Abstract
Slowing down aging-associated accumulation of molecular damage or its prevention represents a promising therapeutic paradigm to combat aging-related disease and death. While several chemical compounds extend lifespan in model organisms, their mechanism of action is often unknown, reducing their therapeutic potential. Using a systematic approach, here we characterize the impact of the GMP pathway on yeast lifespan and elucidate GMP synthesis inhibition as a lifespan extension mechanism. We further discover that proteasome activation extends lifespan in part through the GMP pathway. GMP synthesis inhibition exerts its lifespan extension effect independently of the canonical nutrient-sensing pathway regulating lifespan. Exposing longitudinally aging yeast cells to GMP pathway inhibition in an age-dependent manner, we demonstrate that the lifespan extension is facilitated by slowing, rather than reversing, the aging process in cells. Using a GUK1 mutant with lower GMP-to-GDP conversion activity, we observe lifespan extension, suggesting that reduced GDP level by itself can also extend yeast lifespan. These findings elucidate the involvement of nucleotide metabolism in the aging process. The existence of clinically-approved GMP pathway inhibitors elicits the potential of a new class of therapeutics for aging-related disorders.
Keywords: aging, yeast, mycophenolic acid, GMP, GDP, proteasome, replicative lifespan
Introduction
Aging is the primary risk factor for human morbidity and mortality throughout the developed world1–3. Prevention or elimination of the molecular damage that causes aging therefore represents a promising therapeutic paradigm to combat the plethora of diseases that manifest late in life. Target-based drug discovery has advanced several therapeutic candidates to human trials, including metformin4 and mTOR inhibitors5. While these treatments hold promise, additional interventions are necessary to fully minimize the health effects of advanced age.
In recent years, phenotype-based discovery approaches6,7 have identified many compounds capable of extending lifespan in model organisms1. These compounds are a starting point for therapeutic development, but the mechanism of action for many of them remains unknown, reducing their therapeutic potential. One such compound is mycophenolic acid (MPA), an FDA-approved immunosuppressant that extends the replicative lifespan (RLS) of the yeast Saccharomyces cerevisiae6.
MPA’s primary, but not exclusive, effect is to reduce cellular GMP pools through inhibition of inosine monophosphate dehydrogenase (IMD), the rate-limiting enzyme in de novo GMP synthesis8,9 (Fig. 1A). The de novo GMP synthesis involves the production of xanthosine 5’-phosphate (XMP) from inosine 5’-phosphate (IMP) via the action of IMP Dehydrogenase (IMD). Conversion of IMP to XMP is accompanied by the reduction of NAD+ to NADH10. This reaction is followed by the production of GMP from XMP through the action of GMP synthase GUA1. Conversion of XMP to GMP is accompanied by the conversion of ATP to AMP11,12. GMP can also be synthesized via a salvage pathway in the presence of exogenous guanine8,13. This reaction is catalyzed by the dimeric hypoxanthine-guanine phosphoribosyltransferase HPT1. GMP is converted to GDP by the guanylate kinase GUK1.
Figure 1. Inhibition of GMP synthesis extends yeast RLS. A.
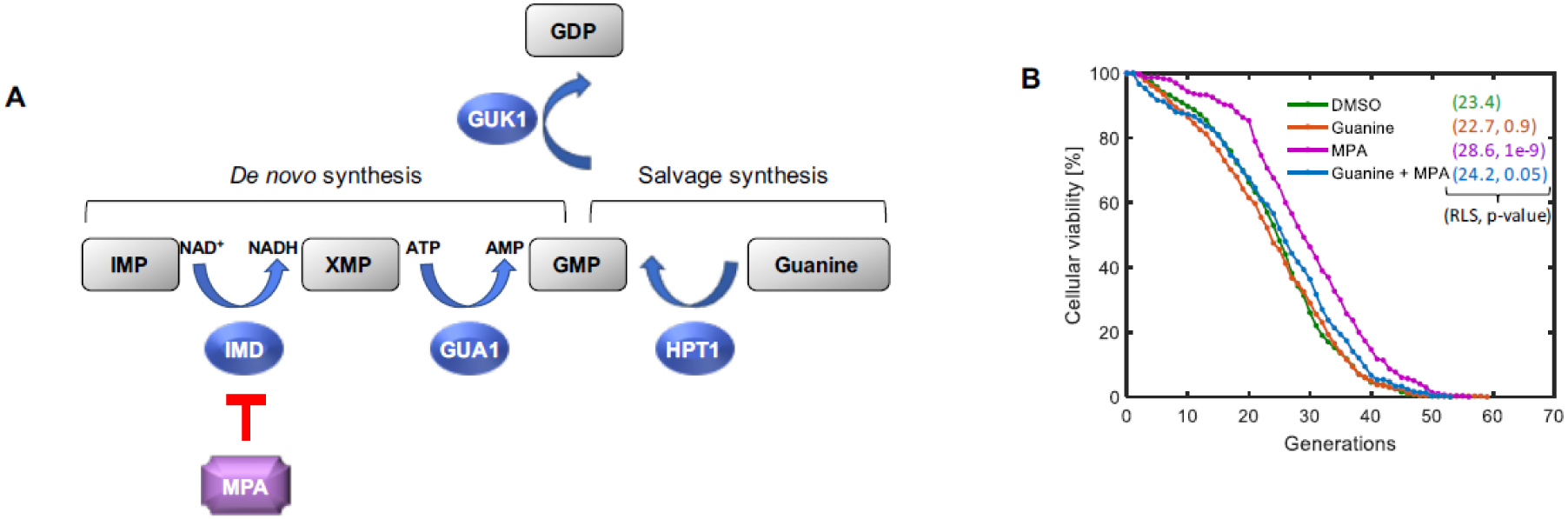
Simplified schematic representation of GMP synthesis pathways in S. cerevisiae. MPA limits de novo GMP synthesis via inhibition of IMD genes. Conversion of IMP to XMP is accompanied by the reduction of NAD+ to NADH. Conversion of XMP to GMP is accompanied by the conversion of ATP to AMP. GMP is also synthesized via the salvage pathway in the presence of exogenous guanine. GMP is converted to GDP by the guanylate kinase GUK1. B. Lifespan curves for wild-type, haploid yeast (BY4741) in the presence or absence of MPA and guanine. N = 400 cells each for the DMSO and guanine conditions, pooled from four independent experiments of 100 cells each; N = 300 cells each for the MPA and guanine&MPA conditions, pooled from three independent experiments of 100 cells each. Average RLS values associated with all growth conditions are reported in colored numbers in parentheses. Next to each average RLS value reported, p-value obtained from log-rank test is reported; each lifespan curve is compared to the curve obtained in the DMSO condition.
