Abstract
Purpose:
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease with dismal survival rates. Tumor microenvironment (TME), comprising of immune cells and cancer-associated fibroblasts, plays a key role in driving poor prognosis and resistance to chemotherapy. Herein, we aimed to identify a TME-associated, risk-stratification gene biomarker signature in PDAC.
Experimental Design:
The initial biomarker discovery was performed in The Cancer Genome Atlas (TCGA, n=163) transcriptomic data. This was followed by independent validation of the gene signature in The International Cancer Genome Consortium (ICGC, n=95), E-MTAB-6134 (n=288), and GSE71729 (n=123) datasets for predicting overall survival (OS), and for its ability to detect poor molecular subtypes. Clinical validation and nomogram establishment was undertaken by performing multivariate cox regression analysis.
Results:
Our biomarker discovery effort identified a 15-gene immune, stromal and proliferation (ISP) gene signature that significantly associated with poor OS (HR: 3.90, 95% CI, 2.36–6.41, p<0.0001). This signature also robustly predicted survival in 3 independent validation cohorts ICGC (HR:2.63 [1.56–4.41], p<0.0001), E-MTAB-6134 (HR:1.53 [1.14–2.04], p=0.004), and GSE71729 (HR:2.33 [1.49–3.63], p<0.0001). Interestingly, the ISP signature also permitted identification of poor molecular PDAC subtypes with excellent accuracy in all 4 cohorts; TCGA (AUC=0.94), ICGC (AUC=0.91), E-MTAB-6134 (AUC=0.80), and GSE71729 (AUC=0.83). The ISP-derived high-risk patients exhibited significantly poor OS in a clinical validation cohort (n=119; HR:2.62 [1.50–4.56], p=0.0004). A nomogram was established which included the ISP, CA19–9, T and N-stage for eventual clinical translation.
Conclusions:
We report a novel gene signature for risk-stratification and robust identification of PDAC patients with poor molecular subtypes.
Keywords: Stroma, tumor immunity, pancreatic ductal adenocarcinoma, overall survival, molecular subtypes, prognosis, risk-stratification
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer related deaths worldwide, with the 5-year survival rates of less than 9%1. Despite recent improvements in treatment modalities, both in adjuvant and neoadjuvant settings, the post-surgical prognosis of PDAC patients remains dismal1. More recently, a variety of chemotherapeutic regimens targeting pancreatic cancer cells have been developed, among which a large majority are based upon the backbone of FOLFIRINOX; however, their overall efficacy and survival benefits remain minimal at best2–6.
Our recent understanding of the molecular underpinnings of pancreatic cancer biology has revealed that in addition to cancer cells, the stromal component within the pancreatic tumor microenvironment (TME) plays a pivotal role in the disease biology and confers resistance to various treatment modalities. The TME within the pancreatic cancer is quite complex, and comprises of a variety of cell types including the epithelial cancer cells, the stromal cells, cancer-associated fibroblasts (CAFs), immune cells and other components of extra cellular matrix (ECM)7,8. Furthermore, not only the interactions between cancer and stromal cells play a critical role in pancreatic cancer progression, the stromal abundance results in an elevated production of various growth factors, secretion of ECM proteins, and the activation of fibroblasts, which in turn promote proliferation, invasion, metastasis, and treatment-resistance in pancreatic cancer9–11. In this context, recently, three major oncological mechanisms involving cancer-associated fibroblasts (CAFs) - anti-tumor immunity, immune gene signature, and mesenchymal phenotype were reported to be associated with poor prognosis in pancreatic and other cancers12–17. In addition, higher expression of c-Myc, and several other proliferation-associated genes controlled by this oncogene, were found to be associated with a mesenchymal phenotype and poor prognosis in pancreatic cancer patients18,19.
