Abstract
Purpose of review:
Many studies have suggested low nephron endowment at birth contributes to the risk of developing hypertension and chronic kidney disease (CKD) later in life. Loss of nephrons with age and disease is largely a subclinical process. New technologies are needed to count nephrons as glomerular filtration rate (GFR) is a poor surrogate for nephron number.
Recent findings:
Cortical volume, glomerular density, and % globally sclerotic glomeruli are imperfect surrogates for nephron number. The disector-fractionator method is the most accurate method to count nephrons but is limited to autopsy settings. Glomerular density plus kidney imaging and ultrafiltration coefficient based methods require a kidney biopsy, and have been applied in living humans (kidney donors). Low nephron number predicts a higher post-donation urine albumin. Contrast enhanced magnetic resonance imaging (MRI) has detected glomeruli without a biopsy, but so far, not in living humans.
Summary:
Currently, there is no accurate and safe method for determining nephron number in living humans. A clinically useful method may allow GFR to be replaced by its more relevant determinants: nephron number and single nephron GFR. This could revolutionize nephrology by separating the measurement of chronic disease (nephron loss) from more reversible hemodynamic effects (nephron hyper/hypo-filtration).
Keywords: Nephron number, glomeruli, kidney biopsy, glomerular density, kidney volume, magnetic resonance imaging
Introduction
Older studies have suggested low nephron endowment (nephron number at birth) contributes to the development of hypertension and chronic kidney disease later in life (1). The proposed mechanism has been that low nephron endowment causes compensatory glomerular hyperfiltration and glomerulomegaly, which over time, leads to glomerulosclerosis with further loss of nephrons, and as a culmination of a vicious cycle, eventually leads to kidney failure (2). Indeed, there is evidence to support some aspects of this purported mechanism (3). Several studies have linked low birth weight (a surrogate for low nephron endowment) to an increased risk of developing chronic kidney disease (CKD) or hypertension in adulthood (4, 5). An animal model with 30% fewer nephrons was more likely to develop maladaptive changes in the kidney (higher blood pressure and albuminuria) and cardiac fibrosis on a high salt diet (6).
Despites its perceived importance, determining nephron endowment or nephron number in clinical practice has been challenging.
Limitations of GFR as a surrogate for nephron number
Glomerular filtration rate (GFR) is the product of nephron number and mean single nephron GFR (snGFR). The GFR is not an ideal surrogate for nephron number because snGFR can vary across states of health and disease (7). Only in setting where snGFR remains stable is GFR is a good surrogate for nephron number. In particular, the age-related decline in nephron number is detected by the decline in GFR with age, (8) due to a stable single nephron GFR (snGFR) with age (7). However, when nephrosclerosis (i.e., chronic changes on histopathology) with nephron loss exceeds those expected for age, there is a compensatory increase in the snGFR of the remaining nephrons (7). In the short term, increased snGFR preserves renal function (total GFR); however, these nephrons are under stress due to hyperfiltration. Notably, glomerular enlargement and hyperfiltration often results in proteinuria (3, 7, 9). Proteinuria is a useful complementary biomarker to total GFR, in part, because it reflects the increase in snGFR even when total GFR is decreased from nephron loss. Increased snGFR with resultant glomerular enlargement can itself lead to glomerulosclerosis and progressive CKD (9). As CKD progresses, interstitial fibrosis appears to constrain further compensatory glomerular enlargement and may contribute to glomerular ischemia (9).