Yeast RLS is the most rapid aging model: most cells die within several days after birth. Due to the evolutionarily-conserved aging mechanisms, yeast replicative lifespan is closely related to healthspan and lifespan in humans. Several interventions14,15 that extend yeast RLS promise to improve general health in humans. For example, a rapamycin analog improves immune response in the elderly16. Moreover, dietary restriction reduces biomarkers for diabetes, cardiovascular disease, and cancer17.
RLS is quantified by the number of mitotic division events experienced by a newborn yeast cell until its death; it is measured by counting the daughter cells budded off from the tracked/aging mother cell. Differently from RLS, a second commonly-used lifespan metric for yeast, chronological life span (CLS), measures the chronological time a cell can remain viable in a post-replicative state.
Aging and lifespan are multifactorial processes whose comprehensive quantitative characterization at the whole-genome level is still missing. For example, the full range of pathways and how their dynamic interactions regulate lifespan are not definitely known, yet. Despite this reality, three major lifespan-extension pathways (nutrient sensing pathway, proteasome pathway and sirtuin pathway) have been previously identified18 for S. cerevisiae. These pathways may not be fully orthogonal to each other, as the sirtuin pathway has been thought19 to act downstream of the nutrient-sensing pathway.
Excluding our previous work6 which elucidated MPA’s lifespan-extending effect on yeast, no previous study directly and empirically implicated MPA as a longevity-enhancing chemical compound. Therefore, MPA’s action mechanism has been unknown. Here, we seek to understand the mechanism behind MPA’s ability to extend replicative lifespan. We find that guanine reverses MPA’s pro-longevity effect, suggesting that reduction in cellular GMP, or its downstream metabolites, is responsible for the lifespan extension. We then investigate if MPA’s RLS extension mechanism overlaps with two of the previously-described aging pathways (nutrient sensing and proteasome pathways), elucidating that it partially overlaps with the effect exerted by activated proteasome. Using a GUK1 mutant with lower guanylate kinase activity, we again observe lifespan extension, suggesting that reduced GDP level by itself contributes to the lifespan extension in a major way.
Results
Inhibition of GMP synthesis extends yeast RLS
We hypothesized that the reduction in cellular GMP pools was responsible for MPA’s pro-longevity effect. Using our microfluidic “yeast replicator” platform20, we quantified the number of daughter cells produced by mother cells individually tracked throughout their replicative lifespan. These single-cell RLS measurements were made in minimal media environments containing MPA (at standard 10 μM) with or without supplemental guanine.
While the average lifespan of the cells grown in the guanine-supplemented environment was slightly reduced compared to DMSO control, the difference was not statistically significant (Table 1). Importantly, we found that the longevity effect of MPA was prevented by the supplementation of exogenous guanine (Fig. 1B). These results confirm that MPA extends replicative lifespan in S. cerevisiae through inhibition of GMP synthesis; however, further experimental characterizations are needed to determine if it is decreased levels of GMP and/or its downstream metabolites that cause the lifespan extension. In this study, for the determination of statistical significance, we used the log-rank test which is a hypothesis test to compare two survival distributions. It is important to note that the log-rank test takes into account the entire survival distribution when assessing significance, instead of simply comparing means from the two distributions.
Table 1.
Summary of lifespans and log-rank test results.
| Condition 1 | Condition 1 Mean Lifespan (Generations) | Condition 2 | Condition 2 Mean Lifespan (Generations) | Percent Change | p-value obtained from log-rank test |
|---|---|---|---|---|---|
| BY4741-DMSO | 23.39 | BY4741-Guanine | 22.7 | −2.949978623 | 0.9034 |
| BY4741-DMSO | 23.39 | BY4741-MPA | 28.553 | 22.0735357 | 1.43E-09 |
| BY4741-DMSO | 23.39 | BY4741-MPA-Guanine | 24.183 | 3.390337751 | 0.0463 |
| BY4741-DMSO | 23.39 | ΔHXK2-DMSO | 26.845 | 14.77126977 | 6.60E-04 |
| ΔHXK2-DMSO | 26.845 | ΔHXK2-Guanine | 27.6 | 2.812441795 | 0.45 |
| ΔHXK2-DMSO | 26.845 | ΔHXK2-MPA | 31.35 | 16.78152356 | 0.0000269 |
| BY4741-MPA | 28.553 | ΔHXK2-MPA | 31.35 | 9.795818303 | 0.0039 |
| BY4741-DMSO | 23.39 | ΔTOR1-DMSO | 28.805 | 23.1509192 | 4.27E-08 |
| ΔTOR1-DMSO | 28.805 | ΔTOR1-Guanine | 28.935 | 0.451310536 | 0.87 |
| ΔTOR1-DMSO | 28.805 | ΔTOR1-MPA | 32.525 | 12.91442458 | 0.000634 |
| BY4741-MPA | 28.553 | ΔTOR1-MPA | 32.525 | 13.91097258 | 2.40E-06 |
| BY4741-DMSO | 23.39 | ΔUBR2-DMSO | 28.8 | 23.12954254 | 2.32E-13 |
| ΔUBR2-DMSO | 28.8 | ΔUBR2-Guanine | 27.28 | −5.277777778 | 0.0147 |
| ΔUBR2-DMSO | 28.8 | ΔUBR2-MPA | 31.5 | 9.375 | 0.0122 |
| BY4741-MPA | 28.553 | ΔUBR2-MPA | 31.5 | 10.32115715 | 2.35E-04 |
| BY4741-Guanine | 22.7 | ΔUBR2-Guanine | 27.28 | 20.17621145 | 7.99E-08 |
| BY4741-DMSO | 23.39 | ΔPRE9-DMSO | 23.005 | −1.646002565 | 0.5019 |
| ΔPRE9-DMSO | 23.005 | ΔPRE9-MPA | 27.84 | 21.01717018 | 0.0000684 |
| BY4741-MPA | 28.553 | ΔPRE9-MPA | 27.84 | −2.497110636 | 0.1524 |
| BY4741-DMSO | 23.39 | BY4741-MPA(0–24) | 24.37 | 4.189824711 | 0.0013 |
| BY4741-DMSO | 23.39 | BY4741-MPA(24+) | 25.55 | 9.23471569 | 0.0066 |
| BY4741-DMSO | 23.39 | BY4741-MPA(24–30) | 23.755 | 1.560495938 | 0.2429 |
| BY4741-MPA | 28.553 | BY4741-MPA(0–24) | 24.37 | −14.64994922 | 0.0436 |
| BY4741-MPA | 28.553 | BY4741-MPA(24+) | 25.55 | −10.51728365 | 0.0122 |
| BY4741-MPA | 28.553 | BY4741-MPA(24–30) | 23.755 | −16.80383848 | 4.83E-04 |
| BY4741-MPA(0–24) | 24.37 | BY4741-MPA(24–30) | 23.755 | −2.523594584 | 0.15 |
| BY4741-MPA(0–24) | 24.37 | BY4741-MPA(24+) | 25.55 | 4.842018876 | 0.66 |
| BY4741-MPA(24+) | 25.55 | BY4741-MPA(24–30) | 23.755 | −7.025440313 | 0.279 |
| BY4741-DMSO | 23.39 | guk1–1-DMSO | 28.3 | 20.99187687 | 2.83E-05 |
| BY4741-MPA | 28.553 | guk1–1-DMSO | 28.3 | −0.886071516 | 0.1155 |
Inhibition of GMP synthesis extends lifespan independent of the nutrient sensing pathway
We next asked how lifespan extension via GMP-synthesis inhibition could be placed among the known lifespan pathways. To answer this question, we used a systematic approach to categorize longevity interventions to genetic regulators of lifespan. A longevity intervention can act either within or independent from any known longevity pathway. If within a longevity pathway, the intervention must act upon, upstream from, or downstream of a given pathway component. We aimed to conclusively identify the placement of the lifespan extension mechanism through GMP-synthesis inhibition relative to the known genetic lifespan pathways using the two-step Longevity Placement Test (LPT) (Fig. 2A, Fig. S1, Supplementary Information). In Step 1, we ask if the longevity intervention extends lifespan in a strain lacking a critical lifespan pathway component, the probe gene. In Step 2, we ask whether an epistatic agent that prevents lifespan extension from the longevity intervention can also prevent lifespan extension conferred by modulation of the probe gene. By combining this information, one can definitively classify the relationship of a longevity intervention to a known lifespan regulation pathway.