In support of these exciting scientific discoveries, whole transcriptomic analysis by multiple groups has led to the identification of distinct molecular subtypes highlighting the involvement of TME in driving poor PDAC subtypes. In this regard, Collison and colleagues identified quasi-mesenchymal subtype, Moffitt et al., discovered basal and activated stromal subtype, Bailey and coworkers reported squamous subtype, and most recently Puleo’s group identified pure basal and stromal activated subtypes that are associated with poor overall survival outcomes in PDAC patients14–16,20. However, the clinical translation of these molecular subtypes has been hampered by two key challenges. First, although each of these molecular subtyping efforts have led to the discovery of unique subsets of PDAC patients with poor survival outcomes, there is a lack of consensus on the nomenclature, as well as the panel of genes included within this subtype. Second, these gene expression-based signatures for the identification of poor molecular subtypes require analysis of hundreds of genes with sophisticated bioinformatic approaches, which poses a challenge for its routine implementation in clinical settings. Nevertheless, in view of the increased recognition for the role of tumor stroma and immune components in pancreatic cancer, we hypothesized that the gene expression signature related to CAFs, cellular proliferation and immune cells might provide an attractive platform for the identification and selection of PDAC patients with poor molecular subtypes exhibiting unfavorable prognosis. Such biomarkers will allow a more robust risk-stratification and will allow the clinicians to tailor more personalized treatment regimens for improving treatment outcomes in PDAC patients.
In this study, by including various components of pancreatic TME into consideration, we undertook a systematic and comprehensive biomarker discovery and validation effort to identify and develop a gene expression signature for a robust prognostication of PDAC patients. This was achieved by using simple, inexpensive and quantitative PCR-based assays that can be readily translated into the clinic. Herein, we report a novel 15-gene immune, stromal and proliferation-associated (ISP) signature, which not only offers excellent accuracy in identifying patients with poor a molecular subtype, but is also unique in the sense that it allows interrogation of all poor PDAC subtypes previously reported in the literature14–16,20. Taken together, we believe ISP gene signature offers an attractive platform for risk-stratification in PDAC patients, which has important significance in the clinical management of patients suffering from this fatal malignancy.
MATERIALS AND METHODS
Study design and patient cohorts
This study employed a two-phase design - an initial biomarker discovery phase, where we systematically analyzed transcriptomic data from PDAC patients available in TCGA, ICGC, E-MTAB-6134, and GSE71729, for the identification and independent in-silico validation of a clinically translatable gene signature that captures tumor cell proliferation and the microenvironment, specifically immune and stromal components; hereafter referred to as immune, stromal and proliferative (ISP) gene signature. In the second phase, we performed validation of discovered biomarkers in formalin-fixed paraffin-embedded (FFPE) tissues from multiple clinical cohorts of PDAC patients. In the initial discovery phase, normalized RNA sequencing data from 163 PDAC patients was downloaded from the Broad GDAC Firehose (http://gdac.broadinstitute.org/) to identify a 15-gene ISP signature. We excluded the 8 neuroendocrine pancreatic cancer cases from the TCGA cohort in developing the ISP signature. Subsequently, transcriptomic data from ICGC (N=95; validation cohort 1), microarray gene expression data from E-MTAB-6134 (N=288; validation cohort 2) and GSE71729 (N=123; validation cohort 3) were used for an initial, in-silico validation. While the normalized ICGC RNA-sequencing data for PDAC was downloaded from the ICGC Data Portal (https://dcc.icgc.org/), the clinical and microarray-based gene expression data from 288 PDAC patients belonging to the E-MTAB-6134 and 123 PDAC patients from GSE71729 were downloaded from ArrayExpress and Gene Expression Omnibus (GEO), respectively.
In the subsequent clinical validation phase, we performed quantitative reverse-transcription PCR (qRT-PCR) assays to evaluate the expression levels of candidate genes using FFPE tissues specimens (N=119) from PDAC patients who had undergone surgery at the Kumamoto University Hospital, Japan (clinical validation cohort). The study was conducted in accordance with the Declaration of Helsinki, wherein a written informed consent was obtained from all patients and the study were approved by the institutional review boards of all participating institutions. Patient demographics and clinicopathological characteristics for all patient cohorts used in our study are shown in Table 1.
