.6 (30.4)e | 46.8 (25.4) | 52.5 (22.7)f | 62.5 (22.5)g | 55.8 (26.3)g | −3.8 (−14.9 to 7.2) | .50 | |
| Quality of life | 44.0 (16.0) | 48.7 (17.5) | 49.4 (15.7) | 49.3 (19.1)e | 47.5 (16.0) | 46.9 (13.6)f | 54.7 (14.7)g | 54.4 (14.6)g | 1.4 (−5.0 to 7.9) | .66 | |
| Partners in Health (0-96; higher=better) | 76.8 (11.0) | 76.7 (11.5) | 78.9 (9.0) | 81.0 (7.0)e | 78.0 (12.0) | 81.6 (9.6)f | 82.6 (9.3)g | 82.6 (10.0)g | −2.3 (−6.6 to 1.9) | .28 | |
| Patient Health Questionnaire-9 (0-27; lower=better) | 5.2 (4.6) | 4.0 (3.0) | 3.6 (3.3) | 4.2 (4.1)e | 5.4 (5.3) | 4.7 (4.9)f | 4.5 (5.2)g | 4.5 (5.3)g | −0.4 (−1.7 to 0.8) | .47 | |
| Self-Reported Habit Index (1-7; higher=stronger habit) | |||||||||||
| Sitting at work subscale | 5.0 (1.4) | 5.2 (1.6) | 4.7 (2.1) | 5.0 (1.9)e | 4.5 (2.1) | 4.4 (2.0)f | 4.3 (2.1)g | 4.1 (2.1)g | 0.7 (0.2 to 1.2) | .004 | |
| Sitting at leisure subscale | 4.8 (1.1) | 5.1 (1.1) | 5.0 (1.1) | 5.5 (1.1)e | 5.1 (1.4) | 4.7 (1.6)f | 4.4 (1.9)g | 4.7 (1.7)g | 0.7 (0.2 to 1.2) | .006 | |
| Walking subscale | 4.3 (1.8) | 4.4 (1.6) | 4.6 (1.8) | 4.7 (1.8)e | 4.8 (2.0) | 4.6 (2.0)f | 4.8 (1.6)g | 4.6 (1.8)g | 0.3 (−0.3 to 0.9) | .27 | |
| Theory of Planned Behavior Questionnaire (1-7; higher=more positive) | |||||||||||
| Attitude toward physical activity | 6.0 (0.6) | 5.9 (0.7) | 6.0 (0.5) | 6.0 (0.5)e | 6.1 (0.6) | 6.1 (0.7)f | 6.2 (0.6)g | 6.1 (0.7)g | −0.1 (−0.4 to 0.2) | .63 | |
| Subjective norm | 6.2 (0.6) | 6.2 (0.8) | 6.1 (0.8) | 6.2 (1.1)e | 6.3 (0.8) | 6.2 (0.7)f | 6.3 (0.8)g | 6.3 (0.7)g | 0.2 (−0.1 to 0.5) | .13 | |
| Perceived control | 5.8 (1.0) | 5.6 (1.4) | 5.6(1.5) | 5.7 (1.4)e | 6.1 (0.7) | 5.8 (1.2)f | 6.2 (1.0)g | 6.2 (0.9)g | 0.1 (−0.6 to 0.8) | .80 | |
| Intention | 6.2 (0.8) | 5.9 (1.0) | 5.7 (1.1) | 5.8 (0.8)e | 6.3 (0.8) | 6.4 (0.6)f | 6.4 (0.6)g | 6.3 (0.9)g | 0.1 (−0.1 to 0.3) | .22 | |
aMVPA: moderate-to-vigorous physical activity.
bMVPA was performed at ≥3 metabolic equivalent of tasks and in bouts ≥10 min.
cPurposeful activity was performed at ≥4 METs and in bouts ≥10 min.
dSedentary behavior was performed at ≤1.5 METs in bouts ≥20 min.
en=19.
fn=22.
gn=24.
Figure 2.
