Abstract
Background
The N6-methyladenosine (m6A) RNA modification of mRNA mediates various cellular functions and cancer progression. However, the roles of m6A RNA modification in the regulation of esophageal squamous cell carcinoma (ESCC), the dominant subtype of esophageal cancer in Asia, were unclear.
Materials and Methods
Here, we analyzed the mRNA expression level of methyltransferase like 3 (METTL3) in the public available datasets of ESCC tissues and matched adjacent normal tissues. We also performed immunohistochemistry (IHC) assays to detect the protein expression of METTL3 in human ESCC tissue specimen. In our study, we also analyzed the association between METTL3 expression and prognosis using Cox proportional hazard regression in 207 ESCC patients.
Results
The results of public available datasets and IHC assays showed that METTL3 was upregulated in tumor compared with adjacent nonmalignant esophageal mucosal tissues. The IHC results indicated that higher expression level of METTL3 was associated with worse survival. We also found that METTL3 expression level was an independent predictor for disease-free survival and overall survival of ESCC patients.
Conclusion
Our results revealed that the METTL3 expression level could be used as an independent prognostic biomarker for ESCC prognosis.
Keywords: esophageal squamous cell carcinoma, METTL3, prognosis, survival
Introduction
N6-methyladenosine (m6A) RNA modification, which was discovered in the 1970s, occurs across different regions of mRNA transcripts and is particularly enriched near the stop codons. The transcriptome-wide m6A sequencing results suggest the consensus motif DRA*CH (D denotes A, G or U; R denotes G or A; A* denotes methylatable A; and H denotes A, C or U).1–4 Many m6A-associated proteins have been identified by comprehensive and systematic mass-spectrometry-based screening of m6A interactors.5 The “writer” heterotrimeric complex consists of the enzyme methyltransferase like 3 (METTL3) and the two co-factors called METTL14 and WTAP.6–9 The “eraser” proteins FTO and ALKBH5 are RNA demethylases.10,11 YTH domain-containing family proteins (YTHDF1, YTHDF2, YTHDF3, YTHDC1 and YTHDC2) are the most studied “reader” proteins, which regulate RNA metabolism in many aspects.12–18 Importantly, recent studies found that METTL3, having m6A enzymatic activity, regulated various tumorigenesis.19–22 However, whether METTL3 could affect esophageal squamous cell carcinoma (ESCC) progression is unclear.
Esophageal cancer is the 8th most common cancer and the 6th most common cause of cancer-related deaths.23 ESCC is the dominant subtype of esophageal cancer in Asia, accounting for 90% of the cases in China.24 A notably high incidence is observed in China.24 So far, no effective ESCC prognostic factors have been characterized, and the overall 5-year survival rate for ESCC is less than 15%.25 It is worth to investigate whether METTL3 could regulate ESCC progression and whether it could be an effective predictor for ESCC prognosis.
Here, we detected the expression of METTL3 in a total of 207 ESCC patients by immunohistochemistry (IHC) assays and found that increased expression of METTL3 was associated with poor prognosis for ESCC patients.
Materials and Methods
Human ESCC Tissue Specimens
The Internal Review and Ethics Board of Sun Yat-sen University Cancer Center, Guangzhou, China approved this study. This study was conducted in accordance with the Declaration of Helsinki. Patients whose tissues and medical records were used for this study had provided written informed consent. This study included 207 ESCC patients, who were diagnosed from October 2000 to April 2007 in Sun Yat-sen University Cancer Center and underwent surgical resection without neoadjuvant/adjuvant therapy. We used the 7th edition of the TNM classification of the International Union Against Cancer (2009) to record the histologic grade and the clinical stage of the tumors. Patients’ age, sex, tumor location, histopathology grade, histopathology tumor status, N categories, TNM stage were recorded by reviewing the patient medical records after surgery. Follow-up assessments were performed every 3 months postoperatively for the first years, and followed by every 6 months later.