Potential clinical uses of nephron number
Many medications used for treating chronic kidney disease have an early effect that lowers GFR (by decreasing snGFR), and a long-term effect that slows GFR decline (by preventing nephron loss). In particular, angiotensin blockers (ACE inhibitors or angiotensin receptor blockers) may be beneficial in slowing progression of eGFR decline by lowering single nephron GFR (reversing hyperfiltration) (10). However, angiotensin blockers are also associated with acute kidney injury (AKI) as they can “overshoot” and cause single nephron hypofiltration, especially in volume depleted states (11). Dosing of these medications could potentially be optimized to prevent CKD progression, but without risking AKI, by targeting a specific snGFR level as determined by GFR divided by nephron number. Another potential use of nephron number would be to detect CKD or CKD progression that is currently not detected. For example, a patient with 500,000 nephrons per kidney may lose 20% of his or her nephrons, but if the snGFR also increases by 25% there will be no discernable change in total GFR. The increase in snGFR may be indirectly detected by a concurrent increase in proteinuria or it may go undetected.
Structural surrogates for nephron number
Kidney biopsy and kidney imaging structural findings have been used as surrogates for nephron number. However, these have important limitations. The percentage of glomeruli that are globally-sclerosed does identify nephron loss. However, globally sclerotic glomeruli continue to atrophy to a point where they are no longer detected such that the degree of nephron loss with glomerulosclerosis is underappreciated (8). Glomerular density on biopsy may also be useful as a surrogate for nephron number, as more densely packed glomeruli on biopsy are suggestive of more nephrons in the kidney. But it is better viewed as an inverse measure of nephron size. Indeed, factors such as obesity and albuminuria associate with larger nephrons (lower glomerular density) (12) but not with nephron number (8). Nonetheless, more glomerulosclerosis and lower glomerular density on biopsy predict kidney failure in a variety of patient settings, and this is likely due, to some extent, their reflection of lower nephron number (13, 14).
Kidney volume has long been considered a surrogate for nephron number, but often measures of kidney volume are not limited to parenchyma but include renal pelvis, sinus fat, and cysts. Cortical volume is also a better surrogate for nephron number than kidney volume, as medullary volume only reflects tubules of deep nephrons and does not decline with age in a pattern consistent with nephron loss (15). However, cortical volume under detects nephron loss because of a compensatory increases in the volume occupied by remaining tubules; tubules account for up to 96% of the volume of the cortex (8). Potentially toxic contrast agents are also needed to distinguish cortex from medulla with computed tomography or magnetic resonance imaging.
Nephron number in human kidneys at autopsy
The disector-fractionator method based on stereology provides an unbiased measurement of nephron number in an autopsy kidney (16, 17). This method requires extensive sectioning of the whole kidney in a series of steps, ending with a known sampled fraction of representative cortical tissue (“fractionator”) (Figure 1A) (18). Adjacent sampled sections are then analyzed in pairs, and glomeruli counted using the “disector” principle (counted when present in one section but not in the adjacent section) (19). By this method, every glomerulus regardless of its size has the same chance of being counted, thus, making this method completely unbiased despite the known variability in number of glomeruli and their size. The glomeruli counted in the samples and the known fraction of the samples for the total kidney allows for calculation of the total number of glomeruli in the kidney. By this method, the number of nephrons per kidney across three continents and five racial groups averages about 900,000 with a 13-fold range from 210,000 to 2.7 million nephrons (20–26). Significant variability in nephron number has also been shown in kidneys from children less than 3 years of age (27). While the disector-fractionator is an unbiased method, it is still unclear the extent measurement error (imprecision) may inflate the range of nephron number in the population. Another important caveat is that autopsy studies have counted all glomeruli, without distinguishing normal (functioning) glomeruli from globally-sclerosed glomeruli.
Figure 1. Different methods for estimation of nephron number.