Figure 2. Inhibition of GMP synthesis extends lifespan independent of the nutrient sensing pathway.
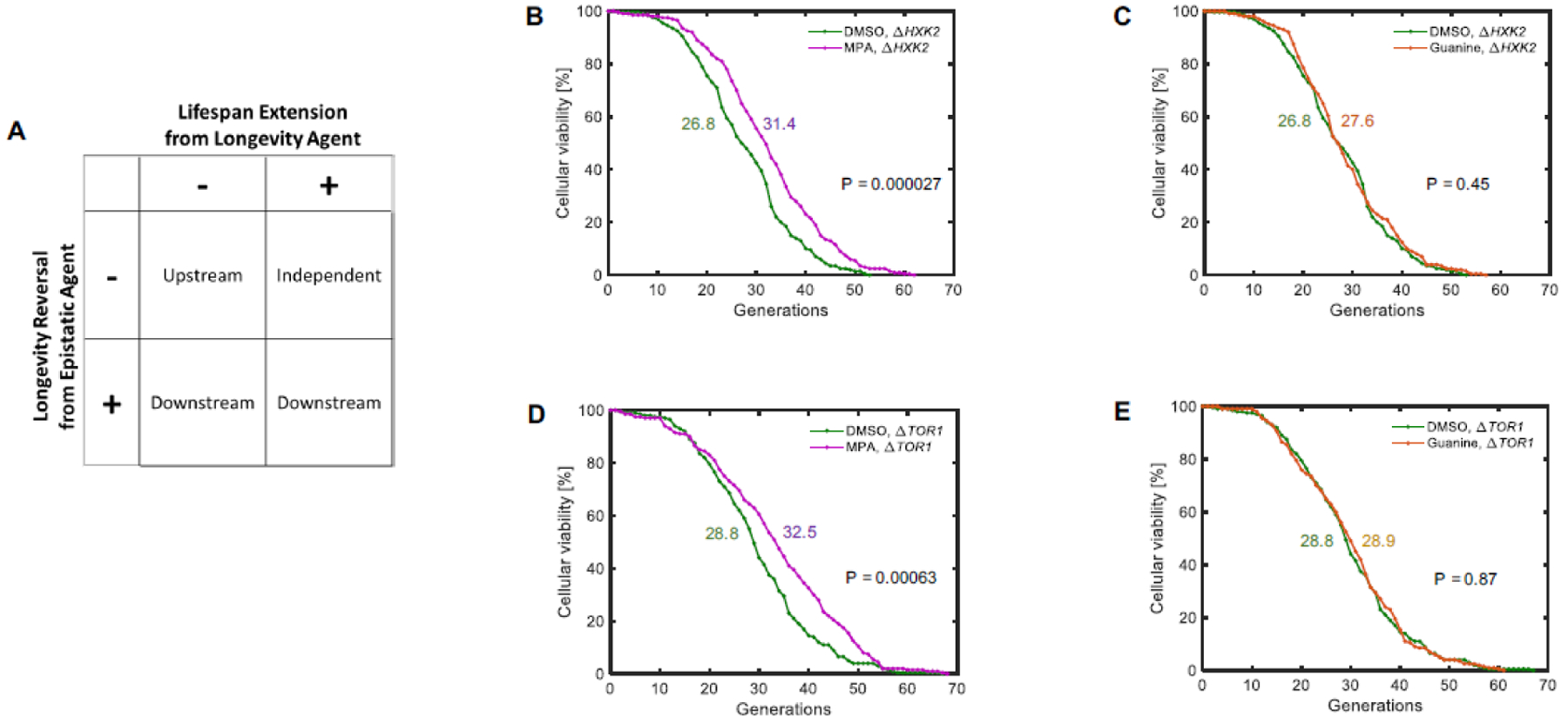
A. Schematic representation of the LPT test and its interpretation. B-E. Lifespan curves corresponding to Step 1 (B, D) or Step 2 (C, E) of the LPT test for the nutrient sensing pathway, including a dietary restriction mimetic (B, C) and TOR inhibition (D, E). N = 200 cells for each condition, pooled from two or more independent experiments. Colored numbers next to the lifespan curves indicate the average replicative lifespan values corresponding to the lifespan curve matching its color.
We used the LPT to determine the relationship of GMP synthesis inhibition to two major lifespan-extension pathways known for S. cerevisiae18,19. For this purpose, MPA was used as the longevity intervention, and guanine was used as the epistatic agent. The first pathway we tested was the nutrient sensing pathway21, which encompasses dietary restriction15 and target of rapamycin (TOR) inhibition22. As the LPT probe genes, we chose TOR1 and HXK2, whose individual deletion is known to extend yeast lifespan. TOR1 is a protein kinase subunit of the TORC1 complex that controls cell growth in response to nutrient availability. HXK2, on the other hand, is a hexokinase whose deletion provides a genetic mimicry of nutrient limitation because it phosphorylates intracellular glucose as part of glucose metabolism. MPA further extended lifespan in the long-lived ΔTOR1 (p=0.000634) and ΔHXK2 (p=0.0000269) strains, while guanine did not suppress gene-deletion-caused lifespan extension in these strains (Fig. 2B–E, Table 1). These results indicate that inhibition of GMP synthesis exerts its longevity effect independent of the nutrient-sensing pathway.