Table 1:
Clinicopatholgical characteristics of the patient cohorts
| In-silico discovery and validation | Clinical validation | ||||
|---|---|---|---|---|---|
| TCGA | ICGC | E-MTAB-6134 | GSE71729 | Cohort | |
| (n=163) | (n=95) | (n=288) | (n=143) | (n=119) | |
| Sex | |||||
| Men | 90 | 49 | 166 | 55 | 60 |
| Women | 73 | 46 | 122 | 67 | 59 |
| Age (years) | |||||
| Mean ± SD | 64.6 ± 11.1 | 66.5 ± 11.2 | NA | NA | 67.4 ± 10.5 |
| Histological type | |||||
| PDAC | 154 | 81 | 288 | NA | 119 |
| Neuroendocrine | 8 | 0 | 0 | NA | 0 |
| IPMC | 0 | 12 | 0 | NA | 0 |
| Acinar cell carcinoma | 0 | 2 | 0 | NA | 0 |
| Others/Not specified | 1 | 0 | 0 | NA | 0 |
| Location | |||||
| Head | 132 | 75 | NA | NA | 73 |
| Body | 13 | 5 | NA | NA | 27 |
| Tail | 15 | 15 | NA | NA | 9 |
| Others/Not specified | 0 | 0 | 10 | ||
| Differentiation | |||||
| Well | 29 | 1 | NA | 16 | 52 |
| Mod | 86 | 55 | NA | 49 | 47 |
| Poor | 45 | 34 | NA | 34 | 9 |
| Others/Not specified | 2 | 5 | NA | NA | 11 |
| T-stage | |||||
| Tis | 0 | NA | 0 | NA | 2 |
| T1 | 8 | NA | 12 | 2 | 14 |
| T2 | 19 | NA | 39 | 20 | 2 |
| T3 | 132 | NA | 237 | 91 | 84 |
| T4 | 3 | NA | 0 | 1 | 11 |
| N-stage | |||||
| N0 | 46 | NA | 72 | 36 | 43 |
| N1 | 115 | NA | 216 | 80 | 71 |
| NX | 1 | NA | 0 | NA | 0 |
| M-stage | |||||
| M0 | 159 | NA | NA | 115 | 111 |
| M1 | 3 | NA | NA | 2 | 7 |
| AJCC Stage | |||||
| 0 | 0 | 0 | NA | NA | 2 |
| IA | 7 | 4 | NA | NA | 11 |
| IB | 13 | 5 | NA | NA | 2 |
| IIA | 25 | 25 | NA | NA | 24 |
| IIB | 110 | 54 | NA | NA | 57 |
| III | 4 | 1 | NA | NA | 9 |
| IV | 3 | 6 | NA | NA | 7 |
| R- status | |||||
| R0 | 99 | NA | 235 | NA | 114 |
| R1 | 51 | NA | 49 | NA | 4 |
| R2 | 2 | NA | 0 | NA | 1 |
| RX | 4 | NA | 4 | NA | 0 |
Abbreviations: NA, Not Available; IPMC, Intra Papillary Mucinous Adenocarcinoma
PDAC transcriptomic subtype information from TCGA, ICGC and E-MTAB-6134 datasets
TCGA and ICGC subtype labels for squamous subtype defined by Bailey14, quasi-mesenchymal (QM) subtype defined by Collisson15, and basal-like subtype defined by Moffitt16, as well as the subtype information of E-MTAB-6134 and GSE71729 datasets were derived from the original publications16,20.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from the micro-dissected FFPE tissues using the All Prep DNA/RNA/ FFPE kit (Qiagen, Hilden, Germany). A High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used to convert RNA into cDNA. Quantitative reverse transcriptase polymerase chain reactions (qRT-PCR) were performed using a SensiFAST SYBR Lo-ROX Kit (Bioline, London, UK) and the QuantiStudio 7 Flex Real Time PCR System (Applied Biosystems, Foster City, CA). Cycling conditions were as follows: polymerase activation at 95°C for 2 minutes, 50 cycles of denaturing at 95°C for 5 seconds, followed by annealing and extension at 60°C for 20 seconds. The relative expression of the target genes was normalized to the expression levels of GAPDH using the 2−ΔCt method. Each PCR reaction was performed in duplicates, and the mean values were used to calculate the expression level. The sequences of the primers for the target genes used in the present study are shown in Supplementary Table 1.
Statistical analysis
A total of 170 immune17, stromal16, and proliferation19 associated genes were identified from the published datasets. To identify a clinically translatable immune, stromal and proliferative (ISP) gene signature, we performed Lasso penalized Cox regression analysis21 on these genes. We used Cox proportional hazard regression analysis to evaluate the association of the 15-gene ISP signature in predicting overall survival (OS) in PDAC patients. A logistic regression model with this ISP signature was built to identify the poor PDAC subtypes. Kaplan-Meier (KM) analysis was performed to determine survival outcomes. The median values were used as cut-off thresholds to plot the KM curves, and the statistical significance was evaluated by the log rank test. The Cox proportional hazard regression model was employed to perform univariate and multivariate analysis for OS. A nomogram for predicting the OS was built using the R library “rms” package. The survival probabilities were predicted for both 3- and 5-year survival. The validation of the nomogram-based prediction model was accessed via bootstrapped calibration curves and quantified as a C-index. The “B” and “m” parameters were set as 200 and 30, respectively. All statistical calculations were performed using R (version 3. 4. 1; http//www.r-project.org/), and Medcalc version 16.1. (MedCalc Software, Belgium). All P values were two-sided, and a value of less than 0.05 was considered statistically significant.
