The testing of patients for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been proposed as a mechanism for protecting patients and healthcare workers during the current Coronavirus disease 2019 (COVID-19) pandemic.1 However, when detected by nucleic acid amplification using polymerase chain reaction (PCR), there is the possibility of false negative results with these tests.2 In addition, the test only assesses for virus at a single point in time, and if a patient is still early in the incubation phase the presence of virus can be missed. Both of these factors may lead healthcare workers to be falsely reassured by a negative PCR test result. Finally, there is little published evidence that testing patients who are not considered persons of interest (PUI) or not exhibiting symptoms of COVID-19 infection increases the safety of the hospital environment.
Despite this, testing of pre-surgical patients, both emergent and elective, has quickly become common practice due to fear of exposure, the desire to reassure patients that areas of the hospital are “COVID-free”, and competitive pressures within markets. Several organizations, including the American College of Surgeons, have recommended that testing via PCR/nucleic acid amplification be conducted or considered prior to surgery.3
A systematic review of 39 studies found that 29 of those recommend screening for all surgical patients prior to surgery.4 Most recommendations consist of testing at least 48–72 hours prior to surgery, with some recommendations including quarantine until test results arrive or concurrent chest imaging.4 In some instances, testing is recommended only for patients with clinical symptoms or those undergoing high-risk surgeries, such as transplant or lung resection.5 Testing of asymptomatic patients, particularly in high prevalence areas, has also been proposed, although the rate of perioperative complications and postoperative fatality has ranged widely, from 0% to 66.6%.6
As elective and non-emergent procedures resume amid COVID-19, providers are challenged with determining the best pre-surgical testing processes to balance safety of patients and healthcare workers with other factors, such as delays to needed procedures and the cost of testing, and frameworks have been proposed for evaluating the changing regulatory guidelines and societal pressures.7 As testing is only one part of the necessary considerations and precautions in the perioperative period during this pandemic,8 HCA Healthcare has approached the return to elective surgery and other deferred care by implementing a series of universal protections in our hospitals. The universal protection framework consisted of a new standard to promote patient safety and confidence across all sites of care. This framework had four main areas: infection prevention, access control, distancing and patient flow. Infection prevention areas of focus included universal masking for all within the hospital, execution of personal protective equipment guidance and policies, and continued adherence to or expansion of infection prevention policies to reduce the possibility of transmission. Access control included separate entrances for different patient groups, screening of colleagues and patients, and limits on visitation. To promote distancing, patient cohorting was implemented to reduce overlap with high-risk populations. Patient flow was also adjusted to promote cohorting, conduct prescreening, and expedite patient movement throughout the facilities.
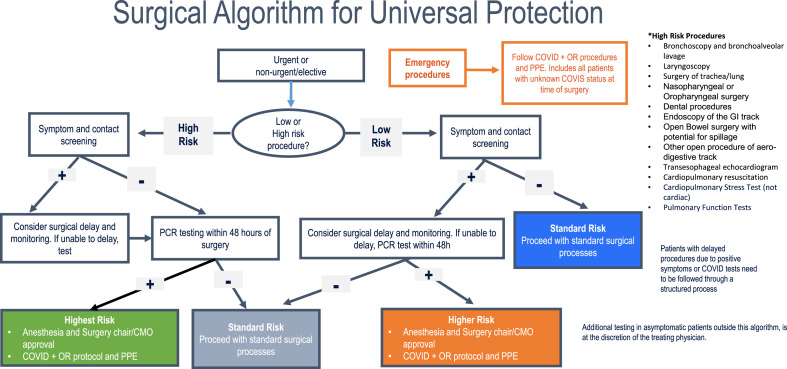
Alongside these efforts, we deployed two pre-surgical testing processes for COVID-19 to support and expand the universal protections. The two testing processes consisted of the following: 1) algorithm-based testing according to risk of exposure (Fig. 1 ); 2) expanded testing of all pre-surgical and pre-procedural cases. The algorithm was developed based on a previously published protocol.9 That protocol was adapted, with minor modifications, by the HCA Healthcare clinical leadership team based on feedback from clinical leaders in the facilities in order to enhance adoption and align with local workflows (Fig. 1). At the same time, COVID-19 testing was always available for all pre-surgical and pre-procedural cases at the provider’s request.
