Abstract
Inadequate supply of filtering facepiece respirators (FFRs) for healthcare workers during a pandemic such as the novel coronavirus outbreak (SARS-CoV-2) is a serious public health issue. The aim of this study was to synthesize existing data on the effectiveness of ultraviolet germicidal irradiation (UVGI) for N95 FFR decontamination. A systematic review (PROSPERO CRD42020176156) was conducted on UVGI in N95 FFRs using Embase, Medline, Global Health, Google Scholar, WHO feed, and MedRxiv. Two reviewers independently determined eligibility and extracted predefined variables. Original research reporting on function, decontamination, or mask fit following UVGI were included. Thirteen studies were identified, comprising 54 UVGI intervention arms and 58 N95 models. FFRs consistently maintained certification standards following UVGI. Aerosol penetration averaged 1.19% (0.70–2.48%) and 1.14% (0.57–2.63%) for control and UVGI arms, respectively. Airflow resistance for the control arms averaged 9.79 mm H2O (7.97–11.70 mm H2O) vs 9.85 mm H2O (8.33–11.44 mm H2O) for UVGI arms. UVGI protocols employing a cumulative dose >20,000 J/m2 resulted in a 2-log reduction in viral load. A >3-log reduction was observed in seven UVGI arms using >40,000 J/m2. Impact of UVGI on fit was evaluated in two studies (16,200; 32,400 J/m2) and no evidence of compromise was found. Our findings suggest that further work in this area (or translation to a clinical setting) should use a cumulative UV-C dose of 40,000 J/m2 or greater, and confirm appropriate mask fit following decontamination.
Keywords: PPE, Decontamination, Masks, N95, UVGI
Introduction
The global spread of the novel coronavirus (SARS-CoV-2) threatens public health worldwide, and healthcare professionals are at increased risk due to their close contact with infected patients. Physicians have reported that National Institute for Occupational Safety and Health (NIOSH)-certified N95 filtering facepiece respirators (FFRs), which filter 95% of airborne particles [1], were the personal protective equipment (PPE) that they felt protected them most during the last serious coronavirus outbreak in 2003 (SARS-CoV-1) [2]. Correspondingly, N95 FFRs are currently recommended by the US Centers for Disease Control and Prevention (CDC) to be donned by healthcare professionals treating patients with the coronavirus disease (COVID-19) [3]. Unfortunately, PPE shortages are characteristic of large epidemics, and COVID-19 is no exception [4,5]. Consequently, rationing FFR supply has become a matter of increasing urgency at many hospitals worldwide.
A potential approach to extending the use of existing FFRs would be to decontaminate and reuse the masks. This has prompted the question of whether FFRs can be safely decontaminated for reuse without compromising their structural integrity and efficacy [6]. Previously studied FFR decontamination methods have included bleach, ethanol, hydrogen peroxide, autoclaving, microwaving, and UV light [[7], [8], [9]]. Ultraviolet germicidal irradiation (UVGI) using short-wave ultraviolet-C light (UV–C, usually 245 nm) has a long history of antiseptic applications in medicine and is used for air disinfection in hospitals [10]. Recent reviews highlight the growing popularity of UV-C light for microbial decontamination of objects from toothbrushes to stethoscopes [[11], [12], [13]], and its scalable nature makes it suitable for large-scale decontamination during a pandemic. However, any decontamination process may limit the functionality of the mask if it alters its fit, filter efficiency (aerosol penetration), and/or airflow resistance [14]. Therefore, while some institutions have already implemented UVGI FFR-decontamination protocols during the COVID-19 pandemic [15], FFR producers continue to recommend disposal of contaminated FFRs due to the lack of an evidence-based protocol that addresses these important functional parameters [16].
To help inform FFR-reuse policies and procedures, our team has conducted three systematic reviews to synthesize existing published data regarding the effectiveness of UVGI, heat, microwave irradiation, and chemical disinfectants for N95 FFR decontamination. This review will focus on UVGI, with the following objectives: (1) to assess the impact of the UVGI method on FFR performance, with a specific focus on aerosol penetration and airflow resistance; (2) to determine the effectiveness of the UVGI decontamination method at removing viral or bacterial load; and (3) to describe measures or observations related to fit or physical degradation.
