Abstract
Background
Study characteristics influence vaccine effectiveness (VE) estimation. We examined the influence of some of these on seasonal influenza VE estimates from test-negative design (TND) studies.
Methods
We systematically searched bibliographic databases and websites for full-text publications of TND studies on VE against laboratory-confirmed seasonal influenza in outpatients after the 2009 pandemic influenza. We followed the Cochrane Handbook for Systematic Reviews of Interventions guidelines. We examined influence of source of vaccination information, respiratory specimen swab time, and covariate adjustment on VE. We calculated pooled adjusted VE against H1N1 and H3N2 influenza subtypes, influenza B, and all influenza using an inverse-variance random-effects model.
Results
We included 70 full-text articles. Pooled VE against H1N1 and H3N2 influenza subtypes, influenza B, and all influenza was higher for studies that used self-reported vaccination than for those that used medical records. Pooled VE was higher with respiratory specimen collection within ≤7 days vs ≤4 days of symptom onset, but the opposite was observed for H1N1. Pooled VE was higher for studies that adjusted for age but not for medical conditions compared with those that adjusted for both. There was, however, a lack of statistical significance in almost all differences in pooled VE between compared groups.
Conclusions
The available evidence is not strong enough to conclude that influenza VE from TND studies varies by source of vaccination information, respiratory specimen swab time, or adjustment for age/medical conditions. The evidence is, however, indicative that these factors ought to be considered while designing or evaluating TND studies of influenza VE.
Keywords: seasonal influenza, vaccine effectiveness, test-negative design, outpatients, systematic review, meta-analysis
Vaccination is the most effective prevention for seasonal influenza. Observational studies, rather than randomized controlled trials, are used to examine seasonal influenza vaccine effectiveness (VE) due to feasibility and ethical considerations. Continuous changes that occur in influenza viruses (antigenic drift) [1] mean that influenza vaccines have to be re-formulated every influenza season and that vaccine virus strains may be mismatched with circulating virus strains. Influenza VE studies are conducted each season in many jurisdictions worldwide to assess vaccine performance and to inform subsequent influenza season vaccine development.
Studies on influenza VE often have differences in their design. Studies approach participant recruitment differently, and influenza vaccination status may be determined by either self-report or medical record ascertained. Clinic presentation and timing of respiratory specimen swab collection differ across study participants. The characteristics of study participants, such as age and health status, also vary and may impact VE [2]. Adjustment in analysis of VE varies across studies, and adjustment for specific potential confounders such as age and medical conditions may lead to differences in VE estimations. Due to these variations and other factors, influenza VE estimates vary between jurisdictions.
The test-negative design (TND), an observational study design type, is an increasingly popular design for estimating influenza VE [3, 4]. In a TND study, patients presenting with influenza-like symptoms are tested for influenza. Those with a positive test result become the cases, and those with a negative test result become the controls. Influenza VE (represented as a percentage) is calculated as 1 minus the adjusted ratio of the odds of vaccination in those with positive test results to the odds of vaccination in those with negative test results, multiplied by 100. The TND has been credited with reducing biases due to differential health care–seeking behavior between vaccinated and unvaccinated individuals and differential misclassification of influenza infection status [3]. However, if stringent methods for study participants’ enrollment and influenza testing are not applied, the TND may fail to correct for differential health care–seeking behavior among vaccinated and unvaccinated individuals [5].
We systematically identified, critically appraised, and summarized the findings of published TND studies that examined seasonal influenza VE in primary care settings since the 2009 pandemic influenza. We conducted a systematic review and meta-analysis following the Cochrane Handbook for Systematic Reviews of Interventions guidelines [6], and we reported our findings following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [7].
METHODS
Search Strategy and Selection Criteria
We developed and registered a review protocol in the international prospective register of systematic reviews (PROSPERO) before commencement of this review (registration number CRD42017064595). We searched the MEDLINE (Ovid), Embase (Ovid), PubMed, Scopus (Elsevier), Web of Science, and Google Scholar bibliographic databases. Our literature search strategy (Supplementary Table 1) was reviewed by a knowledge synthesis librarian using the PRESS checklist [8]. The literature search was first conducted in April 2017 and updated in July 2018. Corresponding authors of regional influenza surveillance studies were contacted to check if our searches missed any relevant studies. Identified literature citations were imported and screened in a specially designed Microsoft Access 2016 database (Microsoft Corporation, Redmond, WA, USA).
We were interested in community-based TND studies conducted in primary care settings (outpatients) after the 2009 pandemic influenza (from influenza season 2010/2011 onwards). Only studies that reported multivariable-adjusted influenza VE estimates against laboratory-confirmed influenza of any type or subtype were considered for inclusion in the review. We included only studies with influenza confirmation based on reverse transcriptase polymerase chain reaction (RT-PCR) assay or viral culture of a respiratory specimen and only full-text study publications, irrespective of language of publication. We also included only studies in which patients deemed to have received influenza vaccination did so at least 14 days before their symptom onset, and their symptom onset must not have been >7 days before medical consultation, specimen collection, and study enrollment. Studies involving only hospitalized patients and studies that reported results from mixed hospitalized patients and outpatients without reporting separate results for the 2 patient groups were excluded. We also excluded studies based on retrospective analysis of respiratory samples obtained for clinical diagnostic testing. Furthermore, we excluded studies conducted in military barracks, prisons, care homes, schools, and in subgroups such as individuals with chronic diseases. The outcomes of our interest were adjusted influenza VE against the H1N1 and H3N2 influenza subtypes, influenza B, and all influenza. Two reviewers independently screened the identified citations against the eligibility criteria using a 2-stage sifting approach to review titles/abstracts and full-text articles. Disagreements during this process were resolved through discussion between the 2 reviewers or by involvement of a third reviewer. The number of ineligible citations at the title/abstract screening stage and both the number and reasons for ineligibility at the full-text article screening stage were documented.
Data Extraction
We extracted data in MS Excel 2016 (Microsoft Corporation, Redmond, WA, USA). One reviewer independently extracted data from the included articles, and a second reviewer independently checked the extracted data for errors. Disagreements during this process were resolved through discussion between the 2 reviewers or by involvement of a third reviewer. We extracted study details such as name of the first author, publication year, country, and funding source; study characteristics such as influenza season, participant recruitment strategy, number of participants, source of vaccination information, respiratory specimen swab time, influenza vaccine type, influenza diagnostic test, and the adjusted covariates in analysis; study outcome: influenza VE against the H1N1 and H3N2 influenza subtypes, influenza B, and all influenza; and study results: multivariable-adjusted influenza VE and associated 95% confidence interval (CI). Vaccine antigenic similarity with circulating virus strains was determined from articles, where reported. Where incidence of confirmed influenza was reported, we considered the season’s vaccine to be antigenically similar if the strain that caused a majority of the cases (at least 75%) was similar to that contained in the vaccine, antigenically partially similar if there was modest similarity with strains covered in the vaccine, and antigenically dissimilar if circulating strains were not similar to the strains covered in the vaccine.
Data Synthesis and Analysis
The main study characteristics were synthesized in tabular form. We pooled reported multivariable-adjusted influenza VE estimates and their associated 95% CIs using inverse-variance random-effects models implemented in STATA (version 13; StataCorp LP, Texas, USA). Heterogeneity between the pooled adjusted VE estimates was assessed and quantified statistically using the I2 statistic [9]. The chi-square statistic (χ 2) was used to assess the statistical significance (P value) of the difference between 2 groups of pooled adjusted results. We assessed publication bias (where appropriate) visually using funnel plots and, statistically, using the Egger’s regression test [10]. Subgroup analysis was conducted according to the source of participants’ influenza vaccination status, respiratory specimen swab time, and whether studies included age or age and medical conditions in their multivariable adjustment models. Subgroup analyses were conducted for all patients, and for each of the following age groups: <5 years, 5 to 17 years, 18 to 49 years, 50 to 64 years, and ≥65 years. We included only results for age groups that clearly fell within these predefined age groups without overlapping with another age group.
