Abstract
Purpose:
Although platinum compounds are the first-line treatment for ovarian cancer, the majority of patients relapse and develop resistance to treatment. However, the mechanism underlying resistance is unclear. The goal of our study is to decipher the mechanism by which a metabolic kinase, diacylglycerol kinase alpha (DGKA), confers platinum resistance in ovarian cancer.
Experimental Design:
Metabolic kinase RNAi synthetic lethal screening was used to identify a cisplatin resistance driver in ovarian cancer. DGKA variants were used to demonstrate the need for DGKA activity in cisplatin resistance. Phospho-proteomic and genomic screens were performed to identify downstream effectors of DGKA. Therapeutic efficacy of targeting DGKA was confirmed and clinical relevance of DGKA signaling was validated using ovarian cancer patient-derived tumors that had different responses to platinum-based therapy.
Results:
We found that platinum resistance was mediated by DGKA and its product, phosphatidic acid (PA), in ovarian cancer. Proteomic and genomic screens revealed that DGKA activates the transcription factor c-JUN and consequently enhances expression of a cell cycle regulator, WEE1. Mechanistically, PA facilitates JNK recruitment to c-JUN and its nuclear localization, leading to c-JUN activation upon cisplatin exposure. Pharmacological inhibition of DGKA sensitized ovarian cancer cells to cisplatin treatment and DGKA-c-JUN-WEE1 signaling positively correlated with platinum resistance in tumors derived from ovarian cancer patients.
Conclusions:
Our study demonstrates how the DGKA-derived lipid messenger, PA, contributes to cisplatin resistance by intertwining with kinase and transcription networks, and provides preclinical evidence for targeting DGKA as a new strategy in ovarian cancer treatment to battle cisplatin resistance.
Keywords: Diacylglycerol kinase (DGKA), platinum-based chemotherapy, drug resistance, ovarian cancer, lipid metabolism, c-JUN, mitosis inhibitor protein kinase WEE1, phospho-proteomics, transcription profiling
INTRODUCTION
Platinum-based compounds have been the most actively used front-line clinical drug for the treatment of ovarian cancer for decades. The prognosis of patients with ovarian cancer can be defined by the response of the tumor to cisplatin; patients whose tumors are intrinsically resistant to platinum at the time of initial treatment or acquire resistance during treatment have very poor prognosis (1-3). Therefore, understanding of the crucial factors driving platinum resistance is needed to enhance the therapeutic outcomes of platinum-based chemotherapy. Although efforts have been made to identify platinum resistance factors including HOXD8 (4), ATP11B (5), Foxo3a (6), and Xiap (7) in ovarian cancer, most studies lack a clinical correlation approach or a detailed explanation for how these factors intertwine with signaling networks in ovarian cancer cells to control platinum-resistant survival.
Emerging evidence suggests that metabolite levels are altered in cancer cells and control of these changes may provide metabolic vulnerabilities that can be exploited for cancer therapy (8). The altered metabolites are reported to function as critical signaling molecules that contribute to cellular actions needed for proliferative and metastatic potential in cancer cells. For instance, ribulose-5-phosphate, an intermediate in the oxidative pentose phosphate pathway, inhibits AMPK signaling by disrupting the interaction of AMPK with its activating kinase LKB1 and eventually promotes lipogenesis by activating a downstream effector acetyl-CoA carboxylase 1 (9). We found Fumarate, an intermediate metabolite produced by glutamine metabolism, binds to and activates a reactive oxygen species (ROS) scavenging enzyme glutamate peroxidase 1 to promote redox balance in cancer cells (10). In line with these findings, recent studies demonstrate that the response to first-line chemotherapy is influenced by the metabolic state of cancer cells (11). Cisplatin-resistant ovarian cancer cells are reported to have higher levels of reducing equivalents such as glutathione than parental cells and are vulnerable to ROS inducing agents (12,13). The Bcl-2 inhibitor ABT-737 is known to reverse cisplatin resistance in ovarian cancer cells by targeting glucose metabolism (14). Cisplatin-resistant ovarian cancer cells were found to have higher rates of glycolysis and lower mitochondrial activity compared to the parental cells. In contrast, lung cancer cells have lower levels of hexokinase and glycolytic rates but instead rely on oxidative phosphorylation and have higher dependence on glutamine metabolism. Therefore, inhibition of glutaminase sensitized cisplatin-resistant lung cancer cells to cisplatin-based chemotherapy, which reflects the tumor specific heterogeneity of metabolic alterations (15,16). The current findings focus on an observation of metabolic alterations and related therapeutic strategies. However, comprehensive mechanisms by which cellular metabolites are intertwined with cellular signaling networks to confer platinum resistance specifically in ovarian cancer and whether these signaling pathways are virtually relevant in patients are not explored. Here we identify that phosphatidic acid (PA), a lipid metabolite produced by the diacylglycerol kinase alpha (DGKA) enzyme reaction, acts as a critical signaling effector in cisplatin resistance of ovarian cancer.
PA is a vital cellular phospholipid that is typically metabolized from a membrane lipid, diacylglycerol (DAG), by diacylglycerol kinases (DGKs) (17,18). DGKs are a large family of conserved membrane lipid kinases (19). Among ten known DGK enzymes, DGKA has been implicated in human cancers (20,21). DGKA and its product PA are involved in ovarian cancer cell invasion that is mediated through α5β1 integrin trafficking. DGKA is involved in NF-κB or RAS-Raf-MEK activation, which suppresses TNFα mediated apoptosis in melanoma or enhances hepatocellular carcinoma progression, respectively (22,23). In addition, DGKA has been linked to activation of HIF-1α, c-MET, ALK, and VEGF (20,24-27). DGKs are also appreciated as physiological regulators of immune cell function through PA serving as second messengers in T cell receptor signaling (28). DGKA is known to be overexpressed in CD8+ tumor-infiltrating T cells. Treatment with R59949 and R59022, small molecule compounds that inhibit DGKA by binding to its catalytic domain, was reported to recruit CD8+ T cells to the anti-tumor response and may prevent inactivation of T cells (29,30).
In this study, we report a novel role of DGKA and its product PA, connecting metabolic and cellular kinase signaling to gene regulatory machinery that provides resistance to platinum-based chemotherapy uniquely in ovarian cancer.
MATERIALS and METHODS
Reagents
Lentiviral plasmids containing short hairpin RNA (shRNA) for DGKA, LPAAT2, PLD2, c-JUN, WEE1, ATX, PLA1A, PLA2G4A, DGKB, DGKI, and Human Kinase shRNA Gene Family Library were purchased from Dharmacon. The shRNA sense strand sequences were GCTCTGGAAGTTCCAGTATAT (clone #1), and GCTAAATATGTCCAAGGAGAT (clone #2) for DGKA, CGGACCTTATGGCTACAGTAA (clone #1) and CGCAAACCTCAGCAACTTCAA (clone #2) for c-JUN, CCACCCAGAGTAATAGAACAT (clone #1) and CTAGAAAGAGTGCAGAACAAT (clone #2) for WEE1, CGTGCATCATACATGAAGAAA for ATX, GCCAGATAAACCAAGTGAAAT for PLA1A, CCGACTTATTTGGAAGCAAAT for PLA2G4A, CCTGAACTGCATGATTTATTA for DGKB, GCACCCTAATTGACTTGGAAA for DGKI, CGAGGGTACTCGCAACGACAA for LPAAT2, and CCAAGAAGAAATACCGTCATT for PLD2. Image clones for human DGKA, c-JUN, and WEE1 were obtained from DNASU. Primers for DGKA shRNA-resistant silent mutant, DGKA kinase-dead mutant G434A, and real-time qPCR were obtained from Integrated DNA Technologies. The mutant constructs were generated using site-directed mutagenesis kit from Agilent Technologies. 1,2-dioctanoyl-sn-glycero-3-phosphate, the phosphatidic acid (PA), was obtained from Avanti Polar Lipids. 1-oleoyl-2-acetyl-sn-glycerol, an analog of the PKC-activating second messenger diacylglycerol (DAG) as a DGKA substrate, lysophosphatidic acid (LPA), and DGK Inhibitor II (R59949) were obtained from Sigma Aldrich. Gemcitabine, paclitaxel, bortezomib, rapamycin, and MK-1775 were obtained from Selleckchem. Recombinant human proteins c-JUN and JNK1 were from Abcam and Invitrogen, respectively. NE-PER Nuclear and Cytoplasmic Extraction Reagent was from Thermo Fisher Scientific. Fluorometric PicoProbe Phosphatidic Acid Assay Kit was obtained from BioVision. LPA Bioassay ELISA Kit was from US Biological Life Sciences. Chromatin immunoprecipitation assay kit was from Millipore. Proteome Profiler Human Phospho-Kinase and Human Cell Stress Array Kits was from R&D System.