Immunohistochemistry Staining Analysis
Total 207 ESCC specimens were selected for IHC staining to determine METTL3 expression. First, the 4-μm FFPE ESCC samples were placed in an oven for 2 h at 65 °C, and then deparaffinized in xylene and rehydrated with ethanol alcohol followed by distilled water. To block endogenous peroxidase activity, the sections were immersed in 3% hydrogen peroxide at room temperature for 10 min. After that, the sections were boiled in Citrate Antigen Retrieval Solution (pH = 6.5) for 4 min in an electric pressure cooker for antigen retrieval. The sections were then incubated with rabbit monoclonal antibody against METTL3 (1:500 dilution; ab195352, Abcam) at 37°C for 1 h in a moist chamber and a goat anti-rabbit secondary antibody at 37°C for half an hour. Subsequently, the tissue sections were stained with DAKO Liquid 3,’3-diaminobenzidine tetrahydrochloride (DAB) for 10 min, counterstained with 10% Mayer’s hematoxylin, then were dehydrated and mounted. Normal rabbit IgG was used as a negative control. Each section was independently evaluated by two pathologists.
The staining results were scored based on two criteria: (1) the proportion of tumor cells staining positive: 0 (none), 1 (1% to 10%), 2 (11% to 50%), 3 (51% to 75%), and 4 (76% to 100%); and (2) the average intensity of the positive cells: 0 (none), 1 (weak), 2 (moderate) and 3 (strong). The proportion and intensity scores were then multiplied to obtain a total score (range, 0 to 12). All these patients were divided into two groups according to the median total score, and the score higher than 8 was considered as high METTL3 expression.
Bioinformatic Analysis
Differential expression analysis of METTL3 in ESCC tissues versus matched normal tissues was performed by using public available microarray datasets GSE23400, GSE38129 and GSE75241.
Statistical Analysis
Data analyses were performed using SPSS v-23 (IBM), R v3.3.3 and GraphPad Prism 6. The Student’s t-test was used to compare different METTL3 expression between normal and ESCC groups. The change of METTL3 expression was showed with the log2FC (fold change of METTL3 expression from tumor to normal tissues). The Benjamini–Hochberg approach for the false discovery rate (FDR) was used for the multiple testing correction. We defined the results as statistical significant when the adjusted p-value <0.05. Chi-square test was used to determine the correlation between METTL3 expression and the clinicopathological variables. Disease-free survival was defined as the time from surgery to cancer progression or metastasis. Overall survival was defined as the time from surgery to death. Cumulative survival time was calculated using the Kaplan-Meier method, and compared using the Log-rank test. The effects of various clinicopathological features and METTL3 expression on survival were assessed using Cox proportional hazard regression analysis. For all analysis, p-value <0.05 indicates statistical significance.