RESULTS
Identification of a 15-gene risk stratification signature for PDAC patients with poor prognosis
We first analyzed a panel of 170 immune, stromal, and proliferation-associated genes (Supplementary Table 2), using the Lasso penalized Cox regression analysis in the TCGA discovery cohort. Using the lambda.min cut-off threshold, we identified a 15-gene signature (hereafter referred to as, Immune Stromal Proliferation, ISP) that significantly associated with poor OS in PDAC patients. These genes included AHR, CD160, CDC20, FOXP3, IFIT3, IL4, IL32, INHBA, KDM6B, PLD4, PVRL3, RFC4, TNFSF10, TNFSF18, and TNFRSF4. The 5-year OS rates for ISP-derived high and low risk patients were 0% and 51%, respectively, in the TCGA discovery cohort stratified by the median score ([HR: 3.90 (2.36–6.41], p<0.0001, Figures 1A).
Figure 1.

Performance of the ISP signature in predicting OS as well as identifying poor PDAC subtypes in the TCGA discovery and three independent validation cohorts. (A, B, C & D) 5-year OS in ISP high vs. low-risk patients depicted by KM plots in the TCGA discovery and three validation cohorts respectively, and (E, F, G & H) ROC curves to depict the accuracy of ISS signature in identifying poor PDAC subtypes in TCGA discovery and three external validation cohorts, respectively.
These findings were subsequently validated in multiple additional datasets, which were in agreement with the initial discovery cohort findings, and the 5-year OS for ISP high and low-risk patients were significantly discriminatory; cohort-1 (ICGC; HR: 2.63 [1.56–4.41]; p<0.0001), cohort-2 (E-MTAB-6134; HR: 1.53 [1.14–2.04]; p=0.004), and cohort-3 (Moffit; HR: 2.33 [1.49–3.63]; p<0.0001; Figures 1B, C and D). These results demonstrate and highlight the robustness of our ISP gene signature in predicting unfavorable prognosis in PDAC patients.
The ISP gene signature robustly identifies poor molecular subtypes in PDAC
In addition to predicting survival, we next determined the performance of our ISP signature for its ability to identify recently identified transcriptomic molecular subtypes with poor survival outcomes in PDAC patients. These include the squamous subtype defined by Bailey14, the quasi-mesenchymal (QM) subtype by Collisson15, the basal-like subtype by Moffitt16, and the pure basal-like/stroma activated subtype reported by Puleo22 – all of which associated with significantly poor survival in PDAC patients.
In the TCGA discovery cohort, our ISP gene signature was markedly robust in identifying each of the three molecular PDAC subtypes with poor survival, as evidenced from excellent AUC values for each of the PDAC subtypes: Squamous (AUC=0.94 [0.90–99]), Quasi-mesenchymal (0.91 [0.86–96]), and Basal-like (0.80 [0.72–87]; Figures 1E). Likewise, in our additional validation cohorts, the diagnostic accuracy of ISP signature in identifying Squamous subtype in cohort-1 yielded an AUC value of 0.91 (0.85–97; Figures 1F). Likewise, in the validation cohort-2, the diagnostic accuracy for Pure Basal-like/Stroma Activated subtype was also quite remarkable (AUC=0.80 [0.75–0.85]; Figures 1G), which was subsequently confirmed in the validation cohort-3 for detecting PDAC patients with Basal (AUC=0.83 [0.74–0.92]) and Activated stromal subtypes (AUC=0.85 [0.78–0.92]; Figures 1H). These results were quite encouraging and highlight the potential clinical significance of our ISP signature for the identification of all the previously reported molecular subtypes with poor outcomes in PDAC patients, by utilizing just a 15-gene signature.
The ISP signature serves as an independent predictor of risk and survival outcomes in PDAC patients
To further evaluate the performance of our 15-gene ISP signature into a clinically translatable risk-stratification assay, we next analyzed the expression of these genes in a clinical cohort of 119 PDAC patients by performing qRT-PCR based assays. Once again, it was reassuring to note that in support of our discovery and in-silico validation cohort findings, the Kaplan-Meier survival analysis revealed that 5-year OS rates for ISP-derived high and low-risk patients were 18.5% and 52%, respectively (HR: 2.62 [1.50–4.56], p=0.0004, Figures 2A).