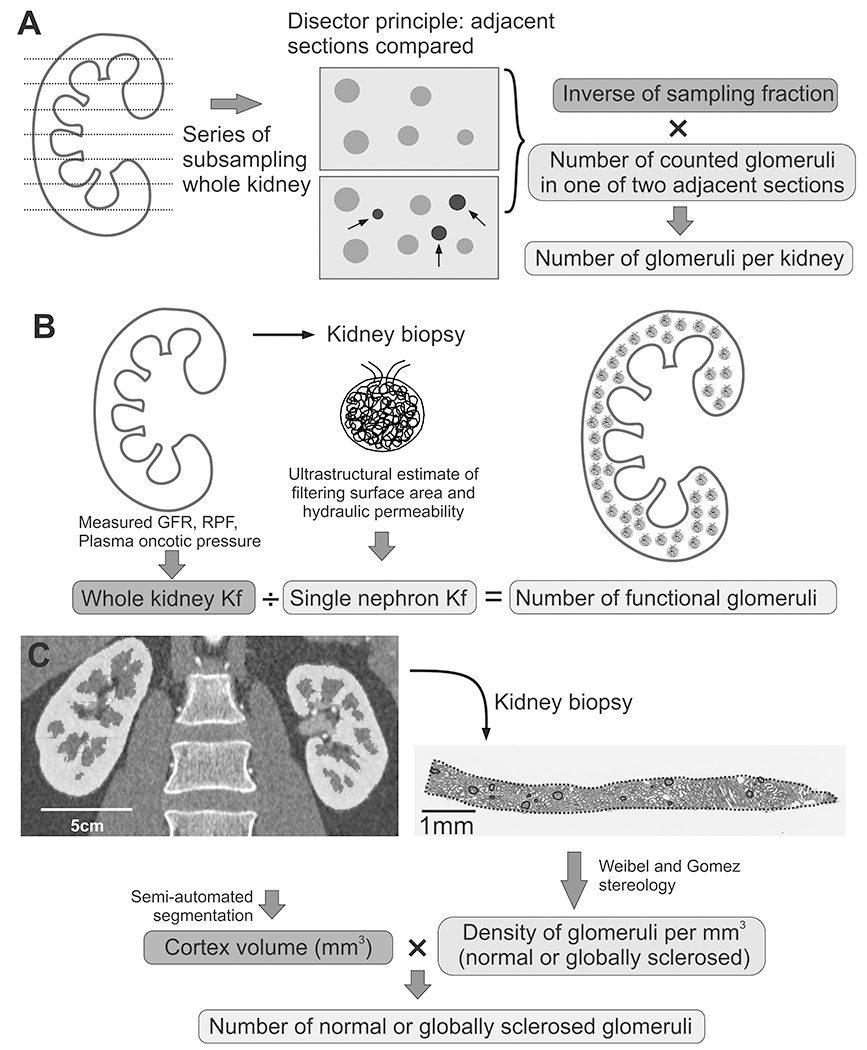
A) Disector/Fractionator method requires extensive sectioning of the whole kidney. In a series of subsampling steps, a known fraction of tissue is processed and sectioned. Glomeruli are then counted using section pairs (disector principle). In a schematic example of 2 pairs, only the three glomeruli in blue color indicated with arrows would be counted. Finally, total number of counted glomeruli is multiplied with an inverse of known sampling fractions to obtain the total number of glomeruli in the whole kidney. B) Measured GFR, renal plasma flow and plasma oncotic pressure are used to estimate the whole kidney Kf. Following kidney biopsy, an ultrastructural analysis of glomeruli provides estimates of filtering surface area and hydraulic permeability, used to calculate single nephron Kf. The number of functional glomeruli is then calculated by dividing whole kidney Kf by single nephron Kf. C) Semi-automated segmentation of predonation CT scans provides measure of cortical volume. A time zero biopsy is performed following kidney implantation, and Weibel and Gomez stereology models are used to estimate volumetric glomerular density. A product of cortical volume and volumetric density provides the total number of glomeruli.
There are also significant challenges with using the disector/fractionator method. The requirement of precise and careful sectioning of the whole kidney is labor intensive. Counting glomeruli on the sampled sections is time consuming and tedious, taking up to 8 hours for a human kidney. These steps require expertise and consistency in tissue processing. Few laboratories are also equipped to handle glycolmethacrylate, a chemical used for tissue embedding in most disector/fractionator studies (28). However, the biggest limitation is that this method requires destruction of the kidney. Nonetheless, having a “gold-standard” unbiased method for testing new methods against is helpful.