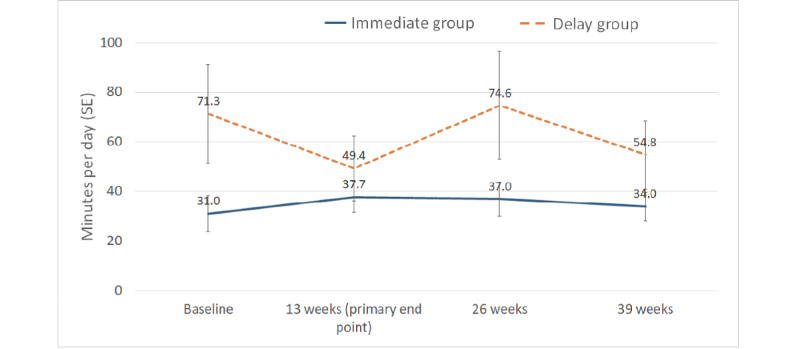
Time in moderate-to-vigorous physical activity.
Figure 3.
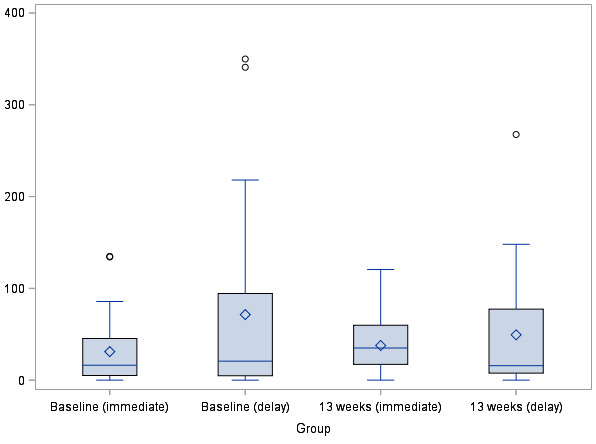
Boxplot of time in moderate-to-vigorous physical activity at baseline and 13 weeks.
Table 3.
Results of mixed effects models.
| Measures | Intervention effect postprogram initiation (95% CI) | Secular trend (95% CI) | |||||
|
|
12 weeks | 25 weeks | 38 weeks | T2a effect vs T1b | T3c effect vs T1 | ||
| Time in MVPAd,e (min) | 9.4 (−3.0 to 21.7) | −3.0 (−34.9 to 29.0) | 0.2 (−44.0 to 44.4) | 11.9 (−12.4 to 36.1) | 6.4 (−29.7 to 42.5) | ||
| Time in purposeful activityf (min) | 1.5 (−8.4 to 11.5) | −9.0 (−33.5 to 15.5) | −6.8 (−42.3 to 28.6) | 10.0 (−8.0 to 28.0) | 6.9 (−21.3 to 35.1) | ||
| Daily steps | 1461.2 (433.8 to 2488.6) | 715.0 (−1995.9 to 3425.9) | 71.4 (−4063.6 to 4206.3) | 101.7 (−2142.6 to 2346.0) | 336.9 (−3261.7 to 3935.4) | ||
| Sedentary timeg (min) | −32.2 (−78.1 to 13.7) | −62.6 (−162.8 to 37.6) | −63.4 (−217.6 to 90.8) | −19.8 (−83.7 to 44.1) | −7.6 (−117.0 to 101.9) | ||
|
|
Symptoms | −2.2 (−8.2 to 3.4) | −12.4 (−22.6 to −2.2) | −21.1 (−36.8 to −5.4) | 6.1 (0.2 to 11.9) | 16.5 (6.2 to 26.9) | |
|
|
Pain | 0.3 (−6.2 to 6.8) | −7.8 (−19.7 to 4.1) | −15.1 (−33.1 to 3.0) | 7.5 (1.3 to 13.8) | 13.7 (2.0 to 25.4) | |
|
|
Activity of daily living | 1.1 (−5.0 to 7.1) | −4.6 (−16.2 to 7.0) | −13.2 (−32.0 to 5.6) | 8.1 (2.7 to 13.6) | 13.5 (2.1 to 24.9) | |
|
|
Sports and recreation | −7.4 (−18.7 to 3.9) | −21.2 (−42.4 to 0.1) | −27.2 (−59.2 to 4.9) | 16.5 (5.1 to 27.9) | 23.6 (2.5 to 44.7) | |
|
|
Quality of life | 2.4 (−4.6 to 9.3) | −2.1 (−13.6 to 9.4) | −6.9 (−24.8 to 11.0) | 4.8 (−3.2 to 12.7) | 9.0 (−3.2 to 21.2) | |
| Partners in Health (0-96; higher=better) | −1.7 (−5.7 to 2.4) | −3.8 (−10.9 to 3.3) | −4.2 (−15.2 to 6.8) | 3.4 (−1.1 to 7.9) | 5.6 (−2.7 to 13.9) | ||
| Patient Health Questionnaire-9 (0-27; lower=better) | −0.9 (−2.4 to 0.6) | −1.3 (−4.0 to 1.3) | −1.3 (−5.5 to 2.9) | 0.1 (−1.2 to 1.5) | 0.6 (−2.2 to 3.3) | ||
|
|
Sitting at work subscale | 0.4 (−0.3 to 1.1) | 0.3 (−1.1 to 1.6) | 0.6 (−1.5 to 2.7) | −0.5 (−1.2 to 0.3) | −0.5 (−1.9 to 0.9) | |
|
|
Sitting at leisure subscale | 0.6 (0.1 to 1.0) | 1.4 (0.5 to 2.4) | 2.4 (0.9 to 3.9) | −1.0 (−1.6 to −0.4) | −1.5 (−2.5 to −0.4) | |
|
|
Walking subscale | 0.3 (−0.3 to 0.9) | 0.4 (−0.6 to 1.4) | 0.7 (−1.1 to 2.4) | 0 (−0.6 to 0.5) | −0.3 (−1.4 to 0.8) | |
|
|