Figure 2.
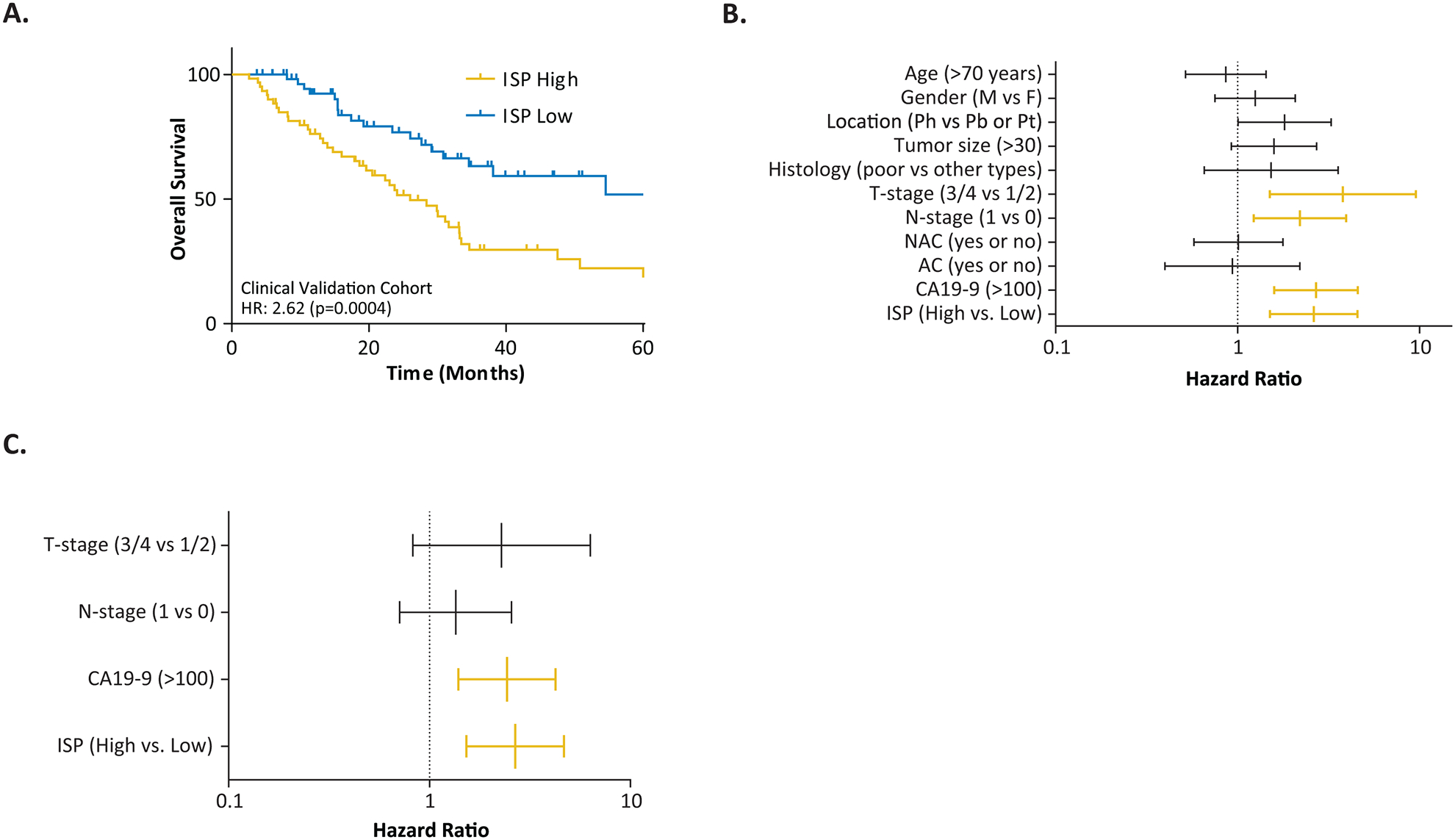
Performance of the ISP signature in predicting OS in the in-house clinical validation cohort. (A) 5-year OS in ISP high vs. low-risk patients depicted by KM plots, (B) Forest plot showing the Hazard Ratios of ISP signature as well as clinicopathological variables in the univariate CoxPH analyses (C) Multivariate CoxPH analyses and Hazard Ratios of ISP signature as well as clinicopathological variables.