Pre-clinical studies to determine nephron number by imaging alone
Imaging glomeruli in ex vivo rodent kidneys
Cationic ferritin can be used to visualize glomeruli by MRI. Ferritins are a superfamily of proteins, highly conserved among animals, with a primary role to store iron. A single ferritin molecule consists of 24 subunits that create a shell with a central cavity, where up to 4,300 iron ions can be stored (29). The outer surface of this shell can easily be cationized,(30) thus, creating cationic ferritin. Following intravenous injection, cationic ferritin binds to anionic sites of proteoglycans on the glomerular basement membrane (31, 32). Then, during the MRI scan, the iron present in the cationic ferritin causes local distortion of the magnetic field, allowing visualization of all perfused glomeruli.
The cationic ferritin intravenously injected into a rat accumulated only in the glomerular basement membrane of healthy individual glomeruli (31). However, in a model of focal and segmental glomerulosclerosis, due to basement membrane breakdown and leak, there is diffuse accumulation of cationic ferritin in the tubules, and reduced accumulation in glomeruli (31). Thus, in diseases with basement membrane disruption, leakage of cationic ferritin into tubules will likely interfere with the ability to detect and count glomeruli. In the ex vivo healthy rat kidney, post-processing of the three-dimensional MRI images has been used to count the number of glomeruli (33, 34). The number of glomeruli obtained via MRI method was lower than measurements based on the disector-fractionator method (33).
An alternative imaging modality is the use of micro-computed tomography with barium sulfate contrast. By this method, one study found adriamycin-treated mice had significantly fewer glomeruli compared to control animals (35).
Cationic ferritin-enhanced MRI in deceased human kidneys
In humans, cationic ferritin-enhanced MRI has been used to count and measure the size of glomeruli in deceased donor kidneys that were declined for use in transplantation (32). Notably, a control kidney without cationic ferritin perfusion still detected nearly 60,000 glomeruli-like particles, assumed to originate from traces within residual blood. This number translates into about 6% false detection rate, similar to that reported in a cationic ferritin-enhanced MRI study in rats (33). For 2 of 3 kidneys tested, the number of glomeruli obtained via MRI was in excellent agreement with the stereology measurements based on disector-fractionator method (32). In the third kidney, the MRI-measurement of number of glomeruli was about 25% higher than by stereology, although about a fifth of the cortex did not have cationic ferritin labeled glomeruli. Review of clinical information revealed that this subject had high and uncontrolled hypertension with accompanying vascular and glomerular pathology. Histological analysis of the regions with no cationic ferritin labeled glomeruli revealed severe glomerulosclerosis, ischemia, and arteriosclerosis, which likely hindered perfusion of glomeruli in these regions. This not only included globally sclerosed glomeruli, but ischemic-appearing glomeruli that were likely under-perfused due to severe vascular changes. However, the reason for overestimated glomerular number was the accumulation of cationic ferritin in regions of vascular remodeling, erroneously detected as glomeruli.
Recent advances in this field are encouraging, but more work is still needed. This includes improved image processing tools to remove artifacts and false positive particles, and developing new contrast agents that have better sensitivity and specificity for glomeruli and at low doses. Tungsten-iron nanoparticles are a potential option (36).
In vivo cationic ferritin-enhanced MRI in rodents
More recently, an in vivo cationic ferritin MRI method with a custom built radio frequency surface coil was used to count glomeruli and measure their size in healthy rats, using presumably non-toxic doses of cationic ferritin (37). With MRI methods, the proximity of the radio frequency coil to the kidney is important for improving spatial resolution. In an attempt to achieve a better spatial resolution, a small wireless amplified NMR detector (WAND) was inserted into the rat colon near the kidneys, allowing high resolution imaging of both cortex and medulla (38). Thus, a WAND placed via colonoscopy, may potentially be useful in assessing number of glomeruli in the human kidney.