Beliefs toward physical activity | −0.1 (−0.4 to 0.2) | −0.2 (−0.7 to 0.3) | −0.3 (−1.1 to 0.4) | 0.2 (−0.1 to 0.4) | 0.2 (−0.4 to 0.7) | |
|
|
Subjective norm | 0.1 (−0.3 to 0.5) | 0.0 (−0.6 to 0.6) | 0.1 (−0.9 to 1.1) | 0.0 (−0.4 to 0.4) | 0.0 (−0.6 to 0.6) | |
|
|
Perceived control | 0.0 (−0.6 to 0.7) | −0.4 (−1.6 to 0.7) | −0.7 (−2.5 to 1.1) | 0.3 (−0.3 to 1.0) | 0.8 (−0.3 to 2.0) | |
|
|
Intention | −0.4 (−0.7 to −0.1) | −1.1 (−1.7 to −0.4) | −1.5 (−2.6 to −0.5) | 0.4 (0.1 to 0.7) | 1.0 (0.3 to 1.7) | |
aT2: 26-week assessment.
bT1: 13-week assessment.
cT3: 39-week assessment.
dMVPA: moderate-to-vigorous physical activity.
eMVPA was performed at ≥3 metabolic equivalent of tasks and in bouts of ≥10 min.
fPurposeful activity was performed at ≥4 METS and in bouts of ≥10 min.
gSedentary behavior was performed at ≤1.5 METS in bouts of ≥20 min.
For the secondary outcome, a trend favoring the IG, but not statistically significant, was found from baseline to 13 weeks in purposeful activity time (1.6 min per day; 95% CI −3.0 to 6.1), step count (1106.5; 95% CI −19.9 to 2232.9), and sedentary time (−29.5 min per day; 95% CI −75.8 to 16.7). The results from the KOOS, PIHS, and PHQ-9 were also not statistically significant (Table 2). We found a small effect in perceived sitting habit while at work (0.7; 95% CI 0.2 to 1.2) or during leisure activities (0.7; 95% CI 0.2 to 1.2).
Secondary analysis demonstrated an effect attributable to being in the program for daily steps at 12 weeks (1461.2; 95% CI 433.8 to 2488.6; Table 3). Knee symptoms (measured by the KOOS symptoms subscale), perceived sitting habit during leisure activities, and intention to be physically active also showed statistically significant effects attributable to being in the program for different durations, although statistically significant secular trends were also observed over the assessment period.
Intervention Adherence and Adverse Events
Intervention adherence in the IG was 100% (26/26) for education session attendance, 96% (25/26) for PT counseling phone calls, and 81% (21/26) for Fitbit use (Table 4). In all, 81% (21/26) of participants met all 3 fidelity criteria. Adherence rates were similar in the DG when participants received the program in week 13. During the 4 weeks when the PT counseling ended, 2 participants each from the IG and the DG contacted their study PT via email with further questions regarding their physical activity.
Table 4.