We next performed univariate and multivariate cox regression analyses on this gene panel by including various clinicopathological features, the CA19–9 levels, as well as the neoadjuvant and adjuvant therapy status of PDAC patients. We assigned patients with CA-19–9 levels greater than 100 U/mL as high-risk group since the range of 37–50 U/mL is usually considered as a moderate risk-population23,24. In the univariate analysis, advanced T-stage (T1/2 vs. T3/T4 (p=0.005), positive lymph node metastasis (p=0.008), CA19–9 levels greater than 100 U/mL (p<0.0001), and the ISP signature (p=0.001) emerged as significant predictors of poor OS (Figure 2B). Subsequent multivariate analysis using only the features that were statistically significant in the univariate model, revealed that both the ISP gene signature (p=0.001) and higher CA19–9 levels (p=0.002) were independent risk factors for predicting poor OS in PDAC patients (Figure 2C). Taken together, our results validate and confirm that our ISP gene signature is quite robust, superior than currently used clinicopathological features in the clinic, and can serve as an independent predictor of survival in PDAC patients.
Establishment of a risk-nomogram for predicting survival in PDAC patients
To further improve the predictive accuracy of our ISP gene signature by examining its performance in combination with other univariate significant features (e.g. T-Stage, N-Stage and CA19–9 levels), we established an easy-to-use and clinically adaptable, risk-nomogram for predicting the survival probability in PDAC patients. As depicted in Figure 3A, a higher total score based on the sum of the assigned numbers for each of the factors in the nomogram was associated with a worse 3-year and 5-year OS rates. For instance, a patient with higher CA19–9 levels and a higher ISP score would yield a total of 190 points (90 points for high CA19–9 levels, and 100 points ISS High), with a predicted 3-year and 5-year OS rates of 39.0% and 27.0%, respectively.
Figure 3.

Nomograms Predicting Survival in PDAC Patients. (A) The nomogram to predict 3- and 5-year overall survival was created based on four independent prognostic factors (B) Kaplan-Meier curves demonstrating 5-year overall survival according to the nomogram derived high vs. low-risk patients stratified by median risk-score (C, D) Calibration plots comparing predicted and actual overall survival probabilities at 3- and 5-year follow-up. The 30-patient bootstrapped calibration plot for the prediction of 3- and 5-year overall survival is shown. The dotted line represents the ideal fit; circles represent nomogram-predicted probabilities; stars represent the bootstrap-corrected estimates; and error bars represent the 95% CIs of these estimates.
To assess the risk discriminatory potential of this nomogram, we performed Kaplan-Meier analysis by stratifying all patients based upon the median risk-scores derived from the nomogram consisting of ISP as well as T-Stage, N-Stage and CA19–9 levels (Figure 3B). The 5-year OS rates of high-risk patients exhibited substantially poor survival outcomes (15.7%) compared to low-risk patients (56%; HR: 3.72 [2.21–6.26]; p<0.0001). To validate the prediction model, the predictive performance of the nomogram was assessed by computing the discrimination index and the calibration plot of the model for the 3- and 5-year survival. The predictive accuracy of the nomogram was higher than the ISP signature alone with an accompanying C-statistic discriminatory index value of 0.70. This higher C-statistic reveals that ISP signature including CA19–9 and T/N-stage is quite robust in distinguishing subjects with different outcomes. The 30-patient bootstrapped calibration plots for the prediction of 3 and 5-year OS rates are shown in Figures 3C and 3D, respectively. The calibration plots of the ISP signature including CA19–9 levels and T/N-stages show excellent agreement between observed outcomes and predicted survival probabilities. These results further strengthen the clinical significance of our ISP signature in conjunction with CA19–9 levels and T/N-stages, with an overall superior predictive power for determining survival outcomes in PDAC patients.