Antibodies
Anti-DGKA antibody for immunoblotting and immunohistochemistry (IHC) staining was purchased from Proteintech. Antibodies against PLD2 (13904), LPAAT2 (14937) phospho-c-JUN Ser63 (9261 for immunoblotting and 2361 for IHC), c-JUN (9165/60A8), phospho-JNK Thr183/Tyr185 (9255/G9), JNK (9252), His-tag (2366/27E8), myc-tag (2278/71D10), PARP (5625/D64E10), WEE1 (13084/D10D2), phospho-S6 Ser235/236 (4858/D57.2.2E), S6 (2217/5G10), phospho-cdc2 Tyr15 (4539/10A11), and cdc2 (9112) were obtained from Cell Signaling Technology. Anti-β-actin antibody (A1978/AC-15), anti-GST antibody (G1160/GST-2), and anti-flag antibody (F7425) were obtained from Sigma Aldrich. Antibody against Ki-67 (ab92742/EPR3610) and phospho-Serine/Threonine (ab17464) were from Abcam.
RNAi, phospho-proteomic, and genomic screens
Primary synthetic lethal RNAi screen was performed using the TRC Human Kinase shRNA Gene Family Library (32). Among the 100 top ranking candidates from the primary screen, 16 kinases that use small molecular metabolites as substrates were selected out and further investigated for synthetic lethality with cisplatin in cisplatin-resistant ovarian cancer cell lines. In brief, A2780cisR and SK-OV-3cisR cells were infected with lentivirus pools targeting individual genes and treated with sublethal doses that result in 15% cell death for each cell line (SK-OV-3cisR: 2 μg/ml, A2780cisR: 5 μg/ml) of cisplatin. For phospho-proteomic screen, A2780cisR derivatives were treated with PBS or cisplatin for 48 hr. Cell lysates were obtained and applied to the Proteome Profiler Human Phospho-Kinase Array and the results were quantified by image J software. For targeted genomic screen, probes for ENCODE-based 44 potential DGKA-c-JUN target genes were designed and the gene expression profile for each gene was assessed by real-time qPCR using A2780cisR cells with DGKA or c-JUN knockdown.
Cell culture and generation of cell lines
A2780cisR cells were cultured in RPMI 1640 medium with 10% FBS. 293T cells were cultured in Dulbecco Modified Eagle Medium (DMEM) with 10% FBS. SK-OV-3cisR cells were cultured in McCoy’s 5A medium with 10% FBS. A2780, A2780cisR, COV318, COV504, and PEO1 cells were obtained from Sigma Aldrich. BG-1 and 1A9 cells are as described (32). 293T, CAOV3, SW626, OV90, and SK-OV-3 cells were from the American Type Culture Collection (ATCC). SK-OV-3cisR cells were generated by constantly exposing SK-OV-3 cells to increasing concentrations (3 ~ 10 μM) of cisplatin. A2780cisR and SK-OV-3cisR cells are 6.8 and 3.5-fold more resistant to cisplatin, respectively, than their parental cell lines in terms of cisplatin IC50. Lentivirus and retrovirus production, RNAi, and protein overexpression in human cancer cells were as described (10,31). In brief, shRNA lentivirus was generated in 293T cells using pLKO.1 vector encoding shRNA, psPAX2, and pMD2.G. A2780cisR cells were infected with lentivirus for 2 days and selected using 2 μg/ml of puromycin. Human DGKA, JNK, c-JUN, and WEE1 were flag or myc tagged by PCR and cloned into pLHCX or pDEST-27 for overexpression. Selection of cells with stable DGKA expression was carried out using 50 μg/ml of hygromycin.
DGKA kinase activity assay
GST-fused DGKA wild-type or kinase-dead mutant proteins enriched from 293T cells were mixed with 100 ug/ml of 1-oleoyl-2-acetyl-sn-glycerol (DAG) in lipid dilution buffer (25 mM HEPES [pH 7.5], 0.5 mM EGTA). The kinase activity of DGKA was assessed by ADP-Glo kinase assay (Promega). GST protein was used as a negative control for normalization.
Cell viability assay and colony formation assay
Cells were seeded at 7000 cells/well in 96-well plates and compounds were added at the indicated concentrations for 48 hr. Cell viability was determined using CellTiter-Glo Luminescent Viability Assay (Promega). For colony formation assay, 500 cells were seeded in 35 mm dishes and treated with sublethal doses of cisplatin for 24 hr. The cells were cultured in complete media for 10 additional days before the colonies were stained and counted by image J software.
Metabolic assays
For anabolic biosynthesis analyses, cells were spiked with 4 μCi/ml of D-[U-14C]-glucose for 2 hr. Lipids were extracted using hexane and isopropanol (3:2 v/v), dried, and re-suspended in chloroform. The amount of labeled lipid was quantified by liquid scintillation counting and normalized to the total protein amount. For RNA synthesis assay, total RNA was isolated from the spiked cells. The labeled RNA was quantified by liquid scintillation counting and normalized to the total RNA content. Intracellular ATP levels were determined using ATP bioluminescent somatic cell assay (Sigma Aldrich). The total ATP levels were normalized to cell number. Intracellular ROS levels were determined by staining cells with CM-H2DCFDA.
Apoptosis assay and cell cycle assay
3 × 105 cells were treated with cisplatin for 48 hr and stained with FITC-conjugated annexin V labeling reagent (BD Pharmingen) and propidium iodide followed by FACS analysis for apoptotic cell population analysis. Cell cycle was assessed by propidium iodide flow cytometric assay and analyzed with FlowJo Software V10.
Cellular thermal shift assay
Cellular thermal shift assay was performed as previously described with a modification(33,34). In brief, 293T cells were transfected with c-JUN or JNK expressing vectors for 24 hr. Cell lysates were treated with or without 10 μM of PA, equally aliquoted, and heated at 55, 57, 60, 63, 66, 69, 72, and 75 degrees for 3 min. Cells were lysed and c-JUN or JNK in the soluble fraction was quantified by Western blot analyses.
Surface plasmon resonance (SPR)
SPR assays were performed using a Biacore X100. A series of increasing concentrations of recombinant JNK in running buffer (0.01 M HEPES (pH 7.4), 0.15 M NaCl, 0.005% v/v surfactant P20) were injected over immobilized c-JUN in the presence of 10 uM PA, LPA or vehicle at 30 μl/min for 180 seconds at 20°C. Multiple-cycle kinetics analyses were used to quantify c-JUN and JNK interaction. Sensorgrams were obtained by subtracting values of vehicle alone and dissociation constants (Kd) were calculated using BIAevaluation Software.
WEE1 promoter reporter assay
Cells with knockdown or overexpression of DGKA or c-JUN were transfected with WEE1 promoter reporter construct followed by cisplatin treatment. Activity of WEE1 promoter was assessed using luciferase assay kit. Cypridina luciferase reporter was used for normalization.
Immunofluorescence staining
A2780cisR cells were seeded on coverslips and fixed in PHEMO buffer (68 mM PIPES, 25 mM HEPES, 3 mM MgCl2, 15 mM EGTA, 0.05% glutaraldehyde, 3.7% formaldehyde, 0.5% Triton X-100). Samples were blocked in 10% goat serum and stained with anti-c-JUN antibody followed by Alexa 488-conjugated anti-rabbit IgG antibody. The coverslips were mounted and imaged on a Zeiss LSM 510 META confocal microscope.
Real time-qPCR
The total RNA of A2780cisR and SK-OV-3cisR were isolated using RNeasy Mini Kit (Qiagen). To detect the expression levels of target genes, reverse transcription of RNA was performed using High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher) followed by qPCR using Universal SYBR Green Supermix (Bio-Rad). The relative expression levels of the target genes were computed by the 2−ΔΔCt method. The primers for RT-qPCR were obtained from Integrated DNA Technologies.
Human studies
Approval to use human-derived tissue specimens was given by the Institutional Review Board of Emory University. Clinical samples were collected with informed consent under Health Insurance Portability and Accountability Act approved protocols. Formalin fixed and paraffin embedded tissues derived from patients with ovarian cancer receiving platinum-based chemotherapy were kindly provided by Champions Oncology.
