Abstract
Pulmonary marginal zone lymphoma (PMZL) is the most common non‐Hodgkin lymphoma affecting the lung. PMZL is usually an indolent disease. Clinical and radiological variables associated with shorter survival are largely unknown and no consensus exists on preferred treatment strategy in PMZL. Herein we aimed to identify clinical and radiological features associated with shorter survival and inferior treatment outcomes. Forty patients with PMZL were analyzed. FDG‐avid disease was evident in most patients (93%) with staging PET/CT (n = 15). With a median follow‐up in treated patients (n = 38) of 8.4 years (range 0.07‐18.44), the median progression‐free survival (PFS) and overall survival (OS) were 7.5 years (95% CI 1.8‐9.5) and 15.7 years (95% CI 9.3‐NE) respectively. Shorter PFS was observed in patients who presented at diagnosis with elevated LDH, B symptoms, advanced stage and failed to achieve complete response (CR) after initial treatment. Patients with multifocal lung disease, extrapulmonary MZL and cavitary lesions on CT scans exhibited shorter PFS. Nevertheless, no clinical or radiologic findings were associated with shorter OS. All patients treated with surgery (n = 4) and radiation therapy (n = 3) achieved and remained in CR. No higher grade transformations occurred during the follow‐up period. PMZL exhibited excellent outcomes with a 15‐year PMZL‐related OS of 94.9% (95% CI: 81.25%‐98.7%). Radiation therapy and surgery are potentially curative strategies in localized PMZL.
Keywords: Pulmonary marginal zone lymphoma, radiographic findings, treatment outcomes
Elevated LDH, B symptoms, advanced stage and failure to achieve complete response after initial treatment are clinical variables associated with shorter progression‐free survival (PFS) in pulmonary marginal zone lymphoma (PMZL). Multifocal lung disease, extrapulmonary MZL and cavitary lesions on computed tomography scans have been identified as radiologic features associated with shorter PFS. Radiation therapy and surgery are potentially curative strategies in PMZL.
1. INTRODUCTION
Extranodal marginal zone B‐cell lymphoma of mucosa associated lymphoid tissue (MALT), also known as MALT lymphoma, comprises 6% to 8% of all non‐Hodgkin lymphomas (NHL). 1 MALT lymphomas most commonly affect stomach; however, it has been reported in virtually all tissues. 2 , 3 , 4 MALT lymphoma originating in the lung, also known as pulmonary marginal zone lymphoma (PMZL), is a rare disease representing 9%–14% of MALT lymphomas. 4 However, it is the most common NHL affecting the lung. PMZL arises from bronchial associated lymphoid tissue (BALT) which is absent in normal healthy adults. Continuous antigen stimulation as occurs in association with inflammation, smoking, and autoimmune disorders (eg Sjögren's syndrome), leads to BALT appearance. 5 This chronic antigenic stimulus is considered the underlying cause in PMZL. 6 PMZL is associated with smoking in 35%–45% of patients 6 , 7 , 8 and a possible association with Achromobacter xylosoxidans infection was described in Europe. 9
Respiratory symptoms are usually present at the time of PMZL diagnosis and diverse patterns of lung abnormalities are observed on imaging studies. 10 On computed tomography (CT) scans, PMZL usually manifests with bilateral (60%–70%) and multiple (70%–77%) lesions, without a clear topographic predominance. 10 The most frequent patterns are lobar or segmental consolidation followed by nodules and masses. 6 , 10 The role of 18F‐deoxyglucose‐positron emission tomography/CT (FDG‐PET/CT) in MALT lymphoma is not well‐established. Several studies have shown FDG avidity in 80% of PMZL cases with an average SUVmax ranging from 3.3 to 7.5. It was suggested that PET/CT may detect previously unrecognized lesions during staging of PMZL. 10 , 11
Pulmonary marginal zone lymphoma carries a favorable outcome with a 5‐year overall survival (OS) rates of 90%. 10 , 12 Presence of mediastinal lymphadenopathy, extrapulmonary MZL, and use of chemotherapy regimens including anthracyclines or cyclophosphamide have been associated with shorter progression‐free survival (PFS) and time to progression. 6 , 10 The latter may reflect a more advanced disease needing systemic therapeutic approaches. Overall, clinical and radiological variables associated with worse outcome remain poorly understood.
Herein we report a single‐institution experience with a large cohort of patients with PMZL focusing on clinical features, radiological presentation, therapeutic approaches, and outcomes.
2. MATERIALS AND METHODS
All patients presenting with MALT lymphoma involving the lung and/or pleura diagnosed and treated at our institution between January 1995 and September 2019 were included in this analysis. Twenty‐seven patients presented with respiratory presentation and during staging were found to have extranodal MZL limited to the lungs. Additional 13 patients presented with respiratory complains with or without additional complains related to other organs and were found to have concomitant pulmonary and extrapulmonary extranodal MZL during staging. Patients with higher grade transformation (HGT) at presentation were excluded (n = 2) from this analysis. The patients were identified by a review of the Florida Cancer Registry database. The institutional review board approved this study, which followed the tenets of the Declaration of Helsinki. All specimens were reviewed by expert hematopathologists and MZL diagnosis was confirmed using the morphologic and immunophenotypic features defined by the WHO classification. 13 Concomitant amyloid was detected at diagnosis in one patient only. Medical records were reviewed to obtain information on patients’ demographics, laboratory findings, smoking, staging, treatments, dates of diagnosis, relapse, transformation, and last follow‐up or death.
Staging evaluation was not standardized during the study interval, but included a complete physical examination; hematological and chemical survey with LDH; chest X‐rays and computerized tomography (CT) of the chest, abdomen, and pelvis in all the patients. PET‐CT, endoscopies, orbital or other magnetic resonance imaging (MRI), and ultrasound of thyroid or salivary glands were performed if clinically indicated. The decision to perform a staging bone marrow (BM) biopsy was left to the discretion of the treating oncologist. Based on the Ann Arbor staging classification, 14 bilateral lung involvement was classified as stage IV. All the available chest X‐rays, CT scans, and FDG‐PET/CT scans were reviewed and revised by a board‐certified radiologist and nuclear medicine physician (RK).
