Abstract
Background
Oscillometry is a tool to measure respiratory impedance that requires minimal patients’ effort. In patients with chronic obstructive pulmonary disease (COPD), the correlation of respiratory impedance at rest with exertional ventilatory parameters, including exercise tolerance, has scarcely been reported. In addition, the utility of oscillometric parameters might differ between the inspiratory and expiratory phases due to airflow obstruction during expiration, but the hypothesis had not been validated. The aim of the present study was to investigate whether oscillometric parameters are associated with exertional ventilatory parameters in patients with COPD.
Methods
Fifty-five subjects with COPD who attended clinics at the National Hospital Organization Osaka Toneyama Medical Center performed spirometry, oscillometry, and cardiopulmonary exercise testing (CPET) within 2 weeks. The correlations between parameters of spirometry, oscillometry, and CPET were analyzed using Spearman’s rank correlation coefficient, univariate, and multivariate analyses.
Results
Respiratory reactance had better correlations with the CPET parameters than respiratory resistance. Moreover, inspiratory reactance at rest correlated with the CPET parameters stronger than expiratory reactance. In particular, inspiratory resonant frequency (Fres-ins) correlated with peak oxygen uptake (rS=−0.549, p<0.01) and dead space to tidal volume ratio at peak exercise (rS=0.677, p<0.01) and the best predicted expiratory tidal volume (VT ex) at peak exercise of all the oscillometric parameters (rS=−0.679, p<0.01). However, the correlation between Fres-ins and VT ex at peak exercise became weak in subjects with severe and very severe COPD during exercise.
Conclusion
Measurement of respiratory reactance is useful for the effortless evaluation of not only exertional ventilatory parameters but exercise tolerance in patients with COPD. The correlation of respiratory impedance with exertional ventilatory parameters can become weak in patients with advanced COPD; thus, the measurement of oscillometry might not be appropriate for evaluating exertional ventilatory parameters of patients with advanced COPD.
Keywords: dyspnea, dynamic hyperinflation, airflow obstruction, resonant frequency, tidal volume
Introduction
Globally, chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death and a major cause of morbidity.1 Improving exercise tolerance or better physical activity would result in the better prognosis in COPD patients.2 Exercise tests, including 6-minute walk testing (6MWT) and cardiopulmonary exercise testing (CPET), are the gold standard for evaluation of exercise tolerance.3–5 6MWT is common and easier to perform than CPET, but CPET can evaluate exertional ventilatory parameters more precisely.4,5 Often, however, the tests cannot be performed in patients with dysmobility, making the evaluation of exertional ventilatory parameters difficult. Therefore, effortless evaluations of exertional ventilatory parameters are needed in the patients. As methods easy to evaluate exertional ventilatory parameters, pulmonary function tests, including spirometry and oscillometry, might be important.6,7
Oscillometry, which involves measurements of within-breath changes in respiratory impedance, measures respiratory resistance (Rrs) and respiratory reactance (Xrs). Rrs represents the sum of airway resistance and viscous resistance of the lung and thoracic tissue,8 while Xrs is considered to reflect the elastance and inertia of the respiratory system.9 Measurement of respiratory impedance using oscillometry is less time-consuming and requires minimal patients’ cooperation.10 In fact, oscillometry can be used to evaluate patients with severely advanced COPD who are unable to perform even spirometry.10
In patients with COPD, Rrs and resonant frequency (Fres) correlate negatively and Xrs at a lower frequency of oscillation correlates positively with forced expiration volume in 1 second (FEV1)/forced vital capacity (FVC) ratio.11 Since peripheral airway obstruction becomes more severe during expiration,12 these oscillometric parameters change dynamically during respiratory cycle. In particular, Rrs becomes higher and Xrs shifts to a more negative direction in the expiratory phase.13,14 Moreover, when airflow obstruction develops, expiratory flow at a given lung volume reaches its maximal value despite increasing expiratory driving pressure; this is called expiratory flow limitation (EFL).15–17 EFL during exercise promotes dynamic hyperinflation and contributes to a rapid and shallow breathing pattern.15,18 Xrs is normally thought to reflect the elastic and inertial properties of the respiratory system in patients without EFL.19 In patients with EFL, however, oscillatory signals cannot pass through the points of airway obstruction and reach the alveoli; thus, Xrs reflects the mechanical properties of airways proximal to the choke points which are much stiffer than the periphery and becomes more negative.19
As other pathophysiology in COPD patients, ventilatory inefficiency during exercise are reported.16,20 In patients with ventilatory inefficiency, dead space to tidal volume ratio (VD/VT) becomes higher during exercise.20–22 Ventilatory inefficiency is one of the components that affect exercise tolerance in COPD,23 but the correlation between ventilatory inefficiency and oscillometric parameters had not been investigated.
The above observations suggest that the utility of oscillometric parameters might differ between the inspiratory and expiratory phases. However, the association between each inspiratory and expiratory oscillometric parameter and ventilatory CPET parameters has never been thoroughly investigated, although the association of oscillometry with 6MWT and CPET has been previously reported.24,25 In addition, the utility of each oscillometric parameter for evaluating and predicting exertional ventilatory parameters, including ventilatory inefficiency and exercise tolerance, has not been investigated in patients with COPD. The information obtained from oscillometry might be clinically useful for assessing the mechanisms of exercise impairment and the effects of interventions.
The aims of the present study were as follows: (1) to investigate inspiratory and expiratory oscillometric parameters that are associated with exertional ventilatory parameters in COPD patients; and (2) to confirm the utility of oscillometry in COPD patients with various airflow obstructions.
Methods
Subjects
A total of 105 patients with COPD who attended clinics at National Hospital Organization Osaka Toneyama Medical Center or participated in several ethically approved research studies on COPD, between August 2015 and July 2019 were screened in the study. The patients were confirmed to have a FEV1/FVC ratio of <0.70 after using a bronchodilator and were diagnosed with COPD based on their spirometry, smoking history, and symptoms including chronic cough, sputum, and dyspnea. The patients therefore satisfied the definition of the Global Initiative for Chronic Obstructive Lung Disease (GOLD).26 Only COPD patients who underwent spirometry, oscillometry, and CPET were included. Their drug regimens were not changed within 4 weeks before the measurements in this study. Patients were excluded if they were not clinically stable or had exacerbations, defined as increased dyspnea associated with a change in the quality and quantity of sputum, for at least three months before the present study. Patients were excluded if they had malignant tumors, severe heart disease, or bronchial asthma (ie a history of bronchial asthma, or respiratory symptoms that i) occur variably over time and vary in intensity, ii) occur at night or on waking, or iii) are triggered by exercise, including CPET). The qualified 55 symptomatic subjects who met the study criteria [modified Medical Research Council (MRC) dyspnea scale ≥1] were evaluated using the examinations described in the following sections.
Study Design
The subjects were evaluated using all three evaluation methods, oscillometry, spirometry, and CPET, within, at most, a 2-week time period when the subjects’ clinical symptoms were stable. If oscillometry and spirometry were performed on the same day, spirometry was performed after oscillometry. Short-acting β2-agonists were not used for more than 12 hours before these tests in all subjects. Long-acting antimuscarinic agents (LAMA) and long-acting β2-agonists (LABA) were not withdrawn before the measurements of oscillometry, spirometry, and CPET. The Institutional Review Board of National Hospital Organization Osaka Toneyama Medical Center approved the study protocols and chose an opt-out system to obtain subjects’ informed consent (approval number, TNH-2,019,037).