DISCUSSION
Although several studies have recently reported existence of distinct molecular subtypes in PDAC patients that associate with poor prognosis14–16,19, their clinical application, especially for individual risk assessment has thus far been not been possible. Furthermore, an unequivocal consensus indicates that tumor microenvironment (TME) plays a central role in the pathogenesis of pancreatic cancer. In view of these facts, we designed and focused this study on the identification and development of a gene expression signature that intimately associates with three major cell types within pancreatic TME (immune cells, cancer-associated stroma and cell proliferation genes), and can help provide a more individualized risk-assessment in PDAC patients. Our efforts led us to discover a novel 15-gene ISP signature, which was successfully validated in multiple, large, publicly-available datasets from the ICGC, E-MTAB-6134 and Moffitt cohorts, followed by an independent validation of this panel in a clinical cohort of PDAC patients. In addition, our ISP gene signature was extremely robust in identifying all the previously reported molecular subtypes in PDAC; hence, offering a smaller panel of genes that can be easily translated into an easy-to-use clinical assay. Furthermore, the predictive potential of our ISP signature was further improved, when we combined it with a few selected clinicopathological features, as well as CA19–9 levels in a risk-assessment nomogram. We observed that the 5-year overall survival rates for ISP-derived high-risk patients are better in our clinical validation cohort compared to the TCGA discovery cohort. This could be attributed to the multi-disciplinary treatments including neo-adjuvant chemotherapy or chemo-radio therapy given for resectable PDAC patients in the clinical validation cohort.
Recent advances in combination therapies includes multiple chemotherapeutic regimens based upon either FOLFIRINOX (composed of folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin) or GnP (gemcitabine and nab-paclitaxel), in neoadjuvant (for resectable or borderline resectable) and palliative (unresectable) treatment of PDAC patients; however, in each scenario, the therapeutic efficacy of such modalities remains subpar5,25. This highlights the imperative to identify robust prognostic and predictive risk-stratification biomarkers that can help in the selection of appropriate patient subsets for the available therapies. Several clinicopathological factors such as tumor size, histologic grade, vascular invasion, perineural invasion, lymph node metastases, and distant metastases have been recognized as prognostic factors, but their prognostic potential in PDAC patients remains inadequate26. One of the reasons for the lack of availability of molecular markers that can be translated into the clinic is their inability to capture the intrinsic complexity within the TME in PDAC, which plays a central role in regulating the underlying disease biology27,28. The TME in pancreatic cancer comprises primarily of epithelial cells, stromal component, immune cells, cancer associated fibroblasts (CAFs) and extra cellular matrix. Both CAFs and immune cell infiltration plays a crucial role in PDAC development and progression27–29. Furthermore, evidence in recent years has repeatedly highlighted the role of CAFs and stromal activation in driving poor prognosis in PDAC30,31, as well as interactions between cancer cells and TME affects tumorigenesis, angiogenesis, therapy resistance, and the metastatic spread of disease32. In view of these observations, the ISP signature identified in our study deliberately focused on immune, stromal and proliferation-associated genes, which fittingly allowed identification of high-risk PDAC patients with poor survival outcomes.
Effective translation of the transcriptomic-based molecular subtypes reported in PDAC14–16,20 into the routine clinical practice has been hindered by several major challenges. First, all molecular classifications were developed based on gene expression profiles derived from microarray or RNA-seq platforms, which are impractical in actual clinical settings due to their exorbitant cost, long turnaround time, and reliance on bioinformatics expertise. Second, the discovery cohorts used for developing these classifiers contained of mostly fresh frozen samples; hence the performance of these hundreds of genes in FFPE specimens remains questionable. Our discovery and validation of a novel, 15-gene ISP signature offers a promising assay that identifies all poor subtypes previously reported in PDAC, using simple, PCR-based assays, in FFPE-derived RNA; making it attractive for an easier clinical translation.
Another interesting aspect of our gene classifier is that it works independent of the neoadjuvant and adjuvant treatment status. Considering that it is becoming more common to perform EUS-FNA supported collection of biopsy specimens prior to surgery or neo-adjuvant treatment33, it would be ideal to validate our ISP gene-signature in these biopsies; which, if successful, will allow a more prudent clinical-decision making for the selection of appropriate neoadjuvant treatment strategy in PDAC patients. One of the limitations of the study is that several clinicopathological as well as neoadjuvant treatment information is missing across the public cohorts.
In conclusion, using a systematic and comprehensive biomarker discovery and validation approach, we have identified and validated a novel, 15-gene ISP signature, that allows robust risk-stratification and facilitate identification of molecular subtypes in PDAC. Furthermore, we established a risk-nomogram which includes our ISP signature and key clinicopathological features, which offers an easy-to-deploy tool for identifying high-risk PDAC patients and predicting prognosis in patients suffering from this lethal malignancy.