Methods
Study protocol and objectives were established a priori and registered on PROSPERO on 25th March 2020 (CRD42020176156), and reported here according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines of systematic reviews (see Supplementary Data) [17]. The protocol was also uploaded as a pre-print to OSF on 29th March 2020 (https://osf.io/238yh/).
Eligibility criteria
Studies were eligible for inclusion in this systematic review if they satisfied all of the following criteria: (1) original publication or systematic review; (2) study reported on decontamination procedures for N95 (including SN95) FFRs or their components; (3) at least one of the decontamination procedures evaluated used UV light; and (4) the study reported on at least one of the following outcomes of interest: (i) effectiveness of the UVGI method at removing viral or bacterial load; (ii) impact of the UVGI method on filtering face mask performance, with a specific focus on aerosol penetration and airflow resistance (pressure drop); or (iii) measures or observations related to change in mask fit or physical degradation of the mask following UVGI exposure. Only studies published in English or French were included. Studies published prior to 1972, the year that the N95 FFR was invented, were excluded. We also excluded editorials, narrative reviews, book chapters and patents.
Search and selection
The following databases were searched by two health sciences librarians (L.S., M.S.) during the electronic component of the systematic review: Medline (1946–22nd March 2020) and Embase (1947–22nd March 2020) through the Ovid interface and Global Health (1913–20th March 2020) via CAB Direct. A search strategy was developed in Medline, reviewed and then translated into the other databases (see Supplementary Data). There were no language exclusion criteria, nor any other publication restrictions. Google Scholar was searched (24th March 2020). The COVID-19 search concept was adapted from a search developed by Wichor Bramer and posted on Search blocks/Zoekblokken (https://blocks.bmi-online.nl/catalog/397, accessed 18th March 2020). This yielded 2960 hits. The first 1000 were harvested using Publish and Perish, and records were reviewed in order of relevance until a series of 50 consecutive apparently irrelevant records was reached. This generated a set of 208 records, which was then exported to EndNote. The World Health Organization (WHO) COVID-19 record set posted 23rd March 2020 was downloaded from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov. The records were imported into Reference Manager, where topical queries were performed. References from all searches were then entered into an Endnote file and duplicate records were removed.
Citation screening and data extraction
Titles and abstracts were then uploaded to InsightScope (www.insightscope.ca) for title and abstract screening and full-text review. To allow for rapid completion of the systematic review task, the assessments were performed by a team of 13 reviewers recruited from the University of Ottawa, University of Manitoba and McMaster University. Before citation screening was initiated, each reviewer was asked to read the published protocol for this systematic review to familiarize themselves with the review objectives and citation screening criteria and process. Next, to ensure that the reviewers understood the citation eligibility criteria, the study lead (J.D.M.) and co-lead (K.O.) created a test set of 50 citations. The test set included six true positives (i.e. citations that met the eligibility criteria to be included in this systematic review) and 44 true negatives (i.e. citations that did not meet eligibility criteria to be included in this systematic review). Each reviewer (S.G., M.S., R.N., J.G., A.T.L., N.A., A.A., S.B., G.C., E.S., L.S.) was then required to complete the test set by assessing the same 50 citations. Reviewers had to achieve a sensitivity in excess of 80% on the test set before they were given access to title/abstract screening. At both title/abstract and full-text review, citations were assessed in duplicate by two independent reviewers. Citations at both screening levels were removed if two reviewers independently determined that the citation should be excluded. Those cases with conflicts were resolved by review by the study lead (J.D.M.) or co-lead (K.O.). At the completion of full-text review, the study lead reviewed the eligible citations to identify potential duplicates and confirm eligibility. A data extraction tool was developed in REDCap [18,19] by the study lead (J.D.M.) and piloted by the co-lead (K.O.) on five eligible studies. Eligible studies were divided equally among the reviewers for duplicate, independent data extraction, followed by conflict resolution by either the study lead (J.D.M.) or co-lead (K.O.).
Outcome data are reported for N95 (particulate or surgical) masks or their components only. Other FFR types (e.g., R or P) were not included in the analysis. The term ‘components’ was defined as a piece of an N95 mask that had been cut out with all layers still intact. When investigators separated out the layers of an N95 mask and reported outcomes for each individual layer, these data were not included in the analysis but were reported descriptively in the results. When authors did not report the UV-C dose, but reported intensity and time, UV-C dose was calculated. Preference was always given to the actual UV-C dose administered versus the target UV-C dose. In cases where authors only reported the target dose, but did report the intensity and time administered, administered UV-C dose was calculated. For ease of reporting, all doses were converted to J/m2. When necessary, data was extracted from figures using SourceForge Plot Digitizer (http://plotdigitizer.sourceforge.net/).