Measurement of Respiratory Impedance Using Oscillometry
Respiratory impedance was measured at rest with broadband oscillometry using a commercially available device (Mostgraph-01, Chest MI Co, Ltd, Tokyo, Japan). The methods were performed according to the standard recommendations.8 Partitioned (inspiratory and expiratory) and average oscillometric parameters and were measured. Differences between inspiratory and expiratory phases (Δ) were calculated regarding all oscillometric parameters. As indicators of the frequency dependence of Rrs, Rrs at 5 and 20 Hz (R5 and R20, respectively) and the difference between R5 and R20 (R5-R20) were used. In addition, Xrs at 5 Hz (X5), Fres, and a low-frequency reactance area (ALX) were used as indicators of respiratory reactance. Fres indicates the point where Xrs becomes more dominated by inertial rather than elastic forces, and ALX is defined as the integral of X5 to the Fres.
CPET
Symptom-limited exercise tests were performed using an electrically-braked cycle ergometer (CV-1000SS, Lode, Groningen, The Netherlands) using a CPET system (Marquette CASE series T 2001, GE Healthcare, Tokyo, Japan; Aeromonitor AE 310S, Minato Medical Science Co, Ltd, Osaka, Japan).5 First, pre-exercise resting measurements were taken after reaching a steady-state period during at least 3 min of breathing through a mask. Next, incremental testing was performed by increasing 10W per 2 minutes. Before commencing the CPET, subjects were asked to put in maximum effort in the exercises performed during CPET. CPET was then performed until exhaustion without further encouragement, especially during exercise. The subjects were asked to maintain a cycle ergometer speed of about 60 rpm. The following CPET parameters were measured: expiratory tidal volume (VT ex), VD/VT, mean expiratory flow [VT ex/expiratory time (Te)], breathing frequency (fR), minute ventilation (V’E), V’E/V’CO2, inspiratory duty cycle (Ti/Ttot), and oxygen uptake (V’O2). These data were measured breath-by-breath and collected as 30 s averages at rest, during exercise at 2 min intervals, and at the end of exercise. In addition, dyspnea intensity (10-point modified Borg category-ratio scale) was evaluated at rest, at the last 15 s of each exercise stage, and at the end of exercise.
Spirometry
The subjects underwent spirometry using the CHESTAC 8800 spirometer (Chest MI, Inc, Tokyo, Japan) before CPET according to the recommendations of the American Thoracic Society.27 Predicted vital capacity and predicted FEV1 were calculated according to the formula developed by the Japanese Respiratory Society.28
Statistical Analysis
The subjects were grouped into the GOLD stage I and II subgroup (Subgroup A) and the GOLD stage III and IV subgroup (Subgroup B). The Mann–Whitney U-test was used for comparison of various parameters among the subgroups. Spearman’s rank correlation coefficient (rS) and univariate analyses were used for bivariate correlation analysis of the parameters of respiratory impedance and CPET. Multiple stepwise linear regression analysis was performed to detect the variables of oscillometry that were significant determinants of VT ex, VT ex/Te, fR, V’E, and V’O2. After detecting the most significant oscillometric determinants, univariate and multivariate analyses were performed for interaction analyses. These statistical analyses were performed using EZR Version 1.38 (based on R Version 3.5.2 and R commander Version 2.5–1, Saitama, Japan).29 In all analyses, p<0.05 was taken to indicate statistical significance.
Results
Baseline Characteristics
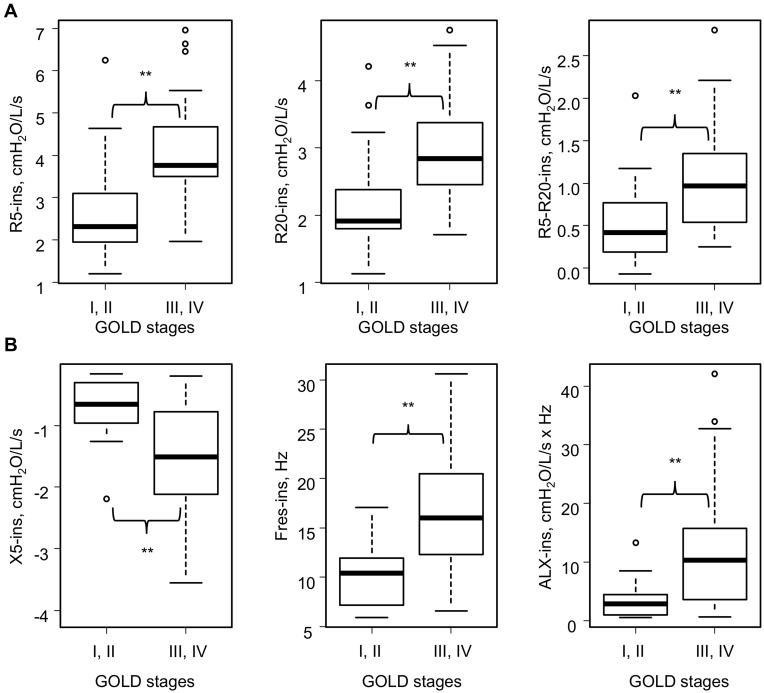
Among 55 subjects included in the present study, 16 subjects were GOLD stage I or II and 39 subjects were GOLD stage III or IV (Table 1). There were no differences between the subgroups in age, body mass index, and smoking history. As for oscillometry, expiratory impedance showed wider variation than inspiratory impedance (Table 2). Resistance parameters, Fres, and ALX were higher and X5 was more negative in Subgroup B (Table 3 and Figure 1). Thus, respiratory impedance variously correlated with the severity of airflow obstruction.
Table 1.
Subjects’ Baseline Characteristics (n=55)
| Age, Years, n | 73.49 (5.56) | 61–83 |
|---|---|---|
| Sex, male/female | 54/1 | |
| BMI, kg m−2 | 20.9 (3.2) | 14.7–25.8 |
| Cigarette smoking, pack years | 56.5 (26.5) | 11–141 |
| GOLD stage I/II/III/IV, n | 6/10/29/10 | |
| GOLD category A/B/C/D, n | 10/29/0/16 | |
| mMRC dyspnea scale 0/1/2/3/4, n | 0/10/22/12/11 | |
| Medications | ||
| LAMA, n | 53 | |
| LABA, n | 43 | |
| ICS, n | 22 | |
| Triple therapy, n | 21 | |
| Spirometry | ||
| FEV1, L | 1.24 (0.62) | 0.41–3.73 |
| % FEV1, % predicted | 47.10 (22.96) | 16.7–141.8 |
| FVC, L | 3.01 (0.89) | 1.24–5.39 |
| % FVC, % predicted | 89.4 (23.7) | 39.4–159.9 |
| FEV1/FVC, % | 40.35 (11.17) | 21.2–69.2 |
| VC, L | 3.05 (0.81) | 1.68–5.31 |
| %VC, % | 84.45 (19.73) | 48.40–146.30 |
| IC, L | 1.92 (0.52) | 0.92–3.08 |
| IRV, L | 0.96 (0.44) | 0.19–1.79 |
| ERV, L | 1.13 (0.47) | 0.33–2.85 |
| VT, L | 0.96 (0.32) | 0.29–1.77 |
Note: Data are presented as the mean (SD) and the minimum and maximum values, unless otherwise stated.
Abbreviations: BMI, body mass index; ERV, expiratory reserve volume; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; ICS, inhaled corticosteroids; IRV, inspiratory reserve volume; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; mMRC dyspnea scale, modified Medical Research Council dyspnea scale; SD, standard deviation; VC, vital capacity; VT, tidal volume.