Fig. 1.
Surgical Algorithm for Universal Protection. Algorithm for pre-surgical SARS-CoV-2 testing according to risk of exposure.
Within the HCA healthcare system, facilities are grouped for operational purposes into 15 regional divisions containing 6 to 18 facilities in each market. Market strategy and clinical operations, as well as responsibility for facility performance, are directed by division-level leadership. Division leadership provide daily operating leadership to facilities; facility leadership is responsible for achievement of division and enterprise goals. The algorithm was provided to leadership at all of the 15 regional divisions; leadership could self-select to use expanded testing based on local conditions, e.g., if requested by the majority of physicians or if required by competitive forces in the local market (i.e., announcement of universal testing by other hospital systems in the area).
Regardless of the testing process used, all facilities had adopted the series of recommended universal protections developed for the COVID-19 pandemic (described above) including universal masking, social distancing, screening and access controls, and patient cohorting. These protections had been adopted by all facilities, with some minor and temporary disruptions reported due to supply availability and local workflows.
We initiated these two testing processes in April 2020. For this comparison of testing processes, data were gathered from the electronic health record regarding all surgical cases between April 19 and May 22, 2020; this includes both elective (where case schedule data was >24 hours prior to surgery complete time) and emergent (case scheduled <24 hours prior to surgery complete time) surgeries. Procedures were included for analysis if they were completed in rooms designated as cardiac catheterization labs, general operating room, cardiovascular surgery operating room, cystoscopy rooms, and endoscopy suites; bedside procedures and interventional radiology were excluded.
Data elements extracted for this study were of two types: patient surgery schedule information and COVID-19 PCR testing data aggregated at the patient level. These data were combined at the patient level to allow for tracking of elective and emergent surgery cases and those patients with COVID-19 test results. PCR-based tests were used for all pre-surgical and pre-procedure testing as a standalone test. Cases were considered to have had pre-procedure COVID-19 testing if there was a COVID-19 test collected within the 96 hours before the procedure. All PCR COVID-19 tests were included (external lab, hospital-based lab, STAT).
Out of the total of 135,858 elective and emergent procedures scheduled between April 19 and May 22, 2020, 51,608 had documentation of a COVID-19 test within 96 hours of the procedure. The overall positive result rate was low; 251 cases had a positive COVID-19 test (overall rate of 0.49% of those tested, and 0.18% of all cases). A total of 93,626 cases meeting the definition of elective were scheduled during this time period. Of these, 36,834 were tested and 138 had a positive test (0.37% of those tested, and 0.15% of all elective cases).
Ten divisions (111 facilities) chose expanded testing for elective cases; five divisions (56 facilities) tested based on the high-risk algorithm (Table 1 ). In the facilities using the expanded testing strategy, there were 61,226 elective cases during the study period. Of these, 29,691 (48.5%) had pre-surgical COVID-19 testing performed within 96 hours of the procedure; 103 (0.35%) of these tests were positive. In the facilities using the high-risk algorithm testing strategy, there were 32,395 elective cases during the study period. Of these, 7143 (22.0%) had pre-surgical COVID-19 testing and 35 (0.49%) of these tests were positive.
Table 1.
Pre-surgical COVID-19 testing, by testing process.
| Expanded | Algorithm | |
|---|---|---|
| Number of facilities | 111 | 56 |
| Median (min, max) beds in operation per facility | 213 (16, 837) | 145 (12, 800) |
| Service areas (states represented) | AK, CA, CO, FL, ID, KS, LA, MO, NV, TX, UT | FL, GA, IN, KY, NC, NH, SC, TN, VA |
| Elective surgery case count during study period | 61,226 | 32,395 |
| Median (min, max) COVID-19 tests 96 hours before procedure per facility during study period | 202 (1, 1581) | 90 (0, 479) |
| Total COVID-19 pre-surgical tests during study period (n, %) | 29,691 (48.5%) | 7143 (22.0%) |
| Positive COVID-19 tests during study period (n, %) | 103 (0.35%) | 35 (0.49%) |
During this time period, 97 (58%) of the 167 participating facilities had none of the pre-surgical COVID-19 tests for elective procedures return a positive result. Of the facilities with zero pre-surgical positive results, 62 were in the expanded testing group and 35 were in the algorithm group. Thus, 56% of the facilities in the expanded group and 63% of the facilities in the algorithm group had zero positive pre-surgical tests.
