Abstract
Purpose
Currently available markers for early detection of diabetic nephropathy (DN), the leading cause of end stage renal disease, have some limitations. There is insufficient evidence from previous studies about the role of several circulating microRNAs (miRNAs) in the early development of DN. This study aimed to describe the expression of miRNA-377, miRNA-93, miRNA-25, miRNA-216a, and miRNA-21 in a sample of type 1 diabetic children and adolescents to explore their association with DN and some indices of kidney injury.
Patients and Methods
Seventy type 1 diabetic patients, with 5 years’ duration of diabetes or more, were recruited from Children’s Hospital, Faculty of Medicine, Cairo University. Quantitative real-time reverse-transcription PCR (qRT-PCR) was used to measure the expression of the above mentioned miRNAs in serum and to assess its association with DN, and the studied risk factors.
Results
There was a significantly higher percentage of up-regulation of miRNA-377 and miRNA-93 (P=0.03, 0.02, respectively) in addition to significant down-regulation of miRNA-25 (P=0.01) in patients with DN than in patients without DN. In patients with DN, expression of miR-216a was significantly negatively correlated with creatinine (r=−0.4, P=0.04) and positively correlated with eGFR using creatinine (r=0.5, P=0.03). In the same group, expression of miR-21 was positively correlated with urinary cystatin C (r=0.6, P=0.01) and was negatively correlated with e-GFR using cystatin c (r=−0.6, P=0.01). miRNA-93 was associated with increased risk (odds ratio=15, 95% CI=12.03–24.63, P=0.01), while miRNA-25 was associated with decreased risk for albuminuria (odds ratio=0.15, 95% CI=0.08–0.55, P=0.03).
Conclusion
miRNA-377, miRNA-93, miRNA-216a, and miRNA-21 may be implicated in the pathogenesis of DN, while miRNA-25 may have a reno-protective role. More studies are needed to document the value of these miRNAs as diagnostic biomarkers as well as therapeutic targets in DN.
Keywords: microRNAs, type 1 diabetes, diabetic nephropathy, albuminuria, children
Video Abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Diabetic nephropathy (DN) is one of the most serious and prevalent complications in diabetic patients leading to end-stage renal disease (ESRD) with high morbidity and mortality.1 It is crucial to identify patients who are more susceptible to develop DN for better control of the disease process. Albuminuria has been one of the standard methods of screening of renal affection; however, it may develop only in advanced stages of DN. In addition, the non-immunoreactive forms of albuminuria may not be detectable by the commonly available immunoassays.2
Cystatin C (CysC), a low molecular weight protein (13.36 KD) produced by all nucleated cells, is filtered freely by the glomeruli and reabsorbed in the tubules.3 In diabetic and pre-diabetic nephropathy, urinary CysC showed an early increase and patients with albuminuria showed elevated levels of urinary CysC compared to those without albuminuria. Consequently, urinary CysC was suggested to be a predictive biomarker for advancement of DN.4,5 MicroRNAs (miRNAs) are 19–25 nucleotide (nt) regulatory RNAs that may be able to regulate up to 30% of the protein-coding genes in the human genome.6 Research has shown that miRNAs may contribute to the development and pathogenesis of DN.7 In a diabetic kidney, up-regulation of certain miRNAs were postulated to bind to renoprotective genes causing the decreased expression of these genes, hence, may influence the progression of DN.8
One of the miRNAs previously reported to be involved in the pathogenesis of DN is miRNA-377 which was found to promote fibronectin expression in mesangial cells (MCs) through the down-regulation of manganese superoxide dismutase and p21-activated kinase.9 A second one is miRNA-93, which is a key controller of the vascular endothelial growth factor (VEGF) signaling in kidneys.10 A third one is miRNA-216a, whose up-regulation leads to mediated tumor growth factor β (TGF-β)-induced collagen expression in kidney cells.11 A fourth one is miRNA-21, which is a pro-fibrotic microRNA, whose role in renal injury was associated with phosphatase and tensin homolog (PTEN) up-regulation as well as TGF-β signaling.12 On the other hand, miRNA-25 was shown to be down-regulated in the MCs of diabetic rat kidneys. In DN, the decrease in miRNA-25 can lead to Nox4 up-regulation and oxidant stress promotion which in turn can result in renal dysfunction. Through inhibition of Nox4, miRNA-25 might be a DN-protective molecule.13
Nevertheless, the exact role of various miRNAs in development and progression of DN has not been fully understood,6,7 and the research evidence is still insufficient to support the effective diagnostic or possible therapeutic roles of different miRNAs in DN.14 The aim of this work was to study the expression of miRNA-377, miRNA-93, miRNA-25, miRNA-216a, and miRNA-21, in a sample of type 1 diabetic children and adolescents to explore their association with risk factors and other indices of DN and markers of acute kidney injury, opening new pathways for earlier diagnosis and effective management of renal affection in diabetes.