RESULTS
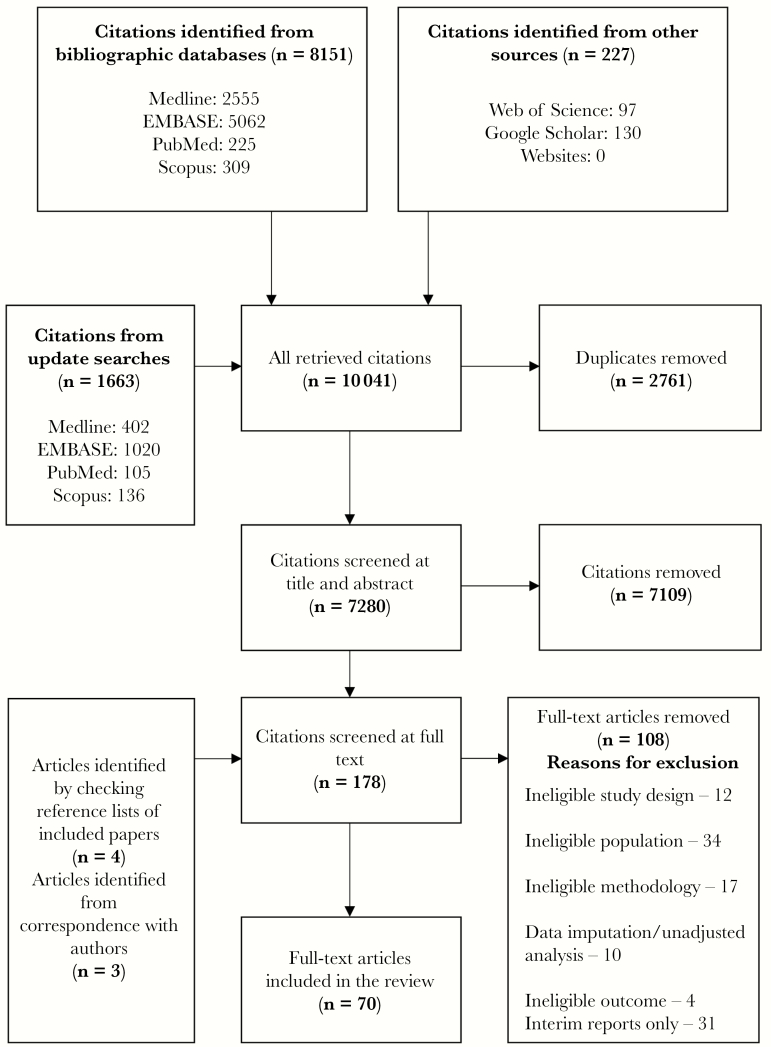
From a total of 10 041 identified citations, 70 full-text articles met our eligibility criteria (Figure 1) [11–80]. The main characteristics of these articles are summarized in Table 1. There were 11 articles each from the United States and Spain, 8 articles from Australia, 7 articles from the I-MOVE group (involving multiple European countries), and 6 articles each from the United Kingdom and Canada. There were 3 articles from China and 2 articles each from Germany, Israel, Netherlands, Romania, and South Africa. One article each was from Austria, Croatia, Italy, Japan, New Zealand, Portugal, Taiwan, and Turkey. The sample size from the studies in these articles ranged from 197 to 11 430 participants. All the studies were funded by nonindustry sources, and 1 study received funding from both industry and nonindustry sources.
Figure 1.
Modified Preferred Reporting Items for Systematic Reviews and Meta-Analysis flowchart.
Table 1.
Summary of Characteristics of Included Studies
| Study | Country | Influenza Season (Study Period) | Respiratory Specimen (Diagnostic Test) | No. of Participants | Circulating Influenza Type(s) | Dominant Influenza Type | VE Outcomes Assessed |
|---|---|---|---|---|---|---|---|
| Kissling et al. (2011) [11] | Europe | 2010/2011 | Nasal or throat swab (PCR & culture) | 3254 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 Influenza B |
| Jimenez-Jorge et al. (2012) [12] | Spain | 2010/2011 | Not reported (PCR) | 1369 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 Influenza B |
| Fielding et al. (2012) [13] | Australia | 2011 | Nose and/or throat swab (PCR) | 529 | H1N1, H3N2, influenza B | H1N1 first half, H3N2 mid to later season, influenza B throughout | All influenza H1N1 H3N2 Influenza B |
| Treanor et al. (2012) [14] | USA | 2010/2011 | Nasal and throat swabs (children aged <2 years provided nasal swabs only; PCR) | 4757 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 H3N2 Influenza A Influenza B |
| Skowronski et al. (2012) [15] | Canada | 2010/2011 | Nasal/nasopharyngeal specimen (PCR) | 1718 | H1N1, H3N2, influenza B | H3N2 | All influenza H1N1 H3N2 Influenza A Influenza B |
| Pitigoi et al. (2012) [16] | Romania | 2010/2011 | Not reported (PCR) | 255 | H1N1, H3N2, influenza B | H1N1 and influenza B | All influenza H1N1 Influenza B |
| Castilla et al. (2013) [17] | Spain | 2011/2012 | Nasopharyngeal and pharyngeal swabs (PCR) | 588 | H3N2, influenza B | H3N2 | All influenza |
| Kelly et al. (2013) [18] | Australia | 2010 & 2011 | Combined nose and throat swab specimens (nose swab specimens were only obtained from children aged <2 years; PCR) | 309 (2010) 398 (2011) | H1N1, H3N2, influenza B | 2010 H1N1, 2011 H3N2 | All influenza H1N1 H3N2 Influenza B |
| Sullivan et al. (2013) [19] | Australia | 2010, 2011, & 2012 | Not reported (PCR) | 420 (2010) 630 (2011) 678 (2012) | H1N1, H3N2, influenza B | 2010 H1N1, 2011 influenza B, 2012 H3N2 | All influenza |
| Martínez-Baz et al. (2013) [20] | Spain | 2010/2011 | Nasopharyngeal swabbing (PCR) | 530 | H1N1, H3N2, influenza B | H1N1 | All influenza |
| Kissling et al. (2013) [21] | Europe | 2011/2012 | Nasopharyngeal swab (PCR & culture) | 4362 | H1N1, H3N2, influenza B | H3N2 | H3N2 |
| Jimenez-Jorge et al. (2013) [22] | Spain | 2011/2012 | Not reported (PCR & culture) | 378 | H1N1, H3N2, influenza B | H3N2 | All influenza H3N2 |
| Pebody et al. (2013) [23] | UK | 2011/2012 | Respiratory samples (PCR) | 3560 | H1N1, H3N2, influenza B | H3N2 | H3N2 |
| Bateman et al. (2013) [24] | USA | 2010/2011 | Nasal and oropharyngeal swab (PCR) | 1549 | H1N1, H3N2, influenza B | H3N2 | H1N1 H3N2 Influenza A |
| Englund et al. (2013) [25] | Germany | 2010/2011 | Nasal or pharyngeal swabs or nasopharyngeal aspirates (PCR) | 1866 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 Influenza B |
| Lo et al. (2013) [26] | Taiwan | 2011/2012 | Throat or nasal swabs (PCR & culture) | 918 | H1N1, H3N2, influenza B | Influenza B | All influenza Influenza A Influenza B |
| Pebody et al. (2013) [27] | UK | 2010/2011 | Mouth swab (PCR) | 7121 | H1N1, influenza B | HINI | H1N1 Influenza B |
| Sullivan et al. (2014) [28] | Australia | 2012 | Nasal and throat samples (PCR) | 600 | H1N1, H3N2, influenza B | H3N2 | All influenza H3N2 |
| Levy et al. (2014) [29] | Australia | 2010 to 2012 | Two nose swabs and 1 throat swab (PCR) | 448 (2010) 351 (2011) 1361 (2012) | H1N1, H3N2, influenza B | H1N1 in 2010 and 2011, H3N2 in 2012 | All influenza H1N1 H3N2 Influenza B |
| Ohmit et al. (2014) [30] | USA | 2011/2012 | Throat swab and nasal swab (or nasal swab only in patients aged <2 years; PCR) | 4771 | H1N1, H3N2, influenza B | H3N2 | All influenza H1N1 H3N2 Influenza A Influenza B |
| Kissling et al. (2014) [31] | Europe | 2012/2013 | Nasopharyngeal swab (PCR & culture) | 6609 | H1N1, H3N2, influenza B | Influenza B | H1N1 H3N2 Influenza B |
| Suzuki et al. (2014) [32] | Japan | 2011/2012 | Nasopharyngeal swab (PCR) | 309 | H1N1, H3N2, influenza B | H3N2 | All influenza Influenza A |
| Skowronski et al. (2014) [33] | Canada | 2011/2012 | Nasal/nasopharyngeal swabs (PCR) | 1507 | H1N1, H3N2, influenza B | Influenza B | All influenza H1N1 H3N2 Influenza A Influenza B |
| Savulescu et al. (2014) [34] | Spain | 2010/2011 | Not reported (PCR & culture) | 5057 | H1N1, H3N2, influenza B | H1N1 and influenza B | H1N1 Influenza B |
| Nunes et al. (2014) [35] | Portugal | 2012/2013 | Nasopharyngeal swab or a combined nasopharyngeal and oropharyngeal swab (PCR & culture) | 335 | H1N1, H3N2, influenza B | H1N1 | All influenza |
| Skowronski et al. (2014) [36] | Canada | 2012/2013 | Nasal or nasopharyngeal swabs (PCR) | 1501 | H1N1, H3N2, influenza B | H3N2 | All influenza H1N1 H3N2 Influenza A Influenza B |
| Yang et al. (2014) [37] | China | 2012/2013 | Pharyngeal swabs (culture) | 1998 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 H3N2 |
| Andrews et al. (2014) [38] | UK | 2012/2013 | Not reported (PCR) | 3286 | H1N1, H3N2, influenza B | Influenza B | H1N1 H3N2 Influenza A Influenza B |
| McAnerney et al. (2015) [39] | South Africa | 2010 to 2013 | Nasopharyngeal swab (PCR) | 5344 | H1N1, H3N2, influenza B | 2010 influenza B, 2011 H1N1, 2012 H3N2, 2013 H1N1 | All influenza |
| Pitigoi et al. (2015) [40] | Romania | 2012/2013 | Not reported (PCR) | 197 | H1N1, H3N2, influenza B | Influenza B | All influenza H1N1 |
| Valenciano et al. (2015) [41] | Europe | 2013/2014 | Nasopharyngeal swab (PCR) | 3020 | H1N1, H3N2, influenza B | H3N2 | H1N1 |
| Helmeke et al. (2015) [42] | Germany | 2012/2013 | Throat or nasopharyngeal swab (PCR) | 834 | H1N1, H3N2, influenza B | Influenza B | All influenza H1N1 H3N2 Influenza B |
| Carville et al. (2015) [43] | Australia | 2013 | Nose or throat swab (PCR) | 262 | H1N1, H3N2, influenza B | Influenza A and B | All influenza H1N1 Influenza B |
| Chen et al. (2015) [44] | USA | 2010/2011 & 2011/2012 | One nasal and 1 throat swab (PCR) | 927 | H1N1, H3N2, influenza B | H1N1 | All influenza |