Immunohistochemistry staining
Formalin fixed and paraffin embedded tissues derived from patients with ovarian cancer receiving platinum-based chemotherapy were kindly provided by Champions Oncology. Tumors collected from cell line-based or patient-derived xenograft (PDX) mice were stained with anti-DGKA (1:500), anti-phospho-c-JUN Ser63 (1:100), anti-WEE1 (1:100), or anti-Ki-67 (1:1000) antibodies. Staining intensity of DGKA, phospho-c-JUN Ser63, WEE1, and Ki-67 in the tumors was scored as 0 to +3.
Xenograft studies
Animal studies were performed according to protocols approved by the Institutional Animal Care and Use Committee of Emory University. Nude mice (Hsd:Athymic Nude-Foxn1nu, female, 6-week old, Envigo) were injected with 1.2 × 106 A2780cisR cells and cisplatin (5 mg/kg) was administered by intraperitoneal injection twice a week when tumor sizes reached 100–150 mm3. Ovarian cancer patient-derived xenograft tumors (Jacksons Laboratory) were implanted in the flank of 6-week old nude mice. The mice were randomly divided into 4 groups when the tumor size reached 100–150 mm3. Cisplatin (5 mg/kg, twice a week) and R59949 (10 mg/kg, every two days) were administered by intraperitoneal and subcutaneous injection, respectively. Tumors were measured blindly, and volumes were calculated as 4π/3 x (width/2)2 x (length/2). Tumor proliferation was assessed by Ki-67 staining.
Statistics
Statistical parameters are indicated in the figure legends and figures. One representative experiment from multiple experiments are shown. Error bars represent mean ± standard error of the mean (SEM) for tumor volume curves and standard deviation (SD) for all others. Statistical significance is based on two-tailed Student’s t test for two group comparisons and 1-way or 2-way ANOVA for multiple comparisons in experiments with more than 2 groups. P values of 0.05 or less were considered statistically significant. Sample size was not pre-determined using statistical methods. For in vivo experiments, animals were randomly chosen and blinding outcome assessment and concealed allocation were used. The in vitro studies were not randomized and allocation and outcome assessment were not blinded. Statistical analyses and graphical presentation and were performed using GraphPad Prism 8.
RESULTS
DGKA contributes to platinum resistance in ovarian cancer
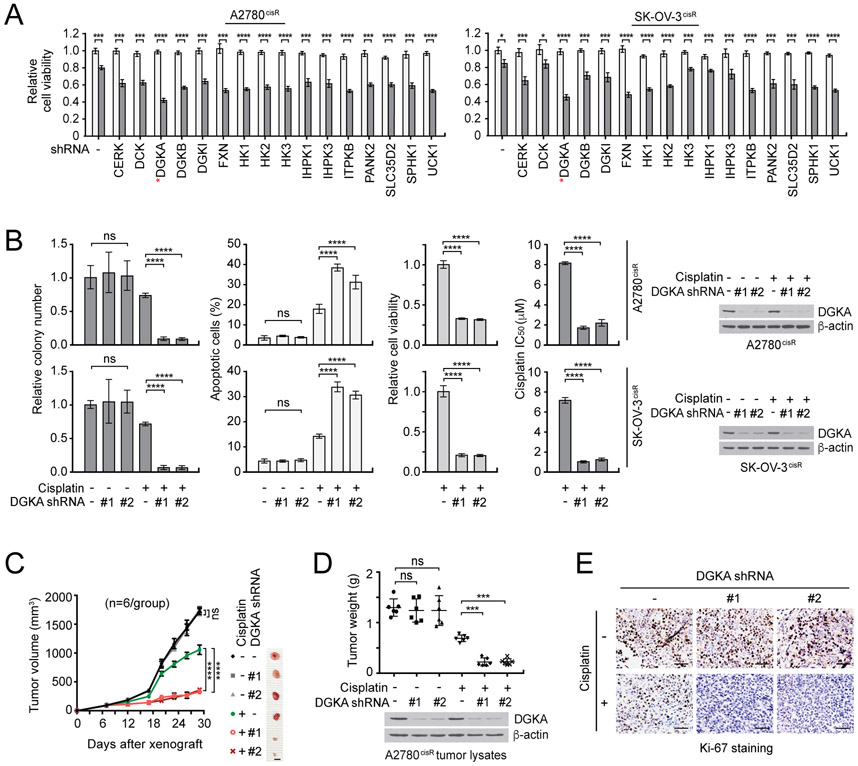
To identify a unique metabolic kinase target that can overcome platinum resistance of human ovarian cancer, we performed targeted RNAi screening in cisplatin-resistant ovarian cancer cell lines with lentiviral shRNA of metabolic kinases selected among the 100 top kinase targets found to sensitize cisplatin response in a kinome-wide RNAi screen. Among 16 enzymes tested, DGKA was identified as the most effective target that commonly sensitizes ovarian cancer cells to cisplatin treatment (Fig. 1A). The RNAi screening result was further validated in vitro and in vivo using individual DGKA shRNA clones. DGKA knockdown using two different shRNA clones significantly attenuated colony formation efficiency, cell proliferation, and cisplatin resistance, and enhanced apoptotic cell death when cells were treated with cisplatin (Fig. 1B). Similar results were obtained when the DGKA knockdown cells were treated with another platinum-based compound, carboplatin, or with chemotherapy agents that impede cell division including gemcitabine and paclitaxel but not with non-chemotherapy agents including the proteasome inhibitor bortezomib or molecularly-targeted inhibitor rapamycin (Supplementary Fig. S1 and S2). Knockdown of DGKA significantly sensitized 10 ovarian cancer cell lines to cisplatin treatment regardless of subtype, suggesting that DGKA commonly provides cisplatin resistant potential to ovarian cancer (Supplementary Fig. S3). Furthermore, tumor growth potential was dramatically decreased in cisplatin-treated xenograft mice bearing ovarian cancer cells with DGKA knockdown (Fig. 1C-E). These results suggest that DGKA contributes to chemotherapy resistance of human ovarian cancer and DGKA could be a potential target in ovarian cancer with treatment of chemotherapy agents such as cisplatin.
Figure 1. DGKA is identified as a critical cisplatin resistance driver in ovarian cancer.
A, Results of synthetic lethality screen targeting top 16 kinases acting on metabolites from a kinome shRNA library with cisplatin in cisplatin-resistant ovarian cancer cell lines. A2780cisR and SK-OV-3cisR cells were infected with pooled shRNA clones and sublethal doses of cisplatin (5 μg/ml A2780cisR and 2 μg/ml SK-OV-3cisR) for 48 hr. Cell viability was determined by CellTiter-Glo luminescent cell viability assay. White bars: no cisplatin treated; Gray bars: cisplatin treated. B, Colony formation potential, apoptotic cell death, cell viability, and cisplatin sensitivity (IC50) in ovarian cisR cancer cells with DGKA knockdown and cisplatin treatment for 48 or 72 hr. Stable DGKA knockdown cells were treated with cisplatin and viability was assessed as in (A). Apoptotic cells were assayed by annexin V staining. DGKA knockdown efficacy is shown by immunoblotting. C-E, Effect of DGKA loss on cisplatin sensitivity in xenograft mice. Mice bearing A2780cisR variants were treated with PBS or cisplatin (5 mg/kg/i.p. twice/week) from 7 days after tumor injection. i.p.: intraperitoneal injection. Tumor size (left), tumor weight (middle), and tumor proliferation rates assessed by Ki-67 staining (right). Scale bars represent 10 mm for (C)and 50 μm for (E). (A and B) n=3 technical replicates. Results of one representative experiment from two (A) and three (B) independent experiments are shown. (C-E) n=6. Error bars represent SEM for (C) and SD for all others. P values were determined by Student’s t-test for (A), one-way ANOVA for (B) and (D), and two-way ANOVA for (C) (ns: not significant; ***P < 0.001; ****P < 0.0001).