Methods to determine nephron number in living humans
Ultrafiltration coefficient based methods
The permeability of the glomerular basement membrane to filtrate is known as the ultrafiltration coefficient (Kf). Adaptive increases in glomerular filtration rate are due to increases in Kf, which may help prevent glomerular hypertension (39). The whole kidney ultrafiltration coefficient (wkKf) divided by the single nephron ultrafiltration coefficient (snKf) provides a count of functional nephrons (Figure 1B) (40). The whole kidney filtration coefficient (wkKf) can be estimated from measured GFR, renal plasma flow (RPF), and plasma oncotic pressure (41). Then, an ultrastructural analysis of a kidney biopsy (at 12,000X magnification) was used to estimate the filtering surface area (by measuring the volume and percentage of filtering capillary surface area of glomeruli), and hydraulic permeability in glomeruli (from measurement of glomerular basement membrane thickness and frequency of filtration slits). The product of the filtering surface area and hydraulic permeability calculates snKf. By this method, lower GFR in older living kidney donors has been linked to reduced nephron number (40). Using this method, one study found that living donors with hypertension had fewer glomeruli than normotensive donors (42). Limitations of this method are potential bias and imprecision with determining snKf , and hemodynamic factors may bias estimation of wkKf (43). The invasiveness of kidney biopsies and the burdensome nature of measuring GFR and RPF limit clinical utility.
Product of glomerular density and cortical volume method
The product of glomerular density (determined from a kidney biopsy) with the volume of cortex (determined from contrast computed tomography (CT) or MRI) can be used to calculate nephron number. Higher nephron number by this method was first shown to associate with younger age and higher GFR in a small study (44). Larger studies in living kidney donors (n=1638) have applied this method to study nephron number by separately counting non-sclerotic (functioning) and globally sclerotic glomeruli (8). The pre-donation angiogram phase CT images are used to segment the volume of kidney cortex with a semi-automated image processing algorithm (15). The kidney biopsy obtained at the time of transplantation are sectioned, stained, and scanned into high resolution images. After outlining the cortex and each individual glomerular profile, the Weibel and Gomez stereological formula (45) calculates the three-dimensional glomerular density (separately for non-sclerosed and globally sclerosed glomeruli). Importantly, this formula accounts for any lower two-dimensional density of smaller glomeruli at the same three-dimensional density as larger glomeruli. To determine nephron number, cortical volume (mm3 per kidney) is multiplied by the three-dimensional glomerular density (per mm3) and further divided by a correction factor for tissue shrinkage from formalin fixation and a coefficient for tissue shrinkage from loss of perfusion pressure (Figure 1C) (46).
Clinical risk factors and outcomes associated with nephron number have been studied by this method. The mean number of non-sclerotic glomeruli in young adults (18-29 years) is 990,000 per kidney and decreases to 520,000 per kidney in older adults (70-75 years). Conversely, the mean number of detectable globally sclerotic glomeruli in young adults (18-29 years) is 17,000 per kidney and increases to 142,000 per kidney in older adults (70-75 years) (8). By this method, clinical risk factors independently associated with lower nephron number were older age, shorter height, family history of ESRD, higher uric acid level, and lower GFR. Mild hypertension also associated with lower nephron number but not after adjusting for these other risk factors. Lower nephron number was also associated with larger nephrons (larger glomeruli and larger tubules) and with nephrosclerosis (glomerulosclerosis and arteriosclerosis) on biopsy (8). At a median 4 months after donation, low nephron number (<5th percentile for age) predicted onset of a detectable urine albumin excretion (≥ 5 mg/24 h) but not hypertension or GFR <60 ml/min/1.73 m2 (47). Applying this method in Japanese living kidney donors identified a 25% lower nephron number than has been reported in white donors,(48) consistent with findings by the disector-fractionator method in autopsy studies (49). This Japanese study also found lower nephron number to associate with older age, lower estimated GFR, and larger glomerular volume (48).