| McLean et al. (2015) [45] | USA | 2012/2013 | Nasal and throat specimens (for children aged <2 years, only nasal specimens were obtained; PCR) | 6452 | H1N1, H3N2, influenza B | H3N2 | All influenza H3N2 |
| Jimenez-Jorge et al. (2015) [46] | Spain | 2010/2011, 2011/2012, & 2012/2013 | Nasal or nasopharyngeal (PCR & culture) | 3180:SISS, 1369:cycEVA (2010/2011) 3484:SISS, 1446:cycEVA (2011/2012) 3357:SISS, 1432:cycEVA (2012/2013) | H1N1, H3N2, influenza B | 2010/2011 H1N1, 2011/2012 H3N2, 2012/2013 influenza B | H1N1 H3N2 Influenza B |
| Jimenez-Jorge et al. (2015) [47] | Spain | 2010/2011, 2011/2012, 2012/2013, & 2013/2014 | Nasal or nasopharyngeal (PCR & culture) | (cycEVA) | H1N1, H3N2, influenza B | 2010/2011 H1N1, 2011/2012 H3N2, 2012/2013 influenza B, 2013/2014 H3N2 and H1N1 | All influenza H1N1 H3N2 Influenza B |
| Kurecic- Filipovic et al. (2015) [48] | Croatia | 2010/2011 | Not reported (PCR) | 495 | H1N1, influenza B | H1N1 | All influenza H1N1 |
| Martinez- Baz et al. (2015) [49] | Spain | 2012/2013 | Nasopharyngeal and pharyngeal swabs (PCR) | 522 | H1N1, H3N2, influenza B | Influenza B | All influenza Influenza B |
| Skowronski et al. (2015) [50] | Canada | 2013/2014 | Nasal/nasopharyngeal specimens (PCR) | 1700 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 |
| Pebody et al. (2015) [51] | UK | 2014/2015 | Not reported (PCR) | 2931 | H1N1, H3N2, influenza B | H3N2 | All influenza H3N2 Influenza A Influenza B |
| Gherasim et al. (2016) [52] | Spain | 2014/2015 | Not reported (PCR) | 5044 | H3N2, influenza B | H3N2 | H3N2 Influenza B |
| Fielding et al. (2016) [53] | Australia | 2015 | Nose/throat swabs (PCR) | 2443 | H1N1, H3N2, influenza B | Influenza B | All influenza H1N1 H3N2 Influenza B |
| Pebody et al. (2016) [54] | UK | 2015/2016 | Respiratory samples (PCR) | 3841 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 Influenza B |
| Rizzo et al. (2016) [55] | Italy | 2014/2015 | Nasal or throat swab (PCR) | 1193 | H1N1, H3N2, influenza B | H1N1 and H3N2 | All influenza H1N1 H3N2 Influenza B |
| Castilla et al. (2016) [56] | Spain | 2014/2015 | Double swabs, nasopharyngeal and pharyngeal (PCR) | 660 | H1N1, H3N2, influenza B | H3N2 and influenza B | All influenza H3N2 Influenza B |
| Redlberger- Fritz et al. (2016) [57] | Austria | 2014/2015 | Nasopharyngeal swabs (PCR) | 815 | H1N1, H3N2, influenza B | H3N2 | All influenza H1N1 H3N2 Influenza B |
| Thompson et al. (2016) [58] | USA | 2011/2012 & 2012/2013 | Nasal and throat specimens (or nasal specimens only for children aged <2 years; PCR) | 1441 (2011/2012) 1327 (2012/2013) | H1N1, H3N2, influenza B | H3N2 in both seasons | All influenza H3N2 Influenza B |
| Pierse et al. (2016) [59] | New Zealand | 2014 | Nasopharyngeal or throat swab (PCR) | 1154 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 H3N2 Influenza A Influenza B |
| Van Doorn et al. (2017) [60] | The Netherlands | 2010/2011, 2011/2012, 2012/2013, & 2013/2014 | Nose and throat swabs (PCR & culture) | Unclear | H1N1, H3N2, influenza B | 2010/2011 H1N1; 2011/2012, 2012/2013, and 2013/2014 H3N2 | All influenza |
| Kelly et al. (2016) [61] | Australia | 2011, 2012, & 2013 | Not reported (PCR) | 642 (2012/2013) 684 (2012) 354 (2013) | H1N1, H3N2, influenza B | Not reported | All influenza |
| Wang et al. (2016) [62] | China | 2011/2012 | Nasopharyngeal specimen (PCR) | 668 | Not reported | Not reported | All influenza |
| Cowling et al. (2016) [63] | USA | 2010/2011, 2011/2012, & 2012/2013 | Nasopharyngeal, oropharyngeal or nasal swab (PCR) | 4208 (2010/2011) 2164 (2011/2012) 4278 (2012/2013) | H1N1, H3N2, influenza B | H1N1, H3N2, and influenza B in 2010/2011; H3N2 in 2011/2012; H3N2 and influenza B in 2012/2013 | All influenza H1N1 H3N2 Influenza B |
| Skowronski et al. (2016) [64] | Canada | 2014/2015 | Nasal/nasopharyngeal specimens | 1930 | H1N1, H3N2, influenza B | H3N2 | All influenza H3N2 Influenza B |
| Zimmerman et al. (2016) [65] | USA | 2014/2015 | Nasal and throat swabs (children aged <2 years provided nasal swabs only; PCR) | 9311 | H3N2, influenza B | H3N2 | All influenza H3N2 |
| Gaglani et al. (2016) [66] | USA | 2013/2014 | Combined nose and throat swab specimens (nose swab specimens were only obtained from children aged <2 years; PCR) | 5637 | H1N1, H3N2, influenza B | H1N1 | H1N1 |
| Valenciano et al. (2016) [67] | Europe | 2014/2015 | Nasopharyngeal specimens (PCR) | 6524 | H1N1, H3N2, influenza B | H3N2 | H1N1 H3N2 Influenza B |
| McAnerney et al. (2017) [68] | South Africa | 2015 | Throat and/or nasal swabs (PCR) | 899 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 H3N2 Influenza B |
| Darvishian et al. (2017) [69] | The Netherlands | 2010/2011, 2011/2012, & 2012/2013 | Throat swab and nose swab (PCR) | Not reported | H1N1, H3N2, influenza B | H3N in 2011/2012, influenza B in 2012/2013, H3N2 in 2013/2014, influenza B in 2010/2011 | All influenza H1N1 H3N2 Influenza B |
| Ma et al. (2017) [70] | China | 2014/2015 | Oral pharyngeal swab (PCR) | 9297 | H3N2, influenza B | H3N2 | All influenza H3N2 Influenza B |
| Pebody et al. (2017) [71] | UK | 2016/2017 | Not reported (PCR) | 2881 | H1N1, H3N2, influenza B | H3N2 | All influenza Influenza A H3N2 Influenza B |
| Skowronski et al. (2017) [72] | Canada | 2015/2016 | Nasal/nasopharyngeal swab (PCR) | 2008 | H1N1, H3N2, influenza B | H1N1 | All influenza Influenza A H1N1 H3N2 Influenza B |
| Jackson et al. (2017) [73] | USA | 2015/2016 | Nasal/oropharyngeal swab (PCR) | 6879 | H1N1, H3N2, influenza B | H1N1 | All influenza H1N1 H3N2 Influenza B |
| Gherasim et al. (2017) [74] | Spain | 2015/2016 | Not reported (PCR & culture) | 661 | H1N1, influenza B | Influenza B | H1N1 Influenza B |
| Stein et al. (2018) [75] | Israel | 2016/2017 | Nasal and throat swabs (PCR) | 1088 | H1N1, H3N2, influenza B | H3N2 | H3N2 |
| Yaron-Yakoby et al. (2018) [76] | Israel | 2014/2015 | Nose and throat swabs (PCR) | 1005 (2014/2015) 1658 (2015/2016) | H1N1, H3N2, influenza B | H3N2 in 2014/2015, H1N1 & influenza B in 2015/2016 | All influenza & H3N2 in 2014/2015 All influenza, H1N1, & influenza B in 2015/2016 |
| Poehling et al. (2018) [77] | USA | 2015/2016 | Nasal swab (PCR) | 1012 | H1N1, influenza B | H1N1 | All influenza H1N1 Influenza B |
| Valenciano et al. (2018) [78] | Europe | 2011/2012 to 2016/2017 | Nasopharyngeal swab (PCR) | Not clear | H1N1, influenza B (2015/16) H3N2 (2016/17) | H3N2 | H1N1 H3N2 Influenza B |
| Hekimoglu et al. (2018) [79] | Turkey | 2014/2015 | Nasal, nasopharyngeal, throat, nasal plus throat, nasopharyngeal plus throat, nasal plus nasopharyngeal (PCR) | 2561 | H1N1, H3N2, influenza B | Influenza B | All influenza H1N1 H3N2 Influenza B |
| Kissling et al. (2018) [80] | Europe | 2015/2016 | Nasopharyngeal or combined naso- and oropharyngeal specimens (PCR) | 11 430 | H1N1, H3N2, influenza B | H1N1 Influenza B | H1N1 Influenza B |
Abbreviations: PCR, polymerase chain reaction; pdm09, pandemic 2009.