Summary of intervention adherence.
| Adherence criterion | Immediate group (n=26), n (%) | Delay group (n=25), n (%) | All (N=51), n (%) |
| Attended the initial session with group education and met with a physiotherapist to set physical activity goals | 26 (100) | 25 (100) | 51 (100) |
| Completed ≥3 of 4 counseling phone calls with a physiotherapist | 25 (96) | 22 (88) | 47 (92) |
| Met Fitbit use criteriaa ≥11 weeks out of the 12-week intervention period | 21 (81) | 20 (80) | 41 (80) |
| Met 2 of 3 criteria | 25 (96) | 24 (96) | 49 (96) |
| Met all 3 criteria | 21 (81) | 18 (72) | 39 (77) |
aParticipants had steps recorded in their Fitbit ≥5 days per week.
After starting the program, 10 of the 51 participants reported adverse events because of physical activity; of those, 7 reported muscle pain (IG: n=5 and DG: n=2). Falls were reported by 3 in the IG; of those, 2 fell while being physically active (1 had an ankle sprain). Three participants from the DG also reported a fall; 1 occurred while being physically active. Of the remaining 2 participants who had a fall, 1 sustained a vertebral compression fracture.
Discussion
Principal Findings
More than 1 in 6 people in the United States are using wearable devices to monitor their health [45], but the integration of these tools in chronic disease management is at an early stage. This study demonstrated the potential of a multifaceted wearable-based program for promoting MVPA in people with knee OA. We found an effect in the adjusted mean difference in time spent in MVPA between groups after the 12-week program. Furthermore, the mixed effects model analysis suggests a significant effect in daily steps attributable to the program.
These results, however, should be viewed in the context that the DG accumulated significantly more daily MVPA time than the IG at baseline, and the observed effect was primarily driven by a decline in daily MVPA time in the DG at week 13. More than 80% of participants rated their health as “good,” “very good,” or “excellent” at baseline, suggesting that they might have few health constraints to be physically active. Nonetheless, the results extend those of our previous RCT on a similar program, whereby a significant improvement in participation in physical activity was found in people with knee OA at the end of an 8-week intervention [18]. We also found a small effect on the awareness of sitting habits at work and during leisure activities at 13 weeks. The reason for this observation is unclear, but it is likely too small to be clinically important.
Our results contribute to the literature on physical activity promotion in arthritis management. Recommendations by the European League Against Rheumatism endorse the use of behavior change techniques to promote physical activity among people with arthritis [46]. The 2019 American College of Rheumatology/Arthritis Foundation guidelines further highlight the involvement of health professionals, including PTs, to deliver nonpharmacological treatment [47]. The optimal approach for supporting an active lifestyle in people with arthritis remains to be unclear, but research in healthy adults has shown that low-dose health coaching had little effect on physical activity behavior [48]. Although PTs are skilled in exercise prescription, a recent survey in Canada revealed up to 71% of the respondents wished to acquire further training in physical activity counseling.[49] This suggests an opportunity for professional development for PTs to master skills in counseling techniques that match their patients’ readiness to acquire a health-related behavior. In our study, training on motivational interviewing and the opportunity to shadow a PT with experience in counseling participants are essential to the program. The PTs’ written record for each interaction with participants allowed us to ensure that counseling followed the Brief Action Planning approach. Participants continuing to use their Fitbits suggests that the behavior of self-monitoring was sustained even after the PT counseling ended. Future research can refine and compare different implementation strategies of physical activity counseling for this population.
Strengths and Limitations
A strength of this study was intervention fidelity, with an overall intervention adherence of 81%. There is no consensus on what constitutes good intervention adherence, but 80% to 100% has been deemed as high fidelity in delivery [50]. Furthermore, more than 80% of participants adhered to Fitbit use over the 12-week period, indicating that it is feasible to transition individuals from a multifaceted program to a wearable-only intervention after the initial 8-week counseling from a PT.
This study has some limitations. With the use of a delay-control design in which the participants in the control arm received the program after a 13-week delay, the efficacy of the counseling program could only be unequivocally assessed at 13 weeks. At 26 and 39 weeks, both groups had already received the intervention, and there was an absence of a control group at these 2 time points, which can cause intervention effect estimates at 26 and 39 weeks to be less robust and more susceptible to small sample bias. Hence, the long-term effects of the program remain unclear. Furthermore, the results may not be generalizable to men because 82% of the participants were women.