2.1. Statistical analyses
Distributions of demographic and clinical characteristics were listed as frequency and percent. Missing values, except for BM involvement and presence of monoclonal gammopathy (MG), were grouped with the “low‐risk category” because after reviewing the clinical information they were felt more likely to be a negative/normal result. The MALT‐lymphoma International Prognostic Index (MALT‐IPI) was calculated as reported. 15
Progression‐free survival was defined as the time from diagnosis to transformation after diagnosis, progression/relapse, or death, whichever occurred first. OS was defined as the time from diagnosis to death. Event‐free patients were censored at the date of last follow‐up. PFS and OS curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Univariable and multivariable analyses using Cox proportional hazards regression were conducted to evaluate the effect of potential prognostic variables on PFS and OS. Multivariable models were derived using stepwise selection among candidate variables with cut‐offs P ≤ 50% to enter and P ≤ 5% to stay in the model. We first derived model 1 testing variables, except the MALT‐IPI. Next, we derived model 2, by forcing MALT‐IPI and testing other variables that were not part of that index.
3. RESULTS
3.1. Clinical characteristics of PMZL patients
Total of 40 patients with PMZL were diagnosed and treated at our institution between January 1995 and September 2019. Additional two patients with concomitant diagnosis of HGT were excluded from this analysis. Clinical characteristic of the 40 patients with PMZL are presented in Table 1. PMZL was diagnosed in 22 men (55%) and 18 women (45%) with a median age of 58.5 years (range 17.0‐80.0). At the time of diagnosis 67.5% had advanced disease (stage III‐IV), 22.5% presented with B symptoms, elevated LDH was detected in 32.5% of the patients and 37.5% presented with a MALT‐IPI score of 2. Staging BM biopsy was negative in 55% (22/30) of the patients with missing data in 10 patients. Five (12.5%) patients had an associated autoimmune disease but none had a history of Sjögren's syndrome. MG was present in 3 (7.5%) patients (IgM lambda, free light chain lambda, IgA kappa, and IgG lambda). Twelve patients (30%) had a history of smoking but data were missing for six (14%). No HGT was observed during the study follow‐up period.
TABLE 1.
Characteristics of the study population
| Variable | N | % |
|---|---|---|
| Total | 40 | 100.0 |
| Age | ||
| <70 | 30 | 75.0 |
| ≥70 | 10 | 25.0 |
| Gender | ||
| Male | 22 | 55.0 |
| Female | 18 | 45.0 |
| Race | ||
| White | 15 | 37.5 |
| Hispanic | 18 | 45.0 |
| Black | 5 | 12.5 |
| Other/Unknown | 2 | 5.0 |
| Tumor stage | ||
| Stage I‐II | 13 | 32.5 |
| Stage III‐IV | 27 | 67.5 |
| LDH | ||
| Normal LDH | 27 | 67.5 |
| Elevated LDH | 13 | 32.5 |
| Anemia (Hg < 12 g/dL) | ||
| No | 29 | 72.5 |
| Yes | 8 | 20.0 |
| Unknown | 3 | 7.5 |
| Bone marrow involvement | ||
| Negative | 22 | 55.0 |
| Positive | 8 | 20.0 |
| Unknown | 10 | 25.0 |
| Autoimmune disease | ||
| No | 35 | 87.5 |
| Yes | 5 | 12.5 |
| Monoclonal gammopathy | ||
| No | 28 | 70.0 |
| Yes | 3 | 7.5 |
| Unknown | 9 | 22.5 |
| B symptoms | ||
| No B symptoms | 31 | 77.5 |
| B symptoms | 9 | 22.5 |
| PET/CT 5PS | ||
| No PET/CT | 25 | 62.5 |
| DS 1‐3 | 1 | 2.5 |
| DS 4‐5 | 14 | 35.0 |
| Smoking status | ||
| Non‐smoking | 22 | 55.0 |
| Smoking | 12 | 30.0 |
| Unknown | 6 | 15.0 |
| Organ involvement | ||
| Extrapulmonary MZL | 13 | 32.5 |
| PMZL | 27 | 67.5 |
| Mediastinal lymphadenopathy (ML) | ||
| Not present | 16 | 40.0 |
| Present | 18 | 45.0 |
| Unknown | 6 | 15.0 |
| Pleural effusion (PE) | ||
| Not present | 27 | 67.5 |
| present | 7 | 17.5 |
| Unknown | 6 | 15.0 |
| Cavitation | ||
| Not present | 31 | 77.5 |
| Present | 3 | 7.5 |
| Unknown | 6 | 15.0 |
| Mass | ||
| Not present | 19 | 47.5 |
| Present | 15 | 37.5 |
| Unknown | 6 | 15.0 |
| Size | ||
| <2 cm | 4 | 10.0 |
| 2‐5 cm | 18 | 45.0 |
| >5 cm | 9 | 22.5 |
| 0/Unknown | 9 | 22.5 |
| Unifocal/Multifocal | ||
| Unifocal | 12 | 30.0 |
| Multifocal | 22 | 55.0 |
| Unknown | 6 | 15.0 |
| MALT‐IPI score | ||
| 0 | 9 | 22.5 |
| 1 | 14 | 35.0 |
| 2 | 15 | 37.5 |
| 3 | 2 | 5.0 |
Abbreviations: 5PS, five‐point scale; DS, Deauville score; Hg, hemoglobin; LDH: lactate dehydrogenase; MALT‐IPI, mucosa‐associated lymphoid tissue lymphoma international prognostic index; MZL, marginal zone lymphoma; PET/CT, positron emission tomography; PMZL, pulmonary marginal zone lymphoma.
3.2. Pulmonary radiological characteristics of PMZL patients
The involvement was unilateral in 29 (72%) and bilateral in 11 (28%) patients. Lung involvement was multifocal in 22 (55%) of patients. Twenty‐seven patients had lung only PMZL while 13 patients had extrapulmonary MZL with lung involvement. From 27 patients with only lung involvement, nine (33.3%) had stage IV due to multiple lung lesions, five (18.5%) due to BM involvement, while 13 (48.2%) had stage I disease.
