Abstract
Purpose
Decaffeinated green tea (GT) and black tea (BT) polyphenols inhibit weight gain in mice fed an obesogenic diet. Since the intestinal microflora is an important contributor to obesity, it was the objective of this study to determine whether the intestinal microflora plays a role in the anti-obesogenic effect of GT and BT.
Methods
C57BL/6J mice were fed a high-fat/high-sucrose diet (HF/HS, 32% energy from fat; 25% energy from sucrose) or the same diet supplemented with 0.25% GTP or BTP or a low-fat/high-sucrose (LF/HS, 10.6% energy from fat, 25% energy from sucrose) diet for 4 weeks. Bacterial composition was assessed by MiSeq sequencing of the 16S rRNA gene.
Results
GTP and BTP diets resulted in a decrease of cecum Firmicutes and increase in Bacteroidetes. The relative pro-portions of Blautia, Bryantella, Collinsella, Lactobacillus, Marvinbryantia, Turicibacter, Barnesiella, and Parabacteroides were significantly correlated with weight loss induced by tea extracts. BTP increased the relative proportion of Pseudobutyrivibrio and intestinal formation of short-chain fatty acids (SCFA) analyzed by gas chromatography. Cecum propionic acid content was significantly correlated with the relative proportion of Pseudobutyrivibrio. GTP and BTP induced a significant increase in hepatic 5′adenosylmonophosphate-activated protein kinase (AMPK) phosphorylation by 70 and 289%, respectively (P < 0.05) determined by Western blot.
Conclusion
In summary, both BTP and GTP induced weight loss in association with alteration of the microbiota and increased hepatic AMPK phosphorylation. We hypothesize that BTP increased pAMPK through increased intestinal SCFA production, while GTPs increased hepatic AMPK through GTP present in the liver.
Keywords: Black tea, Green tea, Polyphenols, Microflora, Obesity, AMPK phosphorylation, Short-chain fatty acids
Introduction
Consumption of green tea (GT) and black tea (BT) polyphenols provides distinct health benefits due to differences in the chemical structure of tea polyphenols in GT and BT [1, 2]. BTPs such as theaflavins and thearubigins are of large molecular weight and are not absorbed in the small intestine and transported to the colon, whereas GTP are rapidly absorbed in the small intestine. Our previous mouse studies demonstrated that the supplementation of a high-fat, high-sucrose (HF/HS), Western diet with decaffeinated GT and BT polyphenols (P) for 20 weeks decreased body and adipose tissue weight [3]. According to a recent review, the two major mechanisms of the antiobesity effect of GTP are (1) stimulation of fat oxidation and decrease in fatty acid synthesis through AMP-activated protein kinase (AMPK) phosphorylation by tissue polyphenols (epigallocatechin gallate, epicatechin, epigallocatechin, and epicatechin gallate) and (2) decrease in digestion and absorption of lipids in the intestine [4–7]. Since BTP, however, are not absorbed into tissues, it is more likely that their primary site of activity is in the intestine. Therefore, we speculate that changes in the microbiota may represent an important mechanism for BTP and possibly GTP to induce weight loss.
The gut microbiota is an important contributor to human health and has been implicated in the development of obesity and obesity-related diseases such as diabetes [8, 9] and cardiovascular disease [10]. The two most abundant bacterial phyla in humans and in mice are Firmicutes (40–60%) and Bacteroidetes (20–40%) with lower abundance of Actinobacteria, Fusobacteria, Proteobacteria, and Verrucomicrobia [11]. It has been reported that consuming a Western-type diet high in fat and sucrose (HF/HS) alters cecum microflora populations and produces a relative increase in Firmicutes, a relative decrease in Bacteroidetes and a reduction in overall microbiota diversity [12, 13].
Recent studies have examined the effect of tea consumption on human intestinal bacteria [14, 15]. However, these studies used in vitro batch fermentation of fecal material and little is known about the effects of dietary intake of tea polyphenols on gut microflora composition and metabolism in vivo [16]. One recent study determined the effect of brewed GT, BT, and oolong tea provided as drinking fluid on the intestinal microbiota in mice fed a high-fat diet [17]. Brewed tea contains tea polyphenols, alkaloids (caffeine), amino acids/peptides, and carbohydrates [2]. Since our investigation was focused on the effect of tea polyphenols on the microbiome, we used a 75% alcohol:water extract that is enriched for tea polyphenols.
One potential link between changes in the microbiota and weight loss might be the formation of short-chain fatty acids, which can be absorbed in the colon, transported to the liver, and have been shown to induce changes via AMPK activation [18]. AMPK plays a key role in regulating carbohydrate and fat metabolism, serving as a metabolic master switch in response to alterations in cellular energy charge [19]. Recently, den Besten et al. demonstrated that short-chain fatty acid (SCFA) formation by the intestinal microflora protected against the metabolic syndrome via a signaling cascade that involved AMP-activated protein kinase (AMPK) activation [18].
To examine whether changes in the intestinal microbiota leading to an increase in intestinal SCFA formation play a role in the anti-obesogenic effect of GT and BT polyphenols, mice were fed a high-fat/high-sucrose Western diet supplemented with GT or BT polyphenols and changes in relative proportion of intestinal microbiota, intestinal concentration of SCFAs, and changes in hepatic AMPK phosphorylation were determined.
Methods
Tea polyphenol extracts
Chemical reagents and plant materials
All solvents were HPLC grade and purchased from Fisher Scientific Co. (Tustin, CA, USA). Gallic acid (> 98%), catechins, black tea extract (> 80% theaflavin), theanine, and caffeine standards were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). GT and BT leaves were collected and purchased in a selected location in Sichuan Province, China. The samples were kept in sealed bags at room temperature before extraction.
Tea polyphenol extract preparation
500 g of tea leaves were extracted with 2 L of 75% ethanol in water at room temperature for 3 h. The leaves were separated and the procedure was repeated twice. The ethanol was evaporated in a rotary evaporator under reduced pressure at 40 °C. The dried extract was suspended in 500 mL of pure water and extracted with chloroform to remove caffeine. The decaffeinated water solution of tea extract was subjected to an XAD-16 resin column, rinsed with five bed volumes of water and eluted with pure methanol. The extract was dried using the rotary evaporator.