Conclusions
Supporting a physically active lifestyle is a core component of physiotherapy practice. The Exercise is Medicine initiative advocates for the creation and worldwide implementation of effective physical activity promotion strategies in treatment plans for patients [51]. With the ubiquitous use of wearables, health professionals can leverage the use of these tools to motivate, monitor, and counsel people with arthritis to reach physical activity goals. To this end, we have shown that a 12-week multifaceted counseling program, with the use of a wearable device, can improve physical activity participation in people with knee OA.
Acknowledgments
The authors would like to thank Navi Grewal, Morgan Barber, Ankit Gupta, Juliane Chien, Eric C Sayre, Hussein Mamdani, and Amrit Sandhu for their important contributions to this project. The authors would also like to thank Jacob McIvor for his support with the SenseWear Mini data processing. LCL was supported by the Harold Robinson/Arthritis Society Chair in Arthritic Diseases award, the Canada Research Chair Program, and the Michael Smith Foundation for Health Research Scholar Award. HX was supported by the Maureen and Milan Ilich/Merck Chair in Statistics for Arthritis and Musculoskeletal Diseases award. JAAZ was supported by the BC Lupus Society Research Scholar and the Walter & Marilyn Booth Research Scholar. DG was supported by the Canada Research Chair Program during the study. SuPRA project was funded by the Canadian Institutes of Health Research (CIHR; KAL-135772) and Preventing Complications from Inflammatory Skin, Joint and Bowel Conditions, a CIHR team grant (THC-135235). The latter provided funding for the development of FitViz and statistical analysis for the RCT. The funders had no role in the study design, collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Abbreviations
- ANCOVA
analysis of covariance
- CIHR
Canadian Institutes of Health Research
- DG
delay group
- IG
immediate group
- KOOS
Knee Injury and Osteoarthritis Outcome Score
- MET
metabolic equivalent of task
- MVPA
moderate-to-vigorous physical activity
- OA
osteoarthritis
- PHQ-9
Patient Health Questionnaire-9
- PIHS
Partners in Health Scale
- PT
physiotherapist
- RCT
randomized controlled trial
- SMART goal
specific, measurable, attainable, relevant, and time-bound goal
- SuPRA
Supporting Physical activity & Reducing sedentary behaviour in Arthritis
Appendix
CONSORT checklist.
Footnotes
Authors' Contributions: LCL, LMF, CDS, DG, AMH, CK, JAAZ, AFT, GN, and CLB obtained funding. LCL, LMF, HX, CDS, DG, AMH, JAAZ, AFT, GN, and CLB contributed to the study design and proposal. LCL contributed to study oversight. LCL, SZ, JT, and ST contributed to data collection. LCL, LMF, HX, and NL analyzed the data. LCL, LMF, HX, NL, and AMH interpreted the data. LCL, LMF, HX, NL, CDS, DG, SZ, JAAZ, AMH, CK, JT, ST, AFT, GN, and CLB reviewed the manuscript.
Conflicts of Interest: None declared.
References
- 1.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. Br Med J. 2001 May 5;322(7294):1079–80. doi: 10.1136/bmj.322.7294.1079. http://europepmc.org/abstract/MED/11337425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health Canada . Arthritis in Canada: An Ongoing Challenge. Ottawa, Canada: Health Canada; 2003. [Google Scholar]
- 3.Bombardier C, Hawker G, Mosher D. The Impact of Arthritis in Canada: Today and Over the Next 30 Years. Arthritis Alliance of Canada. 2011. [2020-06-19]. http://www.arthritisalliance.ca/images/PDF/eng/Initiatives/20111022_2200_impact_of_arthritis.pdf.