Proteasome activation extends lifespan in part through the GMP pathway
Next, we asked whether inhibition of GMP synthesis extended RLS through the second major lifespan-extension pathway, the proteasome pathway. UBR2 is a ubiquitin ligase subunit, and its deletion activates the proteasome by stabilizing RPN4, a transcription factor that promotes expression of proteasome subunits23. Activation of the proteasome via UBR2 deletion extends RLS independent of the nutrient sensing pathway24. Therefore, we chose UBR2 as the LPT probe gene. MPA led to extension (p = 0.012) of yeast RLS in a ΔUBR2 strain (Fig. 3A, Table 1), compared to an extension (p = 1.43*10−9) in wild-type cells (Fig. 1B, Table 1). On the other hand, guanine supplementation, at a saturating concentration, partially but significantly (p = 0.015) suppressed lifespan extension from UBR2 deletion (Fig. 3B, Table 1). These results indicate that UBR2 deletion activates, if not exclusively, the same lifespan extension mechanism as MPA without saturating the target; in other words, UBR2 deletion extends lifespan in part through the GMP pathway. A role for the GMP pathway in the phenotypic effects of UBR2 deletion is reinforced by the observation that IMD proteins are among the most highly upregulated proteins in a ΔUBR2 strain24. We therefore conclude that, downstream of UBR2, activated proteasome exerts its lifespan-extension effect partially through the GMP pathway, as supplementing ΔUBR2 strain’s growth media with excess guanine significantly but only partially reverses the lifespan extension.
Figure 3. Proteasome activation extends lifespan in part through the GMP pathway.
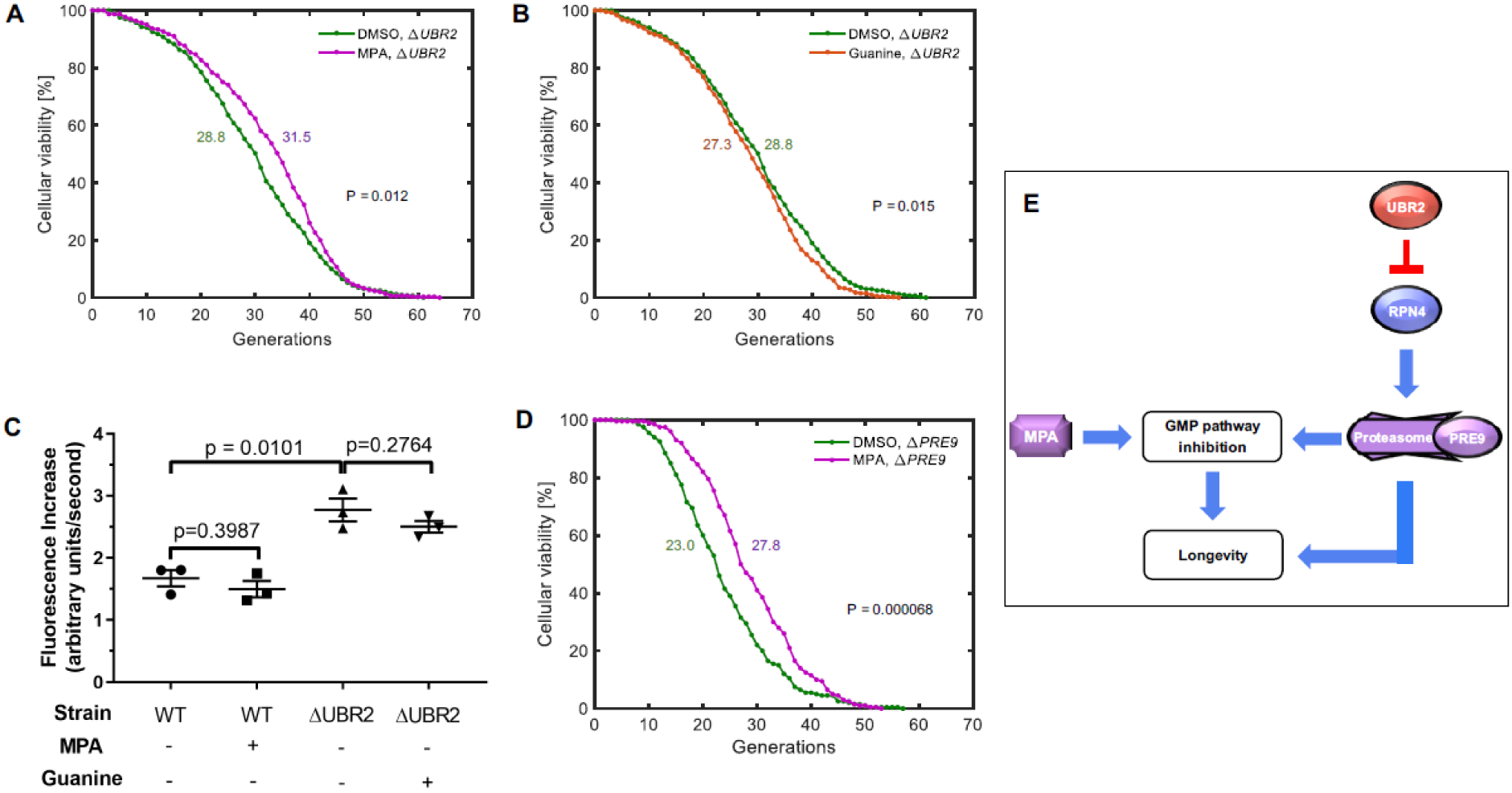
A-B. Lifespan curves corresponding to Step 1 (A) and Step 2 (B) of the LPT test for the proteasome pathway of lifespan extension. N = 300 cells for the MPA condition, pooled from three independent experiments of 100 cells each; N = 400 cells for the DMSO condition, pooled from four independent experiments of 100 cells each; N = 400 cells for the guanine condition, pooled from four independent experiments of 100 cells each. C. Proteasome activity for wild-type (BY4741) cells, or ΔUBR2 cells, in the presence or absence of MPA or guanine. N = 3 biological replicates for each condition. Errors bars are standard error of the mean. D. Lifespan curve for a ΔPRE9 strain in the presence or absence of MPA. N = 200 cells for each condition, pooled from two independent experiments of 100 cells each. In (A, B, D), colored numbers next to the lifespan curves indicate the average replicative lifespan values corresponding to the lifespan curve matching its color. E. A schematic model presenting the relationship of longevity interactions described in this paper. The actions of MPA converge on the actions of the proteasome at the level of GMP pathway inhibition. Inhibition of GMP pathway extends lifespan. Proteasome activation also extends lifespan independently of GMP pathway inhibition.