Supplementary Material
Translational Relevance:
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease with dismal survival rates. Current clinicopathological risk factors are inadequate at identifying high-risk patients. Tumor microenvironment (TME), comprising of immune cells and cancer-associated fibroblasts, plays a key role in driving poor prognosis and resistance to chemotherapy. Herein, using a systematic and comprehensive biomarker discovery and validation approach, we have identified and validated a novel, 15-gene immune, stromal and proliferation (ISP) gene signature, that allows robust risk-stratification and facilitate identification of poor molecular subtypes in PDAC. Since we developed a ‘risk-nomogram’ including our 15-gene signature, CA19–9, T and N-stage in FFPE specimens, this can be readily applied to independent, future retrospective and prospective cohorts to evaluate the translational potential of this classifier in identifying high-risk PDAC patients and predicting prognosis in patients suffering from this lethal malignancy.
Acknowledgments
The present work was supported by the CA72851, CA187956, CA202797 and CA214254 grants from the National Cancer Institute, National Institute of Health, grants from the Sammons Cancer Center and Baylor Foundation, as well as funds from the Baylor Scott & White Research Institute, Dallas, TX, USA awarded to Ajay Goel.
Footnotes
Conflict of interest statement: All authors have no conflicts of interest to declare
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(6):2403–2413. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(15):1960–1966. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013;369(18):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton G, Palmer DH, Greenhalf W, et al. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. The Lancet Oncology. 2017;18(4):486–499. [DOI] [PubMed] [Google Scholar]
- 7.Kota J, Hancock J, Kwon J, Korc M. Pancreatic cancer: Stroma and its current and emerging targeted therapies. Cancer Lett. 2017;391:38–49. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Ehata S, Koinuma D, et al. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37(21):2757–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comen EA, Bowman RL, Kleppe M. Underlying Causes and Therapeutic Targeting of the Inflammatory Tumor Microenvironment. Front Cell Dev Biol. 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary B, Elkord E. Regulatory T Cells in the Tumor Microenvironment and Cancer Progression: Role and Therapeutic Targeting. Vaccines (Basel). 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma In: Grippo PJ, Munshi HG, eds. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India) 2012. [PubMed] [Google Scholar]
- 12.Linnekamp JF, Hooff SRV, Prasetyanti PR, et al. Consensus molecular subtypes of colorectal cancer are recapitulated in in vitro and in vivo models. Cell Death Differ. 2018;25(3):616–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Kandimalla R, Huang H, et al. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin Cancer Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. [DOI] [PubMed] [Google Scholar]
- 15.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine. 2011;17(4):500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nature genetics. 2015;47(10):1168–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1–2):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese G, Carugo A, Tepper J, et al. Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature. 2017;542(7641):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian B, Bigonnet M, Gayet O, et al. Gene expression profiling of patient-derived pancreatic cancer xenografts predicts sensitivity to the BET bromodomain inhibitor JQ1: implications for individualized medicine efforts. EMBO molecular medicine. 2017;9(4):482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puleo F, Nicolle R, Blum Y, et al. Stratification of Pancreatic Ductal Adenocarcinomas Based on Tumor and Microenvironment Features. Gastroenterology. 2018. [DOI] [PubMed] [Google Scholar]
- 21.Tibshirani R The lasso method for variable selection in the Cox model. Statistics in medicine. 1997;16(4):385–395. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki T, Reed JC. A GTP-binding adapter protein couples TRAIL receptors to apoptosis-inducing proteins. Nature immunology. 2001;2(6):493–500. [DOI] [PubMed] [Google Scholar]
- 23.Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3(2):105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballehaninna UK, Chamberlain RS. Serum CA 19–9 as a Biomarker for Pancreatic Cancer-A Comprehensive Review. Indian J Surg Oncol. 2011;2(2):88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(1):23–29. [DOI] [PubMed] [Google Scholar]
- 26.Bilici A Prognostic factors related with survival in patients with pancreatic adenocarcinoma. World J Gastroenterol. 2014;20(31):10802–10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nature reviews Gastroenterology & hepatology. 2012;9(8):454–467. [DOI] [PubMed] [Google Scholar]
- 28.Gore J, Korc M. Pancreatic cancer stroma: friend or foe? Cancer cell. 2014;25(6):711–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the Tumor Stroma for Treatment of Pancreatic Cancer. Gastroenterology. 2018;154(4):820–838. [DOI] [PubMed] [Google Scholar]
- 30.Biffi G, Oni TE, Spielman B, et al. IL-1-induced JAK/STAT signaling is antagonized by TGF-beta to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer discovery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang AY, Yachimski PS. Endoscopic Management of Pancreatobiliary Neoplasms. Gastroenterology. 2018;154(7):1947–1963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