As expected, testing rates increased over the time period of this study as surgical volumes increased and testing capacity grew. Accordingly, the percent of positive results declined as the number of tests performed increased (Table 2 ).
Table 2.
Case and testing volume and percent positive by week, elective cases.
| Week | Elective Case Count |
COVID19 Tested within 96 Hrs of Surgery (n, %) |
COVID19 Positive within 96 Hrs of Surgery (n, %) |
|||
|---|---|---|---|---|---|---|
| Expanded | Algorithm | Expanded | Algorithm | Expanded | Algorithm | |
| April 19, 2020 | 7066 | 4454 | 466 (6.6) | 81 (1.8) | 1 (.21) | 0 (0) |
| April 26, 2020 | 9137 | 4817 | 3652 (40) | 443 (9.2) | 12 (.33) | 4 (.90) |
| May 3, 2020 | 12,680 | 6524 | 7039 (56) | 1540 (24) | 32 (.45) | 6 (.39) |
| May 10, 2020 | 15,568 | 8187 | 8903 (57) | 2394 (29) | 35 (.39) | 13 (.54) |
| May 17, 2020 | 16,775 | 8413 | 9631 (57) | 2685 (32) | 23 (.24) | 12 (.45) |
We also observed that testing rates and percent positive varied by service line. Medical oncology had the highest positive test rate (2.13%) for elective procedures overall, and tested approximately 47% of all elective cases. However, it should be noted that this group only accounted for 0.05% of all surgical cases. In general surgery, which had the highest percentage of total cases (18.55%), the overall testing rate was 42% and the positive test rate was 0.18%. In facilities with expanded testing, the highest positive test rate was again in medical oncology; 2.78% of tests were positive and nearly 56% of this group was tested; these procedures represented 0.06% of the overall surgical volume. In facilities with algorithm based testing, the highest positive test rate was in pulmonology (0.46%); this group represented 0.67% of the total surgical count and had a testing rate of 52.5%.
In total, we found that the prevalence of asymptomatic, COVID positive patients in the pre-surgical population is less than 1% and varied little among facilities in our large healthcare system in the United States. This indicates that, with an estimated prevalence of 0.5% and an estimated cost of $75 per test, the direct cost to identify a single positive COVID-19 test in the elective pre-surgical population is $15,000.
Within the data set, there were a total of 25 patients that had a positive test logged after an elective surgical procedure; 10 of these were in the algorithm group and 15 were in the expanded testing group. However, it appears that only one of post-surgical positive tests was possibly due to hospital-acquired COVID-19: 3 were considered false positives (2 negative tests within 48 hours of the positive test); 4 had positive tests pre-op at a sending facility that was not captured in the original dataset; 2 were known to be exposed at a SNF after discharge from surgical admission but prior to readmission; 8 were readmitted with community acquired COVID after being discharged from the surgical admission; 3 were tested for placement prior to discharge and had positive results with no known exposures and no symptoms; 1 was known to be exposed to infected but asymptomatic staff in the hospital. There has been only 1 reported case of hospital exposure to COVID within our elective surgical population (out of 93,850 for a rate of 0.001%). This occurred in a facility using expanded testing.
Expanded testing for COVID-19 increased the number of patients tested, but decreased the overall positive rate. In this population, the overall positive rate was 0.35% (103 patients). The algorithm-based testing strategy, the overall positive rate was 0.49% (35 patients) but with fewer tests performed. This suggested that the additional tested population in the universal testing group has an equally low, or lower, COVID-19 prevalence rate than those tested via the risk-based algorithm. It should be noted that, during the time frame in question, the geographic areas served by all facilities within the health system were generally considered “non-surge” with low to moderate levels of community transmission.