Since multiple steps exist between UBR2 deletion and proteasome activation, it was possible that MPA acted downstream of UBR2, but upstream of proteasome activation. We sought to differentiate these possibilities. We measured proteasome activity in wild-type cells in the presence and absence of MPA (Fig. 3C, Fig. S2), and found that MPA does not activate the proteasome. Furthermore, guanine does not alter proteasome activity in ΔUBR2 cells (Fig. 3C). This demonstrates that MPA regulates lifespan without modulating the proteasome. We further demonstrated that MPA extends RLS in a ΔPRE9 strain (Fig. 3D, Table 1), in which proteasome activation is not able to increase RLS due to the absence of the proteasome subunit PRE92. These results indicate that MPA acts to extend lifespan downstream of proteasome activation. Since deletion of UBR2 extends lifespan exclusively24 through proteasome activation and excess guanine supplementation only partially reverses the lifespan-extension effect of UBR2 deletion, we conclude that proteasome activation extends lifespan in part through the GMP pathway (Fig. 3E). While the other mechanism(s) through which activated proteasome extends lifespan is still unknown, a previous hypothesis2,3 suggests that proteasome activation leads to lifespan extension via more efficient removal of misfolded and damaged proteins. Future studies will show which proteins are more prone to aging-associated damage and how their removal through activated proteasome impacts cellular lifespan.
MPA slows, rather than reverses, the aging process
Interventions that extend lifespan may mechanistically act to slow the accumulation of aging factors or aging-related damage, reverse aging-related damage, or suppress its effects. We sought to determine through which mechanism MPA extended the lifespan. For this purpose, we assessed the RLS of yeast cells treated with MPA only for the first 24 hours of a Replicator experiment (Fig. 4A), only after the first 24 hours of a Replicator experiment (Fig. 4B), or with a 6-hour pulse treatment between the 24th and 30th hour of the experiment (Fig. 4C). We found that MPA treatment for only part of the lifespan, either early or late, resulted in reduced lifespan extension compared to whole-lifespan treatment (Fig. 4A–B, Table 1). Furthermore, pulse treatment resulted in little to no lifespan extension (Fig. 4C, Table 1). These results indicate that MPA slows, rather than reverses, the accumulation of aging factors in cells.
Figure 4. MPA slows, rather than reverses, the aging process.
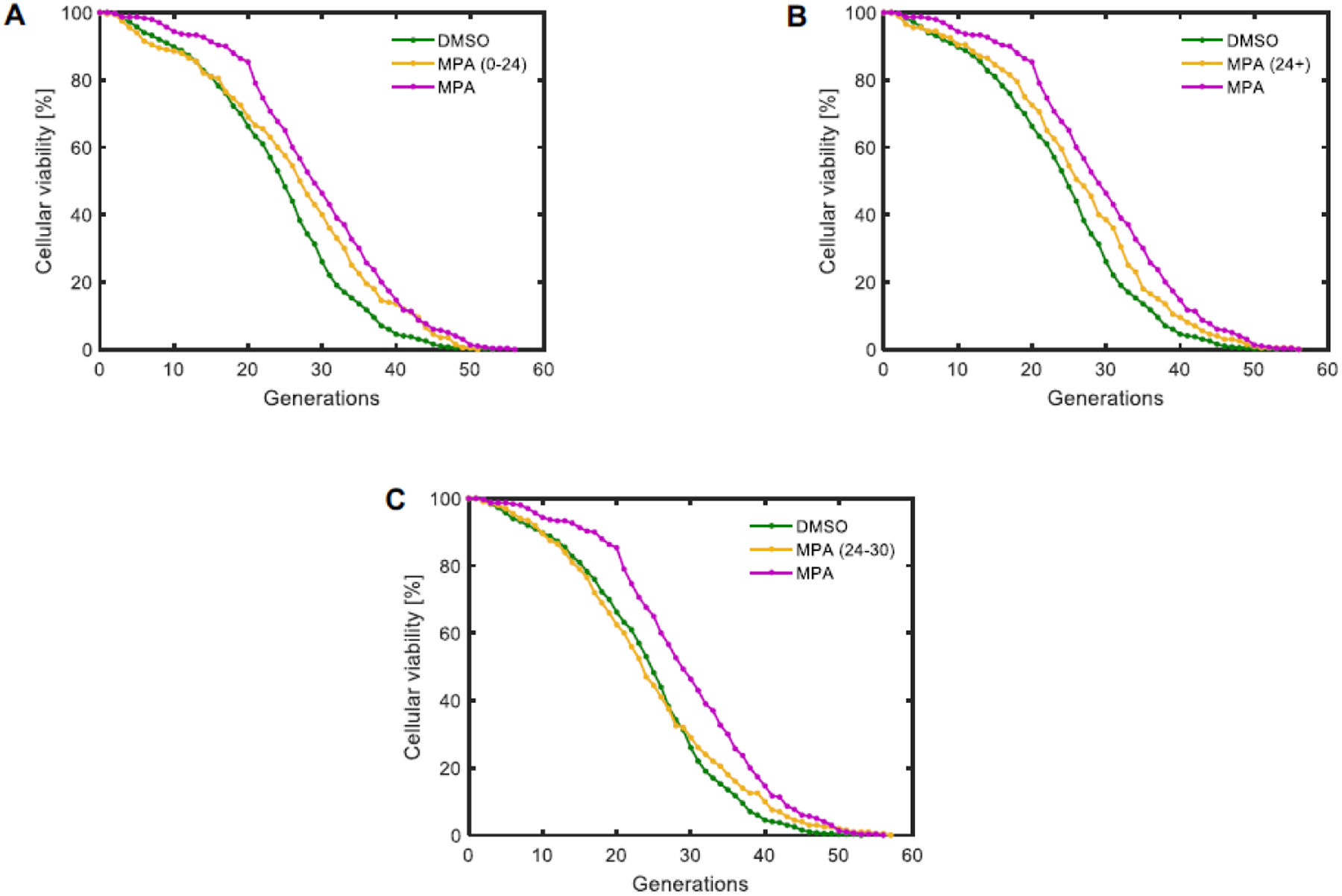
A-C. Replicative lifespan curves for S. cerevisiae treated with 10 μM MPA only during the first 24 hours of a Replicator experiment (A), only after the first 24 hours of a Replicator experiment (B), or between the 24th and 30th hour of a Replicator experiment (C). N = 400 cells for the DMSO condition, pooled from four independent experiments of 100 cells each; N = 300 cells for the full-time MPA condition, pooled from three independent experiments of 100 cells each; N = 200 cells for the partial-time MPA conditions, pooled from two independent experiments of 100 cells each.
Throughout this study, we used MPA at the 10 μM concentration because of the “standard” nature of this concentration based on many compound-screening assays. However, a dose-response characterization we performed showed that 10 μM was indeed the most efficient MPA concentration in terms of its RLS-extending effect (Fig. S3).
Measuring the impact of lowered guanylate kinase activity on replicative lifespan
Is it GMP or one of its downstream metabolites whose reduced production under MPA treatment causes lifespan extension? While we could not directly measure intracellular GMP, GDP, and GTP levels in aging cells due to lack of experimental probes or their integration to our microfluidic platform, we sought to answer this question by focusing on the synthesis of GDP from GMP. Yeast GUK1 gene encodes a guanylate kinase which converts GMP to GDP. Knocking out GUK1 makes cells inviable, however a previous study25 produced a viable variant of the GUK1 gene with lower guanylate kinase activity.