Black tea extract analysis
Theaflavin and thearubigin fractions were extracted following a modification of the method by Xie et al. [20]. 100 mg of BT extract was dissolved in 20 mL boiling water and shaken for 5 min. 10 mL of ethylacetate was added and shaken for 5 min. The mixture was centrifuged at 2050g for 10 min and the ethylacetate fraction (catechins and theaflavins) was transferred into fresh centrifuge vial. The procedure was repeated two more times and all ethylacetate extracts were combined and evaporate to dryness. The water fraction was further extracted with 10 mL butanol and shaken for 5 min. After centrifugation for 10 min at 2050g, the butanol fraction (thearubigins) was transferred into a fresh centrifuge vial. The procedure was repeated two more times and all butanol extracts were combined and evaporate to dryness. The amount of dried residue was determined by weight. The residues were reconstituted in 50% methanol:50% water and analyzed using HPLC and LC–MS/MS.
Liquid chromatography–mass spectrometry (LC–MS)
The HPLC with electrospray ionization mass spectrometry (ESI/MS) system consisted of an LCQ Advantage Finnigan system (ThermoFinnigan, San Jose, CA, USA), equipped with a Surveyor LC system consisting of an autosampler/injector, quaternary pump, column heater, and diode array detector (DAD) with the Xcalibur 1.2 software (Finnigan Corp., San Jose, CA, USA). The HPLC column was an Agilent Zorbax Sb-C18 3.5 um (150 × 2.1 mm). The flow rate was 0.2 mL/min and gradient elution was used. The gradient started at 5% solution A (acetonitrile) and 95% solution B (1% acetic acid/water) increasing over 40 min to 40% solution A and 60% solution B. DAD range 210–600 nm, 440 nm as detection wavelength for the theaflavin fraction, and 400 nm for the thearubigin fraction. MS parameters: ionization mode ESI+ and ESI−; scan range 500–1000 amu; spray voltage, 5 kV; auxiliary gas, 40; and capillary temperature, 275 °C. Peak identities were obtained by matching their molecular M+ and M− with expected theoretical molecular weights from the literature [21].
Gallic acid equivalent (GAE)
The assays were performed as previously reported with some modification using the Folin–Ciocalteau reagent [22]. The absorbance was read at 755 nm in a ThermoMax microplate reader (Molecular Devices, Sunnyvale, CA, USA) at room temperature. The standard curves were used to convert the average absorbance of each sample into mg/g gallic acid equivalent (GAE).
HPLC condition for analysis of GT and BT extracts
A Water Alliance 2695 HPLC system coupled with a photodiode array detector and Empower 2 software was used to analyze the catechins, gallic acid, theaflavins, and thearubigin fraction and caffeine. The separation of compounds was conducted on an Agilent Zorbax SBC18 4.6 × 250 mm column (Agilent) with a gradient of acetonitrile (solution A) and 0.1% phosphoric acid in water (solution B). The gradient started at 5% solution A and 95% solution B, increasing over 40 min to 40% solution A and 60% solution B. The detection wavelength was 280 nm for catechins, 440 nm for theaflavin fraction, and 400 nm for thearubigin fraction. Theanine was determined after reaction with AccQ Fluor reagent (Waters WAT-052880) and measured by Waters 474 fluorescence detector.
Experimental animal and body composition studies
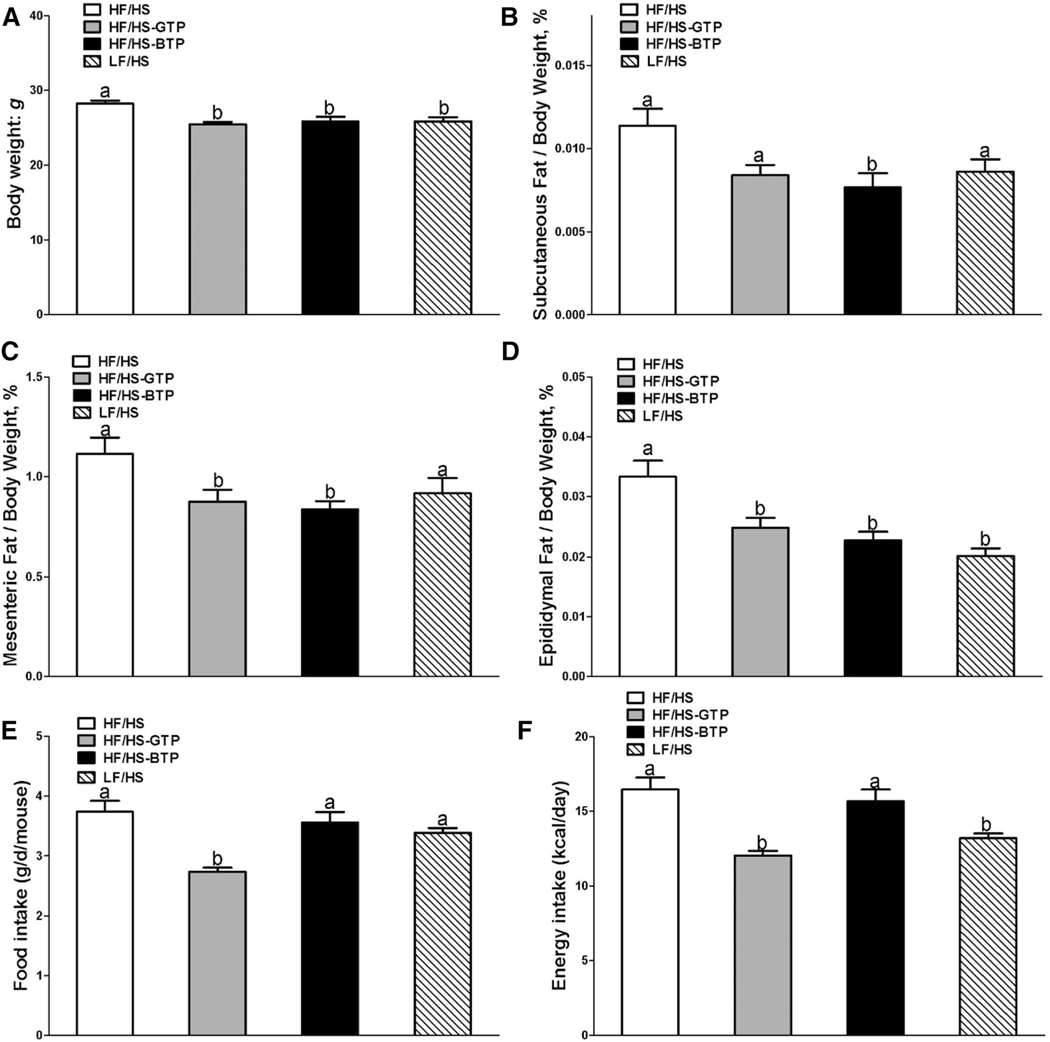
All mouse procedures were approved by the UCLA Animal Research Committee in compliance with the Association for Assessment and Accreditation of Laboratory Care (AAA-LAC) International and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. 48 male C57BL/6J mice (strain JAX 000664) were received from the Jackson Laboratory at 6–7 weeks of age (body weight: 16–18 g). After 1 week of acclimation, 28-day-old male C57BL/6J mice were assigned to four groups with similar body weight distribution in each group and fed either a low-fat/high-sucrose diet (LF/HS) (D12489B from Research Diets Inc., New Brunswick, NJ, USA), high-fat (HF)/HS (D12266B from Research Diets Inc.) (Supplemental Table 1), or HF/HS diet supplemented with GTP and BTP (Table 1) at 0.5 g/100 g of diet providing 0.25 g polyphenols/100 g diet [3]. Based on the food intake, we calculated that mice fed the GTP diet (Fig. 1) in average consumed 240 mg of GTP and 320 mg of BTP per kg body weight. Tea extracts were mixed into the diet by Research Diets Inc. Body weights were recorded weekly and food consumption three times per week. Mice were euthanized after 4 weeks of dietary treatment. Tissues were collected, weighed, and stored at − 80 °C until analysis.