Table 2.
Results of Baseline Respiratory Impedance at Rest (n=55)
| R5, cmH2O/L/s | ||
| Inspiratory | 3.62 (1.30) | 1.20–6.96 |
| Expiratory | 4.88 (2.19) | 1.53–13.83 |
| Average | 4.25 (1.64) | 1.54–10.04 |
| ΔR5 | −1.26 (1.50) | −7.59–1.19 |
| R20, cmH2O/L/s | ||
| Inspiratory | 2.74 (0.80) | 1.13–4.75 |
| Expiratory | 3.32 (1.28) | 1.44–7.94 |
| Average | 3.03 (0.96) | 1.49–6.08 |
| ΔR20 | −0.58 (0.91) | −3.76–0.77 |
| R5-R20, cmH2O/L/s | ||
| Inspiratory | 0.89 (0.60) | −0.07–2.80 |
| Expiratory | 1.56 (0.99) | 0.01–5.89 |
| Average | 1.23 (0.74) | 0.01–3.96 |
| ΔR5-R20 | −0.68 (0.71) | −3.86–0.52 |
| X5, cmH2O/L/s | ||
| Inspiratory | −1.29 (0.85) | −3.55 - −0.16 |
| Expiratory | −3.64 (3.12) | −12.56 - −0.08 |
| Average | −2.47 (1.89) | −7.54 - −0.13 |
| ΔX5 | 2.36 (2.58) | −0.16–10.05 |
| Fres, Hz | ||
| Inspiratory | 14.53 (5.81) | 5.91–30.60 |
| Expiratory | 20.08 (7.81) | 5.56–34.28 |
| Average | 17.31 (6.47) | 5.74–32.18 |
| ΔFres | −5.55 (4.65) | −18.51–1.04 |
| ALX, cmH2O/L/s × Hz | ||
| Inspiratory | 9.32 (9.12) | 0.54–42.08 |
| Expiratory | 33.74 (33.57) | 0.29–145.30 |
| Average | 21.53 (20.14) | 0.46–83.41 |
| ΔALX | −24.41 (28.24) | −123.78–0.92 |
Note: Data are presented as the mean (SD) and the minimum and maximum values, unless otherwise stated.
Abbreviations: ALX, low-frequency reactance area; Δ, difference between inspiratory and expiratory phases; Fres, resonant frequency; R5 and R20, respiratory system resistance at 5 and 20 Hz, respectively; SD, standard deviation; X5, respiratory system reactance at 5 Hz.
Table 3.
Results of Baseline Respiratory Impedance at Rest in the Various GOLD Stages (Stages I–IV) (n=55)
| Parameter | GOLD Stages I, II (n=16) | GOLD Stages III, IV (n=39) | p | ||
|---|---|---|---|---|---|
| R5, cmH2O/L/s | |||||
| Inspiratory | 2.70 (1.25) | 1.20–6.24 | 4.00 (1.14) | 1.96–6.96 | ** |
| Expiratory | 4.26 (2.88) | 1.53–13.83 | 5.14 (1.83) | 2.24–11.82 | * |
| Average | 3.48 (2.03) | 1.54–10.04 | 4.57 (1.36) | 2.10–9.14 | ** |
| ΔR5 | −1.56 (1.82) | −7.59–0.02 | −1.13 (1.36) | −5.64–1.19 | NS |
| R20, cmH2O/L/s | |||||
| Inspiratory | 2.18 (0.73) | 1.13–4.21 | 2.97 (0.71) | 1.71–4.75 | ** |
| Expiratory | 3.01 (1.54) | 1.44–7.94 | 3.45 (1.15) | 1.78–7.36 | NS |
| Average | 2.59 (1.10) | 1.49–6.08 | 3.21 (0.85) | 1.75–5.94 | ** |
| ΔR20 | −0.83 (0.97) | −0.37–0.18 | −0.48 (0.88) | −3.76–0.77 | NS |
| R5-R20, cmH2O/L/s | |||||
| Inspiratory | 0.52 (0.54) | −0.07–2.03 | 1.04 (0.56) | 0.25–2.80 | ** |
| Expiratory | 1.25 (1.38) | 0.01–5.89 | 1.69 (0.77) | 0.14–4.46 | ** |
| Average | 0.89 (0.94) | 0.01–3.96 | 1.04 (0.56) | 0.29–3.20 | ** |
| ΔR5-R20 | −0.73 (0.93) | −3.86–0.52 | −0.65 (0.62) | −2.53–0.42 | NS |
| X5, cmH2O/L/s | |||||
| Inspiratory | −0.69 (0.53) | −2.19 - −0.16 | −1.53 (0.84) | −3.55 - −0.19 | ** |
| Expiratory | −1.72 (2.85) | −11.11 - −0.08 | −4.43 (2.90) | −12.56 - −0.42 | ** |
| Average | −1.20 (1.67) | −6.65 - −0.13 | −2.98 (1.74) | −7.54 - −0.49 | ** |
| ΔX5 | 1.03 (2.39) | −0.15–8.92 | 2.90 (2.48) | −0.16–10.05 | ** |
| Fres, Hz | |||||
| Inspiratory | 9.87 (3.27) | 5.91–17.08 | 16.44 (5.55) | 6.58–30.60 | ** |
| Expiratory | 12.71 (6.37) | 5.56–28.20 | 23.10 (6.20) | 10.67–34.28 | ** |
| Average | 11.29 (4.71) | 5.74–22.64 | 19.77 (5.42) | 9.82–32.18 | ** |
| ΔFres | −2.84 (3.73) | −11.12–1.04 | −6.65 (4.58) | −18.51–0.37 | ** |
| ALX, cmH2O/L/s × Hz | |||||
| Inspiratory | 3.30 (3.43) | 0.54–13.25 | 11.79 (9.59) | 0.60–42.08 | ** |
| Expiratory | 13.41 (26.96) | 0.29–97.59 | 42.07 (32.70) | 2.04–145.30 | ** |
| Average | 8.35 (15.09) | 0.46–55.42 | 26.93 (19.58) | 1.93–83.41 | ** |
| ΔALX | −10.11 (23.80) | −84.34–0.81 | −30.28 (28.08) | −123.78–0.92 | ** |
Notes: Data are presented as the mean (SD) and the minimum and maximum values, unless otherwise stated. *and **p<0.05 and p<0.01 by the Mann–Whitney U-test, respectively.
Abbreviations: ALX, low-frequency reactance area; Δ, difference between inspiratory and expiratory phases; Fres, resonant frequency; GOLD, Global Initiative For Chronic Obstructive Lung Disease; NS, not significant; R5 and R20, respiratory system resistance at 5 and 20 Hz, respectively; SD, standard deviation; X5, respiratory system reactance at 5 Hz.
Figure 1.
Inspiratory impedance at rest in the various GOLD stages (stages I–IV) (n=55). (A) Respiratory resistance and GOLD stages at baseline. (B) Respiratory reactance and GOLD stages at baseline. Sixteen subjects were GOLD stage I or II and 39 subjects were GOLD stage III or IV. **p<0.01 by the Mann–Whitney U-test.
Abbreviations: ALX, low-frequency reactance area; Fres, resonant frequency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; R5 and R20, respiratory system resistance at 5 and 20 Hz, respectively; X5, respiratory system reactance at 5 Hz.