- 4.Brosseau L, MacLeay L, Robinson V, Wells G, Tugwell P. Intensity of exercise for the treatment of osteoarthritis. Cochrane Database Syst Rev. 2003;(2):CD004259. doi: 10.1002/14651858.CD004259. [DOI] [PubMed] [Google Scholar]
- 5.Ottawa Panel Ottawa panel evidence-based clinical practice guidelines for therapeutic exercises and manual therapy in the management of osteoarthritis. Phys Ther. 2005 Sep;85(9):907–71. [PubMed] [Google Scholar]
- 6.Zhang W, Moskowitz R, Nuki G, Abramson S, Altman R, Arden N, Bierma-Zeinstra S, Brandt K, Croft P, Doherty M, Dougados M, Hochberg M, Hunter D, Kwoh K, Lohmander L, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007 Sep;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. http://linkinghub.elsevier.com/retrieve/pii/S1063-4584(07)00234-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Moskowitz R, Nuki G, Abramson S, Altman R, Arden N, Bierma-Zeinstra S, Brandt K, Croft P, Doherty M, Dougados M, Hochberg M, Hunter D, Kwoh K, Lohmander L, Tugwell P. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008 Feb;16(2):137–62. doi: 10.1016/j.joca.2007.12.013. http://linkinghub.elsevier.com/retrieve/pii/S1063-4584(07)00397-4. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay MS, Warburton DE, Janssen I, Paterson DH, Latimer AE, Rhodes RE, Kho ME, Hicks A, Leblanc AG, Zehr L, Murumets K, Duggan M. New Canadian physical activity guidelines. Appl Physiol Nutr Metab. 2011 Feb;36(1):36–46; 47. doi: 10.1139/H11-009. [DOI] [PubMed] [Google Scholar]
- 9.Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, Eaton CB, Hochberg MC, Jackson RD, Kwoh CK, Mysiw WJ, Nevitt MC, Hootman JM. Objective physical activity measurement in the osteoarthritis initiative: are guidelines being met? Arthritis Rheum. 2011 Nov;63(11):3372–82. doi: 10.1002/art.30562. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallis J, Webster K, Levinger P, Taylor N. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis Cartilage. 2013 Nov;21(11):1648–59. doi: 10.1016/j.joca.2013.08.003. http://linkinghub.elsevier.com/retrieve/pii/S1063-4584(13)00906-0. [DOI] [PubMed] [Google Scholar]
- 11.Gyurcsik NC, Brawley LR, Spink KS, Brittain DR, Fuller DL, Chad K. Physical activity in women with arthritis: examining perceived barriers and self-regulatory efficacy to cope. Arthritis Rheum. 2009 Aug 15;61(8):1087–94. doi: 10.1002/art.24697. doi: 10.1002/art.24697. [DOI] [PubMed] [Google Scholar]
- 12.der Ananian C, Wilcox S, Saunders R, Watkins K, Evans A. Factors that influence exercise among adults with arthritis in three activity levels. Prev Chronic Dis. 2006 Jul;3(3):A81. http://www.cdc.gov/pcd/issues/2006/jul/05_0220.htm. [PMC free article] [PubMed] [Google Scholar]
- 13.Henchoz Y, Zufferey P, So A. Stages of change, barriers, benefits, and preferences for exercise in RA patients: a cross-sectional study. Scand J Rheumatol. 2013;42(2):136–45. doi: 10.3109/03009742.2012.724707. [DOI] [PubMed] [Google Scholar]
- 14.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013 Aug;46(1):81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 15.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014 Aug 15;16(8):e192. doi: 10.2196/jmir.3469. https://www.jmir.org/2014/8/e192/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li LC, Sayre EC, Xie H, Clayton C, Feehan LM. A community-based physical activity counselling program for people with knee osteoarthritis: feasibility and preliminary efficacy of the track-OA study. JMIR Mhealth Uhealth. 2017 Jun 26;5(6):e86. doi: 10.2196/mhealth.7863. https://mhealth.jmir.org/2017/6/e86/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons EJ, Swartz MC, Lewis ZH, Martinez E, Jennings K. Feasibility and acceptability of a wearable technology physical activity intervention with telephone counseling for mid-aged and older adults: a randomized controlled pilot trial. JMIR Mhealth Uhealth. 2017 Mar 6;5(3):e28. doi: 10.2196/mhealth.6967. https://mhealth.jmir.org/2017/3/e28/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li LC, Sayre EC, Xie H, Falck RS, Best JR, Liu-Ambrose T, Grewal N, Hoens AM, Noonan G, Feehan LM. Efficacy of a community-based technology-enabled physical activity counseling program for people with knee osteoarthritis: proof-of-concept study. J Med Internet Res. 2018 Apr 30;20(4):e159. doi: 10.2196/jmir.8514. https://www.jmir.org/2018/4/e159/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the american rheumatism association. Arthritis Rheum. 1986 Aug;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 20.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Can J Sport Sci. 1992 Dec;17(4):338–45. [PubMed] [Google Scholar]