Study analysis and statistics
All statistical analyses were performed using the R statistical programming language [20]. Data was meta-analysed using a random-effects model with the R package ‘meta’ [21]. Random effects meta-analyses were employed to present either the pooled absolute value pre-/post-UVGI or relative change (from control or no treatment arm).
For both the particle penetration and airflow resistance outcomes, the data were presented as an absolute value. Appropriateness of pooling was assessed by examining studies heterogeneity using an I 2 statistic. Sample size was calculated as replicate number multiplied by the number of masks tested. Lindsey et al. [22] had more than one UVGI intervention arm and out of concern that this one experimental paradigm might contribute disproportionately to our data, and to reduce within study variation, we took a conservative approach of only including this study's highest-dose experiment. For the outcome UVGI decontamination, all but one study was on viruses; to improve comparability the one study on bacteria decontamination was removed from the germicidal analysis and results of the bacterial decontamination study are instead presented descriptively. Significant heterogeneity was seen with the methods and outcomes related to contamination and decontamination. To account for differences in methods (viral load, application and retrieval from mask) we reported pooled results using log change. For studies which did not report log change, values were calculated based on the data provided. The average log change was taken across mask types within the same dose and medium, in addition to absolute value or change. For the viral load log change, standard error was missing in 20% of study arms. To minimize bias, we imputed standard error for these cases, by substituting the average standard error of all study arms (NB modelling the missing data was disregarded due to poor predictability). Again, prior to pooling the results we performed a test of statistical heterogeneity using I 2 tests. Random effects meta-analysis was used. In the evaluation of UVGI and germicidal activity we also characterized the influence of cumulative doses of UV light (J/m2)
Risk of bias and quality assessment
Risk of bias was ascertained at the study level. The following factors were determined a priori as supportive of low risk of bias: (1) application of UVGI procedures to masks from the same lot to minimize lot-to-lot variation; (2) controlled study or pre-/post-study design; (3) application of identical study procedures between arms other than intervention; and (4) outcome evaluators blinded to study arm or outcome evaluated objectively (result provided by a machine). Based on the anticipated small size of the available literature (<10 studies) and limited variability in study sample sizes generally observed in laboratory studies, no statistical test of publication bias was planned. The cumulative body of evidence regarding the application of UVGI for decontamination and reuse of FFR was evaluated using the following criteria: (1) UVGI was shown to achieve established thresholds for success for pathogen decontamination (≥2-log reduction) and function (NIOSH standards for airflow resistance and aerosol penetration); (2) these findings were demonstrated consistently (≥2 studies); (3) without failing in any other study.
Results
Identification of eligible studies
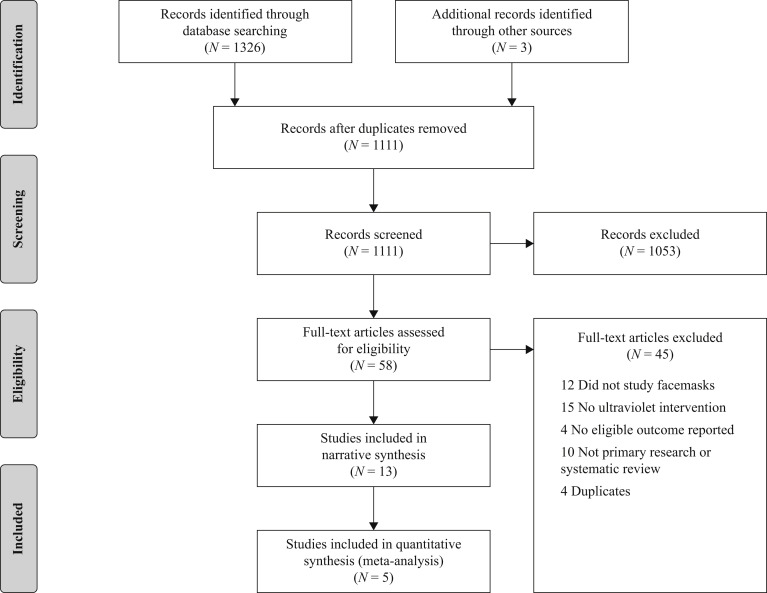