Results
Relationship Between METTL3 Expression and Clinicopathological Variables
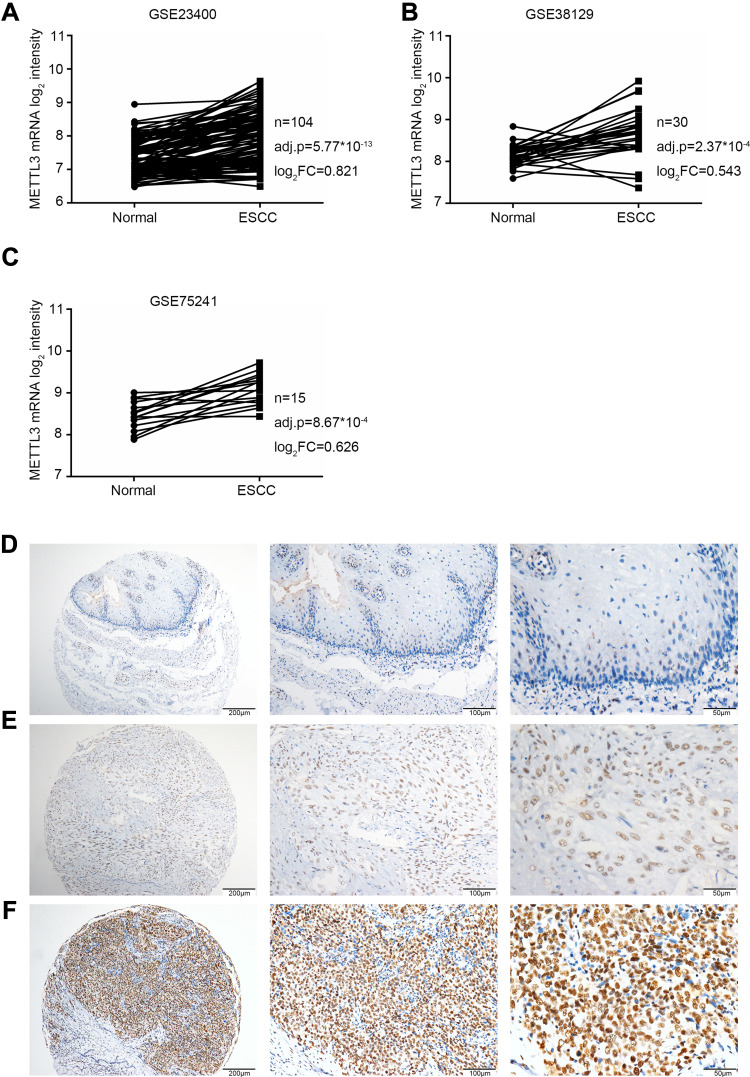
We analyzed METTL3 expression using three microarray datasets of ESCC tissues. We found that METTL3 mRNA expression level was significantly upregulated in ESCC tissues compared with matched adjacent normal tissues (Figure 1A–C). There are a few METTL3 downregulated samples, which might be caused by the effects of the individual differences. These results indicated that METTL3 has a potential oncogenic function for ESCC progression.
Figure 1.
The METTL3 expression in ESCC normal and tumor tissue.
Notes: (A–C) The mRNA expression levels of METTL3 in ESCC tumor and matched normal samples analyzed by three open access microarray data, GSE23400 (A), GSE38129 (B) and GSE75241 (C). Data are significant at adj.p < 0.05. (D–F) The results of immunohistochemistry assay (IHC) in normal esophageal mucosa (D), in ESCC tumor with METTL3 low expression (E) and METTL3 high expression (F).
Abbreviations: adj.p, adjusted p-value calculated by the Benjamini–Hochberg approach for the false discovery rate; ESCC, esophageal squamous cell carcinoma; log2FC, log2 transformation of fold change of METTL3 expression; METTL3, methyltransferase like 3.
We then performed IHC assays in 207 ESCC tissues to detect the expression level of METTL3. We found that METTL3 was expressed in nuclei, shown as brown-yellow granules (Figure 1D–F). METTL3 was lower expressed in normal esophageal mucosa tissues (Figure 1D) than ESCC tissues (Figure 1E and F). We also found that METTL3 was higher expressed in ESCC tissues than adjacent nonmalignant esophageal mucosal tissues (Figure 1E and F). The IHC samples were divided into two subgroups according to the expression level of METTL3: low METTL3 expression subgroup (Figure 1E) and high METTL3 expression subgroup (Figure 1F). Low expression level of METTL3 was detected in 128 of 207 ESCC tissues, whereas high expression level of METTL3 was detected in 79 of 207 ESCC tissues.
We then analyzed the correlation between METTL3 expression and patient clinicopathological variables. As summarized in Table 1, higher METTL3 expression was shown in patients with lymph node metastasis and advanced TNM stage (p=0.045 and p=0.004, respectively). But METTL3 expression was not statistically correlated with age, gender, tumor location, histological grade or histopathology tumor status (p=0.855, 0.905, 0.456, 0.279 and 0.157, respectively).
Table 1.