Materials and Methods
Study Design and Setting
This cross-sectional study was conducted on 70 children and adolescents with type 1 diabetes who were recruited from the Diabetes, Endocrine, and Metabolism Pediatric Unit (DEMPU), Children’s Hospital, Faculty of Medicine, Cairo University.
Patients’ Selection
Patients were included if they had type 1 diabetes for 5 years or more and were aged below 18 years of both sexes. Patients with nephropathy caused by acute inflammation, tuberculosis, autoimmune diseases, cancer, or cardiovascular disease were excluded from the study. Additional exclusion criteria were: active urinary tract infection and thyroid disorders.
Methods
The study protocol was compliant with the guidelines of the ethical committee of the Clinical and Chemical Pathology Department, as well as the Department of Pediatrics, Cairo University. The study and data collection were compliant with all local laws and also with the principles of the Declaration of Helsinki. Written informed consent was obtained from parents/guardians after an explanation of the aim and procedures of the study. The study was approved by the Ethical Committee of Cairo University.
Height and weight were measured and body mass index (BMI) was calculated and expressed as standard deviation scores (SDS). Blood pressure (BP) was measured on three occasions and the mean values were compared to age-specific percentiles for BP.15 Mean Glycosylated hemoglobin (HBA1c) of the previous year, fasting lipid profile (serum triglycerides, cholesterol, LDL cholesterol, and HDL cholesterol), serum creatinine, and albumin creatinine ratio (ACR) were assessed. ACR was estimated from first morning urine samples. Precautions were taken to exclude active urinary tract infection, exercise, hyperglycemia, or menstruation in females at the time of sampling. Two of three urine samples were used as evidence of albuminuria if ≥30 µg/mg in males or ≥42 µg/mg in females over a 3–6 months period.16
Urinary CysC was measured.3 The values of estimated glomerular filtration rate (eGFR) using serum creatinine was calculated by the equation (constant X length/serum creatinine) and the eGFR using CysC was calculated by the equation (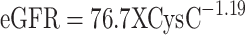 ).17
).17
Diabetic patients were classified as having or not having DN according to the presence of albuminuria: Group without DN (ACR <30 µg/mg in males, <42 µg/mg in females), and group with DN (ACR ≥30 µg/mg in males, ≥42 µg/mg in females).16
Molecular Studies
Quantitation of miRNA-377, miRNA-93, miRNA-25, miRNA-216a, and miRNA-21 in serum was done by real-time quantitative reverse transcription polymerase chain reaction (Real-Time qRT-PCR). miRNA-16 has been used as a reference gene as has been recommended from previous studies to be the most optimal reference to get an accurate result in serum miRNA studies.18,19
RNA Extraction
Tubes were thawed for 2 hours at room temperature and then centrifuged for 10 minutes at 4500x g. The supernatant was removed immediately, and the pellets were resuspended in 4 mL RNase-free water and centrifugated again. 500 μL of QIAzol solution (Qiagen) was added to the obtained pellets. Total RNA, including small RNAs, was isolated from serum using a Qiagen miRNeasy mini kit on QIAcube for automated extraction following the protocol provided by the manufacturer (Qiagen GmbH D-40724 Hilden, Hoffmann-La Roche AG, Max-Volmer-Straße, Germany). The total elution volume was 50 μL of RNAse-free H2O.
Quantification of RNA
Extracted RNA concentration of each sample was measured by Qubit Fluorometer (Life Technologies, 5 71 Van Allen way, Carlsbad, CA 92008. USA). Quality of isolated RNA was determined using Nanodrop ND-2000 (ThermoFisher Scientific). The ratio of absorbance at 260/280 nm was 1.95–2.13 for all samples. Isolated RNA was stored at −80°C until use.
Analysis of circulating MicroRNAs using single-plex TaqMan two step stem-loop real-time quantitative reverse transcription polymerase chain reaction was performed as follows:
Reverse Transcription
cDNA was synthesized starting from 1 ng miRNA using Taqman™ miRNA assays and the Taqman™ miRNA Reverse Transcription Kit (Thermo Fisher Scientific). The following Taqman™ miRNA assays for candidate miRNAs were used in this study: reverse transcription (RT) step: miRNA-216a, miRNA-21, miRNA-377, miRNA-25, and miRNA-93. The total volume of each RT reaction was 15 µL as follows: 7 µL of master mix prepared in the previous step, 5 µL of sample RNA, 3 µL of miRNA-216a, miRNA-21, miRNA-377, miRNA-25, miRNA-93, and miR-16. By the end of this step RNA samples were converted to cDNA using the micro-RNA specific stem loop primer and PCR step: PCR products were amplified from cDNA samples using the TaqMan® Small RNA Assay together with the TaqMan® Universal PCR Master Mix II.
Data Analysis Using Comparative CT Method (ΔΔ CT)
The level of miRNA expression was measured using the cycle threshold (CT). The expression for each miRNA is given by the difference between its CT value and the average CT value of reference genes per sample within a given sample set.