Using its own upstream and downstream intergenic regions, we first cloned one copy of the mutated GUK1 gene into our strain background, followed by the deletion of the endogenous GUK1 gene from yeast genome. Sequencing the open reading frame together with the upstream/downstream intergenic regions showed us the presence of a single G-to-A mutation at the 34th base from the start of the gene, leading to a Glycine to Serine amino acid change.
Next, we measured the replicative lifespan of this guk1–1 strain in the absence of MPA in our microfluidic platform and observed an average lifespan of 28.3 generations (Fig. 5A, Table 1), corresponding to a significant (p = 2.83*10−5) extension of yeast lifespan compared to wild-type. The lifespan extension facilitated by the guk1–1 mutant was very close (p = 0.1155) to the extension caused by the MPA treatment (Fig. 5B, Table 1).
Figure 5. Impact of lowered guanylate kinase activity on replicative lifespan.
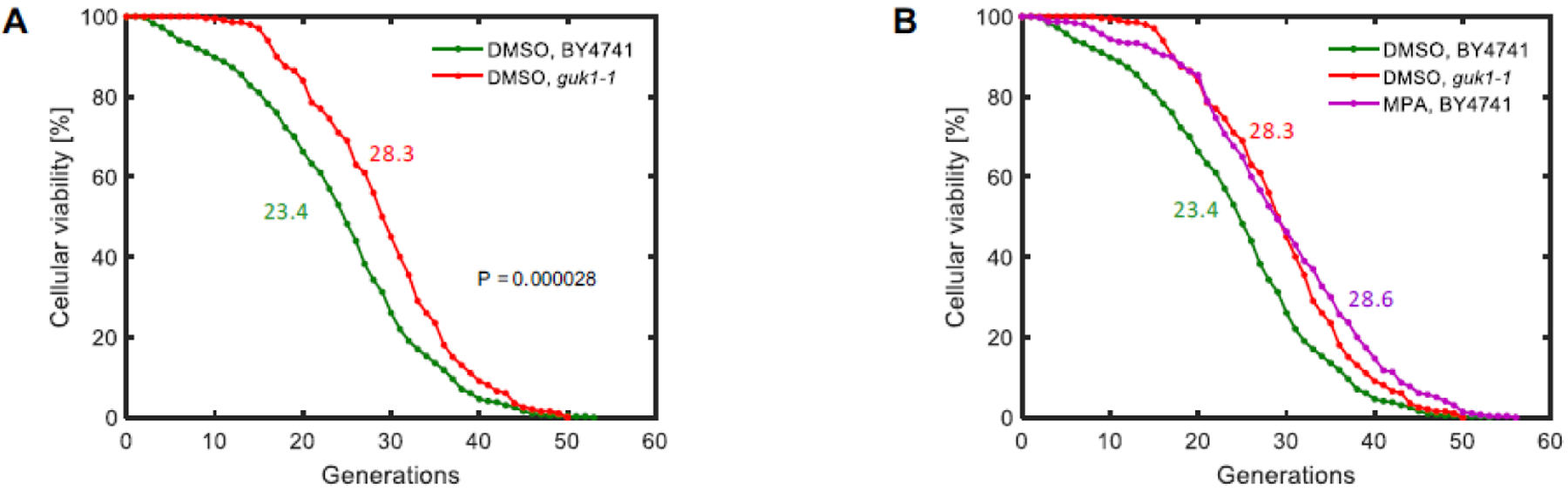
A. Lifespan curves for haploid yeast (BY4741) in wild-type or mutated guk1–1 genetic background. B. Lifespan curves for haploid yeast (BY4741) in wild-type or mutated guk1–1 genetic background, together with wild-type strain under MPA treatment. N = 400 cells for the wild-type DMSO condition, pooled from four independent experiments of 100 cells each; N = 200 cells for the mutated guk1–1 strain in DMSO condition, pooled from two independent experiments of 100 cells each; N = 300 cells for wild-type strain under MPA treatment, pooled from three independent experiments of 100 cells each. Colored numbers next to the lifespan curves indicate the average replicative lifespan values corresponding to the lifespan curve matching its color.
While these results strongly suggest a major role for reduced GDP (and/or its downstream metabolites) to cause lifespan extension, they are not contradictory to the earlier results we observed from yeast cells treated with MPA, as inhibition of GMP synthesis by MPA would already be expected to indirectly lead to a reduction in GDP levels. Moreover, reduced GMP, by itself, may still have a partial effect on lifespan extension. Enabled by the capability to make single-cell level measurements of intracellular GMP and GDP concentrations in aging cells, future studies will show the relative contributions of GMP and GDP reduction on the lifespan extension through the GMP pathway.
Discussion
In this study, we showed how MPA facilitates lifespan extension in yeast. Since MPA has a well-characterized role on the GMP pathway, a direct mechanism for its lifespan extension effect emerged through GMP synthesis inhibition. This was further confirmed by the observation that adding excess guanine into growth media reverses the RLS extension effect of MPA. Here we also showed that activated proteasome extends lifespan in part through the GMP pathway. Furthermore, we found that MPA exerted its lifespan-extending effect by slowing, rather than reversing, the aging process. As part of our characterizations to locate the pathway through which MPA impacts lifespan, we found that the nutrient-sensing pathway was not involved in lifespan extension from the use of MPA. Performing experiments with a GUK1 mutant with lower guanylate kinase activity, we observed lifespan extension, suggesting that reduced GDP levels can extend yeast lifespan in the absence of MPA. Taken together, our work elucidates the involvement of nucleotide metabolism in the aging process.
MPA is an FDA-approved immunosuppressant that is used in the clinic to prevent graft rejection after organ transplant operations. MPA inhibits the production of T-cell cytokines26. This inhibition is facilitated by preventing T-cells to progress to the S-phase of the cell cycle26. It is also known27 that MPA inhibits DNA replication by inducing GTP depletion, which suppresses the synthesis of RNA-primed DNA intermediates. Through which potential mechanism could the inhibition of DNA replication extend lifespan? In general terms, lifespan extension for a cell can be realized by either slowing the accumulation of molecular damage or through its elimination at old age. Our results indicate that MPA exerts its RLS-extending effect through slowing down the aging process. Slower aging, in turn, could be realized by more efficient continuous repair28,29 alleviating accumulation of damage. We therefore propose that MPA extends yeast replicative lifespan via more efficient repair of molecular damage. Among the potential mechanisms directly or indirectly leading to repair pathway activation is stress caused by MPA-induced inhibition of DNA replication. In support of this potential mechanism, genes involved in DNA-damage repair have been shown30,31 to be induced in response to environmental stress mediated by Msn2/432,33. As another layer of support, a recent study showed that a transient pharmacological endoplasmic reticulum stress, imposed early in development on Caenorhabditis elegans, enhanced proteostasis and increased adult lifespan34. Mimicking the transient unfolded protein response up-regulation using the glycosylation inhibitor tunicamycin, the authors saw increased lifespan34. MPA is also known to induce inhibition of glycosylation of proteins through depletion of guanosine nucleotides35.