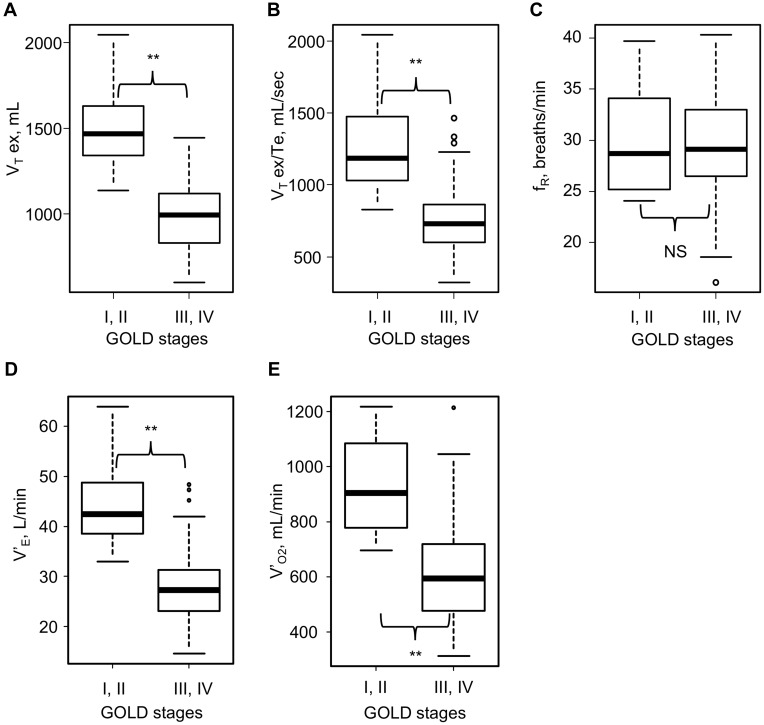
Regarding CPET (Table 4), V’O2 at rest was higher in Subgroup A (Table 5). At peak exercise, VT ex, VT ex/Te, V’E, V’O2, and Ti/Ttot (p<0.01) were higher in Subgroup A (Table 5). These data showed that Subgroup A had better exercise tolerance than Subgroup B. Moreover, given that VD/VT at rest and peak exercise was higher in Subgroup B (Table 5), the results showed ventilatory inefficiency and low oxygen uptake during exercise in subjects with severe airflow obstruction. However, no significant difference was observed in fR between the two subgroups, perhaps because of its wide variation (Figure 2). Whilst, fR correlated positively with VT ex/Te and V’E at peak exercise, but not with VT ex among all subjects (Supplementary Figure 1).
Table 4.
Results of Cardiopulmonary Exercise Testing at Peak Exercise and at Rest (n=55)
| At Peak Exercise | At Rest | |||
|---|---|---|---|---|
| Incremental load testing | ||||
| VT ex, mL | 1132 (332) | 597–2047 | 696 (169) | 354–1192 |
| VT ex/Te, mL/sec | 924 (363) | 322–2043 | 370 (90) | 175–660 |
| fR, breaths/min | 29 (5) | 16–40 | 21 (6) | 8–32 |
| V’E, L/min | 33.0 (10.6) | 14.6–63.9 | 13.7 (2.5) | 6.8–19.1 |
| Ti/Ttot | 0.38 (0.08) | 0.24–0.68 | 0.37 (0.07) | 0.22–0.62 |
| V’O2, mL/min | 711 (236) | 313–1218 | 249 (45) | 171–361 |
| VD/VT | 0.40 (0.05) | 0.31–0.50 | 0.44 (0.04) | 0.38–0.54 |
| V’E/V’CO2 | 46.6 (7.6) | 33.3–72.3 | 61.6 (8.6) | 46.5–81.9 |
Note: Data are presented as the mean (SD) and the minimum and maximum values, unless otherwise stated.
Abbreviations: fR, breathing frequency; SD, standard deviation; Te, expiration time; Ti/Ttot, inspiratory duty cycle; VD, physiological dead space; VD/VT, dead space to tidal volume ratio; V’E, minute ventilation; V’E/V’CO2, ventilatory equivalent for carbon dioxide; V’O2, oxygen uptake; VT, tidal volume; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow.
Table 5.
Results of Cardiopulmonary Exercise Testing (CPET) at Rest and at Peak Exercise in Various GOLD Stages (Stages I–IV) (n=55)
| GOLD Stages I, II (n=16) | GOLD Stages III, IV (n=39) | |||||||
|---|---|---|---|---|---|---|---|---|
| At peak exercise | At rest | At peak exercise | At rest | |||||
| Incremental load testing | ||||||||
| VT ex, mL | 1515 (246)* | 1137–2047 | 739 (145) | 488–935 | 975 (214) | 597–1445 | 679 (177) | 354–1192 |
| VT ex/Te, mL/sec | 1297 (296)* | 927–2043 | 383 (56) | 292–467 | 771 (265) | 322–1464 | 366 (102) | 175–660 |
| fR, breath/min | 29 (5) | 24–40 | 20 (4) | 14–27 | 29 (5) | 16–40 | 21 (6) | 8–32 |
| V’E, L/min | 44.1 (8.3)* | 33.0–63.9 | 14.1 (2.0) | 10.4–17.6 | 28.5 (7.9) | 14.6–48.3 | 13.6 (2.8) | 6.8–19.1 |
| Ti/Ttot | 0.43 (0.06)* | 0.35–0.60 | 0.38 (0.07) | 0.30–0.62 | 0.36 (0.09) | 0.24–0.68 | 0.36 (0.08) | 0.22–0.62 |
| V’O2, mL/min | 928 (168)* | 696–1218 | 270 (45)* | 171–361 | 622 (201) | 313–1214 | 240 (42) | 173–327 |
| VD/VT | 0.35 (0.03)* | 0.31–0.40 | 0.42 (0.04)* | 0.38–0.49 | 0.42 (0.04) | 0.34–0.50 | 0.45 (0.04) | 0.38–0.54 |
| V’E/V’CO2 | 44.9 (6.1) | 36.0–54.4 | 59.5 (8.5) | 46.5–78.3 | 47.2 (8.1) | 37.0–72.3 | 62.5 (8.6) | 46.6–81.9 |
Notes: Data are presented as the mean (SD) and the minimum and maximum values, unless otherwise stated. *p<0.05 by the Mann–Whitney U-test comparing with each CPET parameter of subjects with GOLD stages III and IV.
Abbreviations: CPET, cardiopulmonary exercise testing; fR, breathing frequency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation; Te, expiratory time; Ti/Ttot, inspiratory duty cycle; VD, physiological dead space; VD/VT, dead space to tidal volume ratio; V’E, minute ventilation; V’E/V’CO2, ventilatory equivalent for carbon dioxide; V’O2, oxygen uptake; VT, tidal volume; VT ex, expiratory tidal volume; VT ex/Te; mean expiratory flow.
Figure 2.
Results of cardiopulmonary exercise testing at peak exercise in the various GOLD stages (stages I–IV) (n=55). (A) Expiratory tidal volume at peak exercise in the various GOLD stages. (B) Mean expiratory flow (VT ex/Te) at peak exercise in the various GOLD stages. (C) Breathing frequency (fR) at peak exercise in the various GOLD stages. (D) Minute ventilation (V’E) at peak exercise in the various GOLD stages. (E) Oxygen uptake (V’O2) at peak exercise in the various GOLD stages. **p<0.01 by the Mann–Whitney U-test.
Abbreviations: fR, breathing frequency; GOLD, Global Initiative for Chronic Obstructive Lung Disease; NS, not significant by the Mann–Whitney U-test; V’E, minute ventilation; V’O2, oxygen uptake; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow.
