Abstract
Starting from December 2019 in China, SARS-CoV-2, a novel coronavirus strain has rapidly spread to involve more than 150 countries. SARS-CoV-2 is not only responsible for causing pneumonia, but there are also concerns regarding the involvement of other organs such as the heart, liver, and kidneys. Here, we review kidney involvement in COVID 19, the mechanism of kidney injury, and its impact on mortality. Lastly, we focus on the challenges of COVID19 in dialysis and renal transplant patients.
Keywords: COVID-19, SARS-CoV-2, Kidney, Haemodialysis
1. Introduction
From the first case, which came into view in Wuhan, China, in December 2019 by a novelstrain of human coronaviruses, the severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2), as of March 24, 2020, there had been around 381,761 cases worldwide. On February 11, 2020, WHO termed it as coronavirus disease 2019 (COVID-19). It has now expanded to more than 150 countries across the world and is responsible for 16,558 deaths.1 SARSCoV- 2 is a positive-stranded RNA virus that belongs to the Betacoronavirus category. Often pleomorphic, it has a diameter of 60–140 nm. Coronaviruses are members of Coronavirinae subfamily, which consists of four genera -Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus — theses are based on their phylogenetic relationships and genomic structures. The alphacoronaviruses and betacoronaviruses can infect only mammals. Some strains of gammacoronaviruses and deltacoronaviruses can also infect mammals. Alphacoronaviruses and betacoronaviruses usually responsible for respiratory diseases in humans. The two viruses, SARS-CoV and MERS-CoV are highly pathogenic forms and can lead to severe respiratory syndrome in humans, but other human coronaviruses strains like (HCoV-NL63, HCoV-229E, HCoV-OC43 and HKU1) can also cause mild upper respiratory diseases.2 The origin of SARS-Cov-2 is currently unknown, but after its genomic analyses, there is speculation of its evolution from strain found in bats (BatCov RaTG13).3 Like other respiratory pathogens, human-to-human transmission mainly occurs via aerosol, but transmission by fecal, or direct contact has also been reported. Based on evidence from investigations provided by the China CDC investgations, the incubation time is usually within 3–7 days and can extend up to 2 weeks.4 Clinical reports suggest that the most frequent symptoms of COVID-19 are fatigue, fever, dry cough, sore throat, dyspnoea, and diarrhea.5 Leukopenia is the commonly reported laboratory abnormality.6 Computerized tomography (CT) of the chest is characterized by consolidation or multiple ground-glass opacities involving bilateral sides. Predominantly causing acute respiratory illness, COVID-19 is not confined to only the respiratory system; it may also damage other organs, for instance, the kidneys, heart, gastrointestinal tract, immune, blood, and nervous system.7 This article attempts to highlight the effect of COVID 19 on the kidneys, with a focus on vulnerable patients undergoing dialysis and renal transplantation.
2. Kidney involvement in COVID-19 infection
A concern of kidney impairment was raised in a report of 710 COVID-19 patients by chen et al. which showed proteinuria and hematuria in 44% of patients and hematuria in 26.9 Manuscript (without Author Details) Click here to view linked References percent at the time of admission. Raised serum creatinine was found in 15.5% of patients. An overall incidence of acute kidney injury (AKI) was reported to occur in 3.2% of patients during the study period.8, a), b) Another study by Zhen Li et al., showed a higher prevalence of proteinuria in 63% (32/51) of the patients and raised serum creatinine and blood urea nitrogen in 19% and 27% of the patients respectively. 100% of the patient had abnormalities of the kidneys in CT scans.9 Till now, there is a scarcity of data on kidney histology of COVID-19 patients.
3. Effect of kidney dysfunction on mortality
In recent studies, after adjustment of multiple cofounders like age, sex, leukocyte count and disease severity, AKI, proteinuria, and hematuria were the independent risk factor for inhospital mortality.10
4. Mechanism of kidney injury in COVID-19
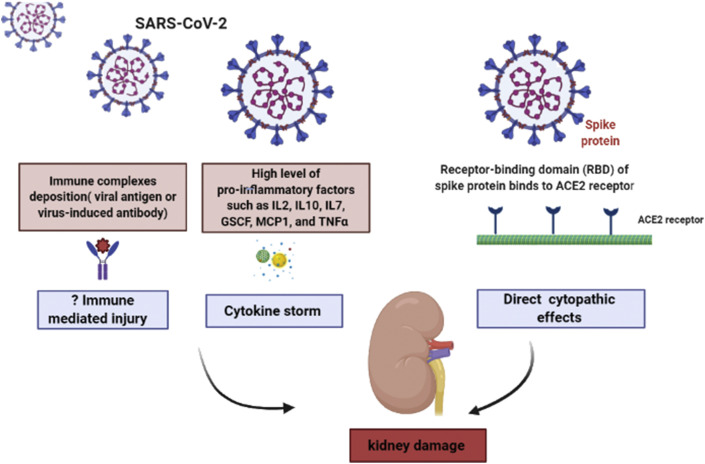
Complete pathogenesis of kidney injury in COVID-19 yet to be elucidated, but it appears to be multifactorial and diverse (shown in Fig. 1 ). Firstly, as some antecodal reports had demonstrated PCR fragments of coronavirus in blood and urine of patients infected with SARS and COVID-19, novel coronavirus may have a direct cytopathic effect of kidney resident cells.11 The spike (S) protein of SARS-CoV-2 uses angiotensin-converting enzyme II (ACE2) and TRMPSS as a cell entry receptor.12 ACE2 is highly expressed in kidneys.