Our work uncovers the possibility of a new class of therapeutics targeting aging-related disease. Future studies will determine the clinical viability of GMP/GDP synthesis inhibitors for delaying the onset of or for the prevention of aging-related disease. The finding that mycophenolic acid extends lifespan in a ΔTOR1 genetic background promotes the idea of combination therapy with the TOR inhibitors currently in clinical trials. Characterization of new targets through which cellular aging36–43 can be regulated represents an important milestone towards the ultimate goal of fully understanding aging and lifespan determinants at single-cell resolution.
Materials And Methods
Yeast strains and media conditions used for determining lifespan characteristics and proteasome activity
All experiments in S. cerevisiae were conducted in a BY4741 haploid strain background (TransOMIC TKY0002). Haploid deletion strains were purchased from the Yeast Knockout Collection maintained by GE Dharmacon, carrying the KanMX selection marker for the deletions. Complete supplement mixture (CSM 2% glucose) media was used for all experiments, with cells maintained in aerobic conditions at 30 °C in 50 mL conical tubes (Becton Dickinson F2070) or sterile 250 mL Erlenmeyer flasks. Cultures were performed in an Innova-42 shaker (New Brunswick Scientific) set to 225 rpm.
To construct the guk1–1 mutant strain in the BY4741 genetic background, we requested from Dr. Bertrand Daignan-Fornier the strain “129” (a strain in the PLY121 genetic background) published in a previous study25. This strain contained the guk1–1 allele25. We PCR-amplified and transformed (using spHIS5 as the selection marker) into the ho locus of the BY4741 strain one copy of the guk1–1 ORF (564bp) and its entire intergenic regions (upstream and downstream of guk1–1 ORF, 1258bp of total length). spHIS5 ORF was flanked by TEF1 promoter and terminator from Ashbya gossypii. Next, in another transformation we performed, the endogenous GUK1 ORF was deleted using KANMX4 selection marker whose ORF was flanked by TEF1 promoter and terminator. PCR checks and local sequencing performed on the isogenic colony collected after the second yeast transformation showed us that the endogenous GUK1 ORF was deleted and the guk1–1 allele was integrated in the ho locus. DNA sequencing also revealed that there was a single G-to-A mutation at the 34th base position of the guk1–1 ORF, leading to a Glycine-to-Serine amino acid change. There were no mutations (compared to the BY4741 genetic background) on the upstream and downstream intergenic regions (PCR-amplified from the strain “129”) around the guk1–1 ORF.
Replicative lifespan measurements for haploid yeast in the Replicator
RLS measurements were performed in the Replicator device for haploids, as described in our previous publication20. Briefly, cells were grown overnight in CSM 2% glucose for 24 hours to an OD600 of 0.1, and then loaded to the microfluidic Replicator device. Media was then flowed through the device, containing DMSO (American Bio AB00435), 10 μM mycophenolic acid (Sigma M5255), and/or guanine (Sigma G11950) at a saturating concentration of 20 mg/L. An automated microscope was used to collect time-lapse images of individual mother cells every 10 min for 120 hours, and RLS was determined by counting the number of daughters produced before death. RLS was scored manually by observing the time-lapse image series produced in a Replicator experiment. Cells were included for measurement if they entered the trap prior to the 10th hour of the experiment. Passage into each generation was denoted by the appearance of a bud.
Protein extraction and proteasome assay
For protein extraction, 50 mL of cells were grown for 18 hours to an OD600 of approximately 0.8, then transferred to a 50 mL conical tube and centrifuged at 4255 xg for 5 minutes at room temperature. The supernatant was discarded, and the cells were resuspended in 150 μL of cold lysis buffer (50 mM Tris-HCl, pH 7.5, 0.5 mM EDTA, 5 mM MgCl2, with cOmplete™ ULTRA mini protease inhibitor tablets, EDTA free) and transferred to a 1.5 mL tube. A ¼ volume of 500–750 μm glass beads (Acros Organics 397641000) was added to each tube. For 10 rounds, the tubes were chilled in ice water for 1 minute, then vortexed at maximum speed for 30 seconds to physically rupture the cells, and returned to the ice water. Samples were then spun for 3 minutes at 2500 xg and 4 °C, and the supernatant was transferred to a fresh tube. The solution was further clarified by centrifugation at 8000 xg for 10 minutes at 4 °C, and the supernatant was transferred to a fresh tube. Protein concentration was measured using a Nanodrop measuring the absorbance of the sample at 280 nm.
The proteasome assay used was previously described24. The assay was performed in a 96-well clear-bottom plate (Costar 3603) with 50 μg of total protein in 200 μL of lysis buffer. The fluorogenic proteasome substrate Suc-LLVY-AMC (Bachem I-1395) was added to a final concentration of 100 μM. Fluorescence intensity with an excitation wavelength of 380 nm and an emission wavelength of 460 nm was recorded at 5-minute intervals using a Neo2 plate reader (BioTek) set to mix constantly and maintain 30 °C. Negative control reactions were performed in the presence of 50 μM MG132 (Sigma 474787), a proteasome inhibitor.
Statistical methods for lifespan characteristics and proteasome activity
Differences in lifespan characteristics were assessed through Log-Rank test using MATLAB with a cut-off value of P = 0.05. A summary of the results is provided in Table 1. The script for Log-Rank test was downloaded from MATLAB central: http://www.mathworks.com/matlabcentral/fileexchange/20388-logrank. Differences in proteasome activity were assessed using the unpaired, two-tailed, parametric t-test function.
Supplementary Material
Acknowledgements
We thank Dr. Bertrand Daignan-Fornier for sending us the yeast strain carrying the guk1-1 allele of the GUK1 gene. We also thank Dr. Shirin Bahmanyar, Dr. Marc Hammarlund, Dr. Gerald Shadel, Dr. Patrick Sung, Guste Urbonaite, Dr. Ruijie Song, Dr. David Moreno Fortuno, and Acar Lab members for comments and feedback on different stages of this work. EAS acknowledges support through an NSF Graduate Research Fellowship and Gruber Science Fellowship. MA acknowledges funding from the Ellison Medical Foundation (AG-NS-1015-13) and US National Institutes of Health (1DP2AG050461-01 and 1R01GM127870-01).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing Financial Interests
The authors declare competing financial interests: EAS and MA have filed a PCT International patent application.