Phosphatidic acid is a key factor for DGKA-mediated cisplatin resistance
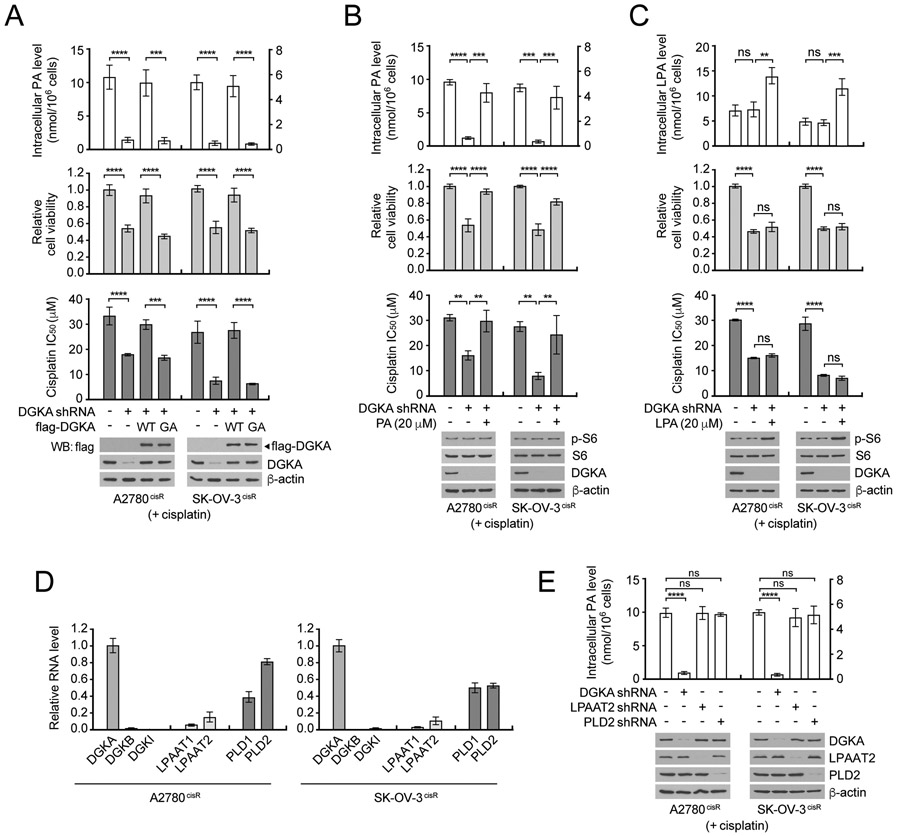
We next examined whether enzyme activity and the product of DGKA are required for cisplatin resistance. Mutation at glycine 434 in the catalytic subunit to alanine in DGKA abrogated its enzyme activity that produces phosphatidic acid (PA) (Supplementary Fig. 4A) (18). Rescue expression of DGKA wild type but not the enzyme-dead mutant G434A (GA) fully restored the decreased level of intracellular PA, cell viability, and cisplatin resistance in cisplatin-resistant ovarian cancer cells with DGKA knockdown (Fig. 2A). We next examined whether the cisplatin resistance is mediated through PA or its downstream lysophosphatidic acid (LPA) signaling by target downregulating enzymes that produce LPA in cells, including phospholipase A1 (PLA1), phospholipase A2 (PLA2), and autotaxin (ATX) (Supplementary Fig. S4B and C). Decrease in intracellular LPA level and LPA signaling, which was assessed by S6 phosphorylation in the mTOR pathway, did not alter cisplatin resistance, whereas accumulated PA levels resulted in enhanced cisplatin resistance (Supplementary Fig. S4D-L). In line with this observation, replenishment of the decreased intracellular PA by external PA significantly rescued the decreased cell viability and cisplatin resistance whereas LPA did not influence these phenotypes in DGKA knockdown cells (Fig. 2B and C). These data suggest that the effect of DGKA on cisplatin resistance is mediated through PA but not downstream metabolite LPA.
Figure 2. Phosphatidic acid mainly derived from DGKA reaction provides cisplatin resistance in ovarian cancer cells.
A, Effect of DGKA modulation on intracellular PA level, cell viability, and cisplatin response. DGKA was knocked down and rescue expressed with shRNA-resistant WT or GA mutant in A2780cisR and SK-OV-3cisR, treated with sublethal doses (5 μg/ml A2780cisR and 2 μg/ml SK-OV-3cisR) of cisplatin for 48 hr. Intracellular PA levels were measured using fluorometric Phosphatidic Acid Assay. Cell viability and cisplatin IC50 were determined by CellTiter-Glo assay. B and C, Effect of external PA (B) or LPA (C) in DGKA-lacking cells on cell viability and cisplatin response. DGKA knockdown cells were treated with 20 μM of PA or LPA and sublethal doses of cisplatin. PA or LPA levels, cell viability, and cisplatin IC50 were assessed as in (A). Activity of LPA-mediated mTOR signaling was assessed by S6 phosphorylation. D, Relative mRNA level of PA producing enzymes in cisplatin-resistant ovarian cancer cells. Isoforms of DGK, LPAAT, and PLD mRNA levels were measured by qRT-PCT using GAPDH as a control. E, Effect of targeting PA producing enzymes on intracellular level of PA in ovarian cancer cells. Major isoforms of DGK, LPAAT, and PLD were stably knocked down, cells were treated with sublethal doses of cisplatin, and PA levels quantified. (A-E) n=3 technical replicates. Results of one representative experiment from three (A, B and E), two (C), and one (D) independent experiments are shown. Error bars represent SD and the P values were determined by one-way ANOVA for all figures (ns: not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).
To identify an essential enzyme that manages PA levels in ovarian cancer cells, gene expression levels of PA producing enzymes including isoforms of DGK, LPAAT, and PLD were examined in ovarian cancer cells (35). Although DGKB and DGKI were additionally identified from the RNAi screen as potential targets to enhance cisplatin response, DGKA was more abundant than the other isoforms in ovarian cancer cells (Fig. 2D). Furthermore, target downregulation study indicated that DGKA is the critical enzyme responsible for PA production among the 3 PA regulating enzymes, DGKA, LPAAT2, and PLD2, in ovarian cancer cells (Fig. 2E). These data suggest that PA, mainly produced by DGKA in ovarian cancer cells, is the key factor that provides DGKA-mediated cisplatin resistance in ovarian cancer cells.
DGKA signals through c-JUN to provide cisplatin resistance
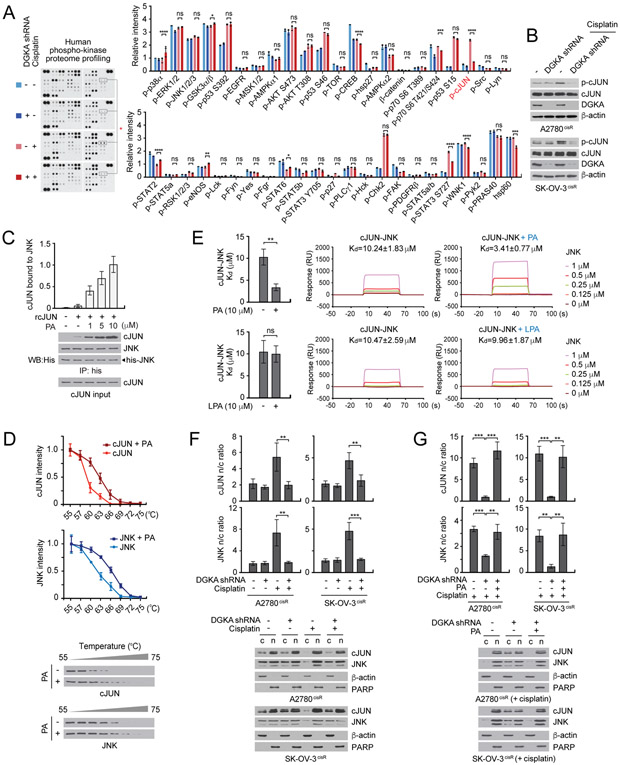
Although DGKA contributes to cisplatin resistance through PA, a key intermediate in lipid metabolism, the knockdown of DGKA along with cisplatin treatment did not induce changes in lipid synthesis, nucleotide synthesis, bioenergetics, or redox status in ovarian cancer cells, suggesting DGKA-PA-induced cisplatin resistance does not occur through metabolic reprogramming (Supplementary Fig. S5). To investigate how DGKA regulates cellular signaling effectors to confer cisplatin resistance in cancer cells, we performed proteome profiling of human phospho-kinase and apoptosis signaling and monitored expression and phosphorylation of 71 factors related to cell survival and apoptosis in ovarian cancer cells with DGKA knockdown and cisplatin treatment. We observed that the phosphorylation of c-JUN at serine 63, which is known to enhance its transcription activity, dramatically decreased when A2780cisR cells lacked DGKA and were treated with cisplatin (Fig. 3A and Supplementary Fig. S6A and B). The array results were further confirmed in SK-OV-3cisR and A2780cisR cells by immunoblotting (Fig. 3B). The decrease in c-JUN phosphorylation in DGKA knockdown cells with cisplatin treatment was not mediated through a change in c-JUN N-terminal kinase (JNK) one of the mitogen-activated protein kinase (MAPK) family (Supplementary Fig. S6C). Target downregulation of c-JUN in these cells mimicked the DGKA knockdown effect, leading to attenuated cell viability and enhanced apoptosis induction when combined with cisplatin treatment, which provides a potential link between DGKA and c-JUN in cisplatin-resistant cell survival (Supplementary Fig. S6D).