Identification of Oscillometric Parameters That Best Predicted CPET Parameters
Univariate analyses showed that respiratory resistance at rest hardly correlated with CPET parameters at rest, and inspiratory reactance at rest correlated with VT ex, VT ex/Te, V’E, and V’O2 at rest (Table 6). However, oscillometric parameters at rest variously correlated with CPET parameters at peak exercise except for fR (Table 7 and Supplementary Table 1). Moreover, VT ex, VT ex/Te, V’E, and V’O2 showed a stronger correlation with parameters of reactance than resistance, and all the inspiratory oscillometric parameters showed stronger correlation with these CPET parameters than expiratory oscillometric parameters. In addition, multiple stepwise linear regression analysis determined Fres-ins as the parameter with the strongest correlation with VT ex, VT ex/Te, V’E, and V’O2 (Supplementary Table 2). Therefore, Fres-ins was detected as the most significant determinant of exertional ventilatory parameters, including exercise tolerance. However, the correlation of exertional ventilatory parameters with Fres-ins was weaker than that with FEV1, which included the correlation of FEV1 with V’O2 (rS=0.823, p<0.01) and that with V’E (rS=0.903, p<0.01) (Table 7).
Table 6.
Spearman’s Rank Correlation Coefficient Between Parameters of Respiratory Impedance at Rest, Spirometry, and Cardiopulmonary Exercise Testing at Rest (n=55)
| VT ex | VT ex/Te | fR | V’E | V’O2 | VD/VT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oscillometry | ||||||||||||
| R5 | ||||||||||||
| Inspiratory | −0.260 | NS | −0.193 | NS | 0.236 | NS | −0.065 | NS | −0.111 | NS | 0.175 | NS |
| Expiratory | −0.176 | NS | −0.048 | NS | 0.222 | NS | 0.050 | NS | −0.105 | NS | 0.108 | NS |
| R20 | ||||||||||||
| Inspiratory | −0.223 | NS | −0.105 | NS | 0.252 | NS | −0.002 | NS | −0.033 | NS | 0.160 | NS |
| Expiratory | −0.133 | NS | −0.051 | NS | 0.150 | NS | 0.008 | NS | −0.050 | NS | 0.062 | NS |
| R5-R20 | ||||||||||||
| Inspiratory | −0.247 | NS | −0.177 | NS | 0.220 | NS | −0.052 | NS | −0.136 | NS | 0.150 | NS |
| Expiratory | −0.174 | NS | −0.140 | NS | 0.182 | NS | −0.031 | NS | −0.189 | NS | 0.094 | NS |
| X5 | ||||||||||||
| Inspiratory | 0.365 | ** | 0.246 | NS | −0.257 | NS | 0.152 | NS | 0.289 | * | −0.338 | * |
| Expiratory | 0.143 | NS | 0.119 | NS | −0.153 | NS | −0.005 | NS | 0.114 | NS | −0.245 | NS |
| Fres | ||||||||||||
| Inspiratory | −0.356 | ** | −0.278 | * | 0.241 | NS | −0.148 | NS | −0.335 | * | 0.385 | ** |
| Expiratory | −0.174 | NS | −0.194 | NS | 0.155 | NS | −0.038 | NS | −0.220 | NS | 0.303 | * |
| ALX | ||||||||||||
| Inspiratory | −0.372 | ** | −0.250 | NS | 0.258 | NS | −0.155 | NS | −0.296 | * | 0.314 | * |
| Expiratory | −0.160 | NS | −0.109 | NS | 0.179 | NS | 0.019 | NS | −0.132 | NS | 0.217 | NS |
| Spirometry | ||||||||||||
| FVC | 0.429 | ** | 0.503 | ** | −0.093 | NS | 0.434 | ** | 0.506 | ** | −0.156 | NS |
| FEV1 | 0.352 | ** | 0.419 | ** | −0.136 | NS | 0.304 | * | 0.467 | ** | −0.253 | * |
| FEV1/FVC | 0.039 | NS | 0.131 | NS | −0.004 | NS | 0.006 | NS | 0.143 | NS | −0.180 | NS |
Notes: *p<0.05; **p<0.01.
Abbreviations: ALX, low-frequency reactance area; FEV1, forced expiratory volume in 1 second; fR, breathing frequency; Fres, resonant frequency; FVC, forced vital capacity; NS, not significant; R5 and R20, respiratory system resistance at 5 and 20 Hz, respectively; Te, expiratory time; V’E, minute ventilation; V’O2, oxygen uptake; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow; X5, respiratory system reactance at 5 Hz.
Table 7.
Spearman’s Rank Correlation Coefficient Between Parameters of Respiratory Impedance at Rest, Spirometry, and Cardiopulmonary Exercise Testing at Peak Exercise (n=55)
| VT ex | VT ex/Te | fR | V’E | V’O2 | VD/VT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oscillometry | ||||||||||||
| R5 | ||||||||||||
| Inspiratory | −0.560 | ** | −0.487 | ** | 0.111 | NS | −0.491 | ** | −0.404 | ** | 0.480 | ** |
| Expiratory | −0.413 | ** | −0.329 | ** | 0.063 | NS | −0.388 | ** | −0.322 | * | 0.358 | ** |
| R20 | ||||||||||||
| Inspiratory | −0.490 | ** | −0.426 | ** | 0.113 | NS | −0.438 | ** | −0.327 | * | 0.384 | ** |
| Expiratory | −0.297 | * | −0.241 | NS | 0.020 | NS | −0.309 | * | −0.207 | NS | 0.252 | NS |
| R5-R20 | ||||||||||||
| Inspiratory | −0.558 | ** | −0.459 | ** | 0.136 | NS | −0.457 | ** | −0.432 | ** | 0.510 | ** |
| Expiratory | −0.522 | ** | −0.427 | ** | 0.063 | NS | −0.486 | ** | −0.439 | ** | 0.422 | ** |
| X5 | ||||||||||||
| Inspiratory | 0.631 | ** | 0.537 | ** | −0.163 | NS | 0.520 | ** | 0.516 | ** | −0.620 | ** |
| Expiratory | 0.488 | ** | 0.446 | ** | −0.049 | NS | 0.463 | ** | 0.419 | ** | −0.463 | ** |
| Fres | ||||||||||||
| Inspiratory | −0.676 | ** | −0.586 | ** | 0.138 | NS | −0.566 | ** | −0.568 | ** | 0.677 | ** |
| Expiratory | −0.577 | ** | −0.533 | ** | 0.027 | NS | −0.564 | ** | −0.514 | ** | 0.554 | ** |
| ALX | ||||||||||||
| Inspiratory | −0.658 | ** | −0.553 | ** | 0.155 | NS | −0.541 | ** | −0.537 | ** | 0.607 | ** |
| Expiratory | −0.517 | ** | −0.463 | ** | 0.057 | NS | −0.489 | ** | −0.445 | ** | 0.437 | ** |
| Spirometry | ||||||||||||
| FVC | 0.770 | ** | 0.798 | ** | 0.115 | NS | 0.756 | ** | 0.718 | ** | −0.550 | ** |
| FEV1 | 0.875 | ** | 0.871 | ** | 0.155 | NS | 0.903 | ** | 0.823 | ** | −0.715 | ** |
| FEV1/FVC | 0.496 | ** | 0.477 | ** | 0.168 | NS | 0.556 | ** | 0.470 | ** | −0.453 | ** |
Notes: *p<0.05; **p<0.01.