Table 1.
Gallic acid equivalent, and chemical composition of GTP and BTP
| GAE (mg/g) | EGCG (mg/g) | ECG (mg/g) | EGC (mg/g) | EC (mg/g) | GA (mg/g) | Theanine (mg/g) | Caffeine (mg/g) | |
|---|---|---|---|---|---|---|---|---|
| GTP | 565 ± 24 | 214 ± 4.5 | 48.7 ± 2.4 | 24.5 ± 2.0 | 21.4 ± 1.6 | 1.7 ± 0.6 | 0.7 ± 0.3 | 0.5 ± 0.1 |
| BTP | 532 ± 25 | 20 ± 0.8 | 7.1 ± 0.4 | 7.1 ± 0.4 | 9.3 ± 0.3 | 4.3 ± 0.2 | 3.1 ± 0.2 | 1.4 ± 0.1 |
Values are means ± SDs. Total phenolic content was expressed as GAE
BTP black tea polyphenol, GTP green tea polyphenol, GAE gallic acid equivalent, EGCG epigallocatechin gallate, ECG epicatechin gallate, EGC epigallocatechin, EC epicatechin, GA gallic acid
Fig. 1.
Effects of tea extracts on (a) body weight, (b) % subcutaneous fat, (c) % mesenteric fat, (d) % epididymal fat normalized to body weight, (e) food intake, and (f) energy intake in male C57BL/6J mice fed an HF/HS, LF/HS, HF/HS-GTP, or HF/HS-BTP diet for 4 weeks. Data are mean ± SEM (n = 11–12). Labeled means of dietary interventions without a common letter differ by diet; P < 0.05
Cecum short-chain fatty acid concentration
Intestinal cecum content was diluted, acidified, and filtered and SCFA (acetic, propionic, butyric, and valeric acids) were quantified by gas chromatography flame ionization detection (Agilent 7890A) and RTX-Stabilwax column (Restek corp. 30 m × 0.25 mm) [23]. SCFA standard mix was purchased from Sigma-Aldrich (St Louis, MO, USA).
Sequencing of bacterial DNA
DNA from cecum content was extracted using QIAamp Stool DNA Extraction Kit (Qiagen, Valencia, CA, USA). The quality of the DNA samples was confirmed using a Nanodrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA). The 16S rRNA gene V4 variable region PCR primers 530/960 with barcode on the forward primer were used in a 30 cycle PCR using the HotStarTaq Plus Master Mix Kit (Qiagen, USA) under the following conditions: 94 °C for 3 min, followed by 28 cycles of 94 °C for 30 s, 53 °C for 40 s, and 72 °C for 1 min, after which a final elongation step at 72 °C for 5 min was performed. After amplification, PCR products are checked in 2% agarose gel to determine the success of amplification and the relative intensity of bands. Sequencing was performed at MR DNA (http://www.mrdnalab.com, Shallowater, TX, USA) on a MiSeq following the manufacturer’s guidelines. Sequence data were processed using a proprietary analysis pipeline (MR DNA, Shallowater, TX, USA). Operational taxonomic units (OTUs) were defined by clustering at 3% divergence (97% similarity). Final OTUs were taxonomically classified using BLASTn against a curated GreenGenes database [24]. β-Diversity was measured by calculating the unweighted UniFrac distances [25] using Quantitative Insights Into Microbial Ecology (QIIME) default scripts. In addition, UniFrac PCoA biplot was visualized using EMPEROR. Statistical difference between different time points was analyzed by PERMDISP.
Western blotting
30 mg of liver tissue was homogenized and lysed in cell lysis buffer containing 20 mM Tris-HCl, 0.5 M NaCl, 0.25% Triton X-100, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycophosphate, 10 m MNaF, 300 μM Na3VO4, 1 mM benzamidine, 2 μM PMSF, and 1 mM DTT. Protein concentrations were determined by a BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Protein was separated by SDS-PAGE, transferred to a nitrocellulose membrane, blocked in 5% BSA, and probed with the following primary antibodies: phospho-AMPKα (T172), AMPKα, and GAPDH (Cell Signaling Technology, Boston, MA, USA). The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma) and visualized with SuperSignal™ West Femto Maximum Sensitivity Substrate (Thermo scientific, Rockford, IL, USA) according to the manufacturer’s recommended protocol.
Statistical analysis
All statistical analyses were conducted using the IBM SPSS Statistics version 21; mean values, standard deviation, and standard errors were calculated using descriptive statistics. Body weight, % epididymal, % mesenteric, % subcutaneous fat/body weight, cecum SCFA content, and hepatic AMPK protein phosphorylation were analyzed with one-way ANOVA, with the factor being diet. Tukey–Kramer multiple comparison procedure was used for post-hoc comparisons. Wilcoxon rank sum test was utilized to evaluate the differences in bacterial relative proportion between study groups. All tests were two sided and all analyses of the microbiota were conducted using the SAS 9.3 (Statistical Analysis System, Cary, NC, USA, 2008) and R (http://www.r-project.org) software. P values < 0.05 were considered statistically significant.
Results
Chemical composition of tea extracts
GTP and BTP extracts were analyzed by HPLC (Table 1). GTP contained tenfold higher concentration of EGCG compared to BTP, while the total phenolic content (GAE) was similar in GTP and BTP extracts. The BTP extract contained more gallic acid and theanine compared to GTP (Table 1). The BTP theaflavin fraction was quantified as 1.49% and thearubigin fraction 18% of solids. Theaflavin content in the ethylacetate fraction was quantified based on the commercial BT extract standard. In the absence of standards, thearubigins in the butanol fraction were quantified by weight. The theaflavin and thearubigin fractions were further analyzed by LC–MS to identify molecular mass of compounds (Supplemental Table 2, Supplemental Figs. 1, 2, 3). The theaflavin fraction contained theaflavin, theaflavin-3′-gallate, theaflavin-3-gallate, and theaflavin-3,3′-digallate (Supplemental Fig. 1). The following compounds were identified in the thearubigin fraction with LC/MS positive mode: theasinensin-digallate, theasinensin, and theaflavin-gallate, and in negative mode: theacitrin, chalcan-flavan dimer-gallate, and theacitrin-gallate based on molecular weight (Supplemental Figs. 2, 3) compared to Kuhnert et al. [21].