METTL3 Expression and Clinicopathologic Variables of 207 ESCC Cases
| Variables | Cases (n=207) | METTL3 Expression | p-valuea | |
|---|---|---|---|---|
| Low (n =128, percent) | High (n =79, percent) | |||
| Ageb (years) | 0.855 | |||
| ≤58 | 111 | 68(53.1) | 43(54.4) | |
| >58 | 96 | 60(46.9) | 36(45.6) | |
| Sex | 0.905 | |||
| Female | 56 | 35(27.3) | 21(26.6) | |
| Male | 151 | 93(72.7) | 58(73.4) | |
| Tumor location | 0.456 | |||
| Upper | 12 | 9(7.0) | 3(3.8) | |
| Middle | 140 | 83(64.8) | 57(72.2) | |
| Lower | 55 | 36(28.1) | 19(24.1) | |
| Histological gradec | 0.279 | |||
| Well differentiated (G1) | 51 | 27(21.1) | 24(30.4) | |
| Moderately differentiated (G2) | 131 | 86(67.2) | 45(57) | |
| Poorly differentiated (G3) | 25 | 15(11.7) | 10(12.7) | |
| pT statusc | 0.157 | |||
| pT1 | 6 | 5(3.9) | 1(1.3) | |
| pT2 | 47 | 34(26.6) | 13(16.5) | |
| pT3 | 151 | 88(68.8) | 63(79.7) | |
| pT4 | 3 | 1(0.8) | 2(2.5) | |
| N categoriesc | 0.045* | |||
| N0 | 110 | 75(58.6) | 35(44.3) | |
| N>0 | 97 | 53(41.4) | 44(55.7) | |
| TNM stagec | 0.004* | |||
| Stage I | 7 | 4(3.1) | 4(3.8) | |
| Stage II | 119 | 85(66.4) | 34(43) | |
| Stage III | 81 | 39(30.5) | 42(53.2) | |
Notes: *p-value <0.05 indicates statistical significance. ap-values were calculated by Pearson’s correlation test. bAge is classified according to the median age of 58-year-old. cThe grading and histopathology stage of ESCC specimens are based on the World Health Organization classification published in 2009.
Abbreviations: ESCC, esophageal squamous cell carcinoma; METTL3, methyltransferase like 3; N categories, lymph node metastasis status; N0, lymph node metastasis negative; N>0, lymph node metastasis positive; pT status, histopathology tumor status; TNM stage, tumor/node/metastasis staging classification.
Higher Expression Level of METTL3 Was Associated with Worse Survival
Using Kaplan-Meier survival analysis (Log-rank test), we investigated the correlation between METTL3 expression and the survival status of ESCC patients. We found that patients with higher METTL3 expression experienced significantly worse overall survival (OS) (p<0.001) and disease-free survival (DFS) (p<0.001) compared with those with lower METTL3 expression level (Table 2, Figure 2A). The mean OS were 74.9 and 44 months in the low and high METTL3 expression groups, respectively (Table 2). The mean DFS were 72.4 and 38.6 months in the low and high METTL3 expression groups (Table 2). We further estimated the effect of METTL3 high expression on OS or DFS by subgroup analysis according to the histopathology tumor status, pT1-2 and pT3-4. We found that worse OS and DFS were observed in patients with higher METTL3 expression in pT3-4 (p<0.001 and p<0.001, respectively, Table 2), but not in pT1-2 (p=0.064 and 0.057, respectively; Table 2). Additionally, worse OS and DFS were observed in patients with higher METTL3 expression in both well-differentiated histologic grade (G1) (p=0.002 and 0.002, respectively; Table 2) and moderately/poorly histologic grade (G2-3) (p<0.001 and p<0.001, respectively; Table 2). METTL3 expression showed no statistically significant relationship with histopathology tumor status or histological grade by the clinicopathologic analysis (Table 1). The sample size, different grouping and analysis methods might affect the statistical results of the relationship between METTL3 expression and histopathology tumor status or histological grade.
Table 2.
