Abstract
Background.
Little is known about how early alterations in white matter relate to clinically relevant behaviors such as emotional dysregulation. Thus, our goal was to examine how the white matter structural integrity of key limbic (i.e., uncinate fasciculus and cingulum) and commissural (i.e., forceps minor) bundles in 3-month-old infants prospectively predicts emotional regulation behaviors at 9 months.
Methods.
Three-month-old infants underwent multishell diffusion-weighted imaging. Following image processing, tractography was performed for each tract within each infant’s native space (n=20). Measures of white matter integrity, including microstructure and morphology, were extracted from each tract. At 9 months, negative emotionality (NE) and positive emotionality (PE) were elicited using Laboratory Assessment of Temperament tasks. Elastic net regressions were performed for variable selection, which included white matter integrity variables from each of the 3 tracts, along with several covariates, including age, sex, use of public assistance, and the mother’s depressive symptoms. Outcome variables were NE and PE composite scores evaluated in two separate models.
Results.
Notably, following hierarchical regression using elastic net-selected variables, uncinate structural integrity was the most robust predictor of NE (ß=−0.631, p=0.005).
Limitations.
The sample size of our study is a limitation, however, as a preliminary study, our goal was to describe our findings to inform future larger studies.
Conclusions.
Greater uncinate structural integrity predicted lower NE, suggesting that greater uncinate structural integrity at 3 months allows greater emotional regulation capacity at 9 months. To our knowledge, this is the first study to demonstrate prospective brain-to-emotional behavior relationships in infants.
Introduction
Rapid development of the human brain in the first years of life establishes important brain-behavior relationships that set the stage for future health outcomes (1). Much of this research has focused on normative neurodevelopmental processes (2–4). Thus, little is known about how early alterations in neural circuits relate to clinically relevant behavioral outcomes, such as emotional regulation. In childhood, emotional dysregulation is an early transdiagnostic risk factor for subsequent behavioral and emotional problems (5). To this end, our goal was to examine a prospective brain-to-emotional behavior relationship in infants, focused on key limbic (uncinate fasciculus and cingulum) and commissural (forceps minor) white matter bundles contributing to these behaviors (6–9). This work is the first to our knowledge to examine how white matter structural integrity (microstructure/morphology) in 3-month-old infants prospectively predicted emotional regulation behaviors at 9 months. As this is a preliminary study, our goal is to describe our findings in order to inform future larger studies.
Methods
This sample comprised 48 mothers (age 19–34) and their 3-month-old infants recruited from the community: 40 from the population-based, longitudinal Pittsburgh Girls Study (PGS) (10) and 8 from postnatal wards at a local University hospital. Following informed consent, mother-infant dyads completed research visits at the Children’s Hospital of Pittsburgh. All procedures were approved by the University of Pittsburgh Institutional Review Board.
Neuroimaging
A MRI scan was performed (Siemens Skyra, 3 Tesla, 32-channel head coil) during nonsedated infant sleep at 3-months. Diffusion-weighted imaging (DWI) data were acquired using one of two multi-shell diffusion schemes with 2mm in-plane resolution and 2mm slice thickness. B-values were 100 (8 directions), 250 (32 directions) and 750 (64 directions) s/mm2 for the first sequence and 750 (50 directions) and 2000 (100 directions) s/mm2 for the second sequence. Diffusion images were manually realigned, motion and eddy current corrected and reconstructed in native space using Generalized Q-sampling Imaging in DSI Studio (11).
Tractography was performed for each tract (e.g., cingulum, uncinate fasciculus and forceps minor) in each infant’s native space using consistent parameters across infants (Fig. 1A & B; Supplement, Table S1). Measures of white matter integrity, including microstructure [quantitative anisotropy (QA), normalized QA (NQA), and fractional anisotropy (FA)] (12) and morphology (tract volume), were extracted from each tract and averaged across hemispheres for cingulum and uncinate. Notably, QA-aided tractography has better resolution and fewer false fibers than FA-aided tractography (12). Images with artifact, signal loss, or distortion were excluded from further analyses. In order to perform elastic net regression (see below), which does not allow missing data, infants in which tractography in all three tracts could not be reliably performed were also excluded. Among the 40 infants scanned with the first multi-shell sequence (100/250/750), 27 were excluded (n=13). To increase sample size and statistical power, 8 infants were included that were scanned with a second, updated multi-shell sequence (750/2000) (13). Among these 8, 1 was excluded (n=7), resulting in a total of n=20 (70% male). (Participant characteristics, Supplement, Tables S2, S3 & S4).