Abbreviations: ALX, low-frequency reactance area; fR, breathing frequency; FEV1, forced expiratory volume in 1 second; Fres, resonant frequency; FVC, forced vital capacity; NS, not significant; R5 and R20, respiratory system resistance at 5 and 20 Hz, respectively; Te, expiratory time; V’E, minute ventilation; V’O2, oxygen uptake; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow; X5, respiratory system reactance at 5 Hz.
Analysis Between Fres-Ins at Rest and CPET Parameters
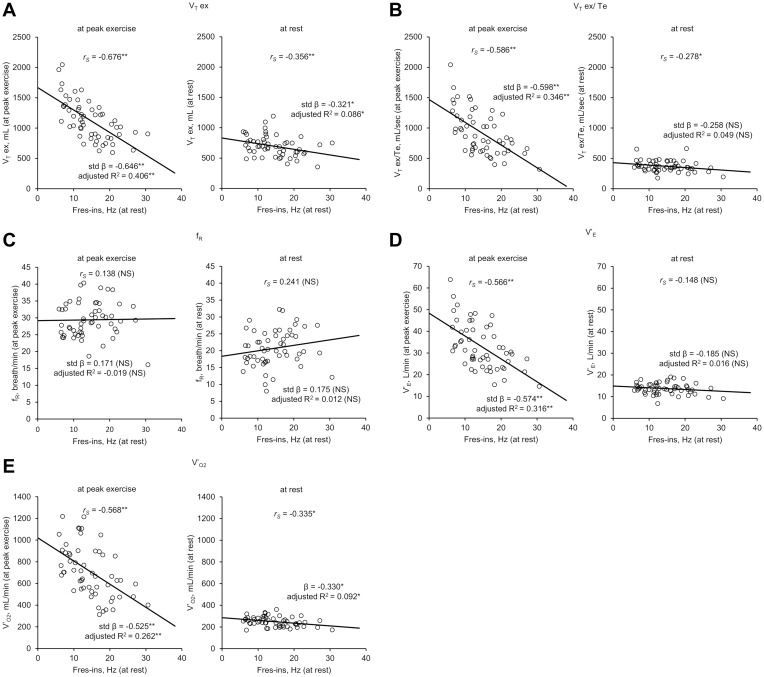
Comparing CPET parameters at rest with those at peak exercise, VT ex, VT ex/Te, V’E, and V’O2 showed stronger correlations with Fres-ins at peak exercise than at rest (Figure 3). Regarding analyses of fR and Fres-ins, statistical significance was not observed at either rest or at peak exercise. Moreover, the differences of CPET parameters between at peak exercise and at rest correlated with Fres-ins, including VT ex (rS=−0.571, p<0.01), VT ex/Te (rS=−0.598, p<0.01), V’E (rS=−0.555, p<0.01), and V’O2 (rS=−0.558, p<0.01). This indicated the statistically more significant difference in correlation coefficients at peak exercise compared with at rest during CPET. Therefore, during exercise, the correlation between Fres-ins at rest and CPET parameters became stronger.
Figure 3.
Results of univariate analyses and Spearman’s rank correlation coefficient analyses between inspiratory resonant frequency (Fres-ins) at rest and ventilatory parameters of cardiopulmonary exercise testing (CPET) (n=55). In each figure, the graphs on the left show the results of Fres-ins at rest and CPET ventilatory parameters at peak exercise, while those on the right show the results of Fres-ins at rest and ventilatory parameters at rest, respectively. (A) Fres-ins and expiratory tidal volume (VT ex). (B) Fres-ins and mean expiratory flow (VT ex/Te). (C) Fres-ins and breathing frequency (fR). (D) Fres-ins and minute ventilation (V’E). (E) Fres-ins and oxygen uptake (V’O2). *p<0.05; **p<0.01.
Abbreviations: CPET, cardiopulmonary exercise testing; fR, breathing frequency; Fres-ins, inspiratory resonant frequency; NS, not significant; R2, R-squared; rS, Spearman’s rank correlation coefficient; std β, standardized regression coefficient estimate; V’E, minute ventilation; V’O2, oxygen uptake; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow.
In addition, Fres-ins correlated with an index of the ventilatory inefficiency: VD/VT at peak exercise (rS=0.677, p<0.01). However, FEV1 correlated with VD/VT at peak exercise stronger than Fres-ins (rS=−0.715, p<0.01) (Table 7).
Correlation Between Fres-Ins at Rest and CPET Parameters at Peak Exercise
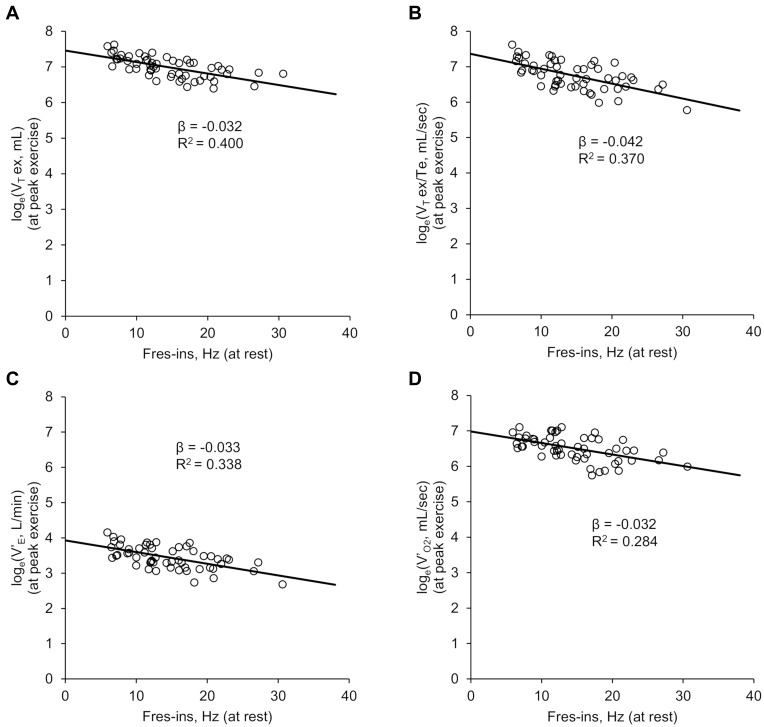
As indicated by the scatter plots of Fres-ins and VT ex, VT ex/Te, V’E, and V’O2 (Figure 3), the correlations between the various parameters can be considered nonlinear and exponential. Therefore, analyses between Fres-ins and the natural logarithms of these CPET parameters were performed. Fres-ins correlated negatively and linearly with the natural logarithms of the CPET parameters (Figure 4). Combined with the results of Figure 1, which indicated that Fres-ins correlated positively with the GOLD stages, these analyses using logarithmic conversion implied that the correlation between Fres-ins and the CPET parameters becomes weak in advanced stages of COPD.
Figure 4.
Results of Spearman’s rank correlation coefficient and univariate analyses of the correlation between inspiratory resonant frequency (Fres-ins) at rest and ventilatory parameters of cardiopulmonary exercise testing (CPET) at peak exercise (n=55).Interaction analyses were performed for Fres-ins and natural logarithms of exertional ventilatory parameters of CPET. All the analyses were statistically significant (p<0.01). (A) Fres-ins and expiratory tidal volume (VT ex). (B) Fres-ins and mean expiratory flow (VT ex/Te). (C) Fres-ins and minute ventilation (V’E). (D) Fres-ins and oxygen uptake (V’O2).