Body, liver, and fat depot weights in mice fed the HF/ HS diet supplemented with tea extracts
The diet contained 0.25% of tea polyphenols. Based on the food intake, average consumption of polyphenols was 240 and 320 mg per kg body weight for mice fed the GTP and BTP diet, respectively. During the 4-week dietary intervention, the HF/HS-treated mice had significantly higher body weight and subcutaneous and epididymal fat by weight (Fig. 1) compared to the LF/HS group. Supplementation of the HF/HS diet with GT and BT polyphenols significantly decreased body weight, subcutaneous, mesenteric, and epididymal fat normalized to body weight compared to HF/HS control equal to the body composition of mice fed the LF/HS diet (Fig. 1). No change in liver weight normalized to body weight was observed among the treatment groups (data not shown).
Cecum microbiota, SCFA concentration, cecum content weight, and total DNA in mice fed the HF/HS diet supplemented with tea extracts
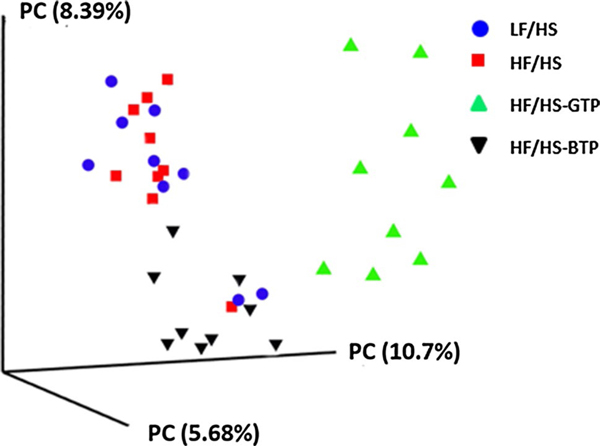
Cecum weight and DNA content normalized to body weight was significantly increased in mice fed the HF/HS-GTP and BTP diets, while feeding the LF/HS diet had no effect compared to the HF/HS diet (Supplemental Fig. 4). The unweighted UniFrac distance metric was calculated and visualized via principle coordinate analyses (PCoA) (Fig. 2). For unweighted UniFrac distance metrics, mice fed the GTP and BTP supplemented diet formed clusters distinctly different from LF/HS and HF/HS microbial patterns (Fig. 2).
Fig. 2.
Beta-diversity analysis of the microbiome of cecum content from mice fed the HF/HS, LF/HS, HF/HS-GTP, or HF/HS-BTP diet for 4 weeks. Three-dimensional principal coordinate analyses (PCoA) based on the unweighted UniFrac distance between samples were performed using QIIME (n = 9)
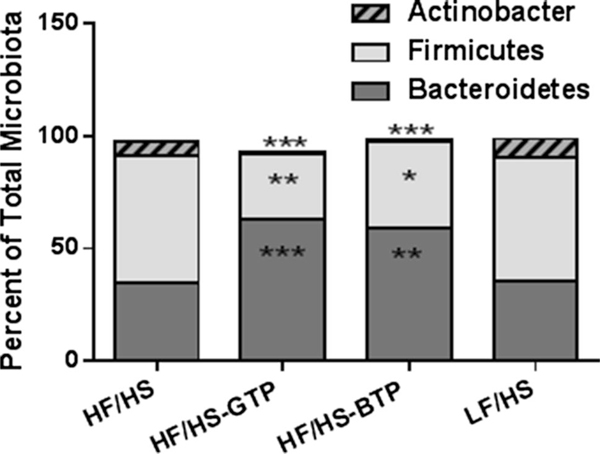
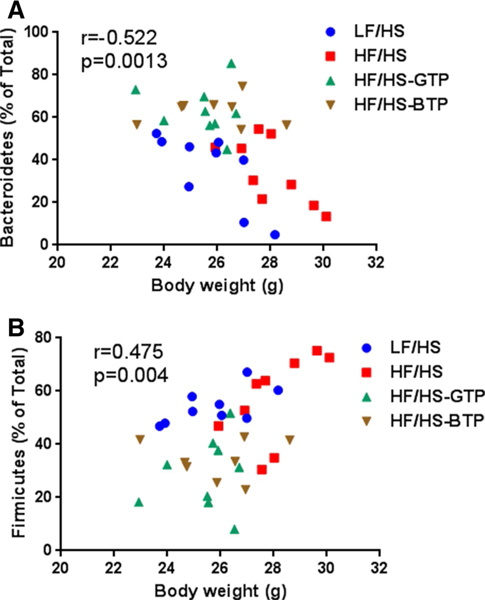
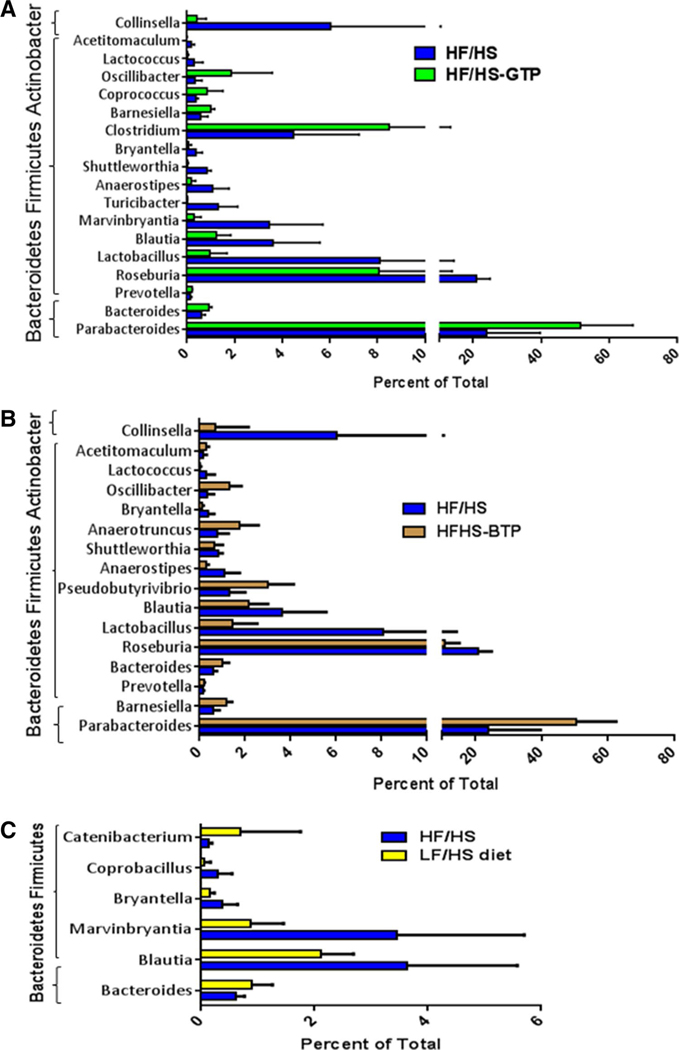
Sequencing of the bacterial DNA demonstrated a significant increase in the relative proportion of cecum phyla Bacteroidetes and decrease in Firmicutes and Actinobacter in mice fed the HF/HS-GTP and BTP diet compared to HF/HS and LF/HS diets (Fig. 3). We observed a significant correlation between Firmicutes and body weight and inverse correlation between Bacteroidetes and body weight (Fig. 4). On the genus level, we found that the relative proportion of Blautia, Bryantella, Collinsella, Lactobacillus, Marvinbryantia, and Turicibacter were positively correlated with body weight (Table 2), while Barnesiella and Parabacteroides were negatively correlated with body weight (Table 2). On the genus level feeding, the HF/HS-GTP and BTP diets were associated with a significant increase in relative proportion of Parabacteroides, Bacteroides, and Prevotella and a significant decrease in several genera from the phylum Firmicutes such as Roseburia, Lactobacillus, Blautia, Anaerostipes, Shuttleworthia, Bryantella, Lactococcus, and Acetitomaculum as well as a significant decrease in Collinsella from the Actinobacter phylum (Fig. 5). Changes only induced by GTP but not BTP consumption included an increase in the genus Clostridium and Coprococcus, and decrease in Turicibacter and Marvinbryantia. Changes only induced by BTP and not GTP consumption were increase in Oscillibacter, Anaerotruncus, and Pseudobutyrivibrio (Fig. 5). In comparison with the HF/HS diet mice fed the LF/HS diet showed an increase in Bacteroides and decrease in Blautia and Bryantella (Fig. 5c).