METTL3 Expression in ESCC Patients by Kaplan–Meier Survival Analysis (Log Rank Test)
| Variables | Cases | DFS (months) | OS (months) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Median | p-value | Mean | Median | p-value | ||
| Total | |||||||
| Low expression | 128 | 72.4 | NR | <0.001* | 74.9 | NR | <0.001* |
| High expression | 79 | 38.6 | 18 | 44 | 25 | ||
| pT statusa | |||||||
| pT1-2 | |||||||
| Low expression | 39 | 66.5 | NR | 0.064 | 70.1 | NR | 0.057 |
| High expression | 14 | 42.3 | 30 | 48.5 | 54 | ||
| pT3-4 | |||||||
| Low expression | 89 | 72.3 | NR | <0.001* | 74.1 | NR | <0.001* |
| High expression | 65 | 37.2 | 17 | 42.1 | 25 | ||
| N categoriesa | |||||||
| N0 | |||||||
| Low expression | 75 | 86.4 | NR | 0.006* | 88.1 | NR | 0.009* |
| High expression | 35 | 58.6 | 58 | 63.2 | 66 | ||
| N>0 | |||||||
| Low expression | 53 | 44.2 | 29 | <0.001* | 48.3 | 40 | <0.001* |
| High expression | 44 | 19.6 | 10 | 25.7 | 15 | ||
| Histologic gradea | |||||||
| G1 | |||||||
| Low expression | 27 | 74.6 | NR | 0.002* | 76.4 | NR | 0.002* |
| High expression | 24 | 37.5 | 26 | 44 | 45 | ||
| G2-3 | |||||||
| Low expression | 101 | 69.7 | NR | <0.001* | 72.4 | 74 | <0.001* |
| High expression | 55 | 38.1 | 14 | 43 | 25 | ||
| TNM stagea | |||||||
| Stage I+II | |||||||
| Low expression | 89 | 83 | NR | 0.02* | 85.2 | NR | 0.025* |
| High expression | 37 | 60 | 58 | ||||
| Stage III | |||||||
| Low expression | 39 | 40 | 27 | 0.001* | 43.2 | 39 | 0.002* |
| High expression | 42 | 17.2 | 9 | 23.1 | 15 | ||
Notes: *p < 0.05, statistically significant; aThe grading and histopathology stage of ESCC specimens are based on the World Health Organization classification published in 2009.
Abbreviations: DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; G1, well differentiated; G2-3, moderately differentiated and poorly differentiated; METTL3, methyltransferase like 3; N categories, lymph node metastasis status; N0, lymph node metastasis negative; N>0, lymph node metastasis positive; NR, not reached; OS, overall survival; pT status, histopathology tumor status; TNM stage, tumor/node/metastasis staging classification.
Figure 2.
High METTL3 expression was associated with poor survival.
Notes: (A) The Kaplan–Meier plots of OS and DFS for 207 ESCC patients classified according to their METTL3 expression level. p<0.05, statistically significant. (B) The Kaplan–Meier plots of OS and DFS for 126 ESCC patients in TNM stage I+II classified according to their METTL3 expression level. p<0.05, statistically significant. (C) The Kaplan–Meier plots of OS and DFS for 81 ESCC patients in TNM stage III classified according to their METTL3 expression level. p<0.05, statistically significant.
Abbreviations: DFS, disease-free survival; ESCC, esophageal squamous cell carcinoma; METTL3, methyltransferase like 3; OS, overall survival; TNM stage, tumor/node/metastasis staging classification.
Consistent results were found in the subgroup analysis of TNM stage. Worse OS and DFS were observed in patients with higher METTL3 expression in both the early stage subgroup (TNM stage I+II) (p=0.025 and 0.02, respectively; Figure 2B) and the advanced stage subgroup (TNM stage III) (p=0.002 and 0.001, respectively; Figure 2C). Similar results were observed in the histopathology subgroups of pT3-4, pN=0, pN=1/2/3, G1 and G2-3 (Table 2).
METTL3 Expression Level Was an Independent Predictor for DFS and OS in ESCC Patients
Using cox proportional hazard model, we performed univariate and multivariate survival analysis to explore the effect of METTL3 expression on OS or DFS of ESCC. As presented in Table 3, the univariate survival analysis revealed that nodal status (HR=3.040, 95% CI: 2.063–4.481, p<0.001) and METTL3 expression level (HR=2.389, 95% CI: 1.652–3.454, p<0.001) are predictors for DFS of ESCC patients. Multivariate survival analysis also showed similar results with HR of 2.932 (95% CI: 1.983–4.335, p<0.001) for nodal status (N>0) and HR of 2.264 (95% CI: 1.563–3.281, p<0.001) for METTL3 high expression. We also performed univariate and multivariate survival analysis for OS of ESCC. As presented in Table 4, the univariate survival analysis revealed higher histopathology tumor status (HR=1.503, 95% CI: 1.014–2.227, p=0.043), lymph node-positive (HR=3.170, 95% CI: 2.150–4.673, p<0.001) and high METTL3 expression level (HR=2.356, 95% CI: 1.629–3.407, p<0.001) are associated with poor OS. Multivariate analysis indicated that nodal status (HR=3.078, 95% CI: 2.080–4.555, p<0.001) and METTL3 expression level (HR=2.164, 95% CI: 1.487–3.151, p<0.001) are predictors for OS of ESCC patients. Here, the univariate survival analysis revealed that histopathology tumor status are associated with OS (HR=1.503, 95% CI: 1.014–2.227, p=0.043, Table 4) and nearly associated with DFS (HR=1.417, 95% CI: 0.957–2.099, p=0.082, Table 3). Multivariate analysis indicated that histopathology tumor status was not recognized as an independent prognostic factor of OS and DFS. The sample size might affect the statistical analysis of histopathology tumor status.