The relative quantity (RQ=2−∆∆CT) of miRNA-216a, miRNA-21, miRNA-377, miRNA-25, and miRNA-93 were normalized to miR-16, which was expressed at high levels in serum and relatively invariant across large numbers of samples and were calculated relative to their expression in serum of age-matched healthy controls.20 Using miR-16 as a reference gene, relative expression (fold change) for each candidate miRNA within each group was then calculated using the equation 2−∆∆CT.20
The ΔCT for each miRNA in each sample was calculated as follows:
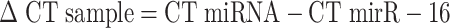 |
Then ∆∆CT was calculated:
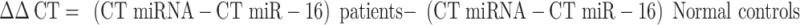 |
The fold change of the target gene expression level = 2−ΔΔCT.
The relative expression (fold change) for each candidate miRNA within each group was then calculated using the equation: 2−∆∆Ct.20
Statistical Analysis
All statistical analyses were conducted using the SPSS 21.0 software (IBM Corporation, Armonk, NY, USA). Quantitative data were normally distributed and were expressed as mean±SD, while qualitative data were expressed as frequencies (percentage). Patients were classified into two groups according to the presence of albuminuria as an evidence of DN. Comparison of categorical variables among groups was performed via chi-squared tests, while Student’s t-test was used to compare the means of the groups for continuous variables. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated by multivariable logistic regression to assess the association between each of the studied miRNAs and the risk factors or indices of DN. Receiver operating characteristic (ROC) analysis was attempted to find the diagnostic accuracy of all studied miRNAs in the assessment of risk of development of DN. P≤0.05 was regarded as statistically significant.
Results
The study included 70 patients; 35 males (50%) and 35 females (50%). The mean age of the study group was 13.21±3.66 years, the mean age at onset of diabetes mellitus (DM) was 5.59±3.36 years, the mean duration of DM was 7.73±3.19 years, and the mean insulin dose was 1.25±0.5 unit/kg/day. Mean weight SDS was −0.28±1.3, mean height SDS was −0.955±1.39, and mean BMI SDS was 0.41±0.98.
Table 1 shows the classification of patients into Group without DN (normal ACR) which included 25 patients (16 males and 9 females), and group with DN (increased ACR), which included 45 patients (19 males and 26 females). There were no statistically significant differences between both groups regarding age, sex distribution, age of onset of diabetes, duration of diabetes, body mass index, BP centiles, serum creatinine, e–GFR using creatinine, or serum lipids.
Table 1.
Demographic, Clinical and Laboratory Data in Patients Without and With Nephropathy
| DM with No Nephropathy (Normal ACR) (n=25) (Mean±SD) | DM with Nephropathy (Increased ACR) (n=45) (Mean±SD) | P-value | |
|---|---|---|---|
| Duration of diabetes (years) | 6.84±2.08 | 8.37±3.70 | 0.09 |
| Age of onset of diabetes (years) | 5.14±2.57 | 5.91±3.82 | 0.51 |
| BMI (SDS) | 0.90±0.40 | 0.80±0.30 | 0.06 |
| BP (percentile) | 85±10 | 87±12 | 0.07 |
| Serum LDL-C (mg/dL) | 105.72±37.73 | 102.09±25.76 | 0.98 |
| Serum HDL-C (mg/dL) | 46.96±13.08 | 53.71±18.19 | 0.11 |
| Serum cholesterol (mg/dL) | 170.44±41.89 | 178.71±37.23 | 0.36 |
| Serum TG (mg/dL) | 95.16±49.22 | 81.49±32.78 | 0.29 |
| Serum creatinine (mg/dL) | 0.66±0.2 | 0.70±0.16 | 0.19 |
| eGFR by Schwartz formula (mL/min/1.73 m2) | 89±8.99 | 79.60±10.86 | 0.03 |
Notes: P≤0.05 is statistically significant. N.B. 2 decimal places were used across all numbers (approximation was done to round to two decimal places). Serum creatinine, lipid profile: converted to mg/dL.
Abbreviations: BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; LDL-C, low density lipoproteins cholesterol; HDL, high density lipoproteins cholesterol; SDS, standard deviation score; TG, triglycerides; eGFR, estimated glomerular filtration rate.
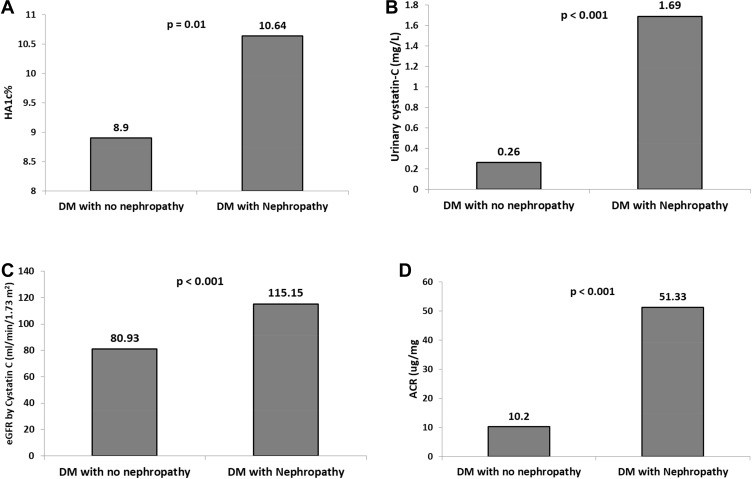
On the other hand, Figures 1A–D show statistically significantly higher values of HBA1c (P=0.01), urinary CysC, e-GFR-Cyst C, and ACR (P<0.001 for each of the latter three variables) in the group with DN than in the group without DN.