In total, 1326 records were identified through the initial database search, resulting in 1108 records for screening following de-duplication in Endnote. Title and abstract screening excluded 1053, with the review team achieving a kappa of 0.5. At full-text level, the reviewers excluded 40 of the records, with a kappa of 0.95. Review of the 15 remaining records by the study lead identified two as duplicates, and two records, a thesis and a government report, that were different reports of the same studies. Of the remaining 11, one study [23] was deemed ineligible during data extraction as only the plastic portion of the facemask was used in the evaluation of UVGI decontamination, and neither of the other two outcomes (aerosol penetration, airflow resistance) were considered. Three additional eligible studies were identified on review of the reference lists of the retained studies. An overview of the search process, results and reason for exclusions are shown in the PRISMA diagram (Figure 1 ).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Study demographics
Geographically, 12 studies were performed in the USA, with the remaining publication originating from East Asia. The studies included 54 total UVGI arms, with a range of 1–22 arms per study. The majority (N = 11) of studies evaluated a single cycle of UVGI, with one study evaluating three cycles [24] and one study evaluating a single 45-min UV exposure that was equivalent to three UV cycles [25]. A summary of the included studies is provided in Table I . The studies included a total of 58 N95 masks (average 4.5) with a range of 1–15 evaluated per study. The most common N95 mask types were 3M 1860 (N = 8), 3M 1870 (N = 8) and 3M 8210 (N = 6). Table I shows the number of masks evaluated in each study. The numbers of studies evaluating the four main study outcomes were: particle penetration (N = 5), airflow resistance (N = 3), germicidal activity (N = 7) and impact on physical characteristics/fit (N = 6).
Table I.
Characteristics of studies included in a systematic review of ultraviolet germicidal irradiation (UVGI) on N95 filtering facepiece respirators
| First author | Year of publication | Country of origin | Number of UVGI arms | Number of UVGI cyclesa | Number of N95 models | Number of conditionsb | Outcomes evaluated |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aerosol penetration | Airflow resistance | Decontamination | Fit | Physical appearance | |||||||
| Bergman | 2010 | USA | 1 | 3 | 6 | 1 | Yes | Yes | No | No | Yes |
| Bergman | 2011 | USA | 1 | 3 | 3 | 1 | No | No | No | Yes | Yes |
| Fisher | 2010 | USA | 22 | 1 | 6 | 1 | No | No | MS2 | No | No |
| Heimbuch | 2011 | USA | 1 | 1 | 6 | 2 | No | No | H1N1 aerosols and droplets | No | Yes |
| Lin | 2018 | East Asia | 10 | 1 | 1 | 2 | No | No | Bacillus subtilis prototype strains | No | No |
| Lindsley | 2015 | USA | 5 | 1 | 4 | 1 | Yes | Yes | No | No | No |
| Lore | 2012 | USA | 1 | 1 | 2 | 1 | Yes | No | Influenza A/H5N1 (VNH5N1) | No | No |
| Mills | 2018 | USA | 1 | 1 | 15 | 2 | No | No | H1N1 influenza | No | No |
| Viscusi | 2007 | USA | 2 | 1 | 1 | 1 | Yes | No | No | No | Yes |
| Viscusi | 2009 | USA | 1 | 1 | 6 | 1 | Yes | Yes | No | No | Yes |
| Viscusi | 2011 | USA | 1 | 1 | 6 | 1 | No | No | No | Yes | Yes |
| Vo | 2009 | USA | 5 | 1 | 1 | 1 | No | No | MS2 | No | No |
| Woo | 2012 | USA | 3 | 1 | 1 | 9 | No | No | MS2 | No | No |
Refers to the number of cycles of ultraviolet applied to the N95 filtering facepiece respirator model(s).
Number of conditions refers to the number of conditions under which decontamination of infectious pathogens was assessed, e.g., different relative humidity conditions, or different inoculation conditions.