Table 3.
Univariate and Multivariate Analysis of Disease-Free Survival of ESCC Patients
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Agea (years) (>58 vs ≤58) | 0.984 | 0.681–1.421 | 0.930 | |||
| Gender (male vs female) | 1.066 | 0.706–1.611 | 0.761 | |||
| Tumor location (upper/middle/lower) | 0.833 | 0.582–1.192 | 0.319 | |||
| Histological gradeb (G3/G2/G1) | 1.213 | 0.888–1.657 | 0.225 | |||
| pT statusb (pT4/pT3/pT2/pT1) | 1.417 | 0.957–2.099 | 0.082 | |||
| N categoriesb (N>0/N0) | 3.040 | 2.063–4.481 | <0.001* | 2.932 | 1.983–4.335 | <0.001* |
| METTL3 expression (High/Low) | 2.389 | 1.652–3.454 | <0.001* | 2.264 | 1.563–3.281 | <0.001* |
Notes: *p < 0.05, statistically significant. aAge is classified according to the median age of 58-year-old. bThe grading and histopathology stage of ESCC specimens are based on the World Health Organization classification published in 2009.
Abbreviations: ESCC, esophageal squamous cell carcinoma; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiate; HR, hazard ratio; METTL3, methyltransferase like 3; N categories, lymph node metastasis status; N0, lymph node metastasis negative; N>0, lymph node metastasis positive; pT status, histopathology tumor status; 95% CI, the 95% confidence interval.
Table 4.
Univariate and Multivariate Analysis of Overall Survival of ESCC Patients
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| Agea (years) (>58 vs.≤58) | 0.980 | 0.678–1.416 | 0.914 | |||
| Gender (male vs female) | 1.120 | 0.741–1.692 | 0.591 | |||
| Tumor location (upper/middle/lower) | 0.862 | 0.600–1.239 | 0.423 | |||
| Histological gradeb (G3/G2/G1) | 1.190 | 0.872–1.624 | 0.272 | |||
| pT statusb (pT4/pT3/pT2/pT1) | 1.503 | 1.014–2.227 | 0.043* | 1.312 | 0.879–1.960 | 0.184 |
| N categoriesb (N>0/N0) | 3.170 | 2.150–4.673 | <0.001* | 3.078 | 2.080–4.555 | <0.001* |
| METTL3 expression (High/Low) | 2.356 | 1.629–3.407 | <0.001* | 2.164 | 1.487–3.151 | <0.001* |
Notes: *p < 0.05, statistically significant. aAge is classified according to the median age of 58-year-old. bThe grading and histopathology stage of ESCC specimens are based on the World Health Organization classification published in 2009.
Abbreviations: ESCC, esophageal squamous cell carcinoma; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiate; HR, hazard ratio; METTL3, methyltransferase like 3; N categories, lymph node metastasis status; N0, lymph node metastasis negative; N>0, lymph node metastasis positive; pT status, histopathology tumor status; 95% CI, the 95% confidence interval.
These data demonstrated that the expression level of METTL3 was significantly related to the clinicopathological characteristics of the ESCC patients and is an efficient predictor of DFS and OS for ESCC patients. Altogether, METTL3 appears to be a good predictive factor for ESCC patients, supporting its potential clinical application as a prognostic biomarker for ESCC.
Discussion
In this study, we found that METTL3 was upregulated in ESCC, and its expression level might be a good predictive factor for ESCC patients, supporting its potential clinical application as a prognostic biomarker for ESCC.
The m6A writer METTL3 is a core catalytic component of methyltransferase complex. Recent studies have shown that METTL3 could regulate various tumors’ progression via m6A modification. For example, METTL3 plays oncogenic function in breast cancer, liver cancer, acute myeloid leukemia, glioblastoma or others.19–22 However, the expression status of METTL3 in ESCC was not characterized so far. Given that ESCC accounts for about 90% of esophageal cancers in Asia, with a particularly high prevalence in Eastern to Central Asia, it is worth to investigate its role involved in ESCC development and progression.