Radiological scans were available and were reviewed for 34 patients and shown in Table 1 and Figure 1. Consolidation or air‐bronchogram pattern were the most common presenting findings seen on CT scans in 22 (65%) of patients, followed by lung nodules in 18 (53%) patients. Ground glass opacities (GGO) were seen in 14 (41%), while pleural effusion was observed in seven (21%) patients. Mass like lesion was detected in 15 (44%) patients, cavitary lesions in three (9%), and lymphangitic spread was seen on chest CT in three (9%) patients. We measured lesion size and classified patients based on the size of their largest lesion into three categories: <2 cm in 4 (12%) patients, 2‐5 cm in 18 (53%) and more than 5 cm in nine (27%), while lesion size data were unavailable for three (9%) patients.
FIGURE 1.
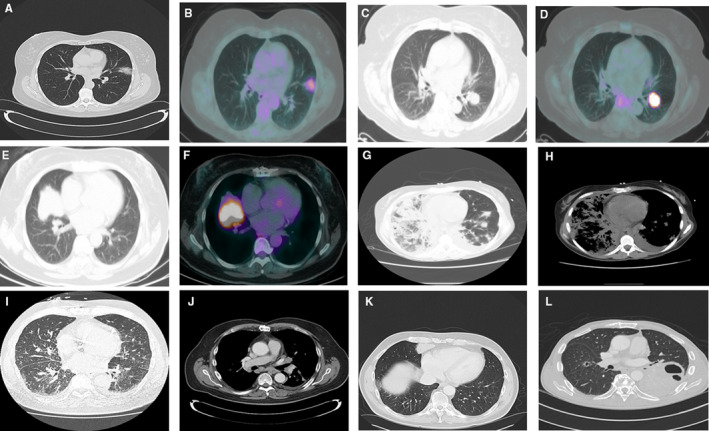
Radiological manifestations of pulmonary marginal zone lymphoma. A and B, focal ground glass opacity with air bronchograms and mild FDG‐uptake in the left upper lobe; (C and D), nodular lesion with intense FDG‐uptake in the left lower lobe; (E and F), pulmonary mass with intense FDG‐uptake in the right upper lobe; (G and H), multifocal bilateral lung parenchymal opacities with small left pleural effusion; (I and J), nodular interstitial thickening indicating lymphangitic spread of lymphoma, also noted to have mediastinal and hilar adenopathy on CT with contrast; (K), focal area of consolidation with homogeneous enhancement in the right middle lobe on CT with contrast; (L), cavitary consolidation in the left lower lobe with an additional cavitary nodule in the right middle lobe
FDG‐PET/CT scan data were available in 15 patients, with 14 (93.3%) patients having FDG‐avid disease, as demonstrated by presence of hypermetabolic lesions corresponding to the areas of PMZL. Fourteen patients demonstrated a Deauville score of 4‐5, one patient demonstrated a Deauville score of 1 (Table 1). The patient with low FDG‐avidity presented with ground‐glass opacities, air‐bronchogram, and nodular lesions of 2‐5 cm on CT scans. SUV max in FDG avid cases ranged from 2.5 to 9.1 with a median SUV max of 4.0. No patient was upstaged based on the PET/CT scan.
3.3. Treatment
Initial treatment at the time of PMZL diagnosis in our cohort of 40 patients consisted of immunotherapy, chemotherapy or both in 26 (65%) patients, radiation therapy only in three (7.5%) patients, combined modality therapy (radiation and immuno/chemotherapy or immuno/chemotherapy and surgery) in five (12.5%) patients, and surgical resection in four (10%) patients (Table 2). Two (5%) of the 40 patients were never treated and remained on active surveillance. The overall response rate in 38 treated patients was 86.9% (25 CR and 8 PR). Overall response per treatment modality was 80.7% (CR 15 and PR 6) for immuno/chemotherapy only, 100% (CR 3) for radiation therapy only, 100% (CR 3 and PR 2) for combined modality, and 100% (CR 4) for surgical resection. It is important to note that all the patients treated with surgical resection, radiation therapy, or combined modality therapy had localized disease only.
TABLE 2.
Treatments and response
| Variable | N | % |
|---|---|---|
| Total | 40 | 100.0 |
| Treatment | ||
| Active surveillance | 2 | 5.0 |
| Surgery only | 4 | 10.0 |
| RT only | 3 | 7.5 |
| Chemo only | 26 | 65.0 |
| Chemo + surgery | 1 | 2.5 |
| Chemo + RT | 4 | 10.0 |
| Chemotherapy regimens | 31 | 100 |
| Rituximab | 10 | 32.2 |
| R‐CVP | 6 | 19.3 |
| R‐CHOP | 5 | 16.1 |
| Ibritumomab Tiuxetan/rituximab | 5 | 16.1 |
| Bendamustine/Rituximab | 2 | 6.4 |
| Rituximab/Lenalidomide | 1 | 3.3 |
| Rituximab/Chlorambucil | 1 | 3.3 |
| Other a | 1 | 3.3 |
| Clinical response (n = 38 excluded 2 untreated) | ||
| CR | 25 | 65.8 |
| PR | 8 | 21.1 |
| SD | 1 | 2.6 |
| PD | 4 | 10.5 |
Abbreviations: CR, complete response; PD, progression of disease; PR, partial response; R‐CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R‐CVP, rituximab, cyclophosphamide, vincristine and prednisone; RT, radiation therapy; SD, stable disease.
Rituximab/Methotrexate/Procarbazine.
3.4. Progression‐free survival and overall survival
With a median follow‐up of 8.4 years (range 0.07‐18.44), 22 PFS events (17 relapses and 5 deaths) were seen among the 38 treated patients. All the PFS events were seen among the 33 patients treated with immuno/chemotherapy or combined modality therapy while there were no PFS events in patients treated with surgery or radiation therapy alone. Table 3 summarizes therapies given to patients upon relapse and patients’ outcome. The seven patients treated with either surgery (n = 4) or radiation therapy (n = 3), as primary modalities, remained alive and free of disease. Two patients on observation also remained alive and stable, not requiring any further therapy after 8.4 and 8.6 years of follow‐up respectively.
TABLE 3.