References
- 1.Harman D The aging process: major risk factor for disease and death. Proc. Natl. Acad. Sci. U. S. A 88, 5360–3 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong X, Milholland B & Vijg J Evidence for a limit to human lifespan. Nature 538, 257–259 (2016). [DOI] [PubMed] [Google Scholar]
- 3.DrugAge: Database of Ageing-Related Drugs. Available at: http://genomics.senescence.info/drugs/index.php. (Accessed: 5th January 2018)
- 4.A Double-Blind, Placebo-Controlled Trial of Anti-Aging, Pro-Autophagy Effects of Metformin in Adults With Prediabetes - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03309007. (Accessed: 5th January 2018)
- 5.Dose Finding Study to Determine if BEZ235 Alone or in Combination With RAD001 Decreases the Incidence of Respiratory Tract Infections in the Elderly - Full Text View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03373903. (Accessed: 5th January 2018)
- 6.Sarnoski EA, Liu P & Acar M A High-Throughput Screen for Yeast Replicative Lifespan Identifies Lifespan-Extending Compounds. Cell Rep. 21, 2639–2646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrascheck M, Ye X & Buck LB An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Escobar-Henriques M & Daignan-Fornier B Transcriptional regulation of the yeast GMP synthesis pathway by its end products. J. Biol. Chem 276, 1523–1530 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Tremblay-Létourneau M, Despins S, Bougie I & Bisaillon M Virtual high-throughput screening identifies mycophenolic acid as a novel RNA capping inhibitor. PLoS One 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPhillips CC, Hyle JW & Reines D Detection of the mycophenolate-inhibited form of IMP dehydrogenase in vivo. Proc Natl Acad Sci U S A 101, 12171–12176 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dujardin G, Kermorgant M, Slonimski PP & Boucherie H Cloning and sequencing of the GMP synthetase-encoding gene of Saccharomyces cerevisiae. Gene 139, 127–132 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Walther T, Novo M, Rössger K, Létisse F, Loret M-O, Portais J-C & François J-M Control of ATP homeostasis during the respiro-fermentative transition in yeast. Mol Syst Biol. 6:344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney MJ, Hoffman DH & Esterman MA Metabolism and biochemistry of mycophenolic acid. Cancer Res. 32, 1803–1809 (1972). [PubMed] [Google Scholar]
- 14.Medvedik O, Lamming DW, Kim KD & Sinclair DA MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 5, 2330–2341 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SJ, Defossez PA & Guarente L Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Mannick JB et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Brandhorst S et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo VD, Shadel GS, Kaeberlein M & Kennedy B Replicative and chronological aging in saccharomyces cerevisiae. Cell Metabolism 16, 18–31 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinkraus KA, Kaeberlein M & Kennedy BK Replicative aging in yeast: the means to the end. Annu. Rev. Cell Dev. Biol 24, 29–54 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Young TZ & Acar M Yeast Replicator: A High-Throughput Multiplexed Microfluidics Platform for Automated Measurements of Single-Cell Aging. Cell Reports 13, 634–644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana L, Partridge L & Longo VD Dietary Restriction, Growth Factors and Aging: from yeast to humans. Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaeberlein M et al. Cell biology: Regulation of yeast replicative life span by TOR and Sch9 response to nutrients. Science 310, 1193–1196 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Mao X, Ju D & Xie Y Rpn4 is a physiological substrate of the Ubr2 ubiquitin ligase. J. Biol. Chem 279, 55218–55223 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Kruegel U et al. Elevated proteasome capacity extends replicative lifespan in saccharomyces cerevisiae. PLoS Genet. 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecoq K, Konrad M & Daignan-Fornier B Yeast GMP kinase mutants constitutively express AMP biosynthesis genes by phenocopying a hypoxanthine-guanine phosphoribosyltransferase defect. Genetics 156, 953–961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lathouder S, Gerards AH, Dijkmans BAC & Aarden LA Two Inhibitors of DNA-Synthesis Lead to Inhibition of Cytokine Production via a Different Mechanism, Nucleosides, Nucleotides & Nucleic Acids, 23:8–9, 1089–1100 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Catapano CV, Dayton JS, Mitchell BS & Fernandes DJ GTP depletion induced by IMP dehydrogenase inhibitors blocks RNA-primed DNA synthesis. Molecular Pharmacology 47, 948–955 (1995). [PubMed] [Google Scholar]
- 28.Young TZ, Liu P, Urbonaite G & Acar M Quantitative insights into age-associated DNA-repair inefficiency in single cells. Cell Reports 28, 2220–2230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song R & Acar M Stochastic modeling of aging cells reveals how damage accumulation, repair, and cell-division asymmetry affect clonal senescence and population fitness. BMC Bioinformatics 20:391 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D & Brown PO Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11, 4241–4257 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutin J, Sadeh A, Rahat E, Aharoni A & Friedman N Condition-specific genetic interaction maps reveal crosstalk between the cAMP/PKA and the HOG MAPK pathways in the activation of the general stress response. Mol Syst Biol 11:829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee M & Acar M Heritable stress response dynamics revealed by single-cell genealogy. Science Advances 4, e1701775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue Y & Acar M Mechanisms for the epigenetic inheritance of stress response in single cells. Current Genetics doi.org/ 10.1007/s00294-018-0849-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matai L, Sarkar GC, Chamoli M, Malik Y, Kumar SS, Rautela U, Jana NR, Chakraborty K & Mukhopadhyay A Dietary restriction improves proteostasis and increases life span through endoplasmic reticulum hormesis. Proc Natl Acad Sci U S A. 116, 17383–17392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heischmann S, Dzieciatkowska M, Hansen K, Leibfritz D & Christians U The Immunosuppressant Mycophenolic Acid Alters Nucleotide and Lipid Metabolism in an Intestinal Cell Model. Sci Rep. 7:45088 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu P, Song R, Elison GL, Peng W & Acar M Noise reduction as an emergent property of single-cell aging. Nat. Commun 8:680 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu P & Acar M The generational scalability of single-cell replicative aging. Sci. Adv 4, eaao4666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnoski EA, Song R, Ertekin E, Kooence N & Acar M Fundamental characteristics of single-cell aging in diploid yeast. iScience doi.org/ 10.1016/j.isci.2018.08.011 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song R, Sarnoski EA & Acar M The systems biology of single-cell aging. iScience doi.org/ 10.1016/j.isci.2018.08.023 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh P & Li R Emerging roles for sphingolipids in cellular aging. Curr Genet 64:761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen ZJ, Postnikoff S & Tyler JK Is Gcn4-induced autophagy the ultimate downstream mechanism by which hormesis extends yeast replicative lifespan? Curr Genet 65:717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senohrabkova L, Malcova I & Hasek J An aggregation-prone mutant of eIF3a forms reversible assemblies escaping spatial control in exponentially growing yeast cells. Curr Genet 65:919 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Harari Y & Kupiec M Mec1ATR is needed for extensive telomere elongation in response to ethanol in yeast. Curr Genet 64:223 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