Abbreviations: β, unstandardized regression coefficient estimate; CPET, cardiopulmonary exercise testing; Fres-ins, inspiratory resonant frequency; R2, R-squared; V’E, minute ventilation; V’O2, oxygen uptake; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow.
Discussion
The present study highlights four major findings regarding the correlation between respiratory impedance and exertional ventilatory parameters: 1) inspiratory rather than expiratory oscillometric parameters correlated with exertional ventilatory parameters stronger; 2) Fres-ins at rest correlated with exertional ventilatory parameters and reflected exercise tolerance in COPD subjects; 3) Fres-ins correlated with ventilatory inefficiency during exercise; and 4) the correlation between respiratory impedance and exertional ventilatory parameters became weak in subjects with severe airflow obstruction. To the best of our knowledge, this is the first study to evaluate exertional ventilatory parameters using respiratory impedance at rest.
In COPD patients, inspiratory reactance at rest, particularly Fres-ins, better correlated with ventilatory parameters at peak exercise than those at rest. During exercise, due to the combination of decreased elastic recoil pressure and increased airway resistance, patients with COPD require a longer expiratory time to enable complete expiration of tidal volume.30 However, the simultaneous increase in fR results in a short expiratory time, which is insufficient for COPD patients to exhale their entire tidal volume due to airflow obstruction, leading to dynamic hyperinflation.18 Accordingly, oscillometric parameters correlate variously with spirometric parameters including FEV1,31 which reflects the severity of airflow obstruction. However, oscillometry has not been considered a surrogate test for spirometry, because these tests do not necessarily evaluate the same breathing conditions and each oscillometric parameter reportedly only fairly or moderately correlates with spirometric parameters.31,32 Therefore, the correlations between oscillometry and exertional ventilatory parameters also seemed to be moderate. Nevertheless, we assessed the utility of oscillometry because it is particularly useful for assessing the respiratory pathophysiology of COPD and for evaluating the exertional ventilatory parameters of patients who are unable to perform even spirometry.
This study suggested that inspiratory impedance at rest correlates with exertional ventilatory parameters of CPET better than does expiratory impedance. Inspiratory rather than expiratory oscillometric parameters were reportedly less variable.7 Airways tend to open during inspiration and the pressure pulses can be transmitted to the periphery, but airflow obstruction during expiration was suggested to occur with inhomogeneity in the lung.7,33 Hence, the more variability of expiratory oscillometric parameters was probably due to the inhomogeneity of airflow obstruction.7 In addition, in patients with COPD, loss of lung elastic recoil causes EFL, and more negative ΔX5 and higher inspiratory X5 were considered to predict the presence of EFL.19,34,35 Therefore, subjects in Subgroup B were estimated to have developed EFL compared with Subgroup A subjects (Table 3). Given that the correlation of Fres-ins with CPET parameters became weak in advanced COPD subjects (Figure 4), EFL and/or dynamic hyperinflation might potentially have associated with the inhomogeneity of airflow obstruction. Therefore, inspiratory impedance might be a more useful parameter for evaluating exertional ventilatory parameters in patients with COPD. In addition, oscillometry might be useful for detecting the inhomogeneity of airflow obstruction in COPD. Further investigations are necessary to validate the hypothesis.
The present study indicated that Fres-ins was the best oscillometric parameter for predicting V’O2 and V’E at peak exercise. Given the stronger correlation between Fres-ins at rest and VT ex at peak exercise than that between Fres-ins at rest and VT ex at rest (Figure 3), respiratory reactance at rest might correlate with the degree of VT expansion. Consequently, Fres-ins might have correlated with V’O2 at peak exercise, which is calculated using V’E: the product of VT ex and fR. Given that changes in X5 and Fres related to small airway bronchodilation in COPD,36 Fres-ins might have reflected airway obstruction and correlated with VT ex. Further studies to assess the correlation of Fres-ins with VT ex might be necessary. The present study suggested that respiratory impedance measured by oscillometry, particularly respiratory reactance at rest, is useful for evaluating exertional ventilatory parameters quantitatively and can be a complementary tool for predicting exercise tolerance, although the utility of oscillometry was not better than that of spirometry.
In addition, the present study suggested that respiratory reactance correlated with ventilatory inefficiency. In Subgroup B, increased VD/VT at peak exercise showed ventilatory inefficiency during exercise (Table 5). This was also supported by the prolonged expiratory time and the shallow breathing pattern (Table 5). Moreover, the correlation between Fres-ins and VD/VT indicated that Fres-ins might be used as a surrogate marker for ventilatory inefficiency during exercise because VD/VT is a common marker of ventilatory inefficiency.16,20 Fres-ins might potentially be useful as an index easy to measure for adding or choosing interventions to improve ventilatory inefficiency in patients with COPD, although further studies are needed.37 The present study first showed that oscillometric parameters correlated with ventilatory inefficiency during exercise and indicated the potential role of respiratory impedance for effortlessly assessing exertional ventilatory parameters qualitatively in COPD patients.
The correlation between respiratory impedance and exertional ventilatory parameters can become weak in patients with advanced COPD. In the present study, the more reduced VT ex/Te in Subgroup B implied that airflow obstruction during exercise became more severe (Table 5). Moreover, as described above, subjects in Subgroup B were estimated to have developed more severe EFL than those in Subgroup A. In addition, the exponential correlations between Fres-ins at rest and CPET parameters at peak exercise showed that the correlations between oscillometry and CPET became weak in COPD subjects with severe airflow obstruction (Figure 4). Given these results, oscillometry might not be appropriate for evaluating exertional ventilatory parameters in advanced COPD patients with EFL because wide variations in respiratory reactance level at rest decrease the accuracy of evaluation. The present study included a small number of subjects and was a single-center study; thus, further investigations are needed to validate the hypothesis and to verify the clinical utility of oscillometry in patients with advanced COPD.
The present study had some limitations. First, it was a single-center investigation and some selection bias might have affected the findings. Second, this study included a small number of subjects. In particular, only one female subject was included in the study. In addition, the number of COPD subjects with each GOLD stage was small. Hence, a larger study population is required to validate the results. Third, this study did not include a healthy control group because only symptomatic subjects who underwent oscillometry, spirometry, and CPET were evaluated retrospectively.
In conclusion, respiratory reactance at rest, particularly Fres-ins, was able to estimate exertional ventilatory parameters of CPET. Inspiratory reactance is useful for effortlessly predicting exertional ventilatory parameters both quantitatively and qualitatively in patients with COPD, but oscillometry might not be appropriate to evaluate exertional ventilatory parameters in patients with advanced COPD with EFL. Further investigations of oscillometry to accurately evaluate exertional ventilatory parameters and to improve the clinical utility are necessary.
Acknowledgments
The authors would like to thank Ms S Ito, Ms S Sakaguchi and Mr T Uenishi for their help with the CPET measurements.
Funding Statement
The authors received no specific funding for the present study.