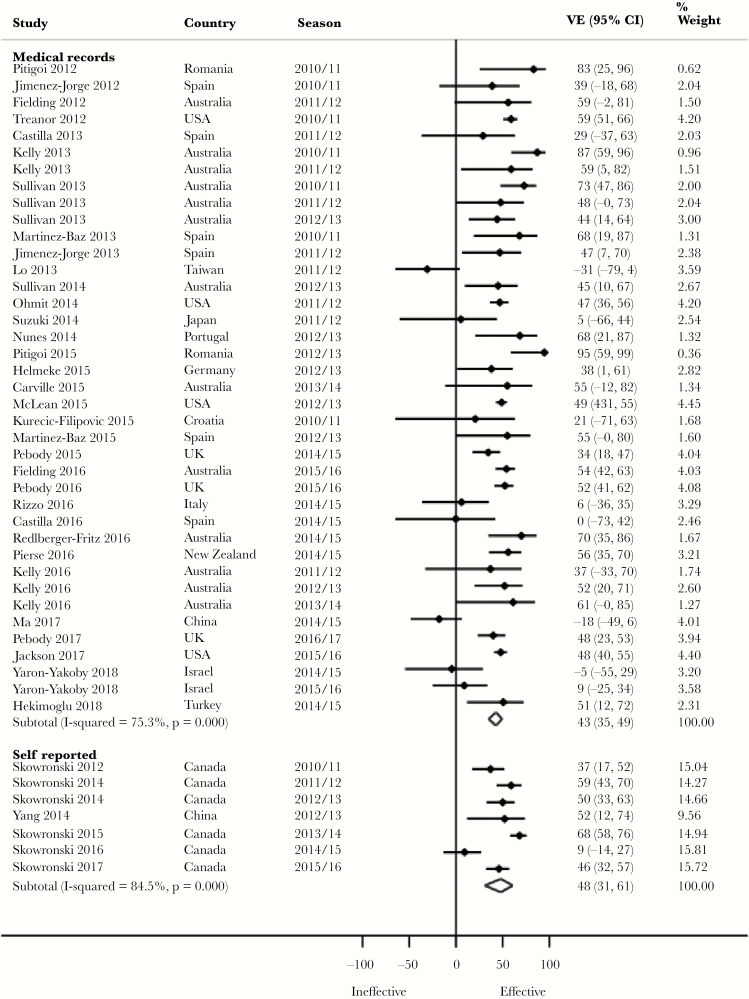
Pooled Adjusted VE by Method of Confirmation of Vaccination Status
Although not statistically significant, we observed a 10% higher pooled VE against H1N1 (P = .191), 7% against H3N2 (P = .626), and 5% against both influenza B (P = .529) and all influenza (P = .554) (Figure 2) for self-reported vaccination compared with medical record vaccination confirmation (Table 2). Almost all of the studies with self-reported vaccination were, however, from 1 research group in Canada. More of the studies with self-reported vaccination compared with those with medical record vaccination confirmation adjusted for both age and medical conditions. Zero percent (for H1N1), 20% (for H3N2, and influenza B), and 14% (for all influenza) of the studies with self-reported vaccination were from seasons in which vaccine virus strains were antigenically dissimilar to the circulating strains. In contrast, 8.3% (for H1N1), 30.8% (for H3N2), 23.1% (for influenza B), and 16% (for all influenza) of the studies with medical record vaccination confirmation were from seasons in which vaccine virus strains were antigenically dissimilar. Similar observations were made against H1N1 in 18- to 49-year-olds and against all influenza in ≥65-year-olds (Supplementary Table 2).
Figure 2.
Forest plot of vaccine effectiveness against all influenza by confirmation of vaccination status. Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
Table 2.
Pooled Adjusted VE for All Patients (Irrespective of Age)
| Influenza Types and Subtypes Analyzed Subgroups | No. of Studies | Pooled VE Across All Seasons (95% CI) | I 2 Statistic, % | Publication Bias, Egger’s Test P Value |
|---|---|---|---|---|
| H1N1 | ||||
| Vaccination status: medical records | 24 | 52 (45–58) | 32.7 | .031 |
| Vaccination status: self-reported | 6 | 62 (46–73) | 55.0 | N/A |
| Respiratory specimen swab: ≤7 d | 39 | 54 (49–58) | 39.5 | .022 |
| Respiratory specimen swab: ≤4 d | 7 | 59 (47–69) | 0.0 | N/A |
| Adjusted age | 26 | 57 (51–63) | 32.1 | .034 |
| Adjusted age & medical conditions | 20 | 53 (46–59) | 43.6 | .148 |
| H3N2 | ||||
| Vaccination status: medical records | 26 | 25 (15–34) | 55.0 | .988 |
| Vaccination status: self-reported | 5 | 32 (-0–53) | 76.9 | N/A |
| Respiratory specimen swab: ≤7 d | 35 | 28 (22–34) | 57.5 | .301 |
| Respiratory specimen swab: ≤4 d | 8 | 18 (-26–47) | 63.3 | N/A |
| Adjusted age | 23 | 34 (28–40) | 11.5 | .794 |
| Adjusted age & medical conditions | 20 | 21 (10–30) | 70.5 | .997 |
| Influenza B | ||||
| Vaccination status: medical records | 26 | 43 (31–52) | 70.3 | .701 |
| Vaccination status: self-reported | 5 | 48 (36–59) | 28.2 | N/A |
| Respiratory specimen swab: ≤7 d | 33 | 48 (43–53) | 28.2 | .974 |
| Respiratory specimen swab: ≤4 d | 10 | 38 (4–60) | 77.5 | .070 |
| Adjusted age | 22 | 50 (44–56) | 26.5 | .893 |
| Adjusted age & medical conditions | 21 | 40 (27–51) | 70.7 | .252 |
| All influenza | ||||
| Vaccination status: medical records | 39 | 43 (35–49) | 75.3 | .807 |
| Vaccination status: self-reported | 7 | 48 (31–61) | 84.5 | N/A |
| Respiratory specimen swab: ≤7 d | 56 | 46 (41–51) | 70.6 | .152 |
| Respiratory specimen swab: ≤4 d | 12 | 38 (15–55) | 77.3 | .009 |
| Adjusted age | 32 | 47 (42–52) | 56.5 | .477 |
| Adjusted age & medical conditions | 37 | 43 (34–51) | 79.8 | .184 |
Abbreviations: CI, confidence interval; N/A, not applicable; VE, vaccine effectiveness.