Detailed characteristics of 17 relapsed PMZL patients
| Patient | Site of disease | Initial treatment | Response 1 | Site of relapse | Treatment | Response 2 | Status | Cause of death |
|---|---|---|---|---|---|---|---|---|
| 1 | Lung, pleural effusion | Rituximab | CR | Lung, Bone, BM | R‐CVP | CR | Alive | NA |
| 2 | Lung, pleural effusion | Rituximab | CR | Lacrimal gland, lung, LNs | BR | SD | Alive | NA |
| 3 | Lung, gastric, skin | Ibritumomab Tiuxetan/rituximab | CR | Skin, subcutaneous soft tissue with skin amyloidosis | R‐CVP | SD | Deceased | Unknown |
| 4 | Lung, LNs, adrenal, Horner syndrome | Rituximab | PD | Lung, LNs, adrenal, Horner syndrome | R‐CHOP, HDMTX | CR | Alive | NA |
| 5 | Lung | R‐CHOP | CR | Lung, LNs | BR | CR | Alive | NA |
| 6 | Pleural effusion, LNs | Rituximab | PD | Pleural effusion | BR | PR | Alive | NA |
| 7 | Lung, spinal cord, Bone, +CSF | Rituximab, procarbazine, IT‐MTX‐AraC | PD | New brain lesion | DHAP, HDMTX and IT‐MTX, AraC | CR | Alive | NA |
| 8 | Lung, spleen, bone | Ibritumomab Tiuxetan/rituximab | PR | Lung | RCVP | PR | Alive | NA |
| 9 | Lung | Rituximab | CR | Lung | BR, RT | CR | Deceased | Unknown |
| 10 | Lung and BM | Rituximab + Surgery | CR | Parotid | Rituximab, RT | CR | Deceased | Unknown |
| 11 | Lung and BM | Rituximab | CR | Lung | BR | CR | Deceased | Unknown |
| 12 | Lung, pelvic mass | Rituximab | CR | Lung, Gastric, perineal | Rituximab | PR | Alive | NA |
| 13 | Lung, Renal, LNs | R‐CVP | PR | Lung, Renal | Ibritumomab Tiuxetan/rituximab | PR | Alive | NA |
| 14 | Lung, LNs | R‐CVP | CR | Lung | R‐CHOP | PR | Alive | NA |
| 15 | Lung, gastric, BM | R‐CVP | PD | Gastric and lung | Surveillance | SD | Deceased | SCC of head and neck |
| 16 | Lung | R‐CHOP | PR | Lung | Rituximab Chlorambucil, Gamma knife | CR | Alive | NA |
| 17 | Lung, LNs | BR | CR | LNs | Surveillance | SD | Alive | NA |
Boldface indicates significance at P < .05. Abbreviations: BM, Bone marrow; BR, bendamustine, rituximab; CR, complete response; DHAP, dexamethasone, cytarabine, cisplatin; HDMTX, high dose methotrexate; IT MTX, intrathecal methotrexate; LNs, lymph nodes; NA, not applicable; PD, disease progression; PMZL, pulmonary marginal zone lymphoma; PR, partial response; R‐CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R‐CVP, rituximab, cyclophosphamide, vincristine, prednisone; SCC, squamous cell carcinoma; SD, stable disease.
There were 10 deaths during the follow‐up period, including two attributed to PMZL (n = 1) or PMZL treatment (n = 1). A patient with the shortest follow‐up of 1.5 months died of PMZL. Four patients died secondary to other causes: squamous cell carcinoma of head and neck (n = 1), peripheral T cell lymphoma that developed after renal transplant (n = 1), autoimmune hemolytic anemia (n = 1), and stab wound to the chest (n = 1). The cause of death was unknown in four patients who died in CR (n = 3) or with SD (n = 1) at the time of last follow‐up.
In treated patients, the median PFS was 7.5 years (95% CI 1.8‐9.5) and the median OS was 15.7 years (95% CI 9.3‐NE; Figure 2A). The 10‐year PFS and OS were 32.9% (95% CI 16.4%‐50.5%) and 70.2% (95% CI 49.9%‐83.6%) respectively.
FIGURE 2.
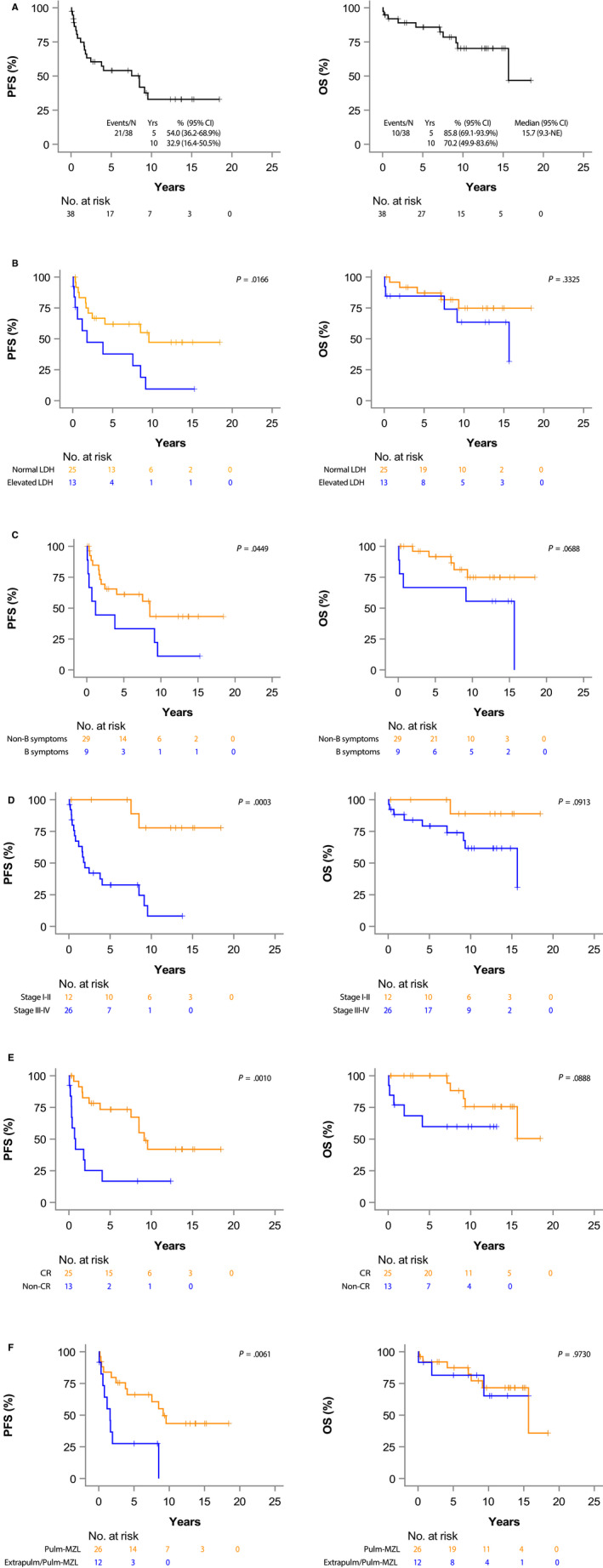
Kaplan‐Meier curves for progression free survival (PFS) and overall survival (OS) in 40 treated patients with pulmonary marginal zone lymphoma based on clinical features present at diagnosis. A, in all the patients; (B), by lactate dehydrogenase (LDH); (C), by B symptoms; (D), by stage (I‐II vs III‐IV); (E), by complete response (CR) after initial therapy; (F), by presence of extrapulmonary (Extrapulm) marginal zone lymphoma (MZL)
In the nine patients who initially were treated with rituximab alone, the mPFS was 7.5 (1.3‐9.1) years and in patients achieving CR, the median duration of response was 5.2 years (range 0.6‐9.5).