Limitations of this method are potential bias and imprecision with determining glomerular density from a limited tissue biopsy sample using a stereological model that assumes glomeruli are uniform spheres and that cortex and medulla were accurately distinguished on a biopsy. The invasiveness of kidney biopsies (hard to justify in absence of a significant nephropathy) and the invasiveness of contrast imaging by CT or MRI (hard to justify with presence of a significant nephropathy) limit clinical utility.
Estimation of nephron number
Given the challenges with directly measuring nephron number, methods that indirectly estimate nephron number may be of interest. A formula was developed to estimate nephron number from birthweight and age (50). However, this formula will not detect loss of nephrons due to an acquired disease, limiting its clinical usefulness. Birthweight data may be difficult to obtain in patients further limiting the clinical utility of this method. Nonetheless, lower estimated nephron number by this method associates with post-donation lower GFR, hypertension, and proteinuria (50, 51).
Conclusion
There are methods to calculate nephron number in living humans, but they have largely been studied in living kidney donors, a population at low risk for kidney disease. These methods have helped gain important insights into kidney physiology and pathophysiology, but have significant limitations that limit their practical use. Ideally, an imaging modality that can safely detect the average density of non-sclerosed glomeruli is needed to make nephron number an accessible tool for clinical practice. Nephron number and snGFR are fundamental properties of the kidney that if measured, may help distinguish nephron loss from more reversible hemodynamic effects on the kidney.
Key points:
Glomerular filtration rate is determined by the product of nephron number and mean single nephron GFR.
Increases in single nephron GFR can mask the loss of nephrons with disease.
Current methods for calculating nephron number in living humans require a kidney biopsy and either cortical volume on imaging or measurement of GFR and renal plasma flow.
Cationized ferritin-enhanced MRI has counted glomeruli without a tissue biopsy, but only in vivo with rodents and ex vivo with human kidneys.
Acknowledgements
Financial support and sponsorship
This study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358).
Footnotes
Conflict of Interest
There are no conflicts of interest.
References
- 1.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. 1988;1(4 Pt 1):335–47. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy: The fetal flaw in essential hypertension? Kidney Int Suppl. 1996;55:S30–4. [PubMed] [Google Scholar]
- 3.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, et al. Glomerular Aging and Focal Global Glomerulosclerosis: A Podometric Perspective. J Am Soc Nephrol. 2015;26(12):3162–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58(2):770–3. [DOI] [PubMed] [Google Scholar]
- 5.Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertens (Greenwich). 2003;5(2):133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlote J, Schroder A, Dahlmann A, Karpe B, Cordasic N, Daniel C, et al. Cardiovascular and renal effects of high salt diet in GDNF+/− mice with low nephron number. Kidney Blood Press Res. 2013;37(4-5):379–91. [DOI] [PubMed] [Google Scholar]
- **7.Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med. 2017;376(24):2349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single nephron GFR can be calculated from nephron number and associates with various clinical and histological findings in healthy adults.
- **8.Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, et al. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J Am Soc Nephrol. 2017;28(1):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nephron number declines with aging at a faster rate than is detected by either glomerulosclerosis on biopsy or cortical volume loss on CT scan.