Figure 1.
Comparisons of laboratory markers between groups with and without diabetic nephropathy. (A) HBA1c, (B) urinary Cystatin C, (C) e-GFR using cystatin c . (D) ACR.
Abbreviations: ACR, albumin creatinine ratio; DM, diabetes mellitus; e-GFR, estimated glomerular filtration rate; HBA1c, glycosylated hemoglobin.
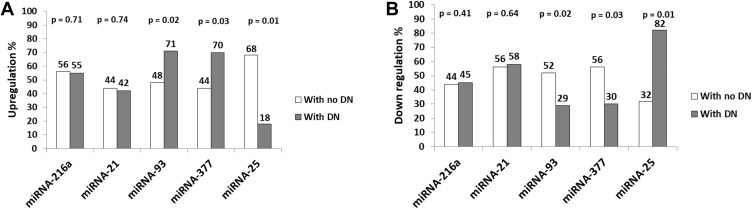
As regards miRNAs’ expression, Figure 2A shows that there was a statistically significant higher percentage of up-regulation of miRNA-377 and miRNA-93 in the group with DN than the group without DN (P=0.03, 0.02, respectively), in addition to a statistically significant higher percentage of upregulation of miRNA-25 in the group without DN than the group with DN (P=0.01). On the other hand, Figure 2B shows that there was a statistically significant higher percentage of downregulation of miRNA-25 in the group with DN than the group without DN (P=0.01), in addition to a statistically significant higher percentage of downregulation of miRNA-377 and miRNA-93 in the group without DN than the group with DN (P=0.03, 0.02, respectively).
Figure 2.
Prevalence of up-regulation and down-regulation of expression of studied miRNAs in groups with and without diabetic nephropathy. (A) Upregulation of miRNAs, (B) downregulation of miRNAs.
Abbreviations: DN, diabetic nephropathy; miRNA, micro RNA.
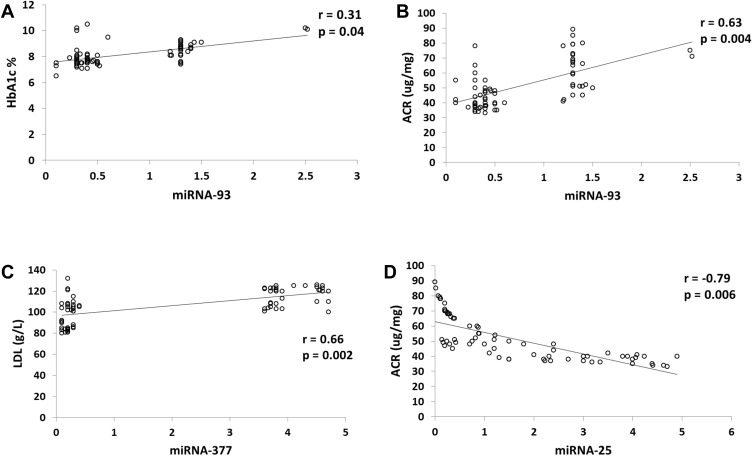
On studying the total group of cases, Figure 3A shows that there was a significant positive correlation between miRNA-93 and HbA1c (r=0.31, P=0.04), Figure 3B shows that there was a significant positive correlation between miRNA-93 and ACR (r=0.63, P=0.004). Figure 3C shows a significant positive correlation between miRNA-377 and LDL (r=0.66, P=0.002), while Figure 3D shows there was a significant negative correlation between miRNA-25 and ACR (r=−0.79, P=0.006).
Figure 3.
Significant correlations between fold change of miRNA expression and laboratory markers in all cases (n=70). (A) miRNA-93and HbA1c, (B) miRNA-93 and ACR, (C) miRNA-377 and LDL-cholesterol, (D) miRNA-25 and ACR.
Abbreviations: ACR, albumin creatinine ratio; HBA1c, glycosylated hemoglobin; LDL, low density lipoproteins.