- 21.Gupta A, Tong X, Shaw CD, Li LC, Feehan LM. FitViz: A Personal Informatics Tool for Self-Management of Rheumatoid Arthritis. Proceedings of the International Conference on Human-Computer Interaction; HCI'17; July 9-14, 2017; Vancouver Canada. Springer International Publishing; 2017. https://link.springer.com/chapter/10.1007%2F978-3-319-58753-0_35. [DOI] [Google Scholar]
- 22.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York, USA: Guilford Press; 2008. [Google Scholar]
- 23.Gutnick D, Reims K, Davis C, Gainforth H, Jay M, Cole S. Brief action planning to facilitate behavior change and support patient self-management. J Clin Outcomes Manag. 2014;21:17–29. https://www.mdedge.com/jcomjournal/article/147101/endocrinology/brief-action-planning-facilitate-behavior-change-and. [Google Scholar]
- 24.Tierney M, Fraser A, Purtill H, Kennedy N. Study to determine the criterion validity of the sensewear armband as a measure of physical activity in people with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013 Jun;65(6):888–95. doi: 10.1002/acr.21914. doi: 10.1002/acr.21914. [DOI] [PubMed] [Google Scholar]
- 25.Holsgaard-Larsen A, Roos EM. Objectively measured physical activity in patients with end stage knee or hip osteoarthritis. Eur J Phys Rehabil Med. 2012 Dec;48(4):577–85. http://www.minervamedica.it/index2.t?show=R33Y2012N04A0577. [PubMed] [Google Scholar]
- 26.Feehan LM, Goldsmith CH, Leung AY, Li LC. Sensewearmini and actigraph GT3X accelerometer classification of observed sedentary and light-intensity physical activities in a laboratory setting. Physiother Can. 2016;68(2):116–23. doi: 10.3138/ptc.2015-12. http://europepmc.org/abstract/MED/27909358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Almeida GJ, Wasko MC, Jeong K, Moore CG, Piva SR. Physical activity measured by the SenseWear Armband in women with rheumatoid arthritis. Phys Ther. 2011 Sep;91(9):1367–76. doi: 10.2522/ptj.20100291. http://europepmc.org/abstract/MED/21719635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008 Jan;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett Jr DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. Compendium of Physical Activities Tracking Guide. Google Sites. 2011. [2020-06-15]. https://sites.google.com/site/compendiumofphysicalactivities. [DOI] [PubMed]
- 30.Owen N. Sedentary behavior: understanding and influencing adults' prolonged sitting time. Prev Med. 2012 Dec;55(6):535–9. doi: 10.1016/j.ypmed.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012 May;35(5):976–83. doi: 10.2337/dc11-1931. http://europepmc.org/abstract/MED/22374636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latouche C, Jowett JB, Carey AL, Bertovic DA, Owen N, Dunstan DW, Kingwell BA. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985) 2013 Feb 15;114(4):453–60. doi: 10.1152/japplphysiol.00978.2012. http://journals.physiology.org/doi/full/10.1152/japplphysiol.00978.2012?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed. [DOI] [PubMed] [Google Scholar]
- 33.Howard BJ, Fraser SF, Sethi P, Cerin E, Hamilton MT, Owen N, Dunstan DW, Kingwell BA. Impact on hemostatic parameters of interrupting sitting with intermittent activity. Med Sci Sports Exerc. 2013 Jul;45(7):1285–91. doi: 10.1249/MSS.0b013e318285f57e. [DOI] [PubMed] [Google Scholar]
- 34.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and osteoarthritis outcome score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998 Aug;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 35.Roos EM, Roos HP, Ekdahl C, Lohmander LS. Knee injury and osteoarthritis outcome score (KOOS)--validation of a Swedish version. Scand J Med Sci Sports. 1998 Dec;8(6):439–48. doi: 10.1111/j.1600-0838.1998.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 36.Petkov J, Harvey P, Battersby M. The internal consistency and construct validity of the partners in health scale: validation of a patient rated chronic condition self-management measure. Qual Life Res. 2010 Sep;19(7):1079–85. doi: 10.1007/s11136-010-9661-1. [DOI] [PubMed] [Google Scholar]