Particle penetration and airflow resistance
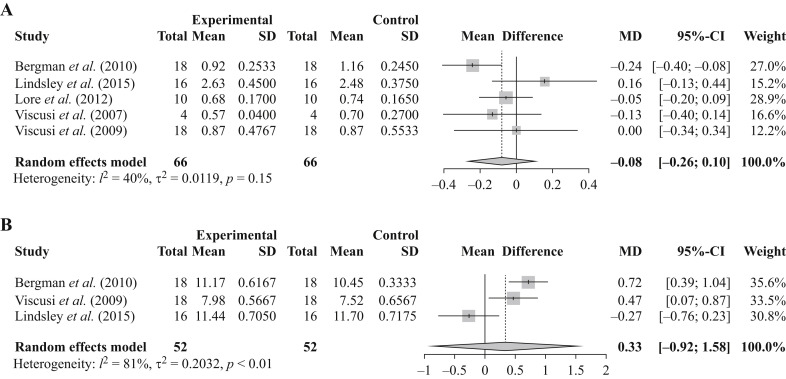
There were five studies identified that evaluated particle penetration post UVGI [9,22,[25], [26], [27]] (Table II ). Four of the studies evaluated a single UVGI protocol, with Lindsley et al. [22] having five UVGI intervention arms. Of these, all but one of the UVGI protocols represented a single decontamination cycle; with the protocol used by Bergman et al. representing three decontamination cycles [25]. The particle penetrations in masks were on average 1.19%% (ranging from 0.70 to 2.48%) and 1.13% (ranging from 0.57 to 2.63%) for the control and post-UVGI treatment arms, respectively. None of the individual studies average mean difference post UVGI treatment were statistically discernable from zero (Figure 2 ). Further the random effects meta-analysis (that weights the evidence by the size of the study) calculated a mean difference of -0.08% (95% confidence interval (CI) -0.26 to 0.10; Figure 2). Statistical heterogeneity was moderate with an I 2 of ∼40%. Fisher et al. reported aerosol penetration for the individual layers of six N95 FFR models [28]. The filter efficiency of the individual filtering layers examined ranged from 87.2 to 99.5% following UVGI. The majority of filter layers had an efficiency of >94%.
Table II.
Ultraviolet germicidal irradiation (UVGI) interventions and N95 filtering facemask respirators used to evaluate UVGI decontamination on aerosol penetration
| First author and year | Wavelength (nm) | Watts (W) | Duration (min)a | Dose (J/m2) | Intensity (mW/cm2) | Distance (cm)b | UVGI study arms | N95 masks evaluated |
|---|---|---|---|---|---|---|---|---|
| Bergman, 2010c | 254 | 40 | 45 | 48,600 | 1.8 | 25 | Single UVGI arm | 3M 8210 3M 8000 Moldex 2201 Kimberly Clark KC PFR95-174 3M 1870 3M 1860 |
| Lindsley, 2015 | 254 | 15 | NR | 1,200,000 to 9,500,000 | NR | 6.2 | Arm 1: 1,200,000 J/m2 Arm 2: 2,400,000 J/m2 Arm 3: 4,700,000 J/m2 Arm 4: 7,100,000 J/m2 Arm 5: 9,500,000 J/m2 |
3M 1860 3M 9210 GE 1730 Kimberley-Clark 46727 |
| Lore, 2012 | 254 | 15 | 15 | 18,000 | 1.6–2.2 | 25 | Single UVGI arm | 3M 1860 3M 1870 |
| Viscusi, 2007c | 254 | 40 | 30–480 | NR | NR | NR | Arm 1: 30 min Arm 2: 480 min |
3M 8000 |
| Viscusi, 2009c | 254 | 40 | 30 | 3520–3620 | 0.18–0.20 | NR | Single UVGI arm | 3M 8210 3M 8000 Moldex 2200 Kimberly Clark PFR95-270 3M 1870 3M 1860 |
NR, not reported.
Refers to the duration for which the N95 filtering facepiece respirator (FFR) models were exposed to ultraviolet.
Refers to the distance from the ultraviolet light to the N95 FFR model(s).
Mask models for the following studies obtained through private correspondence: Bergman (2010), Viscusi (2007), Viscusi (2009).
Figure 2.