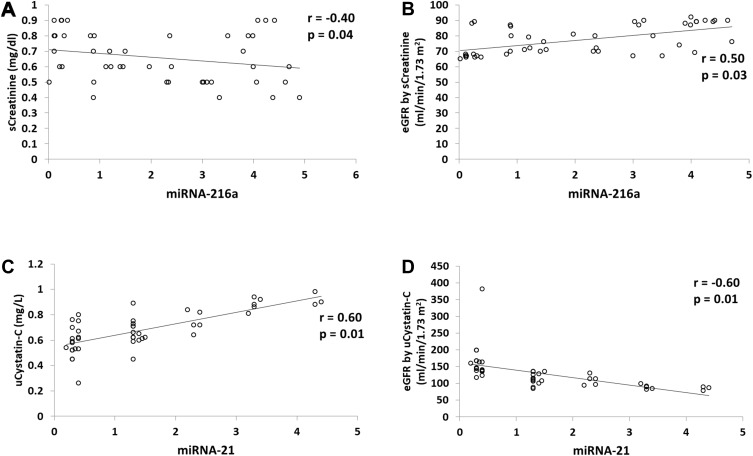
In the group of patients with DN, Figures 4A and B show that expression of miR-216a was significantly negatively correlated with creatinine (r=−0.4, P=0.04), and significantly positively correlated with eGFR when estimation was done using creatinine (r=0.5, P=0.03). In the same group, Figures 4C and D show that expression of miR-21 was positively correlated with urinary CysC levels (r=0.6. P=0.01) and negatively correlated with e-GFR using CysC (r= −0.6, P=0.01) respectively.
Figure 4.
Significant correlations between fold change of miRNA expression and laboratory markers in cases with diabetic nephropathy (n=45). (A) miRNA-216a and creatinine, (B) miRNA-216a and eGFR using creatinine, (C) miRNA-21 and urinary cystatin C, (D) miRNA-21 and and e-GFR using cystatin c.
Abbreviations: e-GFR, estimated glomerular filtration rate; u, urinary.
Table 2 shows the results of multiple regression analysis: miRNA-93 was associated with a statistically significant increased risk for the development of albuminuria (odds ratio=15, 95% CI=12.03–24.63, P=0.01), while miRNA-25 was associated with a statistically significant decreased risk for development of albuminuria (odds ratio=0.15, 95% CI=0.08–0.55, P=0.03).
Table 2.
Risk Assessment for Studied Micro RNAs for Development of Albuminuria
| Albuminuria | ||
|---|---|---|
| OR (95% CI) | P-value | |
| miRNA-216a | 0.79 (0.3–3.5) | 0.99 |
| miRNA-21 | 0.69 (0.19–2.47) | 0.58 |
| miRNA-93 | 15 (12.03–24.63) | 0.01* |
| miRNA-377 | 0.89 (0.11–7.11) | 0.91 |
| miRNA-25 | 0.15 (0.08–0.55) | 0.03* |
Note: *P-value<0.05 is statistically significant.
Abbreviations: CI, confidence interval; miRNA, micro RNA; OR, odds ratio.
ROC analysis was attempted to find the diagnostic accuracy of all studied miRNAs in the assessment of risk of development of diabetic nephropathy. All tests were not performing well, area under the curve (AUC) was <0.65, P>0.05 for all miRNAs.
Discussion
Early identification of DN is crucial for its effective diagnosis and treatment to decrease morbidity and mortality.16 Although albuminuria is considered as the gold standard for the diagnosis of DN, renal affection can also be studied through other parameters including urinary albumin excretion rates, urinary albumin creatinine ratios, glomerular filtration rate, or serum creatinine.2 However, none of the above parameters is sensitive enough to early detect all cases of renal injury in diabetes.4,5
In the present study, there were no significant differences between the two groups (with and without DN) regarding demographic, anthropometric, and clinical data which ensures the comparability of both groups for further comparisons. As regards laboratory data, the lack of difference regarding serum creatinine or e-GFR (calculated using creatinine) between both groups may be because serum creatinine is a late indicator of impairment of kidney function.5 Moreover, duration of diabetes in the study group was not long enough to show the progression of this impairment, although one of the groups had albuminuria, which is evidence of an early stage of DN.