Fig. 3.
Effects of tea extracts on cecum bacteria relative proportion in male C57BL/6J mice fed an HF/HS, LF/HS, HF/HS-GTP, HF/HSOTP, or HF/HS-BTP diet for 4 weeks. Data are means (n = 9). Wilcoxon rank sum test was utilized to evaluate the differences in bacterial relative proportion comparing each tea and LF/HS intervention with HF/HS control diet. Labeled means are different from the HF/HS group with *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
Fig. 4.
Correlation of body weight and bacteria relative proportion of a Bacteroidetes and b Firmicutes phyla including data from all intervention groups. The correlation was evaluated using the GraphPad Prism6 software (San Diego, CA, USA)
Table 2.
Correlation between body weight and relative proportion of bacteria on the genus level
| Pearson R | P value | Phylum | |
|---|---|---|---|
| Barnesiella | − 0.62 | < 0.0001 | Bacteroidetes |
| Parabacteroides | − 0.44 | 0.0087 | Bacteroidetes |
| Blautia | 0.39 | < 0.0001 | Firmicutes |
| Bryantella | 0.37 | 0.029 | Firmicutes |
| Lactobacillus | 0.48 | 0.0037 | Firmicutes |
| Marvinbryantia | 0.57 | 0.0004 | Firmicutes |
| Turicibacter | 0.46 | 0.0053 | Firmicutes |
| Collinsella | 0.39 | 0.0225 | Actinobacter |
Pearson correlation coefficient and P value were calculated using the Prism GraphPad software
Fig. 5.
Effects of tea extracts on microbiome on the genus level in cecum content from male C57BL/6J mice fed an HF/HS, LF/HS, HF/HS-GTP, or HF/HS-BTP diet for 4 weeks. Comparison of relative proportion of bacteria between a HF/HS to HF/HS-GTP, b HF/HS to HF/HS-BTP, and c HF/HS to LF/HS fed mice. Data are mean ± SD; N = 9. The difference in relative proportion compared to mice fed the HF/HS control diet was significant for all bacteria included in the figure (P < 0.05)
The consumption of HF/HS-BTP diet was associated with a significant increase in concentration of the fecal SCFA propionic acid, i-butyric acid, and a trend to increase in butyric acid (P = 0.07) with the sum increased by 24% compared to the HF/HS diet (Table 3). The cecum concentration of propionic and i-butyric acids was significantly correlated with the relative proportion of Pseudobutyrivibrio (Table 4). Propionic acid formation was correlated with Oscillibacter and Maryell (Table 4). To confirm the sequencing results, we also performed pPCR using duodenum bacterial DNA (Supplemental Fig. 5). We confirmed the significant increase in the abundance of Bacteroidetes and decrease in Firmicutes.
Table 3.
Cecum short-chain fatty acid content
| Acetic acid (μmol/g) | Propionic acid (μmol/g) | i-Butyric acid (μmol/g) | n-Butyric acid (μmol/g)* | i-Valeric acid (μmol/g) | n-Valeric acid (μmol/g) | Sum (μmol/g) | |
|---|---|---|---|---|---|---|---|
| HF/HS | 3.46 ± 0.53 | 0.34 ± 0.05b | 0.06 ± 0.01b | 0.62 ± 0.06a | 0.16 ± 0.02ab | 0.12 ± 0.02b | 4.75 ± 0.67 |
| HF/HS-GTE | 2.97 ± 0.41 | 0.42 ± 0.07b | 0.06 ± 0.01b | 0.65 ± 0.12a | 0.15 ± 0.01ab | 0.09 ± 0.03b | 4.35 ± 0.61 |
| HS/HS-BTE | 3.91 ± 0.57 | 0.59 ± 0.07a | 0.14 ± 0.02a | 0.86 ± 0.09a | 0.21 ± 0.03a | 0.18 ± 0.03a | 5.88 ± 0.78 |
| LF/HS | 3.55 ± 0.19 | 0.40 ± 0.04b | 0.05 ± 0.01b | 0.68 ± 0.08a | 0.10 ± 0.01c | 0.16 ± 0.01a | 4.94 ± 0.29 |
Data were analyzed by one-way ANOVA, followed by Tukey–Kramer multiple comparison procedure. Labeled means without a common letter differ, P < 0.05. n-Butyric acid: comparison between HF/HS and HF/HS-BTP diets showed trend (P = 0.071). Values are means ± SEMs (n = 6)
Table 4.