Figure 1.
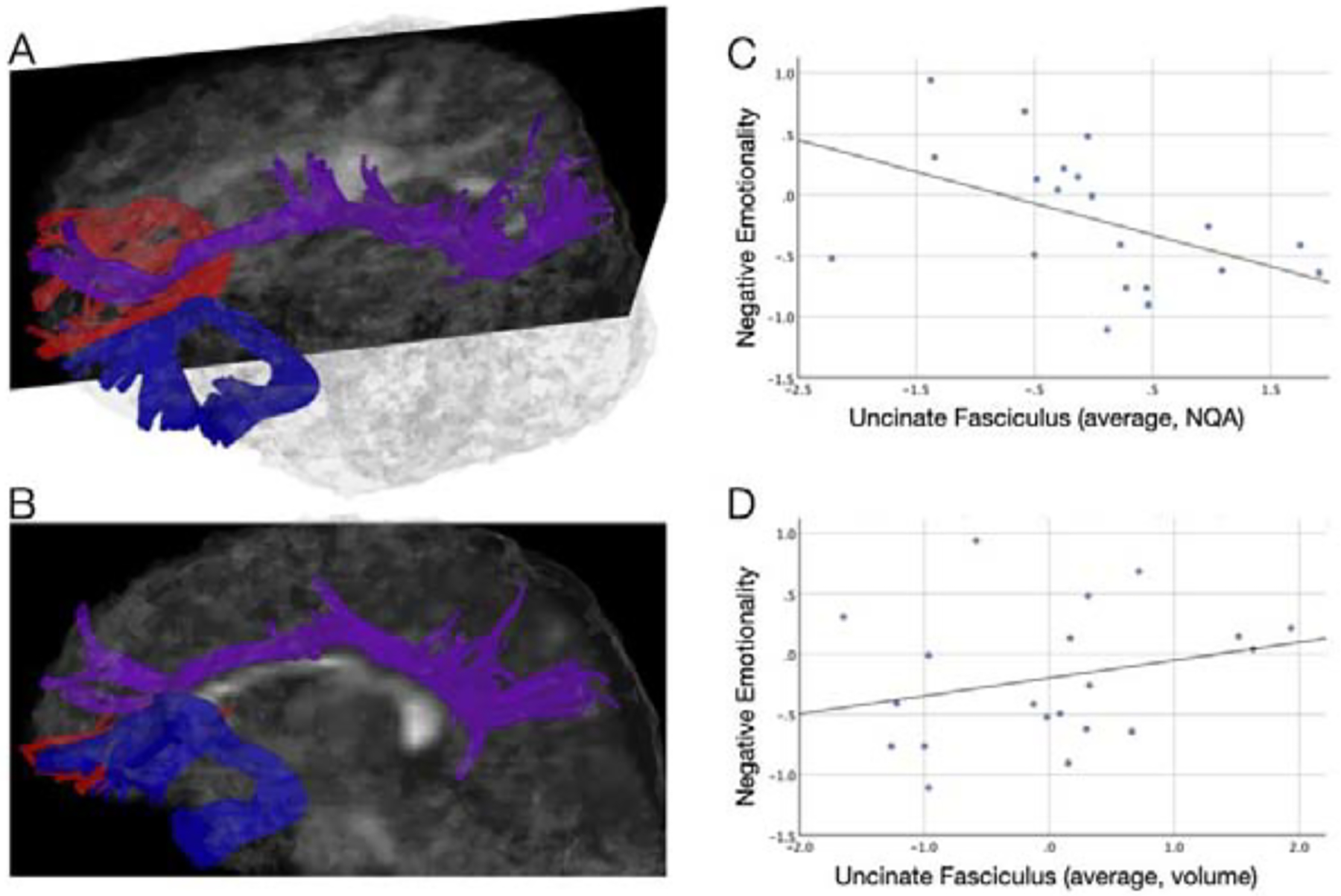
A. A 3-dimensional and B. a sagittal view of uncinate fasciculus (blue), cingulum (violet) and forceps minor (red) tractography in a representative 3-month old infant. C. The prospective relationship between uncinate fasciculus NQA (Z-scored) and negative emotionality (NE); greater uncinate NQA was associated with lower NE. Uncinate NQA was the most robust, significant predictor of NE (ß = −0.631, p = 0.005). C. The prospective relationship between uncinate fasciculus volume (Z-scored) and negative emotionality (NE); greater uncinate volume was associated with greater NE (ß = 0.383, p = 0.002).