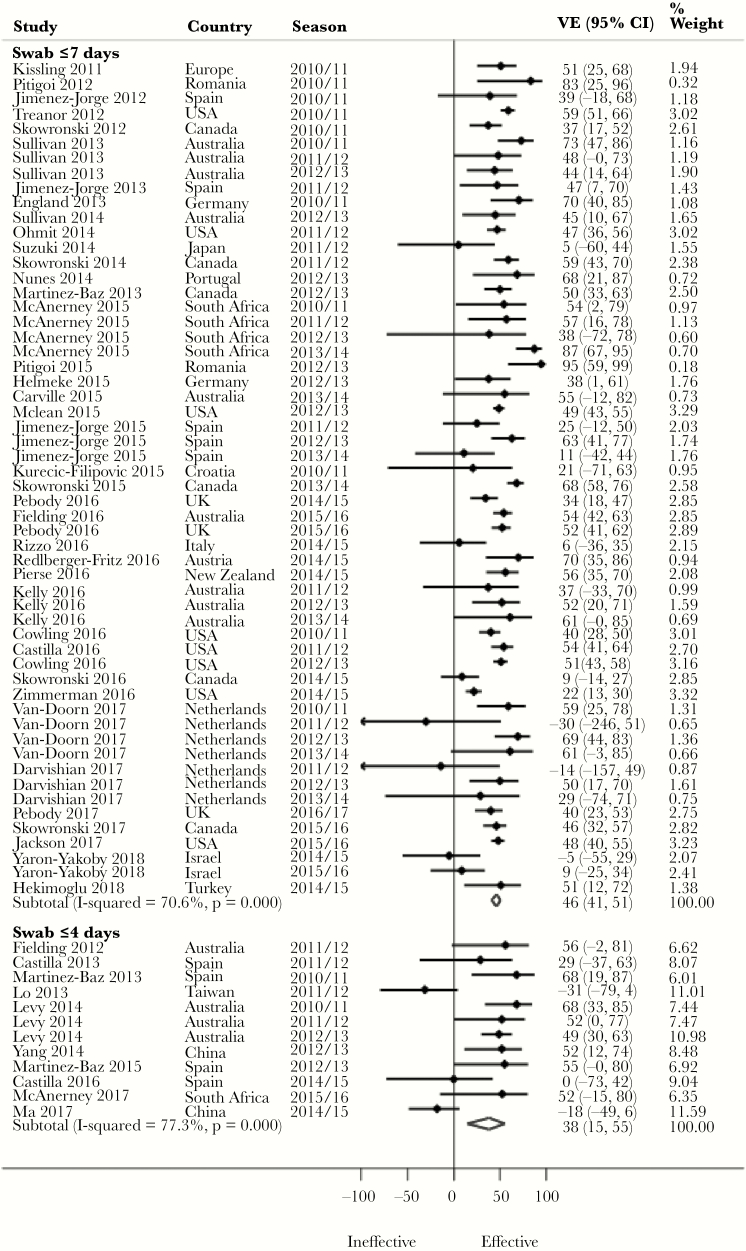
Pooled Adjusted VE by Timing of Respiratory Specimen Swab Collection
Despite a lack of statistical significance, we observed a 10% higher pooled adjusted VE against H3N2 (P = .596) and influenza B (P = .491), and 8% against all influenza (P = .447) (Figure 3), for swab collection within ≤7 days compared with ≤4 days of symptom onset (Table 2). In contrast, a 5% higher pooled adjusted VE was observed against H1N1 (P = .410) for swab collection within ≤4 days compared with swab collection within ≤7 days of symptom onset. There was no meaningful difference between studies with swab collection within ≤7 days and ≤4 days with regards to adjustment for both age and medical conditions in their analyses. Fifteen percent (for influenza B) and 18.5% (for all influenza) of the studies with swab collection ≤7 days were, however, from seasons in which vaccine virus strains were antigenically dissimilar to the circulating strains. In contrast, 22.2% (for influenza B) and 27.3% (for all influenza) of the studies with swab collection within ≤4 days were from seasons in which vaccine virus strains were antigenically dissimilar. Similarly, 5% (for H1N1) of the studies with swab collection within ≤7 days were from seasons in which vaccine strains were antigenically dissimilar, whereas 0% of the studies with swab collection within ≤4 days were from seasons in which vaccine strains were antigenically dissimilar. Evidence was conflicting across age groups (Supplementary Table 2).
Figure 3.
Forest plot of vaccine effectiveness against all influenza by timing of respiratory specimen swab collection. Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
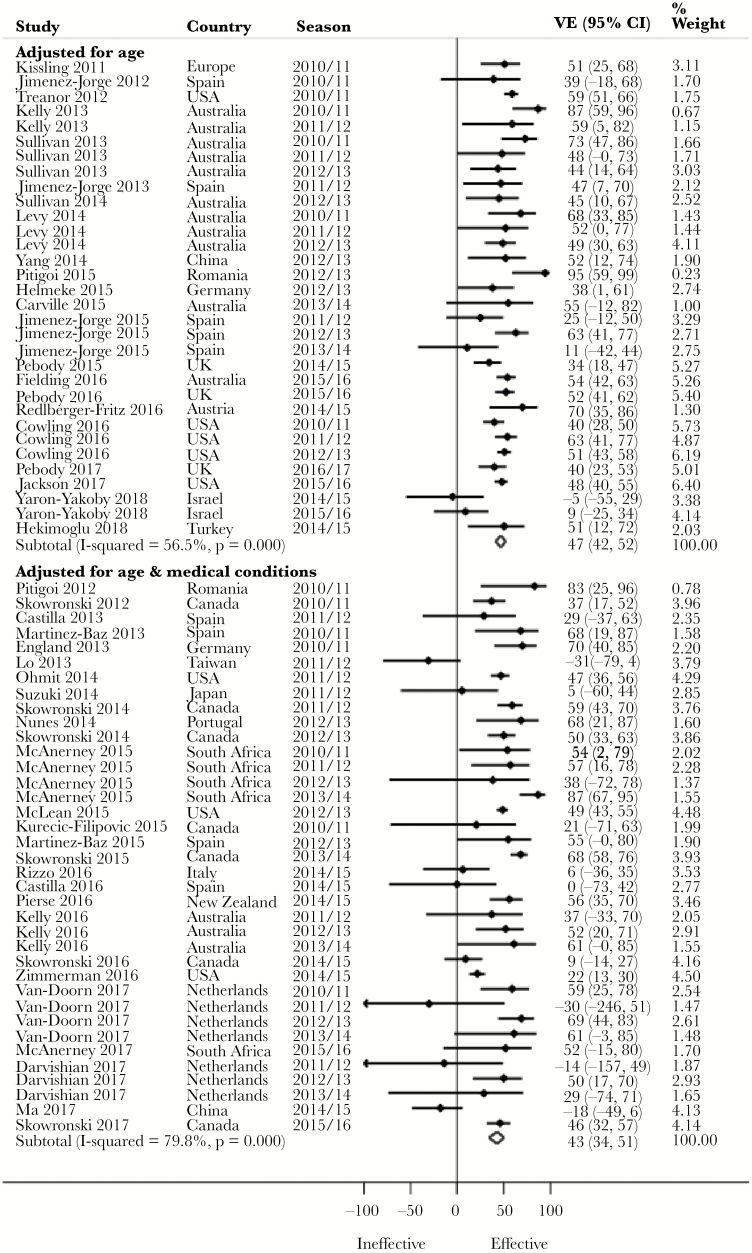
Pooled Adjusted VE by Covariate Adjustment
Notwithstanding a lack of statistical significance apart from for H3N2, we observed a 4% higher pooled adjusted VE against H1N1 (P = .375), 13% against H3N2 (P = .029), 10% against influenza B (P = .144), and 4% against all influenza (P = .427) (Figure 4) for studies that included age among the adjusted covariates compared with those that included both age and medical conditions (Table 2). Three point eight percent (for H1N1), 13% (for H3N2), 13.6% (for influenza B), and 6.7% (for all influenza) of the studies that included age but not medical conditions were, however, from seasons in which vaccine virus strains were antigenically dissimilar to the circulating strains. In contrast, 5.3% (for H1N1), 36.8% (for H3N2), 20% (for influenza B), and 30.6% (for all influenza) of the studies that included age and medical conditions among the adjusted covariates were from seasons in which vaccine virus strains were antigenically dissimilar. Evidence was conflicting across age groups (Supplementary Table 2).
Figure 4.
Forest plot of vaccine effectiveness against all influenza by covariate adjustment. Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
DISCUSSION
Despite a lack of statistical significance, we observed differences in pooled adjusted influenza VE between sources of influenza vaccination confirmation, respiratory specimen swab timing, and adjustments for 2 key confounders in study analysis. In our analysis of all study participants (irrespective of age), small differences were found between self-reported and medical record–confirmed influenza vaccinations, with higher pooled VE observed for self-reported vaccination, contrary to our expectations. However, almost all of the studies for self-reported vaccination were conducted in Canada and by the same group of researchers. We found substantial differences between respiratory specimen swab within ≤7 days and ≤4 days, with higher pooled VE observed for swab within ≤7 days. We also found substantial differences between studies that adjusted for age and those that adjusted for both age and medical conditions, with higher pooled VE observed for studies that adjusted for age. The above findings differed across age groups.