Correlation between cecum concentration of short-chain fatty acids and relative proportion of bacteria in the cecum
| Propionic acid |
Iso-butyric acid |
Phylum | |||
|---|---|---|---|---|---|
| Pearson R | P value | Pearson R | P value | ||
| Bacteroidetes | 0.190 | 0.374 | 0.38 | 0.067 | Bacteroidetes |
| Parabacteroides | 0.136 | 0.525 | 0.41 | 0.045 | Bacteroidetes |
| Moryella | 0.435 | 0.034 | 0.04 | 0.85 | Firmicutes |
| Oscillibacter | 0.566 | 0.004 | 0.34 | 0.101 | Firmicutes |
| Pseudobutyrivibrio | 0.556 | 0.005 | 0.67 | 0.0003 | Firmicutes |
Pearson correlation coefficient and p value were calculated using the Prism GraphPad software
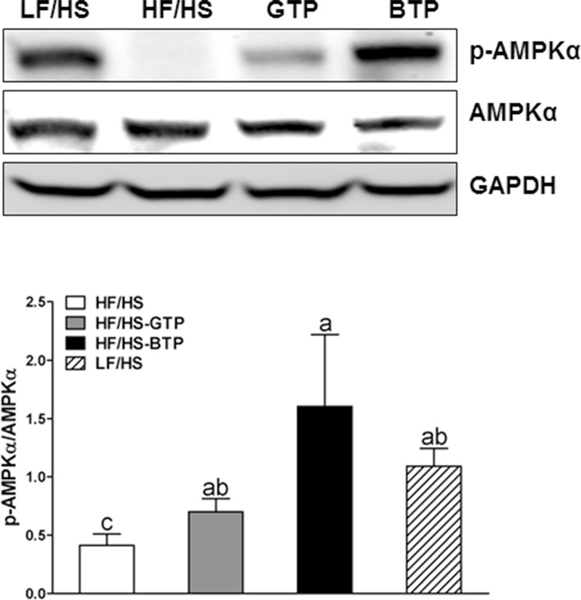
Hepatic AMPK phosphorylation in mice fed the HF/HS diet supplemented with tea extracts
Feeding the LF/HS, HF/HS-GTP and HF/HS-BTP diets increased AMPK phosphorylation compared to the HF/HS diet (Fig. 6). Increased pAMPK/tAMPK ratio was significantly correlated with the relative proportion of Anaerococcus (P = 0.0054).
Fig. 6.
Effects of tea extracts on protein expression of hepatic AMPK phosphorylation in liver from male C57BL/6J mice fed an HF/HS, LF/HS, HF/HS-GTP, or HF/HS-BTP diet for 4 weeks. Data are mean ± SEM (n = 5). Data were analyzed by one-way ANOVA, followed by Tukey–Kramer multiple comparison procedure. Labeled means without a common letter differ, P < 0.05
Discussion
Our data demonstrate the basic differences in the mechanism of the anti-obesogenic activity between GT and BT polyphenols that involve complex changes in the intestinal microbiota. Principal coordinates analysis of unweighted UniFrac distances revealed a distinct separation of the gut microbial communities between GTP, BTP, and HF/HS-LF/HS diet treatments. Although both teas induced similar changes on the phylum level of the intestinal microbiota, on the genus level, we observed strong differences between GT and BT polyphenol supplementation.
In the present study, the addition of GTP and BTP to the HF/HS diet significantly decreased the ratio of Firmicutes to Bacteroidetes. These changes were significantly correlated with body weight. In support of our findings the study by Seo et al. also found that oral gavage of an extract from fermented GT reversed the obesogenic effect of a high-fat diet (HFD) associated with a decrease in the ratio of Firmicutes/Bacteroidetes ratio compared to HFD fed control mice as determined by RT-PCR [26]. The study by Seo et al. was different in that it used GT produced from dried green tea leaves fermented by Bacillus subtilis, a microorganism used to produce fermented soy-bean products [26]. Another study by Liu et al. did not find an effect on Firmicutes to Bacteroidetes ratio in mice fed an HFD and provided with brewed GT, BT, or oolong tea as drinking fluid [17].
In the presented study, the largest change in relative proportion in the cecal microbiota after GTP and BTP intervention was observed for Parabacteroides, a bile-resistant Gram-negative anaerobic bacteria, which in addition to other Bacteroidetes has been associated with the formation of the SCFA propionic acid in in vitro fermentation of different fiber sources with human fecal microbiota [27]. Fermentation of polysaccharides to SCFA is one of the potential mechanisms through which changes in the microbiota can contribute to inhibition of weight gain. For example, the study by Ridaura et al. demonstrated that transplanted microbiomes from lean co-twins to mice exhibited higher expression of genes involved in polysaccharide fermentation [28]. The microbiome transplant from lean co-twins also showed higher abundance of members of the Bacteroides and Parabacteroides genus and resulted in increased formation of the fecal short-chain fatty acids propionate and butyrate [28]. In the present study, however, we observed that cecum content of SCFA was only increased significantly in mice fed the BTP diet. In difference to the GTP intervention, feeding the HF/HS-BTP diet increased the relative proportion of Pseudobutyrivibrio. The relative proportion of Pseudobutyrivibrio was significantly correlated with the formation of propionic and i-butyric acids. Members of the Pseudobutyrivibrio genus have fructanolytic and saccharolytic enzyme activity [29], which may have contributed to the formation of SCFA.
Other bacteria significantly increased only in mice fed the GTP diet belong to the genus Clostridium and Coprococcus. Similar results were found in batch culture studies by Tzounis et al. showing that the addition of GT polyphenols epicatechin and catechin promoted the growth of members from the Clostridium genus, which have the ability to generate SCFA by saccharolytic metabolism [14]. In addition, a rat study by Zhong et al. linked the abundance of Coprococcus to significant increase in fecal SCFA concentration in rats fed a high-fat diet supplemented with barley [30]. In the presented study, however, SCFA were not increased in mice fed the GTP diet, which may depend on the interaction between multiple bacteria present in the intestinal microbiota [30, 31].