Independent Samples T-Tests were used to examine differences in white matter structural integrity measures across sequences. NQA did not statistically differ between sequences for any tract; additionally, uncinate and cingulum volume did not differ between sequences (see Supplement, Table S5).
Behavioral Assessments
At 9 months, negative emotionality (NE) and positive emotionality (PE) were elicited using four Laboratory Assessment of Temperament (Lab-TAB) tasks (14). Gentle Arm Restraint (2 trials of 30 secs each) and Toy Retraction (3 trials of 15 secs each) typically elicit anger/frustration (NE), whereas Puppet Play (3 trials) and a scripted Peek-a-Boo game (4 trials) with the parent typically elicit joy/pleasure (PE). Indicators of infant NE (i.e., facial distress, vocal distress and behavioral struggle) and PE (i.e., smiling, vocal pleasure and behavioral animation) were coded independently using adapted Lab-TAB coding schemes (15) with high levels of inter-rater reliability (ICC>.70) prior to coding. A random sample of 20% were also double-coded to prevent scoring drift.
Data Analyses
In elastic net regressions performed for variable selection, we included 12 variables representing white matter integrity (Z-scored QA, NQA, FA and volume from each of the 3 tracts). Additionally, we included the infant’s “cohort” or DWI sequence, sex, age at time of scan (3-month exact age in days), age at time of behavioral assessment (9-month exact age in days), use of public assistance (an index of socioeconomic status), and the mother’s Edinburgh Postnatal Depression Scale (EPDS) score at the time of the infant scan and at 9 months.
Outcome variables included: 1) a NE composite score (mean of Z-scored facial and vocal distress from the Toy Retraction and facial distress, vocal distress and struggle from the Gentle Arm Restraint) and 2) a PE composite score (mean of Z-scored smile intensity, positive vocalizations and behavioral animation from the Puppet Play and Peek-a-Boo tasks). These outcome variables were evaluated in two separate models. Elastic net regressions were performed using GLMNET in R with cross validation (16) (Supplement, Figs. S1 & S2). Elastic net regressions were followed by standard hierarchical regressions with sociodemographic factors in step 1 and white matter predictors in step 2. See results below.
Neither of the elastic net regressions selected cohort as a predictor of negative or positive emotionality. To further investigate whether the difference in DWI sequence had an influence on the results, however, we performed additional elastic net and subsequent hierarchical regressions using only the five white matter variables that did not statistically differ across sequences (uncinate, cingulum and forceps minor NQA and uncinate and cingulum volume). These results are presented in the Supplement, Results.
Results
Negative Emotionality
Uncinate NQA (elastic net coefficients, −0.199), forceps minor FA, (−0.176), uncinate volume (.165), infant sex (0.122, females displayed higher NE), cingulum NQA (−0.036), EPDS (3 month, −0.035), cingulum QA (0.031) and infant age at 3 months (0.005) were all selected by the elastic net as predicting NE at 9 months. When entered into a standard hierarchical regression, with sociodemographic factors in step 1 and white matter predictors in step 2, step 1 accounted for 17.6% of the variance (adjusted R Square = 0.176), while step 2 accounted for 87.8% of the variance (adjusted R Square = 0.878, R Square Change = 0.623; F = 18.056, p = 0.000). Uncinate NQA was the most robust, significant predictor of NE (ß = −0.631, p = 0.005; Fig. 1C), followed by forceps minor FA (ß = −0.619, p = 0.000), EPDS (3 month, ß = −0.592, p = 0.000), uncinate volume (ß = 0.383, p = 0.002; Fig. 1D), cingulum QA (ß = 0.327, p = 0.022), and infant age at 3 months (ß = 0.259, p = 0.018). (See Table 1 for the results of the full hierarchical regression.)