We then analyzed prognostic factors associated with PFS and OS (Table 4). PFS was significantly shorter in patients with elevated LDH at presentation (Figure 2B), presence of B symptoms (Figure 2C), advanced stage (Figure 2D), failure to achieve CR after initial treatment (Figure 2E), and presence of extrapulmonary MZL (Figure 2F). Nevertheless, none of these variables affected the OS (Figure 2B‐F). Univariable analyzes (Table 4) showed association between shorter PFS and elevated LDH at presentation (HR = 2.57, 95% CI 1.16‐6.51, P = .022), advanced stage (HR = 9.84, 95% CI of 2.24‐43.3, P = .002), and failure to achieve CR after initial treatment (HR = 3.95, 95% CI 1.64‐9.52, P = .002). Patients without extrapulmonary MZL had longer PFS (HR = 3.41, 95% CI 1.35‐8.60, P = .009). However, none of these variables were associated with shorter OS (Table 4).
TABLE 4.
Univaria Cox models for progression‐free survival (PFS) and overall survival (OS) in 38 treated patients
| Variable | N | PFS (21 events) | OS (10 events) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI) | P | Death | HR (95% CI) | P | ||||||
| Gender | |||||||||||
| Male | 20 | 13 | Reference | 5 | Reference | ||||||
| Female | 18 | 8 | 0.63 (0.26, 1.51) | .299 | 5 | 1.11 (0.32, 3.88) | .872 | ||||
| Race | |||||||||||
| White | 14 | 9 | Reference | 4 | Reference | ||||||
| Hispanic | 17 | 7 | 0.75 (0.28, 2.04) | .574 | 4 | 1.30 (0.32, 5.31) | .711 | ||||
| Black | 5 | 5 | 2.58 (0.85, 7.82) | .095 | 2 | 1.69 (0.30, 9.51) | .553 | ||||
| Other/Unknown | 2 | ‐ | NE | ‐ | NE | ||||||
| Age | |||||||||||
| <70 | 30 | 15 | Reference | 6 | Reference | ||||||
| ≥70 | 8 | 6 | 2.11 (0.81, 5.48) | .126 | 4 | 2.59 (0.72, 9.28) | .144 | ||||
| Tumor stage | |||||||||||
| Stage I‐II | 12 | 2 | Reference | 1 | Reference | ||||||
| Stage III‐IV | 26 | 19 | 9.84 (2.24, 43.3) | .002 | 9 | 4.98 (0.63, 39.3) | .128 | ||||
| LDH | |||||||||||
| Normal LDH | 25 | 11 | Reference | 5 | Reference | ||||||
| Elevated LDH | 13 | 10 | 2.75 (1.16, 6.51) | .022 | 5 | 1.85 (0.52, 6.58) | .339 | ||||
| MALT‐IPI | |||||||||||
| 0‐1 | 22 | 8 | Reference | 4 | Reference | ||||||
| 2‐3 | 16 | 13 | 3.98 (1.63, 9.73) | .002 | 6 | 2.02 (0.56, 7.27) | .280 | ||||
| B symptoms | |||||||||||
| Non‐B symptoms | 29 | 13 | Reference | 5 | Reference | ||||||
| B symptoms | 9 | 8 | 2.41 (0.99, 5.84) | .052 | 5 | 3.02 (0.87, 10.5) | .083 | ||||
| Smoking status | |||||||||||
| Non‐smoking | 21 | 14 | Reference | 8 | Reference | ||||||
| Smoking | 11 | 4 | 0.46 (0.15, 1.41) | .175 | ‐ | NE | |||||
| Unknown | 6 | 3 | 0.51 (0.14, 1.78) | .288 | 2 | 0.81 (0.17, 3.90) | .791 | ||||
| Lung disease | |||||||||||
| Pulm MZL | 26 | 12 | Reference | 3 | Reference | ||||||
| Pulm/Extrapulm–MZL | 12 | 9 | 3.41 (1.35, 8.60) | .009 | 7 | 1.02 (0.26, 3.97) | .973 | ||||
| Mediastinal lymphadenopathy (ML) | |||||||||||
| Non‐ML | 14 | 5 | Reference | 2 | Reference | ||||||
| ML | 18 | 12 | 2.72 (0.94, 7.86) | .065 | 6 | 3.28 (0.64, 16.9) | .155 | ||||
| Unknown | 6 | 4 | 1.41 (0.38, 5.27) | .610 | 2 | 1.27 (0.17, 9.67) | .815 | ||||
| Clinical response | |||||||||||
| CR | 25 | 11 | Reference | 5 | Reference | ||||||
| Non‐CR | 13 | 10 | 3.95 (1.64, 9.52) | .002 | 5 | 2.97 (0.80, 11.1) | .105 | ||||
| Pleural effusion (PE) | |||||||||||
| Non‐PE | 25 | 12 | Reference | 6 | Reference | ||||||
| PE | 7 | 5 | 2.28 (0.80, 6.49) | .124 | 2 | 1.52 (0.30, 7.65) | .608 | ||||
| Unknown | 6 | 4 | 0.94 (0.30, 2.93) | .916 | 2 | 0.72 (0.13, 3.89) | .707 | ||||