SCFAs such as acetate, propionate, and butyrate are the main products of intestinal bacterial fermentation of dietary fiber and complex carbohydrates [27]. GT and BT polyphenols have been shown to inhibit α-amylase and α-glucosidase in saliva and small intestine, which may lead to residual carbohydrate in the large intestine providing substrate for the SCFA generation [32, 33]. SCFA serve as energy source for colonic epithelium (butyrate) and peripheral tissues (acetate and propionate) [28]. SCFA are also absorbed into the bloodstream and travel to the liver, where they play a role in energy metabolism [30]. The addition of SCFAs to the diet has been demonstrated to be efficacious in protection against high-fat diet-induced metabolic syndrome [18]. Potential mechanisms are activation of AMPK phosphorylation and inhibition of PPARγ. For example, Den Besten et al. demonstrated that fiber (guar gum)-derived SCFA protected against metabolic syndrome by AMPK activation and PPARγ inhibition in mice fed a high-fat diet for 12 weeks [34]. AMPK phosphorylation in turn can lead to stimulation of FA β-oxidation and inhibition of gluconeogenesis [35].
Both teas induced hepatic AMPK phosphorylation but most likely through different mechanisms based on the difference in chemical composition. In our previous study, using the same GTP and BTP supplemented diets, we found 540 pmol of polyphenols (EGCG, 4″-meEGCG, EGC, and ECG) per g liver in mice fed the HF/HS-GTP diet, whereas only 20 pmol/g liver of ECG was found in mice fed the HF/HS-BTP diet [3]. We, therefore, suggest that GTP intervention induced weight loss in part by increased hepatic AMPK phosphorylation through polyphenols present in the tissue. The formation of phenolic acids as microbial metabolites by catabolism of tea polyphenols provides another potential mechanism. In one of our previous human tea intervention studies and in a recent study by Pereira–Caro, it was demonstrated that GT and BT polyphenols are metabolized to smaller phenolic acids and are present in serum and urine after the consumption of GT and BT [36, 37]. It is possible that some phenolic acids contribute to the effect on AMPK phosphorylation. In addition, the formation of SCFA may contribute to the increase in AMPK phosphorylation. SCFAs in turn can be absorbed and have been shown to induce hepatic AMPK phosphorylation [34]. Increased AMPK phosphorylation has also been observed by Rocha et al. in obese rats gavaged with GT extract dissolved in water [38] and in obese mice gavaged with EGCG enriched GT extract [39]. In the presented study, a low fiber chemically defined rodent diet was used, and therefore, SCFA formation was relatively low. Future studies using a combination of tea extracts and fiber may enhance the formation of SCFA and increase the effect on AMPK phosphorylation.
In addition, based on our findings that eight bacterial genera (Blautia, Bryantella, Collinsella, Lactobacillus, Marvinbryantia and Turicibacter, Barnesiella, and Parabacteroides) were significantly correlated with body weight, we conclude that additional mechanisms related to the intestinal microbiota are involved in the anti-obesogenic activity of both teas.
In summary, we demonstrated that both GTP and BTP administration were associated with significant changes in the cecum microflora. Based on our results and published data, we conclude that the obesogenic effect of tea polyphenols involves multiple mechanisms such as changes in the composition of the intestinal microbiota, changes in microbial metabolite formation, and increase in residual complex carbohydrate through inhibition of α-amylase and α-glucosidase leading to an increase in SCFA formation. In the present study, only changes in microbiota induced by the BTP diet were associated with a significant increase in cecum concentration of SCFA. Future studies using high-fat diets containing fermentable fiber together with GT and BT polyphenols may shed more light on the role of microflora and SCFA formation in inhibiting weight gain when consuming an HF/HS diet.
Up to date, green tea polyphenols are recognized as more effective agents that offer more health benefits than black tea based on the bioavailability of green tea polyphenols. However, our novel findings that feeding a diet containing black tea polyphenols was associated with an increase in cecum SCFA formation and increased hepatic AMPK phosphorylation provides novel insights in the health benefits of black tea.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health (R03CA171583 and P50CA092131) and departmental funds of the Center for Human Nutrition, Department of Medicine, David Geffen School of Medicine, University of California, Los Angeles.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s00394-017-1542-8) contains supplementary material, which is available to authorized users.
References
- 1.Hayat K, Iqbal H, Malik U, Bilal U, Mushtaq S (2015) Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr 55(7):939–954. doi: 10.1080/10408398.2012.678949 [DOI] [PubMed] [Google Scholar]
- 2.Sharma V, Rao LJ (2009) A thought on the biological activities of black tea. Crit Rev Food Sci Nutr 49(5):379–404. doi: 10.1080/10408390802068066 [DOI] [PubMed] [Google Scholar]
- 3.Heber D, Zhang Y, Yang J, Ma JE, Henning SM, Li Z (2014) Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. J Nutr 144(9):1385–1393. doi: 10.3945/jn.114.191007 [DOI] [PubMed] [Google Scholar]
- 4.Yang CS, Zhang J, Zhang L, Huang J, Wang Y (2016) Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol Nutr Food Res 60(1):160–174. doi: 10.1002/mnfr.201500428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sae-Tan S, Grove KA, Kennett MJ, Lambert JD (2011) (−)-Epigallocatechin-3-gallate increases the expression of genes related to fat oxidation in the skeletal muscle of high fat-fed mice. Food Funct 2(2):111–116. doi: 10.1039/c0fo00155d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LS, Choi JH, Sung MJ, Hur JY, Hur HJ, Park JD, Kim YC, Gu EJ, Min B, Kim HJ (2015) Green tea changes serum and liver metabolomic profiles in mice with high-fat diet-induced obesity. Mol Nutr Food Res 59(4):784–794. doi: 10.1002/mnfr.201400470 [DOI] [PubMed] [Google Scholar]
- 7.Ikeda I, Hamamoto R, Uzu K, Imaizumi K, Nagao K, Yanagita T, Suzuki Y, Kobayashi M, Kakuda T (2005) Dietary gallate esters of tea catechins reduce deposition of visceral fat, hepatic triacylglycerol, and activities of hepatic enzymes related to fatty acid synthesis in rats. Biosci Biotechnol Biochem 69(5):1049–1053 [DOI] [PubMed] [Google Scholar]
- 8.Korpela K, Flint HJ, Johnstone AM, Lappi J, Poutanen K, Dewulf E, Delzenne N, de Vos WM, Salonen A (2014) Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS One 9(6):e90702. doi: 10.1371/journal.pone.0090702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilg H, Moschen AR (2014) Microbiota and diabetes: an evolving relationship. Gut 63(9):1513–1521. doi: 10.1136/gutjnl-2014-306928 [DOI] [PubMed] [Google Scholar]