- 9.Denic A, Mathew J, Nagineni VV, Thompson RH, Leibovich BC, Lerman LO, et al. Clinical and Pathology Findings Associate Consistently with Larger Glomerular Volume. J Am Soc Nephrol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kshirsagar AV, Joy MS, Hogan SL, Falk RJ, Colindres RE. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: a systematic overview of randomized placebo-controlled trials. Am J Kidney Dis. 2000;35(4):695–707. [DOI] [PubMed] [Google Scholar]
- 11.Stirling C, Houston J, Robertson S, Boyle J, Allan A, Norrie J, et al. Diarrhoea, vomiting and ACE inhibitors:--an important cause of acute renal failure. J Hum Hypertens. 2003;17(6):419–23. [DOI] [PubMed] [Google Scholar]
- 12.Elsherbiny HE, Alexander MP, Kremers WK, Park WD, Poggio ED, Prieto M, et al. Nephron hypertrophy and glomerulosclerosis and their association with kidney function and risk factors among living kidney donors. Clin J Am Soc Nephrol. 2014;9(11):1892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuboi N, Kawamura T, Koike K, Okonogi H, Hirano K, Hamaguchi A, et al. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hommos MS, Zeng C, Liu Z, Troost JP, Rosenberg AZ, Palmer M, et al. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int. 2018;93(5):1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Vrtiska TJ, Avula RT, Walters LR, Chakkera HA, Kremers WK, et al. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85(3):677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–72. [DOI] [PubMed] [Google Scholar]
- 17.Bertram JF. Counting in the kidney. Kidney Int. 2001;59(2):792–6. [DOI] [PubMed] [Google Scholar]
- 18.Cullen-McEwen LA, Douglas-Denton RN, Bertram JF. Estimating total nephron number in the adult kidney using the physical disector/fractionator combination. Methods Mol Biol. 2012;886:333–50. [DOI] [PubMed] [Google Scholar]
- 19.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–36. [DOI] [PubMed] [Google Scholar]
- 20.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl. 2003(83):S31–7. [DOI] [PubMed] [Google Scholar]
- 21.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70(1):104–10. [DOI] [PubMed] [Google Scholar]
- 22.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69(4):671–8. [DOI] [PubMed] [Google Scholar]
- 23.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr., Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52(1):18–28. [DOI] [PubMed] [Google Scholar]
- 24.McNamara BJ, Diouf B, Hughson MD, Douglas-Denton RN, Hoy WE, Bertram JF. Renal pathology, glomerular number and volume in a West African urban community. Nephrol Dial Transplant. 2008;23(8):2576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoy WE, Hughson MD, Zimanyi M, Samuel T, Douglas-Denton R, Holden L, et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin Nephrol. 2010;74 Suppl 1:S105–12. [DOI] [PubMed] [Google Scholar]
- 26.McNamara BJ, Diouf B, Douglas-Denton RN, Hughson MD, Hoy WE, Bertram JF. A comparison of nephron number, glomerular volume and kidney weight in Senegalese Africans and African Americans. Nephrol Dial Transplant. 2010;25(5):1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Z, Quinlan J, Hoy W, Hughson MD, Lemire M, Hudson T, et al. A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol. 2008;19(10):2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertram JF. Estimating glomerular number: why we do it and how. Clin Exp Pharmacol Physiol. 2013;40(11):785–8. [DOI] [PubMed] [Google Scholar]
- 29.Ford GC, Harrison PM, Rice DW, Smith JM, Treffry A, White JL, et al. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984;304(1121):551–65. [DOI] [PubMed] [Google Scholar]
- 30.Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972;38(5):500–10. [DOI] [PubMed] [Google Scholar]
- 31.Bennett KM, Zhou H, Sumner JP, Dodd SJ, Bouraoud N, Doi K, et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60(3):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeman SC, Cullen-McEwen LA, Puelles VG, Zhang M, Wu T, Baldelomar EJ, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306(11):F1381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300(6):F1454–7. [DOI] [PubMed] [Google Scholar]
- 34.Heilmann M, Neudecker S, Wolf I, Gubhaju L, Sticht C, Schock-Kusch D, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27(1):100–7. [DOI] [PubMed] [Google Scholar]
- *35.Xie L, Koukos G, Barck K, Foreman O, Lee WP, Brendza R, et al. Micro-CT imaging and structural analysis of glomeruli in a model of Adriamycin-induced nephropathy. Am J Physiol Renal Physiol. 2019;316(1):F76–F89. [DOI] [PubMed] [Google Scholar]; Ex vivo micro CT imaging used to quantify loss of glomeruli with adriamycin in mice.