During this time period, the majority (approximately 89.9%) of inpatient COVID-19 tests were performed on an in-lab platform either at a reference lab or in an HCA Healthcare facility. A minority (approximately 6.7%) of tests were performed by one of two point-of-care (POC) PCR platforms. With any test, there is the risk of false negative from poor collection or other pre-analytic variable. Across the system, best practices were shared to educate on best collection and sample handling practices. While the risk of false negatives is low, this is a potential limitation. At the same time, the potential for false negative results is an operational reality and supports the approach of universal protection.
In total, we piloted two strategies for pre-surgical COVID-19 testing and found that neither universal testing nor testing according to a risk-based algorithm identified a substantial number of asymptomatic COVID-19+ patients. The low prevalence of COVID-19 in both strategies suggests that factors such as differences in patient population, procedure type, or local COVID-19 activity would have a small effect, if any, on the efficiency of pre-surgical testing for COVID-19. Thus, we conclude that expanded testing of pre-surgical patients did not provide additional value during the time period under study. It is likely that these results will generalize to other facilities in non-surge areas, although there may be a level of community prevalence where it becomes appropriate to do more universal testing of pre-surgical patients, and the algorithm also allows for detecting a change in prevalence in the community. In the absence of such changes in community prevalence, it is likely that universal protections are adequate to protect elective surgical patients from in-hospital COVID transmission, and the burden and expense of expanded pre-surgical testing does not enhance safety in a measurable way.
Funding
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Declaration of competing interest
The authors declare they have no conflict of interest.
Acknowledgements
Will Jewell, Matt Herod, Deepak Mahajan, Russell E. Poland PhD.
References
- 1.American Society for Microbiology COVID-19 testing FAQs. 2020. https://www.asm.org/Articles/2020/April/COVID-19-Testing-FAQs [cited 2020 July 6]; Available from:
- 2.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020 doi: 10.1056/NEJMp2015897. https://www.nejm.org/doi/full/10.1056/NEJMp2015897 [DOI] [PubMed] [Google Scholar]
- 3.American College of Surgeons Local resumption of elective surgery guidance. 2020. https://www.facs.org/covid-19/clinical-guidance/resuming-elective-surgery [cited 2020 July 6]; Available from:
- 4.Hojaij F.C., Chinelatto L.A., Boog G.H.P. vol. 75. English; 2020. Surgical practice in the current COVID-19 pandemic: a rapid systematic review. (Clinics (Sao Paulo, Brazil)). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris M, Pierce A, Carlisle B, et al. Pre-operative COVID-19 testing and decolonization. Am J Surg. 2020 2020/05/22. English. [DOI] [PMC free article] [PubMed]
- 6.Nahshon C., Bitterman A., Haddad R. Hazardous postoperative outcomes of unexpected COVID-19 infected patients: a call for global consideration of sampling all asymptomatic patients before surgical treatment. World J Surg. 2020 2020/08;44(8):2477–2481. doi: 10.1007/s00268-020-05575-2. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz A., Sarac B.A., Schoenbrunner A.R. Elective surgery in the time of COVID-19. Am J Surg. 2020;219(6):900–902. doi: 10.1016/j.amjsurg.2020.04.014. PubMed PMID: 32312477. Epub 2020/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Balas M., Al-Balas H.I., Al-Balas H. Surgery during the COVID-19 pandemic: a comprehensive overview and perioperative care. Am J Surg. 2020;219(6):903–906. doi: 10.1016/j.amjsurg.2020.04.018. PubMed PMID: 32334800. Epub 2020/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrester J.D., Nassar A.K., Maggio P.M., Hawn M.T. Precautions for operating room team members during the COVID-19 pandemic. J Am Coll Surg. 2020;230(6):1098–1101. doi: 10.1016/j.jamcollsurg.2020.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]