On the other hand, in the group with DN, there were statistically significant higher levels of urinary CysC and eGFR (calculated using CysC) than in the group without DN. It has to be noted that urinary concentration of CysC is independent of muscle mass and tubular secretion, and has been thus considered as a better alternative marker to creatinine for the evaluation of renal function and the estimation of glomerular filtration rate (GFR).4 It has to be stressed that current methods of measuring eGFR are inaccurate to monitor the decline in kidney function in Diabetic Kidney Disease (DKD). eGFR by using CysC may be an overestimation, while calculation using creatinine is an underestimation of true GFR.5 Furthermore, only one third of type 1 diabetic nephropathy had albuminuria with progression of DKD in previous research work.2 The higher HBA1c values in the DN group in this study confirms the results of previous research that hyperglycemia is a major factor behind the progression of diabetic nephropathy.16
miRNAs and their role in DN have recently become the focus of important research studies as analyzed by a recent systematic review and metanalysis.21 Several miRNAs are related to DN; some of them play a role in the pathogenesis while others serve as preventers of renal affection. Increasing evidence indicates that some miRNAs can affect the progress of DN by regulating various signal pathways in the pathogenesis of DN, such as TGF-β, Akt, and Nuclear Factor-kappa β.22 Thus, miRNAs were proposed as useful biomarkers of DKD, being simple, abundant in urine and plasma, stable, renal tissue-specific,6 and its degree of change is often more sensitive than the currently commonly used clinical indicators, such as urine protein.2 Therefore, miRNAs may be considered as emerging biomarkers to assist in the early diagnosis of DN as well as potential future therapeutic intervention in DN.7
In this study, there was a statistically significant higher percentage of up-regulation of miRNA-377 in patients with DN than the group without. Moreover, there was a significant positive correlation between miRNA-377 expression and LDL-cholesterol; the latter has been considered a risk factor for vascular complications of diabetes.16 This is concordant with previous research which reported that the expression of urinary miR-377 was significantly higher in patients with albuminuria than those with normoalbuminuria or healthy controls.9 Moreover, expression of miRNA-377 has also been reported in the same study to be positively correlated with carotid intima thickness in diabetic patients which points to its possible contribution to the pathogenesis of microvascular and macrovascular complications of diabetes.9 Similarly, Wang et al23 reported that miRNA-377 was upregulated in DN, and explained that overexpression of miRNA-377 could target the inhibition of synthesis of some important mesangial cell proteins, this may dangerously lead to fibronectin accumulation, and might enhance oxidative stress in mesangial cells. Consequently, trials of inhibition of miRNA-377 expression have been initiated as a new approach to treatment of DN.23
In this study, there was a statistically significant higher percentage of up-regulation of miRNA-93 in patients with DN, and miRNA-93 was a significant independent variable for the development of albuminuria. Moreover, there was a significant positive correlation between miRNA-93 and each of HBA1c and ACR in diabetic patients. This may suggest the implication of miRNA-93 in development of diabetic nephropathy. In contrast, other studies reported that miRNA-93 was downregulated in DN and that it showed antiangiogenic and antifibrotic effects.24 Moreover, it was found that overexpression of miR-93 may inhibit TGF-β1 induced endothelial to mesenchymal transition and halt renal fibrogenesis via targeting ORAI 1 expression in HK2 cell lines, which could be implicated in the pathogenesis of DN.24,25 The controversy between the results of this study and that of others deserve further research to elucidate the role this miRNA might play in DN.
In the present study, there was a statistically significant higher percentage of down-regulation of miRNA-25 in patients with DN than the group without. Moreover, there was a significant negative correlation between miRNA-25 and ACR. Previous research demonstrated that decreased levels of miR-25 may suppress NADPH oxidase 4 (Nox 4) gene in mesangial cells, which proposes that miR-25 may be a useful novel therapeutic agent as an anti-oxidant and anti-apoptotic through its activation of the PTEN/Akt pathway.13,26 Moreover, low levels of miRNA-25 were found in diabetic patients which were proposed to result in increased production of reactive oxygen species (ROS); an established contributor to diabetic complications.27 Consequently, upregulation of miRNA-25 in diabetic patients can reverse kidney changes and reduce hypertension in DN.28 Hence, miR-25 may have a reno-protective role, and can be used as a therapeutic intervention for diabetic complications including DN.
In this study, among patients with DN, miRNA-216a was significantly negatively correlated with creatinine and positively correlated with eGFR when estimation was done by creatinine. This is in concordance with previous research which found that urinary miR-216a was significantly lower in all children with type 1 diabetes and the lowest levels were among the albuminuric group.9 Research showed that under diabetic conditions, miR-216a was upregulated, followed by the inhibition of Y box binding protein 1 which led to increased expression of TGF-β stimulated clone 22, eventually resulting in high production of COL1 alpha2 in MCs.11
In the albuminuric group in this study, expression of miR-21 was positively correlated with urinary levels of cystatin c and negatively correlated with eGFR using cystatin c. miRNA-21 is a key player in the pathogenesis of diabetic nephropathy and has been found to be highly expressed in renal tissues of patients with DN.29,30 MiR-21 is a multipotent miRNA that has been frequently studied to promote cell proliferation, inflammation, angiogenesis, and immune destruction.3,8 In recent years, studies have confirmed that miR-21 is one of the most important microRNAs involved in renal fibrosis, and its level is upregulated in renal tissues.31–33 Furthermore, miRNA-21 was found to inhibit proliferation of mesangial cells that were exposed to high glucose and it induced Akt activation, thus leading to mesangial hypertrophy and increased fibronectin production.34 Recent experimental studies revealed that miR-21 inhibitors led to marked improvement of both the structural and functional ability of kidneys in animal models. So, miRNA-21 may be a useful future therapeutic target as an anti-fibrotic agent for the treatment of diabetic nephropathy.35
Strengths and Limitations
Most studies probing the role of miRNAs in DN focused on adults with type 2 diabetes; only a few studies recruited type 1 diabetic children and adolescents. This study evaluated the expression of five miRNAs in a sample of type 1 diabetic children and adolescents to explore their association with several risk factors of DN. Limitations of this study may include the small sample size, and lack of prospective follow-up to assess the association of the studied miRNAs to the degree of progression or prevention of DN.