Table 1.
Hierarchical Regression Results for the Negative Emotionality Model.
| Negative Emotionality | ||||
|---|---|---|---|---|
| Variables | Step | St. Beta | t | p |
| Age (3 month, days) | 1 | .196 | .849 | .408 |
| Infant sex | .079 | .333 | .743 | |
| EPDS (3 month) | −.482 | −2.234 | .040 | |
| Age (3 month, days) | 2 | .259 | 2.763 | .018* |
| Infant sex | .100 | .937 | .369 | |
| EPDS (3 month) | −.592 | −6.424 | .000* | |
| Cingulum NQA | .023 | .126 | .902 | |
| Uncinate Volume | .383 | 3.963 | .002* | |
| Uncinate NQA | −.631 | −3.457 | .005* | |
| Cingulum QA | .327 | 2.676 | .022 | |
| Forceps FA | −.619 | −6.172 | .000* | |
Positive Emotionality
Infant sex (elastic net coefficient, −0.197, females displayed lower PE) was the only variable selected by the elastic net regression as predicting PE at 9 months. Sex accounted for 29.7% of the variance (adjusted R Square = 0.297; F = 9.028, p = 0.008) and was a robust predictor of PE (ß = −0.578, p = 0.008).
Multiple Comparisons
Significant regression findings survived the FDR-corrected threshold for 9 multiple predictor-DV tests across both (NE, PE) regression models (p = 0.018) (17), with the exception of cingulum QA in the NE model.
Discussion
Our findings show for the first time prospective brain-to-emotional behavior relationships in infants. Greater limbic (uncinate NQA) and interhemispheric (forceps FA) structural integrity predicted lower NE. These findings suggest that greater uncinate fasciculus structural integrity at 3 months allows greater emotional regulation capacity at 9 months. Greater forceps minor structural integrity may facilitate this relationship by enhancing interhemispheric communication (although we place less emphasis on this finding as forceps FA differed across DWI sequences). In contrast, greater uncinate volume predicted greater NE, which may reflect aberrant morphological development (e.g., more disorganization and/or dispersion of white matter fibers) potentially compromising emotional regulation capacity. Taken together, these findings may provide insights into the optimal development of uncinate microstructure (e.g., greater myelination and less dispersion) for greater emotional regulation capacity.
Unexpectedly, greater 3-month maternal depression was associated with lower infant NE. Mothers in this sample had EPDS scores below the clinical threshold for depression (>13, Table S1); thus, this finding may reflect an adaptive infant behavioral response to low levels of maternal depression. Greater infant age at 3 months predicted greater NE at 9 months; this finding may reflect other factors at 3 months that were not measured and would need to be replicated in future larger studies.
Sex was a robust predictor of PE, with females displaying lower PE. This is consistent with males displaying more approach behaviors and positive affect from early in infancy while females display more fear (18). This may contribute to females being at greater risk for developing affective disorders throughout life.
Limitations
Our small sample size for this preliminary study is a limitation. In accordance with guidelines for pilot or feasibility studies, our goal was to provide a description of our findings and not to focus on hypothesis testing (19). While our results do meet criteria for statistical significance and withstand multiple comparison correction, they should be considered preliminary and used to guide hypotheses for future larger studies. Use of two DWI sequences is also a limitation of this study, however, two of the three white matter predictors of NE did not differ between sequences. Additionally, a supplemental analysis including only the five white matter variables that did not statistically differ across sequences showed similar findings; an uncinate variable (volume) was the most robust white matter predictor of NE and infant sex remained the most robust predictor of PE. Further, our initial infant sample is primarily male; thus, our sex-related finding must be reproduced and replicated in a larger, more balanced sample.