- 36.Clavijo Jordan MV, Beeman SC, Baldelomar EJ, Bennett KM. Disruptive chemical doping in a ferritin-based iron oxide nanoparticle to decrease r2 and enhance detection with T1-weighted MRI. Contrast Media Mol Imaging. 2014;9(5):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **37.Baldelomar EJ, Charlton JR, Beeman SC, Bennett KM. Measuring rat kidney glomerular number and size in vivo with MRI. Am J Physiol Renal Physiol. 2018;314(3):F399–F406. [DOI] [PMC free article] [PubMed] [Google Scholar]; A custom built surface coil and cationic ferritin-based MRI can be used to quantify glomeruli in rats in vivo.
- *38.Zeng X, Ma S, Kruger JM, Wang R, Tan X, Qian C. High-resolution MRI of kidney microstructures at 7.05T with an endo-colonic Wireless Amplified NMR detector. J Magn Reson. 2019;303:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; A wireless amplifier placed in the rat colon, to achieve proximity to kidneys, allows high resolution imaging of kidney microstructures.
- 39.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest. 2015;125(3):1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan JC, Busque S, Workeneh B, Ho B, Derby G, Blouch KL, et al. Effects of aging on glomerular function and number in living kidney donors. Kidney Int. 2010;78(7):686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deen WM, Robertson CR, Brenner BM. A model of glomerular ultrafiltration in the rat. Am J Physiol. 1972;223(5):1178–83. [DOI] [PubMed] [Google Scholar]
- 42.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. The association of predonation hypertension with glomerular function and number in older living kidney donors. J Am Soc Nephrol. 2015;26(6):1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenihan CR, Myers BD, Tan JC. Glomerular Function and Structure in Living Donors: Lessons from Single Nephron Studies. Curr Transplant Rep. 2016;3:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulladosa X, Moreso F, Narvaez JA, Grinyo JM, Seron D. Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol. 2003;14(10):2662–8. [DOI] [PubMed] [Google Scholar]
- 45.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol. 1962;17:343–8. [DOI] [PubMed] [Google Scholar]
- 46.Lerman LO, Bentley MD, Bell MR, Rumberger JA, Romero JC. Quantitation of the in vivo kidney volume with cine computed tomography. Invest Radiol. 1990;25(11):1206–11. [DOI] [PubMed] [Google Scholar]
- **47.Issa N, Vaughan LE, Denic A, Kremers WK, Chakkera HA, Park WD, et al. Larger nephron size, low nephron number, and nephrosclerosis on biopsy as predictors of kidney function after donating a kidney. Am J Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Low nephron number assessed as a predictor of short-term outcomes in living donors and found to predict a modest increase in urine albumin.
- *48.Sasaki T, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, Koike K, et al. Biopsy-based estimation of total nephron number in Japanese living kidney donors. Clin Exp Nephrol. 2019. [DOI] [PubMed] [Google Scholar]; The kidney biopsy and CT method identified 25% fewer nephrons in Japanese compared to white living kidney donors.
- *49.Kanzaki G, Puelles VG, Cullen-McEwen LA, Hoy WE, Okabayashi Y, Tsuboi N, et al. New insights on glomerular hyperfiltration: a Japanese autopsy study. JCI Insight. 2017;2(19). [DOI] [PMC free article] [PubMed] [Google Scholar]; In persons with low nephron number, glomerular hypertrophy rather than glomerular hyperetension is the primary factor that maintains GFR.
- 50.Schachtner T, Reinke P. Estimated nephron number of the remaining donor kidney: impact on living kidney donor outcomes. Nephrol Dial Transplant. 2016;31(9):1523–30. [DOI] [PubMed] [Google Scholar]
- 51.Schachtner T, Reinke P. Estimated Nephron Number of the Donor Kidney: Impact on Allograft Kidney Outcomes. Transplant Proc. 2017;49(6):1237–43. [DOI] [PubMed] [Google Scholar]