Figure 3. DGKA and PA activate c-JUN through c-JUN-JNK complex formation and nuclear localization upon cisplatin treatment.
A, Phospho-kinase proteome profiling of A2780cisR cells with DGKA knockdown and cisplatin treatment. Cells were treated with a sublethal dose of cisplatin for 48 hr. Density analysis was performed using ImageJ software. B, Phospho-cJUN Ser63 levels in A2780cisR and SK-OV-3cisR cells treated with DGKA shRNA and cisplatin. C, Effect of PA on c-JUN and JNK interaction. Recombinant JNK and c-JUN were incubated with increasing concentrations of PA. D, Cellular thermal shift assay (CETSA) curves for c-JUN or JNK measured in cell lysates harboring myc or flag tagged c-JUN or JNK treated with or without 10 μM PA. E, SPR analysis of interaction between c-JUN and JNK in the presence of PA or LPA. F and G, Localization of c-JUN and JNK in cells with DGKA knockdown and cisplatin treatment in (F) and PA rescue in (G). β-actin and PARP were used as cytoplasmic and nuclear markers, respectively. c: cytosol, n: nucleus. (D) n=3 and (A) n=2 technical replicates. Results of one representative experiment from four (C and D), three (B, F and G), two (E) and one (A) independent experiments are shown. Error bars represent SD. P values were determined by one-way ANOVA (ns: not significant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
We explored the molecular mechanism underlying DGKA and PA-mediated activation of c-JUN. DGKA did not directly phosphorylate c-JUN (Supplementary Fig. S6E). However, in vitro incubation with PA enhanced the complex formation of c-JUN and JNK in a dose-dependent manner (Fig. 3C). Furthermore, cellular thermal shift and surface plasmon resonance (SPR) assays showed that PA binds to the cJUN-JNK complex and PA but not LPA enhances the interaction between c-JUN and JNK (Fig. 3D and E). Nuclear accumulation of c-JUN is associated with transcriptional activation of c-JUN. Interestingly, we found that treatment with cisplatin enhances the nuclear localization of c-JUN and JNK. Loss of DGKA results in reduced nuclear import of c-JUN while treatment with PA restored the accumulation of c-JUN in the nucleus in cisplatin-treated ovarian cancer cells (Fig. 3F and G, and Supplementary Fig. S6F and G). These results suggest that DGKA and its product PA promote c-JUN and JNK complex formation and their nuclear localization, which eventually leads to the transcriptional activation of c-JUN in ovarian cancer cells upon cisplatin exposure.
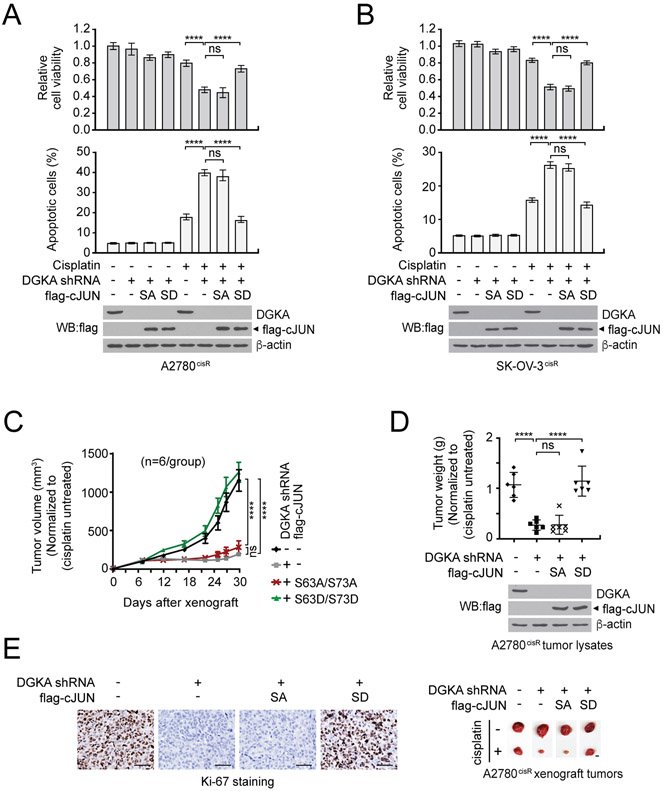
We next examined whether DGKA signals through c-JUN to mediate cisplatin-resistant cancer cell survival and proliferation. Overexpression of phospho-mimetic mutant form (S63D/S73D) but not phospho-deficient mutant form (S63A/S73A) of c-JUN fully restored the decreased cell viability and elevated apoptotic cell death in cisplatin-treated DGKA knockdown cells (Fig. 4A and B). Consistent with the observation in vitro, the phospho-mimetic active mutant form of c-JUN rescued the attenuated tumor growth potential in A2780cisR xenograft mice, suggesting that DGKA mediates cisplatin-resistant cell proliferation and tumor growth by signaling through the transcription factor c-JUN in ovarian cancer (Fig. 4C-E).
Figure 4. DGKA promotes cisplatin resistance through c-JUN activation.
A and B, Cisplatin-dependent cell viability and apoptotic cell death in A2780cisR (A) and SK-OV-3cisR (B) cells with DGKA knockdown and c-JUN SA (S63A/S73A) or SD (S63D/S73D) expression. Cells were treated with sublethal doses of cisplatin for 48 hr followed by CellTiter-Glo assay and annexin V staining. C-E, DGKA knockdown and c-JUN rescue effect on cisplatin-resistant tumor growth. A2780cisR cells with DGKA knockdown and c-JUN SA or SD expression were xenografted into mice and cisplatin (5 mg/kg/i.p. twice/week) was administered when tumors reached 100 mm3. Tumor volume (C), tumor weight and representative tumor images of each group (D), and Ki-67 staining for tumor proliferation rate (E) are shown. Scale bars represent 50 μm for Ki-67 staining images and 10 mm for tumor images. (A and B) n=3 technical replicates and results of one representative experiment from three independent experiments are shown. (C-E) n=6. Error bars represent SEM for (C) and SD for all rest. P values were determined by two-way ANOVA for (C) and one-way ANOVA for others (ns: not significant; ****P < 0.0001).