In this study, we at first identify the expression status of METTL3 in ESCC at the RNA and protein level. We analyzed open available datasets of ESCC and found that mRNA level of METTL3 was upregulated in ESCC tissues compared with adjacent nonmalignant esophageal mucosal tissues. We also performed IHC assays and found that METTL3 was higher expressed in cancer cell of the ESCC tissues. According to the expression level of METTL3, the IHC samples were classified into two subgroups: high and low expression of METTL3. Higher expression level of METTL3 was associated with worse OS and DFS in all samples and TNM stage I+II subgroup or TNM stage III subgroup. After prognostic analysis, we found that high expression of METTL3 was associated with N categories, TNM stages and poor prognosis. Using univariate and multivariate survival analysis, we found that the expression level of METTL3 was an independent factor on DFS and OS of ESCC.
Therefore, our results indicated that METTL3 was more highly expressed in ESCC. The increased expression of METTL3 was significantly positively associated with poor prognosis compared with low METTL3 phenotype. After analyzing of clinicopathological factors, METTL3 expression level was an independent predictor for DFS and OS of ESCC patients. Collectively, our results reveal that the METTL3 expression level could be used as an independent prognostic biomarker for ESCC patient survival. However, the mechanisms of METTL3 in ESCC progression remain to investigate by further studies.
Funding Statement
This study was supported by the National Natural Science Foundation of China (81830090, 81802775), Natural Science Foundation of Guang Dong Province (No. 20181025201938243).
Data Sharing Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDB2020000861.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wei CM, Gershowitz A, Moss B. 5ʹ-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry. 1976;15(2):397–401. doi: 10.1021/bi00647a024 [DOI] [PubMed] [Google Scholar]
- 2.Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–3975. doi: 10.1073/pnas.71.10.3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3ʹ UTRs and near stop codons. Cell. 2012;149(7):1635–1646. doi: 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–206. doi: 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- 5.Edupuganti RR, Geiger S, Lindeboom RGH, et al. N6-methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol. 24:870–878. doi: 10.1038/nsmb.3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz S, Mumbach MR, Jovanovic M, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5ʹ sites. Cell Rep. 2014;8(1):284–296. doi: 10.1016/j.celrep.2014.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–189. doi: 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna. 1997;3(11):1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Yue Y, Han D, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10(2):93–95. doi: 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7(12):885–887. doi: 10.1038/nchembio.687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng G, Dahl JA, Niu Y, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49(1):18–29. doi: 10.1016/j.molcel.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–328. doi: 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Chen YS, Ping XL, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27(3):444–447. doi: 10.1038/cr.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–120. doi: 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Zhao BS, Roundtree IA, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161(6):1388–1399. doi: 10.1016/j.cell.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–519. doi: 10.1016/j.molcel.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m(6)A transcripts by the 3ʹ–>5ʹ RNA Helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68(2):374–387. (). doi: 10.1016/j.molcel.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 18.Hsu PJ, Zhu Y, Ma H, et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27(9):1115–1127. doi: 10.1038/cr.2017.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Wei L, Law CT, et al. RNA N6-methyladenosine methyltransferase METTL3 promotes liver cancer progression through YTHDF2 dependent post-transcriptional silencing of SOCS2. Hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 20.Cai X, Wang X, Cao C, et al. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2017;415:11–19. doi: 10.1016/j.canlet.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 21.Visvanathan A, Patil V, Arora A, et al. Essential role of METTL3-mediated m6A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2017. [DOI] [PubMed] [Google Scholar]
- 22.Vu LP, Pickering BF, Cheng Y, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23(11):1369–1376. doi: 10.1038/nm.4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surgery. 2017;6(2):131–136. doi: 10.21037/acs.2017.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, He YT, Zheng RS, Zhang SW, Chen WQ. Analysis of esophageal cancer time trends in China, 19892008. Asian Pacific j Cancer Prev. 2012;13(9):4613–4617. doi: 10.7314/APJCP.2012.13.9.4613 [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]