Abbreviations
ALX, low-frequency reactance area; β, unstandardized regression coefficient estimate; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CPET, cardiopulmonary exercise testing; Δ, difference between inspiratory and expiratory phases; ERV, expiratory reserve volume; FEV1, forced expiratory volume in 1 second; fR, breathing frequency; Fres, resonant frequency; Fres-ins inspiratory resonant frequency; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; ICS, inhaled corticosteroids; IRV, inspiratory reserve volume; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists; R2, R-squared; R20, respiratory resistance (Rrs) at 20 Hz; R5, Rrs at 5 Hz; R5-R20, difference between R5 and R20; Rrs, respiratory resistance; rS, Spearman’s rank correlation coefficient; SD, standard deviation; std β, standardized regression coefficient estimate; Te, expiratory time; Ti/Ttot, inspiratory duty cycle; VC, vital capacity; VD, physiological dead space; VD/VT, dead space to tidal volume ratio; V’E, minute ventilation; V’E/V’CO2, ventilatory equivalent for carbon dioxide; V’O2, oxygen uptake; VT, tidal volume; VT ex, expiratory tidal volume; VT ex/Te, mean expiratory flow; X5, respiratory reactance (Xrs) at 5 Hz; X5-ins, inspiratory X5; Xrs, respiratory reactance.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its supplementary material file.
Ethics Approval and Consent to Participate
The present study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of NHO Osaka Toneyama Medical Center (approval number, TNH-2,019,037). The Institutional Review Board of NHO Osaka Toneyama Medical Center chose an opt-out system to obtain subjects’ informed consent.
Author Contributions
YY contributed to conceptualizing and designing the study, data collection and analysis, and wrote the manuscript. KM cared for all the subjects, contributed to conceptualizing and designing the study, data analyses, and revision of the manuscript. TM, KF, YO, HK, KT, and KY contributed to collecting subject data and revising the manuscript. MM and HK contributed to data interpretation and design of the study. All the authors reviewed and approved the submission of the final manuscript.
Disclosure
The authors declare no conflicts of interest in association with the present study.
References
- 1.GBD 2017. Causes of death collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 3.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 5.Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–460. doi:doi: 10.1183/13993003.00745-2015 [DOI] [PubMed] [Google Scholar]
- 6.Diaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J. 2000;16(2):269–275. doi: 10.1034/j.1399-3003.2000.16b14.x [DOI] [PubMed] [Google Scholar]
- 7.Kubota M, Shirai G, Nakamori T, Kokubo K, Masuda N, Kobayashi H. Low frequency oscillometry parameters in COPD patients are less variable during inspiration than during expiration. Respir Physiol Neurobiol. 2009;166(2):73–79. doi: 10.1016/j.resp.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi: 10.1183/09031936.03.00089403 [DOI] [PubMed] [Google Scholar]
- 9.Bickel S, Popler J, Lesnick B, Eid N. Impulse oscillometry: interpretation and practical applications. Chest. 2014;146(3):841–847. doi: 10.1378/chest.13-1875 [DOI] [PubMed] [Google Scholar]
- 10.Tse HN, Tseng CZS, Wong KY, Yee KS, Ng LY. Accuracy of forced oscillation technique to assess lung function in geriatric COPD population. Int J Chron Obstruct Pulmon Dis. 2016;11:1105–1118. doi: 10.2147/COPD.S102222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcanti JV, Lopes AJ, Jansen JM, Melo PL. Detection of changes in respiratory mechanics due to increasing degrees of airway obstruction in asthma by the forced oscillation technique. Respir Med. 2006;100(12):2207–2219. doi: 10.1016/j.rmed.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 12.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paredi P, Goldman M, Alamen A, et al. Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax. 2010;65(3):263–267. doi: 10.1136/thx.2009.120790 [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi Y, Kohyama T, Jo T, Nagase T. Dynamic change in respiratory resistance during inspiratory and expiratory phases of tidal breathing in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2012;7:259–269. doi: 10.2147/COPD.S30399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calverley PMA. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–199. doi: 10.1183/09031936.04.00113204 [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, Elbehairy AF, Faisal A, Webb KA, Neder JA, Mahler DA. Exertional dyspnoea in COPD: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2016;25(141):333–347. doi: 10.1183/16000617.0054-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koulouris NG, Dimopoulou I, Valta P, Finkelstein R, Cosio MG, Milic-Emili J. Detection of expiratory flow limitation during exercise in COPD patients. J Appl Physiol. 1997;82(3):723–731. doi: 10.1152/jappl.1997.82.3.723 [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(2):180–184. doi: 10.1513/pats.200508-093DO [DOI] [PubMed] [Google Scholar]
- 19.Dellacà RL, Santus P, Aliverti A, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23(2):232–240. doi: 10.1183/09031936.04.00046804 [DOI] [PubMed] [Google Scholar]
- 20.Elbehairy AF, Ciavaglia CE, Webb KA, et al. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384–1394. doi: 10.1164/rccm.201501-0157OC [DOI] [PubMed] [Google Scholar]
- 21.Neder JA, Berton DC, Arbex FF, et al. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur Respir J. 2017;49(3):1602036. doi: 10.1183/13993003.02036-2016 [DOI] [PubMed] [Google Scholar]
- 22.Neder JA, Berton DC, Müller PDT, et al. Ventilatory inefficiency and exertional dyspnea in early chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(Supplement_1):S22–S29. doi: 10.1513/AnnalsATS.201612-1033FR [DOI] [PubMed] [Google Scholar]
- 23.Caviedes IR, Delgado I, Soto R. Ventilatory inefficiency as a limiting factor for exercise in patients with COPD. Respir Care. 2012;57(4):583–589. doi: 10.4187/respcare.01342 [DOI] [PubMed] [Google Scholar]
- 24.Di Marco F, Terraneo S, Job S, et al. Cardiopulmonary exercise testing and second-line pulmonary function tests to detect obstructive pattern in symptomatic smokers with borderline spirometry. Respir Med. 2017;127:7–13. doi: 10.1016/j.rmed.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann SC, Thamrin C, Chan AS, Bertolin A, Chapman DG, King GG. Relationships between forced oscillatory impedance and 6-minute walk distance after pulmonary rehabilitation in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:157–166. doi: 10.2147/COPD.S225543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD. Available from: https://goldcopd.org/. Accessed April27, 2020.
- 27.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 28.Kubota M, Kobayashi H, Quanjer PH, et al. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig. 2014;52(4):242–250. doi: 10.1016/j.resinv.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gagnon P, Guenette JA, Langer D, et al. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:187–201. doi: 10.2147/COPD.S38934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto S, Miyoshi S, Katayama H, et al. Use of the forced-oscillation technique to estimate spirometry values. Int J Chron Obstruct Pulmon Dis. 2017;12:2859–2868. doi: 10.2147/COPD.S143721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirai T, Kurosawa H. Clinical application of the forced oscillation technique. Intern Med. 2016;55(6):559–566. doi: 10.2169/internalmedicine.55.5876 [DOI] [PubMed] [Google Scholar]
- 33.Goldman MD, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within- and between-day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002;34(4):312–319. doi: 10.1002/ppul.10168 [DOI] [PubMed] [Google Scholar]
- 34.Akita T, Shirai T, Mori K, et al. Association of the forced oscillation technique with negative expiratory pressure in COPD. Respir Physiol Neurobiol. 2016;220:62–68. doi: 10.1016/j.resp.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Dellacà RL, Duffy N, Pompilio PP, et al. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J. 2007;29(2):363–374. doi: 10.1183/09031936.00038006 [DOI] [PubMed] [Google Scholar]
- 36.Borrill ZL, Houghton CM, Tal-Singer R, et al. The use of plethysmography and oscillometry to compare long-acting bronchodilators in patients with COPD. Br J Clin Pharmacol. 2008;65(2):244–252. doi: 10.1111/j.1365-2125.2007.03013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weatherald J, Sattler C, Garcia G, Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: the role of chemosensitivity and dead space. Eur Respir J. 2018;51:2. doi: 10.1183/13993003.00860-2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD. Available from: https://goldcopd.org/. Accessed April27, 2020.