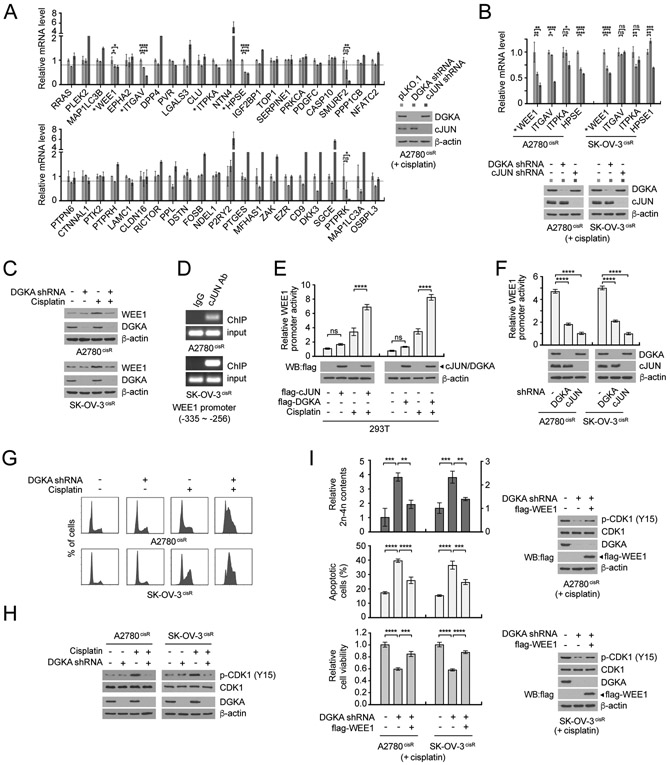
DGKA and c-JUN-mediated WEE1 expression promotes cisplatin-resistant cell survival
C-JUN is involved in various activities in cancer cells via direct regulation of target genes. To further delineate the downstream signaling pathways of DGKA and c-JUN that contribute to cisplatin resistance in ovarian cancer, we assessed gene expression of 44 potential c-JUN transcription targets, which were identified in human melanoma cell lines using ChIP-Seq of the Encyclopedia of DNA Elements (ENCODE), in DGKA or c-JUN knockdown cells treated with cisplatin (36). Among 44 candidates tested, levels of WEE1, ITGAV, ITPKA, and HPSE were significantly decreased in both DGKA and c-JUN knockdown cells (Fig. 5A). Further determination of both gene and protein expression in multiple ovarian cancer cell lines revealed that the level of WEE1, a cell cycle checkpoint regulator, is commonly decreased by DGKA knockdown and cisplatin treatment, suggesting c-JUN and WEE1 as potential downstream effectors of DGKA (Fig. 5B and C). Genetic or pharmacological inhibition of WEE1 mimicked the DGKA or c-JUN knockdown effect, resulting in attenuated cell viability and enhanced apoptosis induction when combined with cisplatin treatment (Supplementary Fig. S6H and I). ChIP assay and WEE1 promoter reporter assay in ovarian cancer cells with c-JUN or DGKA modulation revealed that WEE1 is the direct transcription target of c-JUN, which is activated by DGKA, in ovarian cancer cells upon cisplatin exposure (Fig. 5D-F). Knockdown of DGKA led to aberrant cell cycle progression and decreased inhibitory phosphorylation at Y15 of CDK1, which is essential for the pre-mitotic checkpoint (Fig. 5G and H). Overexpression of WEE1 partially restored the accumulated 2n and 4n DNA content, CDK1 Y15 phosphorylation, enhanced cisplatin-induced apoptosis, and decreased cell viability upon cisplatin exposure in DGKA knockdown cells (Fig. 5I). These data suggest that c-JUN activated by DGKA promotes WEE1 expression, which protects cisplatin-exposed ovarian cancer cells from cell cycle arrest leading to cisplatin-resistant cell survival and proliferation.
Figure 5. DGKA-c-JUN promotes gene expression of WEE1 to control cell cycle upon cisplatin exposure.
A, Gene expression profile of potential DGKA-c-JUN downstream effectors. A2780cisR cells with DGKA or c-JUN knockdown were treated with a sublethal dose (5 μg/ml) of cisplatin for 48 hr. The mRNA level of each target gene was determined by qRT-PCR using GAPDH as a control. The genes significantly decreased in both DGKA and c-JUN knockdown cells are marked with asterisks. B, mRNA levels of top 4 candidates of (a) in A2780cisR and SK-OV-3cisR cells. Genes significantly decreased in both cell lines are labeled with asterisks. C, WEE1 protein levels in ovarian cancer cells with DGKA knockdown and cisplatin treatment were determined by immunoblotting. D, ChIP assay of c-JUN binding to WEE1 promoter. c-JUN antibody was used to enrich endogenous c-JUN in A2780cisR and SK-OV-3cisR cells. E-F, Effect of c-JUN or DGKA overexpression (E) or knockdown (F) on WEE1 promoter activity. G, Cell cycle profile of ovarian cancer cells with DGKA knockdown and cisplatin treatment. H, Levels of CDK1 Y15 phosphorylation in cells with DGKA knockdown and cisplatin treatment. I, Effect of WEE1 overexpression on cell cycle distribution, apoptotic cell population, cell viability, and CDK1 Y15 phosphorylation in cells with DGKA knockdown and cisplatin treatment. n=3 technical replicates. Results of one representative experiment from three (E, F, I), two (B-D, G, H) and one (A) independent experiments are shown. Error bars represent SD. P values were determined by one-way ANOVA (ns: not significant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
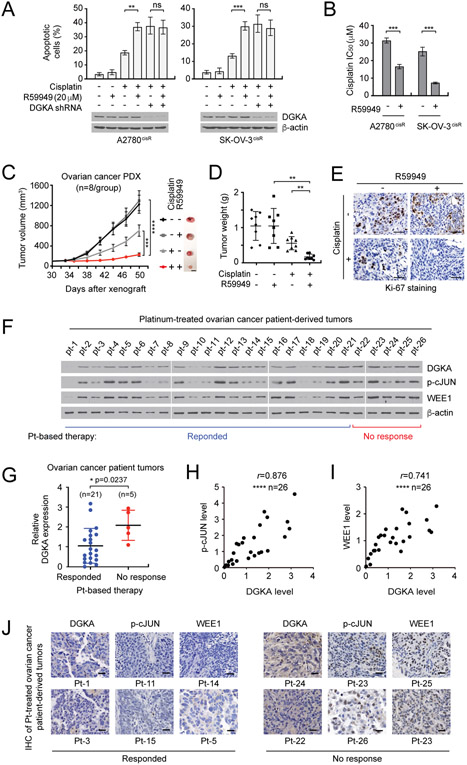
A DGK inhibitor sensitizes ovarian cancer cells to cisplatin treatment
We demonstrated that the knockdown of DGKA replenishes cisplatin sensitivity, implicating DGKA as a promising synthetic lethal target in combination with cisplatin in ovarian cancer. Thus, we examined the effect of targeting DGKA with a small molecule inhibitor on cisplatin sensitization in ovarian cancer cells and patient-derived xenograft (PDX) mouse models of ovarian cancer. Treatment with the DGK inhibitor, R59949, attenuated the activity of DGKA and intracellular PA levels (Supplementary Fig. S7A-C). R59949 enhanced cisplatin-induced apoptotic cell death, whereas cells with knockdown of DGKA but not DGKB or DGKI were resistant to cisplatin-induced apoptosis, indicating that the enhancement of cisplatin-induced cell death by R59949 occurs by targeting DGKA, but not other kinases (Fig. 6A and Supplementary Fig. S7D-F). In line with this observation, treatment with R59949 sensitized cisplatin-resistant ovarian cancer cells to cisplatin treatment (Fig. 6B). Furthermore, treatment with R59949 in combination with cisplatin dramatically attenuated tumor growth in ovarian PDX with no significant changes in body weight or histopathology (Fig. 6C-E and Supplementary Fig. S7G and H). These data collectively suggest that DGKA could be a potent therapeutic target together with cisplatin for ovarian cancer.
Figure 6. DGK inhibitor treatment sensitizes ovarian cancer cells to cisplatin treatment and DGKA-c-JUN-WEE1 signaling correlates with cisplatin resistance in ovarian cancer patient tumors.
A. Effect of DGK inhibitor R59949 on apoptotic cell death in cells with or without DGKA knockdown. B, Cisplatin response in cells treated with R59949 and cisplatin. C-E, Effect of R59949 and cisplatin on ovarian PDX tumor growth. Ovarian PDX mice were treated with cisplatin (5 mg/kg/i.p. twice/week) and R59949 (10 mg/kg/s.c. once every two days) after 30 days of xenograft. s.c.: subcutaneous injection. F, DGKA, p-c-JUN, and WEE1 levels in tumors derived from ovarian cancer patients who received platinum therapy were assessed by immunoblotting. Responded: patients who responded to platinum therapy (pt-1 ~ pt-21). No response: patients who had disease recurrence within 6 months after platinum therapy (pt-22 ~ pt-26). G, Relative DGKA expression in ‘Response’ and ‘No response’ groups. The immunoblots shown in (F) were quantified by ImageJ software. H and I, Correlation between DGKA and p-c-JUN levels (H) or DGKA and WEE1 levels (I) in tumor samples. J, Representative DGKA, p-c-JUN, and WEE1 IHC staining of platinum-treated ovarian cancer patient-derived tumors. Scale bars represent 10 mm (C), 50 μm (E), and 25 μm (J). (A and B) n=3 technical replicates and results of one representative experiment from three independent experiments are shown. (C-E) n=8. Results of one representative experiment from two independent experiments are shown for (F). (G-I) n=26. Error bars represent SEM for (C) and SD for all others. P values were determined by one-way ANOVA for (A) and (D), Student’s t test for (B) and (G), two-way ANOVA for (C), and Pearson correlation for (H) and (I) (ns: not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).