Studies have found that exposure misclassification can lead to significant bias in VE estimation [81, 82]. Self-reported vaccination is susceptible to recall and social desirability (individuals wanting to present a vaccine-compliant image) biases, with the potential for vaccination status misclassification. Smedt and colleagues showed in their simulation study that decreased exposure sensitivity and specificity underestimate true VE when misclassification of exposure (vaccination status) is nondifferential, but that when misclassification is differential, the bias could go in either direction, with the estimated VE deviating largely from the true VE. Compared with vaccination confirmation from medical records, self-reported vaccination usually has a higher sensitivity across various populations [83, 84] but a lower specificity in some population subgroups [85, 86]. Compared with whites, Hispanics were 2.7 times more likely to claim receipt of vaccination (self-report), and compared with younger individuals, self-reported influenza vaccination in the elderly had low specificity [84]. The observed higher pooled adjusted VE for self-reported compared with medical record–confirmed influenza vaccination status in this review, although not expected, may be due to differential misclassification of vaccination status, which Smedt and colleagues showed could either inflate or underestimate the true VE. This becomes more plausible considering that the studies with self-reported vaccination were almost all from Canada and from the same research group. Study center influence such as characteristics of the study participants, participant recruitment strategy, and influenza testing may also explain our findings.
Influenza incubation averages 2 days (range, 1–4 days) [87]. To maximize influenza virus detection from respiratory specimens, it is advocated that, ideally, swabs be collected within <4 days of influenza-like symptom onset. The longer swab collection is from symptom onset, the lower the likelihood of detecting influenza and the greater the potential for false-negative testing. Accurate reporting of symptom onset is therefore important, as a good TND study is predicated on patient symptom onset of ≤7 days. It will also help minimize outcome misclassification bias. False-negative testing among the vaccinated leads to VE overestimation, while false-negative testing among the unvaccinated leads to VE being underestimated. The observed higher pooled adjusted VE for swab collection of ≤7 days compared with ≤4 days in this review may therefore be due to a higher proportion of false negatives among the ≤7 days swab collection group, although this is not confirmable. Additionally, studies that included swab collection within ≤4 days possibly used more stringent swab collection criteria, resulting in reduced precision of VE estimation.
Seasonal influenza VE can vary from person to person. Various individual factors impact the VE [88], and 2 main factors (age and medical conditions) are known to play an important role in determining the likelihood that a vaccine will protect a person against influenza and to what extent. Age-dependent patterns in influenza vaccine protection have been reported from season to season, implicating the potential effect of age-related immune response in seasonal influenza VE [89]. For example, VE in the elderly population is reduced because of lower seroconversion rates that arise due to poorer immunological response to vaccination [90]. How well an individual responds to a vaccine may also be determined by underlying health conditions [91]. The observed higher pooled adjusted VE for studies that included age but not medical conditions compared with those that included both age and medical conditions among adjusted covariates in studies is in line with expectations, as adjusting for both age and medical conditions is likely to diminish VE compared with adjusting for age.
It is widely known that antigenic drift can markedly reduce seasonal influenza VE. For example, Flannery (2016) found that VE against H3N2 was almost 0 for an antigenically drifted genetic group of H3N2 viruses and 44% against a genetic group of H3N2 viruses that were antigenically similar to the seasonal vaccine strains [92]. This may explain the observed higher pooled adjusted VE in the subgroups with lower proportions of studies in which the seasonal influenza vaccine was antigenically dissimilar to the circulating virus strains. Variations in study design, sample size, vaccine type, and the demographic and temporal patterns underlying VE estimates from the included studies may also explain the variations observed in the pooled adjusted VE between compared groups. This, together with vaccine antigenic similarity with the circulating virus strains, may explain the high heterogeneity in many of the pooled adjusted VE. Where there were adequate numbers of studies for exploration of heterogeneity using metaregression, the available covariates tended to be highly collinear, thus limiting the usefulness of metaregression. Second, it was impossible to disentangle the effects of vaccine type and the underlying patient-level variations, as the analysis was conducted at the study level and these were not clearly reported in studies.
To our knowledge, our review is the first to evaluate differences in VE due to source of influenza vaccination status, respiratory specimen swab time, and confounder adjustments in statistical models for analysis. Irving et al. (2009) evaluated influenza vaccination status determined by self-report and by a real-time vaccination registry and found that the sensitivity and specificity of self-reported influenza vaccination compared with vaccination registry records were 95% and 90%, respectively, and that self-reported vaccination status was a sensitive and somewhat specific indicator of actual vaccine status, with misclassification being more common among young people [83]. However, the study did not compare influenza VE from these 2 sources of vaccination. No reviews seem to have compared seasonal influenza VE by respiratory specimen swab time and inclusion of main confounders in statistical models for analysis as we have done.
Our decision to include only influenza seasons after the 2009 pandemic influenza may have limited the number of potentially relevant TND studies for this review. However, it allowed us to focus on studies conducted from when public funding of influenza vaccination increased in most Western jurisdictions. It should be noted that some eligible studies conducted during this stated period may not have been published by the time we conducted our literature search, and therefore would not have been included in this review. Despite growing evidence to suggest that VE may be influenced by prior vaccinations [93, 94], the included studies did not report whether the study participants received the previous season’s influenza vaccination; hence, we could not assess the impact on VE estimates in our analyses. Furthermore, due to insufficient data, we could not examine VE against all outcomes for our subgroup analyses and for all age groups. We could also not separate individual study participants’ effects from study center effects (eg, effectiveness of vaccine policies and programs, participant recruitment strategy, and slight differences in symptom definitions), as the studies were conducted in different jurisdictions with potentially unique jurisdictional characteristics. Finally, we could not assess the reliability of reported estimates from the included studies because we could not ascertain if the studies met all of the assumptions that well-conducted TND studies are expected to meet to ensure that effect size estimates from the studies are not biased [5]. Although many of the studies adjusted for age or age and medical conditions, there were differences in the other covariates adjusted for in the studies. This may have contributed to the high heterogeneity observed in some of our pooled VE estimates.
Our review has many merits. We developed and registered a detailed protocol in PROSPERO before the execution of our search strategy, and we fully complied with the Cochrane Handbook for Systematic Reviews of Interventions guidelines throughout the review. We utilized the expertise of a methodologist trained in evidence synthesis literature searching to develop a comprehensive search strategy for the review, and this was subsequently reviewed by a professional knowledge synthesis librarian using the PRESS checklist. We searched appropriate bibliographic databases for literature and properly screened retrieved citations (against the eligibility) following the standards specified in the Cochrane Handbook for Systematic Reviews of Interventions. Where necessary, we requested additional data from the corresponding authors of the included studies to ensure completeness of the analyzed data. We included only studies in which influenza testing was conducted using the gold standard tests (PCR or viral culture). Furthermore, we examined variations in seasonal influenza VE across all clinically relevant age groups (<5 years, 5 to <18 years, 18 to 49 years, 50 to 64 years, and ≥65 years). We conducted the review to the highest expected standards and have reported in accordance with the PRISMA guidelines.
CONCLUSIONS
The available evidence from TND studies conducted after the 2009 pandemic influenza is not strong enough to conclude that influenza VE varies by source of vaccination status, respiratory specimen swab time, or adjustment for age/medical conditions. However, the evidence is indicative that these factors should be considered while designing or evaluating influenza VE from this study type. There is a need for researchers to ensure that age and medical conditions are both adjusted for in influenza VE estimations from TND studies, while uniformity in covariate adjustments across studies would help reduce heterogeneity and increase precision of pooled VE.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. No external funding was obtained for this study. S.M.M. is supported, in part, by funding from the Canada Research Chairs Program. G.N.O. is a current recipient of the Manitoba Training Program Fellowship Award and the Evelyn Shapiro Award, both for health services research.