Fig. 1.
Pathogenesis of kidney injury by SARS-CoV-2.
Secondly, although no histological evidence is available in the literature, kidney damage may also occur by immune complexes deposition of viral antigen or virus-induced antibody.13
Another postulated mechanism is that in critical cases of COVID-19, a very high level of proinflammatory factors such as IL2, IL10, IL7, GSCF, MCP1, and TNFα was found suggesting the occurrence of the cytokine storm that can result in injury to the kidney, heart, lung and other normal cells of the body.14
5. Diagnosis
The diagnosis of COVID-19 is mainly established by clinical presentation, history of contact (epidemiological data), and laboratory parameters like leukopenia, CT scan, detection of nucleic acid, serology (IgM/IgG), and enzyme-linked immunosorbent assay (ELISA).15 According to CDC recommendations, a nasopharyngeal swab specimen is collected to test SARS-CoV-2. Two important technologies for nucleic acid detection are real-time quantitative polymerase chain reaction (RT-PCR) and gene sequencing. Both in-house and commercially assays for the detection of COVID-19 are currently under development.16
Numerous biomarkers have been studied in patients suffering from COVID19, for example C-reactive protein, interleukin-6, serum amyloid A, lactate dehydrogenase, neutrophil-to-lymphocyte ratio, cardiac troponin, lymphocytes, platelet count, and D-dimer. Out of all these, two, i.e., lower total lymphocytes count and platelet count are seen in severe patients. Neverthless, to discover how different biomarkers behave during the course of illness in COVID19 we need more research and data.17
6. COVID-19 in dialysis patients
Confronting SARS-CoV-2 infection is more challenging in dialysis patients. A less efficient immune system makes dialysis patients more prone to severe infectious diseases as compared to the general population. Dialysis patients with COVID-19 may be asymptomatic or may have may subtle manifestation of the infection. To prevent the COVID19 outbreak, dialysis facilities must be prepared.18 The Chinese Society of Nephrology and the Taiwan Society of Nephrology have provided guidelines for dialysis facilities during the outbreak of the COVID-19. Some essential measures to limit the risk of COVID-19 in dialysis facilities are given in Table 1 .19
Table 1.
General measures to mitigate the risk of COVID19 in dialysis facilities.
|
|
|
|
|
7. COVID-19 in renal transplant patients
Literature regarding impact COVID-19 in renal allograft recipients (RAR) is lacking. Owing to the effect of long-term use of immunosuppressants, they may have an atypical presentation of the infection and are also at high risk of developing severe disease. Recently Guillen et al. reported a case of a COVID-19 infection in a RAR patient, who initially presented with clinical symptoms of gastrointestinal illness with fever, which subsequently developed respiratory symptoms.20 Although there is no consensus for the treatment of COVID-19 in RAR patients, a reduction of immunosuppressants with low dose methylprednisolone is an acceptable strategy.21
8. Conclusion
The occurrence of kidney dysfunction in COVID-19 patients is frequent and is an independent predictor of mortality. The Pathophysiology of kidney involvement in COVID19 is diverse and complex. Early detection is essential to intervene and prevent further kidney impairment. Strict protocols should be followed to mitigate the risk of infection in dialysis patients.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
None.
Authors' contributions
Priti prepared the manuscript. Vinant, Devinder and Anil analyzed and interpreted patient data. Priti and Vinant were the primary contributors in writing the manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
None.
References
- 1.World Health Organization. reportReport of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19).
- 2.Cui J., Li F., Shi Z. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020 Apr;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel 333 coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological 349 characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 8.a) Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients [e-pub ahead of print]. medRxiv 2020.02.18.20023242; b) Li, Z., Wu, M., Guo, J. et al. Caution on Kidney Dysfunctions of 2019-nCoV Patients. medRxiv 2020.02.08.20021212.
- 9.The Team of Zhong Nanshan Responded that the Isolation of SARS-CoV-2 from Urine Remind Us to Pay More Attention to the Cleaning of Individuals and Families. Guangzhou Daily. Published February 22, 2020.
- 10.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W., Moore M.J., Vasilieva N., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Y., He L., Zhang Q., et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu H., Uchimura K., Donnelly E.L., Kirita Y., Morris S.A., Humphreys B.D. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23:869–881. doi: 10.1016/j.stem.2018.10.010. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X.W., Xu D., Zhang H., Zhou W., Wang L.H., Cui X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019- nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu D.K.W., Pan Y., Cheng S.M.S., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020;254:117788. doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naicker S., Yang C.-W., Hwang S.-J., Liu B.-C., Chen J.-H., Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020 Mar doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Expert Team of Chinese Medical Association Nephrology Branch Recommendations for prevention and control of novel coronavirus infection in blood purification center (room) from Chinese Medical Association Nephrology Branch. Chin J Nephrol. 2020;36:82–84. [Google Scholar]
- 20.Guillen E., Pineiro G.J., Revuelta I., et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? [published online ahead of print, 2020 Mar 20] Am J Transplant. 2020 doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phanish M.K., Hull R.P., Andrews P.A., et al. Immunological risk stratification and tailored minimisation of immunosuppression in renal transplant recipients. BMC Nephrol. 2020;21:92. doi: 10.1186/s12882-020-01739-3. 9020 01739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.