| Cavitation | |||||||||||
| Non‐Cavitation | 29 | 15 | Reference | 7 | Reference | ||||||
| Cavitation | 3 | 2 | 49.2 (4.18, 579.00) | .002 | 1 | 2.20 (0.27, 18.1) | .462 | ||||
| Unknown | 6 | 4 | 0.86 (0.28, 2.60) | .787 | 2 | 0.71 (0.14, 3.75) | .690 | ||||
| Mass | |||||||||||
| Non‐Mass | 18 | 9 | Reference | 7 | Reference | ||||||
| Mass | 14 | 8 | 1.07 (0.41, 2.79) | .883 | 1 | 0.16 (0.02, 1.31) | .088 | ||||
| Unknown | 6 | 4 | 0.81 (0.25, 2.66) | .734 | 2 | 0.43 (0.08, 2.14) | .300 | ||||
| Tumor size (cm) | |||||||||||
| <2 cm | 4 | 2 | Reference | 1 | Reference | ||||||
| 2‐5 cm | 16 | 7 | 0.83 (0.17, 4.00) | .813 | 4 | 1.45 (0.13, 16.6) | .767 | ||||
| >5 cm | 9 | 7 | 1.94 (0.40, 9.37) | .411 | 3 | 2.20 (0.18, 26.9) | .537 | ||||
| 0/Unknown | 9 | 5 | 0.61 (0.12, 3.16) | .555 | 2 | 0.64 (0.06, 7.32) | .719 | ||||
| Unifocal/Multifocal | |||||||||||
| Unifocal | 11 | 2 | Reference | ‐ | Reference | ||||||
| Multifocal | 21 | 15 | 7.61 (1.72, 33.7) | .007 | 8 | NE | |||||
| Unknown | 6 | 4 | 3.30 (0.60, 18.1) | .168 | 2 | NE | |||||
Boldface indicates significance at P < .05. Abbreviations: MZL, marginal zone lymphoma; NE, not estimable; Pulm, pulmonary; extrapulm; extrapulmonary.
A multivariable analysis (Table 5) demonstrated that age ≥70 (HR = 3.22, 95% CI 1.04‐9.95, P = .042), elevated LDH (HR = 3.55, 95% CI 1.38‐9.14, P = .009), advanced stage (HR = 6.76, 95% CI 1.48‐31.0, P = .014), not achieving CR (HR = 7.18, 95% CI 2.25‐22.9, P = .001), and MALT‐IPI of 2‐3 (HR of 7.24, 95% CI 2.55‐20.53, P = .001) were associated with shorter PFS. However, none of these variables were associated with shorter OS.
TABLE 5.
Multivariable Cox models for progression‐free survival (PFS) and overall survival (OS) in 38 treated patients
| Variable | Category | PFS (21 events) | OS, event (10 events) | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Model 1 | |||||
| Age | <70 | Reference | Reference | ||
| ≥70 | 3.22 (1.04, 9.95) | .042 | 3.25 (0.76, 13.9) | .111 | |
| Tumor stage | Stage I‐II | Reference | Reference | ||
| Stage III‐IV | 6.76 (1.48, 31.0) | .014 | 2.42 (0.27, 21.7) | .429 | |
| LDH | Normal LDH | Reference | Reference | ||
| Elevated LDH | 3.55 (1.38, 9.14) | .009 | 1.68 (0.44, 6.40) | .450 | |
| Clinical response | CR | Reference | Reference | ||
| Non‐CR | 7.18 (2.25, 22.9) | <.001 | 3.39 (0.76, 15.2) | .110 | |
| Model 2 | |||||
| MALT‐IPI | 0‐1 | Reference | Reference | ||
| 2‐3 | 7.24 (2.55, 20.53) | <.001 | 2.09 (0.58, 7.54) | .260 | |
| Lung disease | Pulm‐MZL | Reference | Reference | ||
| Extrapulm/Pulm‐MZL | 2.49 (0.99, 6.25) | .052 | 0.84 (0.21, 3.30) | .803 | |
| Clinical response | CR | Reference | Reference | ||
| Non‐CR | 7.33 (2.58, 20.85) | <.001 | 3.03 (0.81, 11.32) | .099 | |
Boldface indicates significance at P < .05. Abbreviations: CR, complete response; LDH, lactate dehydrogenase; MALT‐IPI, mucosa‐associated lymphoid tissue lymphoma international prognostic index; MZL, marginal zone lymphoma; Pulm, pulmonary; extrapulm; extrapulmonary.
There were no differences in PFS and OS by lesion size (Figure 3A). Patients with multifocal disease on CT chest tended to have shorter PFS as compared to patients with unifocal disease (HR = 7.61, 95% CI of 1.72‐33.7, P = .007; Figure 3B), as also did patients with cavitary lesions (HR = 49.2, 95% CI 4.18‐579.00, P = .002; Figure 3C). However, radiologic findings were not associated with statistically significant shorter OS. (Figure 3A,C).
FIGURE 3.