Future Research Directions
More research is needed to fully understand the exact and detailed regulation and function of different miRNAs and their role in DN. Experimental verification of target genes of each miRNA is also needed, as miRNA regulations occur basically at a translation level. Better understanding of miRNA biogenesis and function will be beneficial for better application of miRNA-based treatment for DN. Exploration of new techniques to upregulate the renoprotective miRNAs or downregulate the harmful miRNAs should gain more attention in future research. Therapeutic targeting of miRNAs would aim to reverse early cytopathological changes before renal structural alterations take place. Using mimics of renoprotective miRNAs may result in inhibition of harmful miRNAs, TGF-β, and fibronectin accumulation, leading to amelioration of albuminuria, and a decrease of both renal fibrosis and inflammation as has been previously proposed.7,12,21,35 This may help to halt the progression to End Stage Kidney Disease, decreasing the mortality and morbidity and improving the quality-of-life of patients with diabetes.
Conclusion
In conclusion, there was significant up-regulation of miRNA-377 and miRNA-93 in addition to significant down-regulation of miRNA-25 in patients with DN. In patients with DN, expression of miR-216a was significantly negatively correlated with creatinine and positively correlated with eGFR using creatinine. In the same group, expression of miR-21 was positively correlated with urinary cystatin C and was negatively correlated with e-GFR using cystatin c. miRNA-93 was associated with increased risk while miRNA-25 was associated with decreased risk for albuminuria.
There was a significant positive correlation between miRNA-93 and each of HbA1c and ACR as well as a positive correlation between miRNA-377 and LDL-C. On the other hand, a significant negative correlation was found between miRNA-25 and ACR. Thus, it can be deduced that miRNA-216a, miRNA-21, miRNA-377, and miRNA-93 might be associated with DN and its risk factors, while miRNA-25 may have a renoprotective role. Further studies are needed to deepen our understanding of the pathogenesis of DN and the possible diagnostic, prognostic, and therapeutic roles of miRNAs in diabetes-related renal injury.
Disclosure
The authors report no conflicts of interest in this work. There are no financial or non-financial competing interests for any of the authors.
References
- 1.Maggiore U, Budde K, Heemann U, et al.; ERA-EDTA DESCARTES working group. Long-term risks of kidney living donation: review and position paper by the ERA-EDTA DESCARTES working group. Nephrol Dial Transplant. 2017;32(2):216–223. doi: 10.1093/ndt/gfw429 [DOI] [PubMed] [Google Scholar]
- 2.Liu R, Li G, Cui XF, Zhang DL, Yang QH, Mu XY. Methodological evaluation and comparison of five urinary albumin measurements. J Clin Lab Anal. 2011;25:324–329. doi: 10.1002/jcla.20477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SS, Song SH, Kim IJ, et al. Urinary cystatin c and tubular proteinuria predict progression of diabetic nephropathy. Diabetes Care. 2013;36(3):656–661. doi: 10.2337/dc12-0849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou B, Zou H, Xu G. Clinical utility of serum cystatin c in predicting diabetic nephropathy among patients with diabetes mellitus: a meta-analysis. Kidney Blood Press Res. 2016;41(6):919–928. doi: 10.1159/000452593 [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Strengths and limitations of estimated and measured GFR. Nat Rev Nephrol. 2019;15(12):785–786. doi: 10.1038/s41581-019-0213-9 [DOI] [PubMed] [Google Scholar]
- 6.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep. 2016;16(3):35. doi: 10.1007/s11892-016-0724-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Kong L, Zhou S, et al. The role of microRNAs in diabetic nephropathy. J Diabetes Res. 2014;4:920134. doi: 10.1155/2014/920134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622–638. doi: 10.1038/nrd4359 [DOI] [PubMed] [Google Scholar]
- 9.El-Samahy MH, Adly AA, Elhenawy YI, et al. Urinary miRNA-377 and miRNA-216a as biomarkers of nephropathy and subclinical atherosclerotic risk in pediatric patients with type 1 diabetes. J Diabetes Complications. 2018;32(2):185–192. doi: 10.1016/j.jdiacomp.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 10.Tang J, Yao D, Yan H, Chen X, Wang L, Zhan H. The role of MicroRNAs in the pathogenesis of diabetic nephropathy. Int J Endocrinol. 2019;2019:871906. doi: 10.1155/2019/8719060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato M, Wang L, Putta S, et al. Post-transcriptional up-regulation of Tsc-22 by Ybx1, a Target of miR-216a, mediates TGF-β-induced collagen expression in kidney cells. J Biol Chem. 2010;285(44):34004–34015. doi: 10.1074/jbc.M110.165027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the micro RNA perspective. Diabetes. 2011;60:1832–1837. doi: 10.2337/db11-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Zhang Y, Wang Z. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol. 2010;32(6):581–589. doi: 10.1159/000322105 [DOI] [PubMed] [Google Scholar]