- 10.Tuohy KM, Fava F, Viola R (2014) ‘The way to a man’s heart is through his gut microbiota’—dietary pro- and prebiotics for the management of cardiovascular risk. Proc Nutr Soc 73(2):172–185. doi: 10.1017/S0029665113003911 [DOI] [PubMed] [Google Scholar]
- 11.Million M, Lagier JC, Yahav D, Paul M (2013) Gut bacterial microbiota and obesity. Clin Microbiol Infect 19(4):305–313. doi: 10.1111/1469-0691.12172 [DOI] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444(7122):1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 13.Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ, Mehrabian M, Ursell LK, He A, Castellani LW, Zinker B, Kirby M, Drake TA, Drevon CA, Knight R, Gargalovic P, Kirchgessner T, Eskin E, Lusis AJ (2013) Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab 17(1):141–152. doi: 10.1016/j.cmet.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzounis X, Vulevic J, Kuhnle GG, George T, Leonczak J, Gibson GR, Kwik-Uribe C, Spencer JP (2008) Flavanol monomer-induced changes to the human faecal microflora. Br J Nutr 99(4):782–792. doi: 10.1017/S0007114507853384 [DOI] [PubMed] [Google Scholar]
- 15.Lee HC, Jenner AM, Low CS, Lee YK (2006) Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res Microbiol 157(9):876–884. doi: 10.1016/j.resmic.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 16.Jin JS, Touyama M, Hisada T, Benno Y (2012) Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol Immunol 56(11):729–739. doi: 10.1111/j.1348-0421.2012.00502.x [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Chen Z, Guo H, He D, Zhao H, Wang Z, Zhang W, Liao L, Zhang C, Ni L (2016) The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fatinduced obese mice. Food Funct 7(12):4869–4879. doi: 10.1039/c6fo01439a [DOI] [PubMed] [Google Scholar]
- 18.den Besten G, Gerding A, van Dijk TH, Ciapaite J, Bleeker A, van Eunen K, Havinga R, Groen AK, Reijngoud DJ, Bakker BM (2015) Protection against the metabolic syndrome by guar gumderived short-chain fatty acids depends on peroxisome proliferator-activated receptor gamma and glucagon-like peptide-1. PLoS One 10(8):e0136364. doi: 10.1371/journal.pone.0136364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298(4):E751–E760. doi: 10.1152/ajpendo.00745.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie B, Shi H, Chen Q, Ho CT (1993) Antioxidant properties of fractions and polyphenol constituents from green, oolong and black teas. Proc Natl Sci Counc Repub China B 17(2):77–84 [PubMed] [Google Scholar]
- 21.Kuhnert N, Drynan JW, Obuchowicz J, Clifford MN, Witt M (2010) Mass spectrometric characterization of black tea thearubigins leading to an oxidative cascade hypothesis for thearubigin formation. Rapid Commun Mass Spectrom 24(23):3387–3404. doi: 10.1002/rcm.4778 [DOI] [PubMed] [Google Scholar]
- 22.Singleton VL, Esau P (1969) Phenolic substances in grapes and wine, and their significance. Adv Food Res Suppl 1:1–261 [PubMed] [Google Scholar]
- 23.Zhao G, Liu JF, Nyman M, Jonsson JA (2007) Determination of short-chain fatty acids in serum by hollow fiber supported liquid membrane extraction coupled with gas chromatography. J Chromatogr B Anal Technol Biomed Life Sci 846(1–2):202–208. doi: 10.1016/j.jchromb.2006.09.027 [DOI] [PubMed] [Google Scholar]
- 24.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072. doi: 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo DB, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY, Bae IH, Lee SJ (2015) Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food 18(5):549–556. doi: 10.1089/jmf.2014.3265 [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Martinez I, Walter J, Keshavarzian A, Rose DJ (2013) In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 23:74–81. doi: 10.1016/j.anaerobe.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 28.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341(6150):1241214. doi: 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasperowicz A, Stan-Glasek K, Guczynska W, Piknova M, Pristas P, Nigutova K, Javorsky P, Michalowski T (2010) Fructanolytic and saccharolytic enzymes of the rumen bacterium Pseudobutyrivibrio ruminis strain 3—preliminary study. Folia Microbiol 55(4):329–331. doi: 10.1007/s12223-010-0051-4 [DOI] [PubMed] [Google Scholar]
- 30.Zhong Y, Nyman M, Fak F (2015) Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res 59(10):2066–2076. doi: 10.1002/mnfr.201500187 [DOI] [PubMed] [Google Scholar]
- 31.Brahe LK, Astrup A, Larsen LH (2013) Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes Rev 14(12):950–959. doi: 10.1111/obr.12068 [DOI] [PubMed] [Google Scholar]
- 32.Nyambe-Silavwe H, Williamson G (2016) Polyphenol- and fibrerich dried fruits with green tea attenuate starch-derived postprandial blood glucose and insulin: a randomised, controlled, single-blind, cross-over intervention. Br J Nutr 116(3):443–450. doi: 10.1017/S0007114516002221 [DOI] [PubMed] [Google Scholar]
- 33.Striegel L, Kang B, Pilkenton SJ, Rychlik M, Apostolidis E (2015) Effect of black tea and black tea pomace polyphenols on alphaglucosidase and alpha-amylase inhibition, relevant to type 2 diabetes prevention. Front Nutr 2:3. doi: 10.3389/fnut.2015.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.den Besten G, Havinga R, Bleeker A, Rao S, Gerding A, van Eunen K, Groen AK, Reijngoud DJ, Bakker BM (2014) The short-chain fatty acid uptake fluxes by mice on a guar gum supplemented diet associate with amelioration of major biomarkers of the metabolic syndrome. PLoS One 9(9):e107392. doi: 10.1371/journal.pone.0107392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardie DG (2015) AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol 33:1–7. doi: 10.1016/j.ceb.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 36.Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, Lee RP, Carpenter CL, Aronson WJ, Heber D (2013) Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res 57(3):483–493. doi: 10.1002/mnfr.201200646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira-Caro G, Moreno-Rojas JM, Brindani N, Del Rio D, Lean MEJ, Hara Y, Crozier A (2017) Bioavailability of black tea theaflavins: absorption, metabolism, and colonic catabolism. J Agric Food Chem 65(26):5365–5374. doi: 10.1021/acs.jafc.7b01707 [DOI] [PubMed] [Google Scholar]
- 38.Rocha A, Bolin AP, Cardoso CA, Otton R (2016) Green tea extract activates AMPK and ameliorates white adipose tissue metabolic dysfunction induced by obesity. Eur J Nutr 55(7):2231–2244. doi: 10.1007/s00394-015-1033-8 [DOI] [PubMed] [Google Scholar]
- 39.Santamarina AB, Oliveira JL, Silva FP, Carnier J, Mennitti LV, Santana AA, de Souza GH, Ribeiro EB, Oller do Nascimento CM, Lira FS, Oyama LM (2015) Green tea extract rich in epigallocatechin-3-gallate prevents fatty liver by AMPK activation via LKB1 in mice fed a high-fat diet. PLoS One 10(11):e0141227. doi: 10.1371/journal.pone.0141227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.