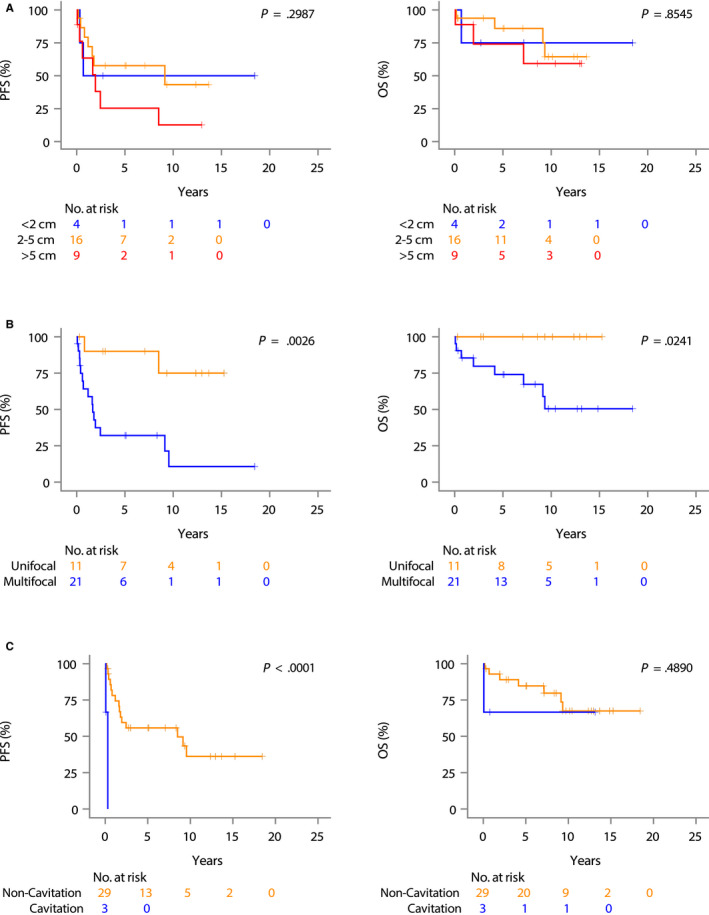
Kaplan‐Meier curves for progression free survival (PFS) and overall survival (OS) in 34 treated patients with pulmonary marginal zone lymphoma based on radiological findings at diagnosis. A, by size of lesion (<2 cm vs 2‐5 cm vs >5 cm); (B), by site of disease (unifocal vs multifocal disease); (C), by presence of cavitation
4. DISCUSSION
Pulmonary marginal zone lymphoma is a rare lymphoma, usually seen in patients older than 60 years old and characterized by an indolent course. 8 The present study aimed to identify clinical and radiological findings associated with shorter survival in a large cohort of patients from a single institution followed for more than 8 years. The overall outcome was excellent with only 10 deaths, most of which were non‐PMZL related.
In line with prior studies, most patients included in our cohort presented with normal LDH (66.7%), absence of B symptoms (78.6%), and negative staging BM biopsy (57.1%); however, advanced stage (66.7%) at diagnosis was more frequent in our cohort compared to prior studies. 8 , 10 Radiologically, consolidation and nodule(s) followed by pulmonary mass were the most common radiological findings, as previously reported 6 , 10 ; however, we also observed lymphangitic spread and cavitations that are not commonly reported. 5 , 8 , 10
Clinical factors associated with shorter survival in PMZL are still largely unknown and controversial. Oh et al reported shorter median time to progression and 5‐year OS in PMZL patients presenting with extrapulmonary (1.1 vs 6.2 years; P = .030 and 65.5% vs 95.95; P = .016, respectively) and lymph node involvement (2.4 vs 5.6 years, P = .024 and 80.7% vs 94.7%; P = .025 respectively). 6 However, extrapulmonary MZL was not associated with inferior prognosis in another study. 10 In our study, extrapulmonary MZL was associated with shorter PFS. Additional clinical factors associated with shorter PFS in the current study were advanced stage, elevated LDH, failure to achieved CR after frontline therapy, presence of cavitary lesions, and multifocal disease. In multivariable analysis, MALT‐IPI>1 and independent variables comprising this prognostic score as well as inability to achieve CR after frontline treatment were associated with shorter PFS. However, we did not identify any clinical or radiological finding associated with shorter OS likely due to the fact that majority of causes of death in our patients were unrelated to lymphoma with only two patients succumbing to lymphoma. These findings indicate excellent prognosis PMZL patients with lymphoma‐related OS of 94.9% (95% CI 81.25%‐98.7%) at 5, 10, and 15 years respectively.
Recent studies have demonstrated the utility of FDG‐PET/CT in small cohorts of PMZL with FDG‐avidity in 80%‐100% of patients. 11 Our study supports prior observations demonstrating FDG‐avidity in PMZL with most patients presenting FDG‐uptake above liver background. Only one patient had disease detected on PET/CT that was not previously seen on CT scans, but not leading to upstaging. Furthermore, PET/CT was not diagnostic for BM involvement in our patients, however, the number of analyzed cases is small.
Higher grade transformation, reported in 3.8%‐8% of patients with MZL, 16 , 17 , 18 is typically associated with shorter survival—a 5‐year OS of 65% after HGT. 17 The incidence of HGT in PMZL patients is largely unknown. In a large study (n = 467) evaluating clinicopathological features of HGT in MALT lymphoma, none of the patients with PMZL transformed to DLBCL. 18 Similarly, none of our patients experienced HGT during follow‐up. This finding in combination with low lymphoma‐associated mortality may suggest a different biology and natural history of PMZL in comparison to MALT lymphoma originating in other locations.
Currently, there is no standard/uniform treatment approach for PMZL patients. This is due to absence of large studies and randomized clinical trials, as well as favorable outcomes with multiple therapeutic modalities. 8 Accepted treatment options include observation, radiation, surgical resection, and immunochemotherapy. Leyfman et al reported a 6‐year event‐free survival (EFS) and OS of 63% (95% CI 50%‐80%) and 88% (95% CI 77%‐100%), respectively, in patients managed with active surveillance. 19 Nevertheless, complete surgical resection have resulted in excellent long‐term disease free survival and may be curative in localized PMZL patients. 20 , 21 Our findings support these prior observations, as all our patients treated with surgery achieved CR and remained without evidence of disease relapse after a prolonged follow‐up. Similarly, patients treated with radiation therapy (median dose 34 Gy, range 30‐36) achieved excellent outcomes providing further evidence that these two modalities may be preferentially considered for treatment of patients with localized PMZL.
Immunochemotherapy is usually reserved for symptomatic patients with extensive disease. Okamura et al evaluated the activity of a single agent rituximab in PMZL (n = 8) that resulted in an ORR of 75% (CR 62.5% and PR 12.5%) and mPFS of 5.5 years (range 0.8‐7.26 years). 22 In our patients treated with rituximab as a single agent, the ORR was 89% and mPFS was 7.5 years (range 1.3‐9.1 years), representing an excellent option in patients requiring systemic therapy.
In summary, our findings confirm excellent outcomes and possible cure in patients with localized PMZL treated with surgery or radiation therapy. Several clinical and radiological features associated with shorter PFS were identified. However, no clinical characteristics associated with shorter OS were detected, likely due to the indolent nature and low PMZL‐related mortality in these patients. Multicenter collaborative studies are needed to define the best therapeutic approach in PMZL patients.
CONFLICT OF INTEREST
ISL has served on advisory boards from Seattle Genetics, Janssen Scientific and Verastem. JPA has received honoraria from Targeted Oncology, OncLive and Oncinfo, and immediate family member has served on advisory boards from Puma Biotechnology, Inovio Pharmaceuticals, Agios Pharmaceuticals, Forma Therapeutics and Foundation Medicine.