Potential conflicts of interest. S.M.M. has received unrestricted research grants from GlaxoSmithKline, Merck, Sanofi Pasteur, Pfizer, and Roche-Assurex for unrelated studies. The other authors declare that they have no conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Boni MF. Vaccination and antigenic drift in influenza. Vaccine 2008; 26(Suppl 3):C8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gomez Lorenzo MM, Fenton MJ. Immunobiology of influenza vaccines. Chest 2013; 143:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine 2013; 31:2165–8. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol 2016; 184:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lewnard JA, Tedijanto C, Cowling BJ, Lipsitch M. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol 2018; 187:2686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Int J Surg 2010; 8:336–41. [DOI] [PubMed] [Google Scholar]
- 8. McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 2016; 75:40–6. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001; 323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kissling E, Valenciano M, Cohen JM, et al. I-MOVE Multi-Centre Case Control Study 2010–11: overall and stratified estimates of influenza vaccine effectiveness in Europe. PLoS One 2011; 6:e27622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jiménez-Jorge S, Savulescu C, Pozo F, et al. ; cycEVA Study Team; Spanish Influenza Sentinel Surveillance System Effectiveness of the 2010-11 seasonal trivalent influenza vaccine in Spain: cycEVA study. Vaccine 2012; 30:3595–602. [DOI] [PubMed] [Google Scholar]
- 13. Fielding JE, Grant KA, Tran T, et al. Moderate influenza vaccine effectiveness in Victoria, Australia, 2011. Eurosurveillance 2012; 17:20115. [PubMed] [Google Scholar]
- 14. Treanor JJ, Talbot HK, Ohmit SE, et al. ; US Flu-VE Network Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis 2012; 55:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skowronski DM, Janjua NZ, De Serres G, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clin Infect Dis 2012; 55:332–42. [DOI] [PubMed] [Google Scholar]
- 16. Pitigoi D, Ivanciuc AE, Necula G, et al. Influenza vaccine effectiveness to prevent medically attended laboratory confirmed influenza during season 2010–2011 in Romania: a case control study. Rev Romana Med Lab 2012; 20:127–34. [Google Scholar]
- 17. Castilla J, Martínez-Baz I, Martínez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Eurosurveillance 2013; 18:20388. [DOI] [PubMed] [Google Scholar]
- 18. Kelly HA, Sullivan SG, Grant KA, Fielding JE. Moderate influenza vaccine effectiveness with variable effectiveness by match between circulating and vaccine strains in Australian adults aged 20-64 years, 2007-2011. Influenza Other Respir Viruses 2013; 7:729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan SG, Kelly H. Late season interim estimates of influenza vaccine effectiveness reliably predict end of season estimates in Victoria, Australia, 2007 to 2012. Euro Surveill 2013; 18:20605. [DOI] [PubMed] [Google Scholar]
- 20. Martínez-Baz I, Martínez-Artola V, Reina G, et al. Effectiveness of the trivalent influenza vaccine in Navarre, Spain, 2010–2011: a population-based test-negative case-control study. BMC Public Health 2013; 13:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Eurosurveillance 2013; 18:20390. [DOI] [PubMed] [Google Scholar]
- 22. Jiménez-Jorge S, de Mateo S, Delgado-Sanz C, et al. ; Spanish Influenza Sentinel Surveillance System Effectiveness of influenza vaccine against laboratory-confirmed influenza, in the late 2011-2012 season in Spain, among population targeted for vaccination. BMC Infect Dis 2013; 13:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pebody RG, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Eurosurveillance 2013; 18:20389. [DOI] [PubMed] [Google Scholar]
- 24. Bateman AC, Kieke BA, Irving SA, et al. Effectiveness of monovalent 2009 pandemic influenza A virus subtype H1N1 and 2010–2011 trivalent inactivated influenza vaccines in Wisconsin during the 2010–2011 influenza season. J Infect Dis 2013; 207:1262–9. [DOI] [PubMed] [Google Scholar]
- 25. Englund H, Campe H, Hautmann W. Effectiveness of trivalent and monovalent influenza vaccines against laboratory-confirmed influenza infection in persons with medically attended influenza-like illness in Bavaria, Germany, 2010/2011 season. Epidemiol Infect 2013; 141:1807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lo YC, Chuang JH, Kuo HW, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011-12 season. PLoS One 2013; 8:e58222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pebody RG, Andrews N, Fleming DM, et al. Age-specific vaccine effectiveness of seasonal 2010/2011 and pandemic influenza A(H1N1) 2009 vaccines in preventing influenza in the United Kingdom. Epidemiol Infect 2013; 141:620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sullivan SG, Komadina N, Grant K, et al. Influenza vaccine effectiveness during the 2012 influenza season in Victoria, Australia: influences of waning immunity and vaccine match. J Med Virol 2014; 86:1017–25. [DOI] [PubMed] [Google Scholar]
- 29. Levy A, Sullivan SG, Tempone SS, et al. Influenza vaccine effectiveness estimates for Western Australia during a period of vaccine and virus strain stability, 2010 to 2012. Vaccine 2014; 32:6312–8. [DOI] [PubMed] [Google Scholar]
- 30. Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kissling E, Valenciano M, Buchholz U, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: The I-MOVE multicentre case-control study, influenza season 2012/13. Eurosurveillance 2014; 19:20701. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki M, Minh LN, Yoshimine H, et al. Vaccine effectiveness against medically attended laboratory-confirmed influenza in Japan, 2011–2012 season. PLoS One 2014; 9:e88813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skowronski DM, Janjua NZ, Sabaiduc S, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. [DOI] [PubMed] [Google Scholar]
- 34. Savulescu C, Jiménez-Jorge S, Delgado-Sanz C, et al. ; Spanish Influenza Surveillance System Higher vaccine effectiveness in seasons with predominant circulation of seasonal influenza A(H1N1) than in A(H3N2) seasons: test-negative case-control studies using surveillance data, Spain, 2003-2011. Vaccine 2014; 32:4404–11. [DOI] [PubMed] [Google Scholar]
- 35. Nunes B, Machado A, Guiomar R, et al. Estimates of 2012/13 influenza vaccine effectiveness using the case test-negative control design with different influenza negative control groups. Vaccine 2014; 32:4443–9. [DOI] [PubMed] [Google Scholar]
- 36. Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One 2014; 9:e92153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang P, Thompson MG, Ma C, et al. Influenza vaccine effectiveness against medically-attended influenza illness during the 2012-2013 season in Beijing, China. Vaccine 2014; 32:5285–9. [DOI] [PubMed] [Google Scholar]
- 38. Andrews N, McMenamin J, Durnall H, et al. Effectiveness of trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2012/13 end of season results. Euro Surveill 2014; 19:5–13. [PubMed] [Google Scholar]
- 39. McAnerney JM, Walaza S, Cohen AL, et al. Effectiveness and knowledge, attitudes and practices of seasonal influenza vaccine in primary healthcare settings in South Africa, 2010-2013. Influenza Other Respir Viruses 2015; 9:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pitigoi D, Necula G, Alexandrescu V, et al. Circulating influenza viruses and the effectiveness of seasonal influenza vaccine in Romania, season 2012–2013. Rev Rom Med Lab 2015; 23:9–20. [Google Scholar]
- 41. Valenciano M, Kissling E, Reuss A, et al. ; I-MOVE Multicentre Case Control Study Team The European I-MOVE Multicentre 2013-2014 Case-Control Study. Homogeneous moderate influenza vaccine effectiveness against A(H1N1)pdm09 and heterogenous results by country against A(H3N2). Vaccine 2015; 33:2813–22. [DOI] [PubMed] [Google Scholar]
- 42. Helmeke C, Gräfe L, Irmscher HM, et al. Effectiveness of the 2012/13 trivalent live and inactivated influenza vaccines in children and adolescents in Saxony-Anhalt, Germany: a test-negative case-control study. PLoS One 2015; 10:e0122910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carville KS, Grant KA, Sullivan SG, et al. Understanding influenza vaccine protection in the community: an assessment of the 2013 influenza season in Victoria, Australia. Vaccine 2015; 33:341–5. [DOI] [PubMed] [Google Scholar]
- 44. Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥50 years. J Infect Dis 2015; 211:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLean HQ, Thompson MG, Sundaram ME, et al. Influenza vaccine effectiveness in the United States during 2012–2013: variable protection by age and virus type. J Infect Dis 2015; 211:1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jimenez-Jorge S, de Mateo S, Delgado-Sanz C, et al. Estimating influenza vaccine effectiveness in Spain using sentinel surveillance data. Euro Surveill Bulletin 2015; 20:21187. [DOI] [PubMed] [Google Scholar]
- 47. Jimenez-Jorge S, Pozo F, Larrauri A. Interim influenza vaccine effectiveness: a good proxy for final estimates in Spain in the seasons 2010–2014. Vaccine 2015; 33:3276–80. [DOI] [PubMed] [Google Scholar]
- 48. Kurečić Filipović S, Gjenero-Margan I, Kissling E, et al. Influenza vaccine effectiveness estimates in Croatia in 2010-2011: a season with predominant circulation of A(H1N1)pdm09 influenza virus. Epidemiol Infect 2015; 143:2596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martínez-Baz I, Navascués A, Pozo F, et al. ; Primary Health Care Sentinel Network and Network for Influenza Surveillance in Hospitals of Navarra Influenza vaccine effectiveness in preventing inpatient and outpatient cases in a season dominated by vaccine-matched influenza B virus. Hum Vaccin Immunother 2015; 11:1626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skowronski DM, Chambers C, Sabaiduc S, et al. Integrated sentinel surveillance linking genetic, antigenic, and epidemiologic monitoring of influenza vaccine-virus relatedness and effectiveness during the 2013–2014 influenza season. J Infect Dis 2015; 212:726–39. [DOI] [PubMed] [Google Scholar]