- 14.Simeoli R, Fierabracci A. Insights into the role of MicroRNAs in the onset and development of diabetic neuropathy. Int J Mol Sci. 2019;20:4627. doi: 10.3390/ijms20184627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Heart, Lung and Blood Institute. The fourth report on diagnosis, evaluation and treatment of high BP in children and adolescents. Pediatrics. 2004;114:555–576. doi: 10.1542/peds.114.2.S2.555 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S1–S2. doi: 10.2337/dc20-Sint [DOI] [PubMed] [Google Scholar]
- 17.Garg V, Kuman M, Mahapatra HS, Chitkora A, Gadpoyle AK, Sekhan V. Novel urinary biomarkers in prediabetic nephropathy. Clin Exp Nephrol. 2015;19(5):895–900. doi: 10.1007/s10157-015-1085-3 [DOI] [PubMed] [Google Scholar]
- 18.Lange T, Stracke S, Rettig R, et al. Identification of miR-16 as an endogenous reference gene for the normalization of urinary exosomal miRNA expression data from CKD patients. PLoS One. 2017;12(8):e0183435. doi: 10.1371/journal.pone.0183435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Zhang X, Yuan J, et al. Evaluation of the performance of serum miRNAs as normalizers in microRNA studies focused on cardiovascular disease. J Thorac Dis. 2018;10(5):2599–2607. doi: 10.21037/jtd.2018.04.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of the relative gene expression data using real time quantitative PCR and the 2−∆∆Ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 21.Wang LP, Gao YZ, Song B, et al. MicroRNAs in the progress of diabetic nephropathy: a systematic review and meta-Analysis. Evid Based Complement Alternat Med. 2019;3513179. doi: 10.1155/2019/351317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava SP, Hedayat AF, Kanasaki K, Goodwin JE. MicroRNA crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front Pharmacol. 2019;10:904. doi: 10.3389/fphar.2019.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Wang Y, Mintoetal AW. MicroRNA-377 is upregulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008;22(12):4126–4135. doi: 10.1096/fj.08-112326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Zhang L, Hao J, Li N, Tang J, Hao L. Up-regulation of microRNA-93 inhibits TGF-β1-induced EMT and renal fibrogenesis by down-regulation of Orai1. J Pharmacol Sci. 2018;136(4):218–227. doi: 10.1016/j.jphs.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 25.Long J, Wang Y, Wang W, Chang BHJ, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285(30):23457–23465. doi: 10.1074/jbc.M110.136168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Zhu X, Zhang J, Shi J. MicroRNA-25 inhibits high glucose-induced apoptosis in renal tubular epithelial cells via PTEN/AKT pathway. Biomed Pharmacother. 2017;96:471–479. doi: 10.1016/j.biopha.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 27.Gholaminejad A, Abdul Tehrani H, Fesharaki MG. Identification of candidate microRNA biomarkers in diabetic nephropathy: a meta-analysis of profiling studies. J Nephrol. 2018;31(6):813. doi: 10.1007/s40620-018-0511-5 [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Li H, Liu J. Variations in MicroRNA-25 expression influence the severity of diabetic kidney disease. J Am Soc Nephrol. 2017;28(12):3627–3638. doi: 10.1681/ASN.2015091017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong X, Chung ACK, Chen HY, et al. MiR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56(3):663–674. doi: 10.1007/s00125-012-2804-x [DOI] [PubMed] [Google Scholar]
- 30.McClelland AD, Herman-Edelstein M, Komers R, et al. miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci. 2015;129(12):1237–1249. doi: 10.1042/CS20150427 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Peng H, Chen J, et al. MicroRNA-21 protects from mesangial cell proliferation induced by diabetic nephropathy in db/db mice. FEBS Lett. 2009;583(12):2009–2014. doi: 10.1016/j.febslet.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Kriegel AJ, Liu Y, et al. Delayed ischemic preconditioning contributes to renal protection by upregulation of miR-21. Kidney Int. 2012;82(11):1167–1175. doi: 10.1038/ki.2012.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JY, Gao YB, Zhang N, et al. MicroRNA-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Mol Cell Endocrinol. 2014;392(1–2):163–172. doi: 10.1016/j.mce.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 34.Fouad M, Salem I, Elhefnawy K, Raafat N, Faisal A. MicroRNA-21 as an early marker of nephropathy in patients with type 1 diabetes. Indian J Nephrol. 2020;30(1):21–22. doi: 10.4103/ijn.IJN_80_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kölling M, Kaucsar T, Schauerteetal C. Therapeutic miR21 silencing ameliorates diabetic kidney disease in mice. Mol Ther. 2017;25(1):165–180. doi: 10.1016/j.ymthe.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]