AUTHOR CONTRIBUTIONS
MH: collected and analyzed the data, and wrote the manuscript; RK: performed review of images; SGI: collected the data; WZ and IMR: analyzed the data and wrote the manuscript; JRC and FV: performed review of diagnostic biopsies and confirmed diagnosis; ISL: conceptualized and designed the study, was involved in the treatment of these patients, analyzed the data, and wrote the manuscript; JPA: collected the data, conceptualized and designed the study, analyzed the data, and wrote the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
ISL is supported by grant 1R01CA233945 from the National Cancer Institute, Florida Health Bankhead‐Coley Cancer Research Award AWD‐005151, the Dwoskin, Recio, and Anthony Rizzo Families Foundations and Jaime Erin Follicular Lymphoma Research Consortium. JPA is a K12 Scholar supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA226330. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Husnain M, Kuker R, Reis IM, et al. Clinical and radiological characteristics of patients with pulmonary marginal zone lymphoma: A single center analysis. Cancer Med. 2020;9:5051–5064. 10.1002/cam4.3096
Izidore S. Lossos and Juan Pablo Alderuccio equal contribution
[Correction added on 12 June 2020, after first online publication: The name of the fourth author has been corrected in this version.]
DATA AVAILABILITY STATEMENT
All the inquiries regarding data will be provided by the corresponding author.
REFERENCES
- 1. Isaacson P, Wright DH. Malignant lymphoma of mucosa‐associated lymphoid tissue. A distinctive type of B‐cell lymphoma. Cancer. 1983;52:1410‐1416. [DOI] [PubMed] [Google Scholar]
- 2. Thieblemont C, Bastion Y, Berger F, et al. Mucosa‐associated lymphoid tissue gastrointestinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997;15:1624‐1630. [DOI] [PubMed] [Google Scholar]
- 3. Zinzani PL, Magagnoli M, Galieni P, et al. Nongastrointestinal low‐grade mucosa‐associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999;17:1254. [DOI] [PubMed] [Google Scholar]
- 4. Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127:2082‐2092. [DOI] [PubMed] [Google Scholar]
- 5. Deng W, Wan Y, Yu JQ. Pulmonary MALT Lymphoma has variable features on CT. Sci Rep. 2019;9:8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oh SY, Kim WS, Kim JS, et al. Pulmonary marginal zone B‐cell lymphoma of MALT type–what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy?: Consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol. 2010;89:563‐568. [DOI] [PubMed] [Google Scholar]
- 7. Zhao S, Zhang L, Gu Z, et al. Clinical manifestations of pulmonary mucosa‐associated lymphoid tissue lymphoma: single‐center experience with 18 patients. Onco Targets Ther. 2018;11:555‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sammassimo S, Pruneri G, Andreola G, et al. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol Oncol. 2016;34:177‐183. [DOI] [PubMed] [Google Scholar]
- 9. Adam P, Czapiewski P, Colak S, et al. Prevalence of Achromobacter xylosoxidans in pulmonary mucosa‐associated lymphoid tissue lymphoma in different regions of Europe. Br J Haematol. 2014;164:804‐810. [DOI] [PubMed] [Google Scholar]
- 10. Borie R, Wislez M, Thabut G, et al. Clinical characteristics and prognostic factors of pulmonary MALT lymphoma. Eur Respir J. 2009;34:1408‐1416. [DOI] [PubMed] [Google Scholar]
- 11. Albano D, Borghesi A, Bosio G, et al. Pulmonary mucosa‐associated lymphoid tissue lymphoma: (18)F‐FDG PET/CT and CT findings in 28 patients. Br J Radiol. 2017;90:20170311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stefanovic A, Morgensztern D, Fong T, et al. Pulmonary marginal zone lymphoma: a single centre experience and review of the SEER database. Leuk Lymphoma. 2008;49:1311‐1320. [DOI] [PubMed] [Google Scholar]
- 13. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues, 4th rev ed. Lyon, France: IARC Press; 2017. [Google Scholar]
- 14. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin's disease staging classification. Cancer Res. 1971;31:1860‐1861. [PubMed] [Google Scholar]
- 15. Thieblemont C, Cascione L, Conconi A, et al. A MALT lymphoma prognostic index. Blood. 2017;130:1409‐1417. [DOI] [PubMed] [Google Scholar]
- 16. Conconi A, Franceschetti S, Aprile von Hohenstaufen K, et al. Histologic transformation in marginal zone lymphomas. Ann Oncol. 2015;26:2329‐2335. [DOI] [PubMed] [Google Scholar]
- 17. Alderuccio JP, Zhao W, Desai A, et al. Risk factors for transformation to higher‐grade lymphoma and its impact on survival in a large cohort of patients with marginal zone lymphoma from a single institution. J Clin Oncol. 2018;36(34):3370‐3380. [DOI] [PubMed] [Google Scholar]
- 18. Maeshima AM, Taniguchi H, Toyoda K, et al. Clinicopathological features of histological transformation from extranodal marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue to diffuse large B‐cell lymphoma: an analysis of 467 patients. Br J Haematol. 2016;174:923‐931. [DOI] [PubMed] [Google Scholar]
- 19. Leyfman Y, Joffe E, Drill E, et al. Expectant management of extranodal marginal zone lymphoma of bronchial‐associated lymphoid tissue (BALT). Blood. 2019;134:2826‐2826. [Google Scholar]
- 20. Lee H, Yang B, Nam B, et al. Treatment outcomes in patients with extranodal marginal zone B‐cell lymphoma of the lung. J Thorac Cardiovasc Surg. 2017;154:342‐349. [DOI] [PubMed] [Google Scholar]
- 21. Wöhrer S, Kiesewetter B, Fischbach J, et al. Retrospective comparison of the effectiveness of various treatment modalities of extragastric MALT lymphoma: a single‐center analysis. Ann Hematol. 2014;93:1287‐1295. [DOI] [PubMed] [Google Scholar]
- 22. Okamura I, Imai H, Mori K, et al. Rituximab monotherapy as a first‐line treatment for pulmonary mucosa‐associated lymphoid tissue lymphoma. Int J Hematol. 2015;101:46‐51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the inquiries regarding data will be provided by the corresponding author.