- 51. Pebody R, Warburton F, Andrews N, et al. Effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 end of season results. Eurosurveillance 2015; 20. [PubMed] [Google Scholar]
- 52. Gherasim A, Pozo F, de Mateo S, et al. ; cycEVA team and the VEVA Working Group Waning protection of influenza vaccine against mild laboratory confirmed influenza A(H3N2) and B in Spain, season 2014-15. Vaccine 2016; 34:2371–7. [DOI] [PubMed] [Google Scholar]
- 53. Fielding JE, Levy A, Chilver MB, et al. Effectiveness of seasonal influenza vaccine in Australia, 2015: an epidemiological, antigenic and phylogenetic assessment. Vaccine 2016; 34:4905–12. [DOI] [PubMed] [Google Scholar]
- 54. Pebody R, Warburton F, Ellis J, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 end-of-season results. Eurosurveillance 2016; 21:30348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rizzo C, Bella A, Alfonsi V, et al. Influenza vaccine effectiveness in Italy: age, subtype-specific and vaccine type estimates 2014/15 season. Vaccine 2016; 34:3102–8. [DOI] [PubMed] [Google Scholar]
- 56. Castilla J, Navascués A, Fernández-Alonso M, et al. ; Primary Health Care Sentinel Network; Network for Influenza Surveillance in Hospitals of Navarra Effectiveness of subunit influenza vaccination in the 2014-2015 season and residual effect of split vaccination in previous seasons. Vaccine 2016; 34:1350–7. [DOI] [PubMed] [Google Scholar]
- 57. Redlberger-Fritz M, Kundi M, Popow-Kraupp T. Detailed report on 2014/15 influenza virus characteristics, and estimates on influenza virus vaccine effectiveness from Austria’s Sentinel Physician Surveillance Network. PLoS One 2016; 11:e0149916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thompson MG, Clippard J, Petrie JG, et al. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013: the importance of two priming doses. Pediatr Infect Dis J 2016; 35:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pierse N, Kelly H, Thompson MG, et al. ; SHIVERS investigation team Influenza vaccine effectiveness for hospital and community patients using control groups with and without non-influenza respiratory viruses detected, Auckland, New Zealand 2014. Vaccine 2016; 34:503–9. [DOI] [PubMed] [Google Scholar]
- 60. van Doorn E, Darvishian M, Dijkstra F, et al. Influenza vaccine effectiveness estimates in the Dutch population from 2003 to 2014: the test-negative design case-control study with different control groups. Vaccine 2017; 35:2831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kelly HA, Lane C, Cheng AC. Influenza vaccine effectiveness in general practice and in hospital patients in Victoria, 2011e2013. Med J Australia 2016; 204:76.e71–5. [DOI] [PubMed] [Google Scholar]
- 62. Wang Y, Zhang T, Chen L, et al. Seasonal influenza vaccine effectiveness against medically attended influenza illness among children aged 6-59 months, October 2011-September 2012: a matched test-negative case-control study in Suzhou, China. Vaccine 2016; 34:2460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cowling BJ, Feng S, Finelli L, et al. Assessment of influenza vaccine effectiveness in a sentinel surveillance network 2010-13, United States. Vaccine 2016; 34:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Skowronski DM, Chambers C, Sabaiduc S, et al. A perfect storm: impact of genomic variation and serial vaccination on low influenza vaccine effectiveness during the 2014–2015 season. Clin Infect Dis 2016; 63:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zimmerman RK, Nowalk MP, Chung J, et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis 2016; 63:1564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaglani M, Pruszynski J, Murthy K, et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013-2014 in the United States. J Infect Dis 2016; 213:1546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Valenciano M, Kissling E, Reuss A, et al. Vaccine effectiveness in preventing laboratory-confirmed influenza in primary care patients in a season of co-circulation of influenza A(H1N1)pdm09, B and drifted A(H3N2), I-MOVE Multicentre Case–Control Study, Europe 2014/15. Euro Surveill 2016; 21:pii=30139. [DOI] [PubMed] [Google Scholar]
- 68. McAnerney JM, Walaza S, Tempia S, et al. Estimating vaccine effectiveness in preventing laboratory-confirmed influenza in outpatient settings in South Africa, 2015. Influenza Other Respir Viruses 2017; 11:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Darvishian M, Dijkstra F, Van Doorn E, et al. Influenza vaccine effectiveness in the Netherlands from 2003/2004 through 2013/2014: the importance of circulating influenza virus types and subtypes. PLoS One 2017; 12:e0169528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma C, Pan Y, Zhang L, et al. Influenza vaccine effectiveness against medically attended influenza illness in Beijing, China, 2014/15 season. Hum Vaccin Immunother 2017; 13:2379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pebody R, Warburton F, Ellis J, et al. End-of-season influenza vaccine effectiveness in adults and children, United Kingdom, 2016/17. Eurosurveillance 2017; 22:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Skowronski DM, Chambers C, Sabaiduc S, et al. Beyond antigenic match: possible agent-host and immuno-epidemiological influences on influenza vaccine effectiveness during the 2015–2016 season in Canada. J Infect Dis 2017; 216:1487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. New Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gherasim A, Martinez-Baz I, Castilla J, et al. Effect of previous and current vaccination against influenza A(H1N1)pdm09, A(H3N2), and B during the post-pandemic period 2010–2016 in Spain. PLoS One 2017; 12:e0179160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stein Y, Mandelboim M, Sefty H, et al. Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66:1383–91. [DOI] [PubMed] [Google Scholar]
- 76. Yaron-Yakoby H, Sefty H, Pando R, et al. Effectiveness of influenza vaccine in preventing medically-attended influenza virus infection in primary care, Israel, influenza seasons 2014/15 and 2015/16. Eurosurveillance 2018; 23:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Poehling KA, Caspard H, Peters TR, et al. 2015–2016 vaccine effectiveness of live attenuated and inactivated influenza vaccines in children in the United States. Clin Infect Dis 2018; 66:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Valenciano M, Kissling E, Larrauri A, et al. Exploring the effect of previous inactivated influenza vaccination on seasonal influenza vaccine effectiveness against medically attended influenza: results of the European I-MOVE multicentre test-negative case-control study, 2011/2012–2016/2017. Influenza Other Respir Viruses 2018; 12:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hekimoglu CH, Emek M, Avci E, et al. Seasonal influenza vaccine effectiveness in preventing laboratory confirmed influenza in 2014–2015 season in Turkey: a test-negative case control study. Balk Med J 2018; 35:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kissling E, Valenciano M, Pozo F, et al. ; I-MOVE/I-MOVE+ study team 2015/16 I-MOVE/I-MOVE+ multicentre case-control study in Europe: moderate vaccine effectiveness estimates against influenza A(H1N1)pdm09 and low estimates against lineage-mismatched influenza B among children. Influenza Other Respir Viruses 2018; 12:423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. De Smedt T, EM, DM, et al. Bias due to differential and non-differential disease- and exposure misclassification in studies of vaccine effectiveness. PLoS One 2018; 13:e0199180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jackson ML, Phillips CH, Benoit J, et al. The impact of selection bias on vaccine effectiveness estimates from test-negative studies. Vaccine 2018; 36:751–7. [DOI] [PubMed] [Google Scholar]
- 83. Irving SA, Donahue JG, Shay DK, et al. Evaluation of self-reported and registry-based influenza vaccination status in a Wisconsin cohort. Vaccine 2009; 27:6546–9. [DOI] [PubMed] [Google Scholar]
- 84. Rolnick SJ, Parker ED, Nordin JD, et al. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine 2013; 31:3928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jackson ML. Use of self-reported vaccination status can bias vaccine effectiveness estimates from test-negative studies. Vaccines 2019; 1:100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zimmerman RK, Raymund M, Janosky JE, et al. Sensitivity and specificity of patient self-report of influenza and pneumococcal polysaccharide vaccinations among elderly outpatients in diverse patient care strata. Vaccine 2003; 21:1486–91. [DOI] [PubMed] [Google Scholar]
- 87. Carrat F, Vergu E, Ferguson NM, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol 2008; 167:775–85. [DOI] [PubMed] [Google Scholar]
- 88. Dhakal S, Klein SL. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol 2019; 93:e00797–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lewnard JA, Cobey S. Immune history and influenza vaccine effectiveness. Vaccines 2018; 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wilhelm M. Influenza in older patients: a call to action and recent updates for vaccinations. Am J Manag Care 2018; 24:15–24. [PubMed] [Google Scholar]
- 91. Zhao L, Stirling R, Young K. Should individuals use influenza vaccine effectiveness studies to inform their decision to get vaccinated? Can Commun Dis Rep 2019; 45:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Flannery B, Zimmerman RK, Gubareva LV, et al. Enhanced genetic characterization of influenza A(H3N2) viruses and vaccine effectiveness by genetic group, 2014-2015. J Infect Dis 2016; 214:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sullivan SG, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis 2013; 57:474–6. [DOI] [PubMed] [Google Scholar]
- 94. McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis 2014; 59:1375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.