The DGKA-c-JUN-WEE1 signaling axis is associated with platinum resistance in ovarian cancer patient-derived tumors
To further validate the clinical relevance of the DGKA-c-JUN-WEE1 signaling axis, we examined levels of DGKA, phosphorylation of c-JUN, and WEE1 expression in tumors originally derived from ovarian cancer patients who received cisplatin or carboplatin containing therapy before tumor excision. Ovarian cancer patients were divided into 2 groups: patients who responded to cisplatin or carboplatin-based therapy for a duration of six months and patients who lost response to the therapy within six months. Expression of DGKA, phosphorylation of c-JUN, and WEE1 expression were assessed in 26 patient-derived tumors that had different responses to platinum-based therapy (Fig. 6F and Supplementary Table S1). DGKA level was significantly higher in tumors collected from ovarian cancer patients who did not respond to platinum-based therapy compared to patient-derived tumors that were sensitive to a platinum-based regimen (Fig. 6G). Primary tumor samples were collected before platinum treatment, suggesting that initial expression of DGKA in tumors contributes to platinum resistance. Moreover, DGKA expression positively correlated with phospho-cJUN and WEE1 levels in these tumors with correlation coefficient r values of 0.876 and 0.741, respectively (Fig. 6H and I). The signaling axis was further confirmed by IHC staining (Fig. 6J). These data suggest that the DGKA-cJUN-WEE1 signaling axis is connected with cisplatin resistance in ovarian cancer patients.
DISCUSSION
Platinum-based chemotherapeutic regimens are the mainstay of treatment for advanced ovarian cancer. However, around 70% of ovarian cancer patients relapse after cisplatin-containing first-line chemotherapy and understanding of the resistance mechanisms in a whole cellular context is needed (37). Our findings provide a comprehensive view of the inter-relationship between metabolic pathways and cellular kinase signaling that confers cisplatin resistance in ovarian cancer. We present a distinct role of the key intermediate in lipid metabolism, PA, as a signaling molecule that activates the transcription factor c-JUN upon cisplatin exposure and consequently enhances transcription of the cell cycle checkpoint regulator WEE1 gene. Mechanistically, we found that PA activates the c-JUN transcriptional machinery by recruiting c-JUN to upstream kinase JNK and translocating the complex to the nucleus for its function. Cisplatin treatment relocates c-JUN and JNK complex into the nucleus. While removal of DGKA impairs this translocation, refueling with PA restored the nuclear accumulation. Therefore, it is possible that PA binds within the c-JUN and JNK complex and induces the conformational change of the complex to facilitate nuclear translocation. Indeed, studies report PA as a cellular translocation mediator (38,39). Further structural and mutational study is warranted to resolve the structure-based mechanism by which PA influences cellular location of c-JUN and JNK in ovarian cancer cells.
Our data suggest that the role of DGKA as a cisplatin resistance driver is prominent in ovarian cancer, as the effects of targeting DGKA in other types of cancer including cervical cancer, head and neck cancer, and lung cancer were not as significant as in ovarian cancer in our series of synthetic lethal RNAi screens using a 781-kinome shRNA library in multiple types of cancer cells (32). In line with these results, targeting DGKA itself effectively attenuates cancer cell proliferation in other types of cancers including melanoma and hepatocellular carcinoma, which is different from observations in ovarian cancer cells (22,23). This implies that metabolic and cellular needs of tumor cells may differ depending on cancer type and their disparate metabolic status.
We recently reported that microtubule-associated serine/threonine kinase 1 (MAST1) and inositol triphosphate kinase B (ITPKB) are commonly upregulated in various types of cisplatin-resistant tumors compared to cisplatin-sensitive tumors from patients with head and neck cancer, lung cancer, and ovarian cancer (32,40,41). MAST1 contributes to cisplatin resistance by rewiring the Raf-MEK pathway, whereas ITPKB balances redox status in cisplatin-treated cancer cells by inhibiting a ROS producing enzyme, NADPH oxidase 4. It is worthwhile to evaluate whether these factors operate in entirely separate signaling axes or are crosslinked with one another to overcome cisplatin resistance in human cancers. If these signaling factors work in parallel by separately contributing to kinase signaling, metabolic balance, and gene regulation, a combinatorial targeting approach of these factors may provide synergistic or additive effects on sensitizing cisplatin response in cancer cells.
Previous studies suggest that JNK-mediated c-JUN activation can subsequently induce both transcription of pro-apoptotic and pro-survival factors. Therefore, c-JUN signaling could be a double-edged sword in cisplatin treatment, simultaneously not only being a significant factor in cell death but also being associated with increased resistance to cisplatin-based chemotherapy through inducing cell survival (42). In the context of ovarian cancer, c-JUN mainly induces pro-survival signaling by regulating the cell cycle progression regulator WEE1 upon cisplatin exposure. WEE1 regulates the G2 checkpoint and prevents entry into mitosis in response to DNA damage (43,44). Therefore, targeting DGKA leads to inhibition of c-JUN and WEE1 and this may result in unrepaired DNA entering mitosis, leading to mitotic catastrophe and consequently to apoptotic cell death in response to cisplatin. In contrast to c-JUN, the transcription factor KLF2 is reported to specifically downregulate WEE1 expression in ovarian cancer (45). It is possible that c-JUN may be a predominant transcriptional regulator of WEE1 induced by DGKA when ovarian cancer cells are exposed to cisplatin.
We also demonstrated the clinical relevance of the DGKA-c-JUN-WEE1 signaling axis in cisplatin resistance using patient-derived tumors from ovarian cancer patients. Our findings support that DGKA signaling could be used as a promising predictive marker of cisplatin-based chemotherapy response. Lastly, genetic and pharmacologic inhibition of DGKA effectively enhanced cisplatin response in ovarian cancer, which provides evidence that the strategy of targeting DGKA signaling has potential efficacy in the treatment of ovarian cancer in combination with platinum-based chemotherapy. Further clinical trials and treatment optimization are warranted to target the DGKA signaling axis to overcome cisplatin resistance in ovarian cancer.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Platinum-based therapy is a main treatment option for ovarian cancer, but resistance often leads to therapeutic failure. Dysregulated kinase signaling and altered metabolism are implicated in human cancers, but how these pathways intertwine and contribute to chemotherapy resistance is largely unknown. Here we report that loss of DGKA selectively rescues cisplatin sensitivity in cisplatin-resistant ovarian cancer in a kinase-dependent manner through its metabolic product, PA. Mechanistically, PA activates a transcription factor c-JUN by binding and translocating it into the nucleus to promote WEE1 expression. DGKA inhibition significantly sensitizes cancer cells to cisplatin in a patient-derived xenograft model. Moreover, the clinical relevance of DGKA signaling in platinum resistance was confirmed in patient tumors. Our findings not only suggest a mechanism by which DGKA provides cisplatin resistance through c-JUN-WEE1 signaling, but also implicate DGKA as a potent therapeutic target to overcome platinum resistance in ovarian cancer.
ACKNOWLEDGEMENT
We acknowledge Dr. Anthea Hammond for critical reading and editing of the manuscript. We thank Champions Oncology team for providing ovarian cancer patient tumor specimen. This study was supported in part by NIH/NCI (R01CA175316, R01CA207768, DRP of P50CA217691 to S.K.), Department of Defense (W81XWH-17–1-0186 to S.K.), American Cancer Society (Winship IRG-17–181-04 to L.J.), Elsa U. Pardee Foundation (Research grant to L.J.), University of Florida (UF Health Cancer Center Pilot Grant to L.J.), and the Emory University Integrated Cellular Imaging Microscopy Core of the Winship Cancer Institute comprehensive cancer center grant, P30CA138292. S.K. is a Georgia Cancer Coalition Scholar, a Robbins Scholar, and an American Cancer Society Basic Research Scholar.
Funding Information: This work was supported by NIH R01 CA175316 (S.K.), NIH R01 CA207768 (S.K.), DoD W81XWH-17–1-0186 (S.K.), NIH DRP of P50CA217691 (S.K.), Developmental Funds from the Winship Cancer Institute of Emory University (S.K.), and #IRG-17–181-04 from the American Cancer Society (L.J.), and the Emory University Integrated Cellular Imaging Microscopy Core of the Winship Cancer Institute grant, P30CA138292.