- 37.Rhodes RE, Courneya KS, Blanchard CM, Plotnikoff RC. Prediction of leisure-time walking: an integration of social cognitive, perceived environmental, and personality factors. Int J Behav Nutr Phys Act. 2007 Oct 31;4:51. doi: 10.1186/1479-5868-4-51. https://ijbnpa.biomedcentral.com/articles/10.1186/1479-5868-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhodes RE, Blanchard CM, Courneya KS, Plotnikoff RC. Identifying belief-based targets for the promotion of leisure-time walking. Health Educ Behav. 2009 Apr;36(2):381–93. doi: 10.1177/1090198107308376. [DOI] [PubMed] [Google Scholar]
- 39.Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the patient health questionnaire (PHQ-9): a meta-analysis. Can Med Assoc J. 2012 Feb 21;184(3):E191–6. doi: 10.1503/cmaj.110829. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=22184363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner B, de Bruijn G, Lally P. A systematic review and meta-analysis of applications of the self-report habit index to nutrition and physical activity behaviours. Ann Behav Med. 2011 Oct;42(2):174–87. doi: 10.1007/s12160-011-9282-0. [DOI] [PubMed] [Google Scholar]
- 41.Gardner B, Abraham C, Lally P, de Bruijn G. Towards parsimony in habit measurement: testing the convergent and predictive validity of an automaticity subscale of the self-report habit index. Int J Behav Nutr Phys Act. 2012 Aug 30;9:102. doi: 10.1186/1479-5868-9-102. https://ijbnpa.biomedcentral.com/articles/10.1186/1479-5868-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ory M, Resnick B, Jordan PJ, Coday M, Riebe D, Garber CE, Pruitt L, Bazzarre T. Screening, safety, and adverse events in physical activity interventions: collaborative experiences from the behavior change consortium. Ann Behav Med. 2005 Apr;29(Suppl):20–8. doi: 10.1207/s15324796abm2902s_5. [DOI] [PubMed] [Google Scholar]
- 43.Rate Limits. Fitbit SDK. 2019. [2019-01-01]. https://dev.fitbit.com/build/reference/web-api/basics/#rate-limits.
- 44.Diggle PG, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data (Oxford Statistical Science Series - 25) Oxford, UK: Oxford University Press; 2013. [Google Scholar]
- 45.iHealth: How Consumers Are Using Tech to Stay Healthy. The Nielsen Company. 2014. Apr 10, [2020-06-15]. http://www.nielsen.com/us/en/insights/news/2014/ihealth-how-consumers-are-using-tech-to-stay-healthy.html.
- 46.Osthoff AR, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP, Hurkmans E, Juhl CB, Kennedy N, Kiltz U, Knittle K, Nurmohamed M, Pais S, Severijns G, Swinnen TW, Pitsillidou IA, Warburton L, Yankov Z, Vlieland TP. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018 Sep;77(9):1251–60. doi: 10.1136/annrheumdis-2018-213585. [DOI] [PubMed] [Google Scholar]
- 47.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020 Feb;72(2):220–33. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellingson LD, Lansing JE, DeShaw KJ, Peyer KL, Bai Y, Perez M, Phillips LA, Welk GJ. Evaluating motivational interviewing and habit formation to enhance the effect of activity trackers on healthy adults' activity levels: randomized intervention. JMIR Mhealth Uhealth. 2019 Feb 14;7(2):e10988. doi: 10.2196/10988. https://mhealth.jmir.org/2019/2/e10988/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien MW, Shields CA, Campbell KL, Crowell SJ, Fowles JR. Perceptions and practices of providing physical activity counselling and exercise prescriptions among physiotherapists in Nova Scotia. Phys Can. 2019 Aug 29;-:1–9. doi: 10.3138/ptc-2018-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, DeFrancesco C, Levesque C, Sharp DL, Ogedegbe G, Resnick B, Orwig D. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. J Consult Clin Psychol. 2005 Oct;73(5):852–60. doi: 10.1037/0022-006X.73.5.852. [DOI] [PubMed] [Google Scholar]
- 51.Lobelo F, Stoutenberg M, Hutber A. The exercise is medicine global health initiative: a 2014 update. Br J Sports Med. 2014 Dec;48(22):1627–33. doi: 10.1136/bjsports-2013-093080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT checklist.