Conclusions
In summary, understanding infant brain-emotional behavior relationships may contribute to early identification of risk for behavioral and mental health problems later in life, and aid in the identification of novel neural targets for early interventions to foster normative development of emotional regulation in childhood.
Supplementary Material
Highlights.
Greater uncinate structural integrity of 3-month-old infants predicted lower negative emotionality at 9 months
Greater uncinate volume of 3-month-old infants predicted greater negative emotionality at 9 months
Sex was the most robust predictor of positive emotionality in 9-month-old infants
Funding & Acknowledgements
This work was supported by the National Institute of Mental Health (M.L.P. & A.E.H, 1R21MH106570 and 5R01MH115466, and L.B., 5K01MH102406) and The Pittsburgh Foundation (M.L.P., Endowed Chair).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gao W, Grewen K, Knickmeyer RC, Qiu A, Salzwedel A, Lin W, et al. A review on neuroimaging studies of genetic and environmental influences on early brain development. NeuroImage. 2019;185:802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi A, Mercure E, Lloyd-Fox S, Thomson A, Brammer M, Sauter D, et al. Early Specialization for Voice and Emotion Processing in the Infant Brain. Current Biology. 2011;21(14):1220–4. [DOI] [PubMed] [Google Scholar]
- 3.Gao W, Gilmore JH, Shen D, Smith JK, Zhu H, Lin W. The Synchronization within and Interaction between the Default and Dorsal Attention Networks in Early Infancy. Cerebral Cortex. 2012;23(3):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Short SJ, Elison JT, Goldman BD, Styner M, Gu H, Connelly M, et al. Associations between white matter microstructure and infants’ working memory. NeuroImage. 2013;64:156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno C Towards understanding and acting on risk factors for developmental psychopathology. European Child & Adolescent Psychiatry. 2018;27(1):1–3. [DOI] [PubMed] [Google Scholar]
- 6.Catani M, Dell’Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neuroscience & Biobehavioral Reviews. 2013;37(8):1724–37. [DOI] [PubMed] [Google Scholar]
- 7.Versace A, Ladouceur CD, Graur S, Acuff HE, Bonar LK, Monk K, et al. Diffusion imaging markers of bipolar versus general psychopathology risk in youth at-risk. Neuropsychopharmacology. 2018;43(11):2212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Versace A, Acuff H, Bertocci MA, Bebko G, Almeida JRC, Perlman SB, et al. White Matter Structure in Youth With Behavioral and Emotional Dysregulation Disorders: A Probabilistic Tractographic Study. JAMA Psychiatry. 2015;72(4):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6(7):533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, et al. The Pittsburgh Girls Study: Overview and Initial Findings. Journal of Clinical Child & Adolescent Psychology. 2010;39(4):506–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh FC, Wedeen VJ, Tseng W. Generalized q-sampling imaging. IEEE transactions on medical imaging. 2010;29(9):1626. [DOI] [PubMed] [Google Scholar]
- 12.Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLOS ONE. 2013;8(11):e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutter J, Tournier JD, Price AN, Cordero-Grande L, Hughes EJ, Malik S, et al. Time-efficient and flexible design of optimized multishell HARDI diffusion. Magnetic Resonance in Medicine. 2018;79(3):1276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery (LAB-TAB). University of Wisconsin. 1993. [Google Scholar]
- 15.Goldsmith H, Rothbart MK. Prelocomotor and locomotor laboratory temperament assessment battery (Lab-TAB; version 3.0, technical manual). Madison: University of Wisconsin, Department of Psychology; 1996. [Google Scholar]
- 16.Friedman J, Hastie T, Simon N, Tibshirani R. Package glmnet: lasso and elastic-net regularized generalized linear models ver 2.0. 2016. [Google Scholar]
- 17.Narum SR. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conservation Genetics. 2006;7(5):783–7. [Google Scholar]
- 18.Planalp EM, Van Hulle C, Gagne JR, Goldsmith HH. The Infant Version of the Laboratory Temperament Assessment Battery (Lab-TAB): Measurement Properties and Implications for Concepts of Temperament. Front Psychol. 2017;8:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Medical Research Methodology. 2010;10(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