Footnotes
DISCLOSURE of POTENTIAL CONFLICTS of INTEREST
The authors declare no competing interests.
REFERENCES
- 1.Damia G, Broggini M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers (Basel) 2019;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012;64:706–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain A, Jahagirdar D, Nilendu P, Sharma NK. Molecular approaches to potentiate cisplatin responsiveness in carcinoma therapeutics. Expert Rev Anticancer Ther 2017;17:815–25. [DOI] [PubMed] [Google Scholar]
- 4.Sun P, Song Y, Liu D, Liu G, Mao X, Dong B, et al. Potential role of the HOXD8 transcription factor in cisplatin resistance and tumour metastasis in advanced epithelial ovarian cancer. Sci Rep 2018;8:13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Smith M, Halder JB, Meltzer PS, Gonda TA, Mangala LS, Rupaimoole R, et al. ATP11B mediates platinum resistance in ovarian cancer. J Clin Invest 2013;123:2119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fei M, Zhao Y, Wang Y, Lu M, Cheng C, Huang X, et al. Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest 2009;27:52–9. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Feng Q, Kim JM, Schneiderman D, Liston P, Li M, et al. Human ovarian cancer and cisplatin resistance: possible role of inhibitor of apoptosis proteins. Endocrinology 2001;142:370–80. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan LB, Gui DY, Vander Heiden MG. Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 2016;16:680–93. [DOI] [PubMed] [Google Scholar]
- 9.Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou L, et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol 2015;17:1484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 2015;27:257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaal EA, Berkers CR. The Influence of Metabolism on Drug Response in Cancer. Front Oncol 2018;8:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catanzaro D, Gaude E, Orso G, Giordano C, Guzzo G, Rasola A, et al. Inhibition of glucose-6-phosphate dehydrogenase sensitizes cisplatin-resistant cells to death. Oncotarget 2015;6:30102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catanzaro D, Nicolosi S, Cocetta V, Salvalaio M, Pagetta A, Ragazzi E, et al. Cisplatin liposome and 6-amino nicotinamide combination to overcome drug resistance in ovarian cancer cells. Oncotarget 2018;9:16847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Gao W, Zhang Y, Wu S, Liu Y, Deng X, et al. ABT737 reverses cisplatin resistance by targeting glucose metabolism of human ovarian cancer cells. Int J Oncol 2018;53:1055–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wangpaichitr M, Wu C, Li YY, Nguyen DJM, Kandemir H, Shah S, et al. Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer. Oncotarget 2017;8:49275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan EJ, Kurtoglu M, Brenneman R, Liu H, Lampidis TJ. Targeting cisplatin-resistant human tumor cells with metabolic inhibitors. Cancer Chemother Pharmacol 2014;73:417–27. [DOI] [PubMed] [Google Scholar]
- 17.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J 2008;409:1–18. [DOI] [PubMed] [Google Scholar]
- 18.Topham MK, Epand RM. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim Biophys Acta 2009;1790:416–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakane F, Imai S, Kai M, Yasuda S, Kanoh H. Diacylglycerol kinases: why so many of them? Biochim Biophys Acta 2007;1771:793–806. [DOI] [PubMed] [Google Scholar]
- 20.Purow B Molecular Pathways: Targeting Diacylglycerol Kinase Alpha in Cancer. Clin Cancer Res 2015;21:5008–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez CL, Floyd DH, Xiao A, Mullins GR, Kefas BA, Xin W, et al. Diacylglycerol kinase alpha is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov 2013;3:782–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagisawa K, Yasuda S, Kai M, Imai S, Yamada K, Yamashita T, et al. Diacylglycerol kinase alpha suppresses tumor necrosis factor-alpha-induced apoptosis of human melanoma cells through NF-kappaB activation. Biochim Biophys Acta 2007;1771:462–74. [DOI] [PubMed] [Google Scholar]
- 23.Takeishi K, Taketomi A, Shirabe K, Toshima T, Motomura T, Ikegami T, et al. Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEK-ERK pathway. J Hepatol 2012;57:77–83. [DOI] [PubMed] [Google Scholar]
- 24.Temes E, Martin-Puig S, Acosta-Iborra B, Castellanos MC, Feijoo-Cuaresma M, Olmos G, et al. Activation of HIF-prolyl hydroxylases by R59949, an inhibitor of the diacylglycerol kinase. J Biol Chem 2005;280:24238–44. [DOI] [PubMed] [Google Scholar]
- 25.Temes E, Martin-Puig S, Aragones J, Jones DR, Olmos G, Merida I, et al. Role of diacylglycerol induced by hypoxia in the regulation of HIF-1alpha activity. Biochem Biophys Res Commun 2004;315:44–50. [DOI] [PubMed] [Google Scholar]
- 26.Baldanzi G, Mitola S, Cutrupi S, Filigheddu N, van Blitterswijk WJ, Sinigaglia F, et al. Activation of diacylglycerol kinase alpha is required for VEGF-induced angiogenic signaling in vitro. Oncogene 2004;23:4828–38. [DOI] [PubMed] [Google Scholar]
- 27.Bacchiocchi R, Baldanzi G, Carbonari D, Capomagi C, Colombo E, van Blitterswijk WJ, et al. Activation of alpha-diacylglycerol kinase is critical for the mitogenic properties of anaplastic lymphoma kinase. Blood 2005;106:2175–82. [DOI] [PubMed] [Google Scholar]
- 28.Noessner E DGK-alpha: A Checkpoint in Cancer-Mediated Immuno-Inhibition and Target for Immunotherapy. Front Cell Dev Biol 2017;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Sakane F, Kanoh H, Walsh JP. Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharma 2000;59:763–72. [DOI] [PubMed] [Google Scholar]
- 30.Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol 2012;188:5990–6000. [DOI] [PubMed] [Google Scholar]
- 31.Jin L, Li D, Lee JS, Elf S, Alesi GN, Fan J, et al. p90 RSK2 mediates antianoikis signals by both transcription-dependent and -independent mechanisms. Mol Cell Biol 2013;33:2574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin L, Chun J, Pan C, Li D, Lin R, Alesi GN, et al. MAST1 Drives Cisplatin Resistance in Human Cancers by Rewiring cRaf-Independent MEK Activation. Cancer Cell 2018;34:315–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez Molina D, Jafari R, Ignatushchenko M, Seki T, Larsson EA, Dan C, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013;341:84–7. [DOI] [PubMed] [Google Scholar]
- 34.Gad H, Koolmeister T, Jemth AS, Eshtad S, Jacques SA, Strom CE, et al. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014;508:215–21. [DOI] [PubMed] [Google Scholar]
- 35.Foster DA. Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol Metab 2013;24:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schummer P, Kuphal S, Vardimon L, Bosserhoff AK, Kappelmann M. Specific c-Jun target genes in malignant melanoma. Cancer Biol Ther 2016;17:486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giornelli GH. Management of relapsed ovarian cancer: a review. Springerplus 2016;5:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiozaki K, Takahashi K, Hosono M, Yamaguchi K, Hata K, Shiozaki M, et al. Phosphatidic acid-mediated activation and translocation to the cell surface of sialidase NEU3, promoting signaling for cell migration. FASEB J 2015;29:2099–111. [DOI] [PubMed] [Google Scholar]
- 39.Yao H, Wang G, Wang X. Nuclear translocation of proteins and the effect of phosphatidic acid. Plant Signal Behav 2014;9:e977711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan C, Chun J, Li D, Boese AC, Li J, Kang J, et al. Hsp90B enhances MAST1-mediated cisplatin resistance by protecting MAST1 from proteosomal degradation. J Clin Invest 2019;129:4110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan C, Jin L, Wang X, Li Y, Chun J, Boese AC, et al. Inositol-triphosphate 3-kinase B confers cisplatin resistance by regulating NOX4-dependent redox balance. J Clin Invest 2019;129:2431–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan D, An G, Kuo MT. C-Jun N-terminal kinase signalling pathway in response to cisplatin. J Cell Mol Med 2016;20:2013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell 1987;49:559–67. [DOI] [PubMed] [Google Scholar]
- 44.Do K, Doroshow JH, Kummar S. Wee1 kinase as a target for cancer therapy. Cell Cycle 2013;12:3159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F, Zhu Y, Huang Y, McAvoy S, Johnson WB, Cheung TH, et al. Transcriptional repression of WEE1 by Kruppel-like factor 2 is involved in DNA damage-induced apoptosis. Oncogene 2005;24:3875–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.