Pooled results assessing particle penetration and airflow resistance. The forest plot in (a) illustrates the mean particle penetration in masks. The experimental arm refers to the ultraviolet germicidal irradiation (UVGI) -treated arm. Mask type examined varied by study; Bergman et al. (3M 8210, 3M 8000, Moldex 2201, Kimberly Clark KC PFR95-174, 3M 1870, 3M 1860), Lindsley et al. (3M 1860, 3M 9210, GE 1730,KC 46727), Lore et al. (3M 1860, 3M 1870), Viscusi et al. 2007 (3M 8000), and Viscusi et al. 2009 (3M 8210, 3M 8000, Moldex 2200, Kimberly Clark PFR95-270, 3M 1870, 3M 1860). The forest plot in (b) illustrates the mean airflow resistance in masks. The experimental arm refers to the UVGI-treated arm. Mask type examined varied by study; Bergman et al. (3M 8210, 3M 8000, Moldex 2201, Kimberly Clark KC PFR95-174, 3M 1870, 3M 1860), Viscusi et al. 2009 (3M 8210, 3M 8000, Moldex 2200, Kimberly Clark PFR95-270, 3M 1870, 3M 1860) and Lindsley et al. (3M 1860, 3M 9210, GE 1730, KC 46727). Total refers to the number of replicates multiplied by the number of masks tested. CI, confidence interval; MD, Mean difference; SD, standard deviation.
Three studies evaluated airflow resistance [22,25,27] (Table III ). Two of the studies evaluated a single UVGI protocol, with Lindsley et al. evaluating five UVGI intervention arms (NB: only the highest-dose arm was included in our meta-analysis). The UVGI protocols used by Lindsley et al. [22] and Viscusi et al. [9] represented a single decontamination cycle, whereas the UVGI protocol used by Bergman et al. [25] represented three decontamination cycles. The airflow resistance in masks were on average 9.89 mm H2O (ranging from 7.52 to 11.70 mm H2O) and 10.20 mm H2O (ranging from 7.98 to 11.44 mm H2O) for the control and post-UVGI treatment arms, respectively. None of the individual studies average mean difference post-UVGI treatment were statistically discernable from zero (Figure 2). The random-effects meta-analysis calculated a mean difference of 0.33% (95% CI -0.92 to 1.58; Figure 2). Statistical heterogeneity was high with an I 2 of ∼81%. Fisher et al. reported airflow resistance for the individual layers of six N95 FFR models [28] following UVGI. The airflow resistance of the individual filtering layers ranged from 2.5 to 7.6 mm H2O.
Table III.
Ultraviolet germicidal irradiation (UVGI) interventions and N95 filtering facemask respirators used to evaluate UVGI decontamination on airflow resistance
| First author and year | Wavelength (nm) | Watts (W) | Duration (min)a | Dose | Intensity (mW/cm2) | Distance (cm)b | UVGI study arms | N95 masks evaluated |
|---|---|---|---|---|---|---|---|---|
| Bergman, 2010c | 254 | 40 | 45 | 48,600 | 1.8 | 25 | Single UVGI arm | 3M 8210 3M 8000 Moldex 2201 Kimberly Clark PFR95-174 3M 1870 3M 1860 |
| Lindsley, 2015 | 254 | 15 | NR | 1,200,000 to 9,500,000 | NR | 6.2 | Arm 1: 1,200,000 J/m2 Arm 2: 2,400,000 J/m2 Arm 3: 4,700,000 J/m2 Arm 4: 7,100,000 J/m2 Arm 5: 9,500,000 J/m2 |
3M 1860 3M 9210 GE 1730 Kimberley-Clark 46727 |
| Viscusi 2009 c | 254 | 40 | 30 | 3520–3620 | 0.18–0.20 | NR | Single UVGI arm | 3M 8210 3M 8000 Moldex 2200 Kimberly Clark PFR95-270 3M 1870 3M 1860 |
NR, not reported.
Refers to the duration for which the N95 filtering facepiece respirator (FFR) models were exposed to ultraviolet.
Refers to the distance from the ultraviolet light to the N95 FFR model(s).
Mask models for the following studies obtained through private correspondence: Bergman (2010), Viscusi (2009).
Germicidal
Seven studies evaluated the germicidal impact of one or more UVGI interventions [7,8,26,[28], [29], [30], [31]], including one study on bacteria, and six studies on viruses (Table IV ). The most common were H1N1 and MS2, used as the viral pathogen in two and three studies respectively. All of the studies that evaluated the germicidal impact of UVGI used a single decontamination cycle. Lin et al. was the only group to use a bacterial pathogen. Using a water medium, they looked at the relative survival of Bacillus subtilis prototype strains following exposure to a range of UV-C doses from 11,340 to 226,800 J/m2. Relative survival following UVGI was 0.8% ± 0.4 for the lowest dose, 0.2% ± 0.14 for the second-lowest dose, and 0% ± 0 for the three higher doses.
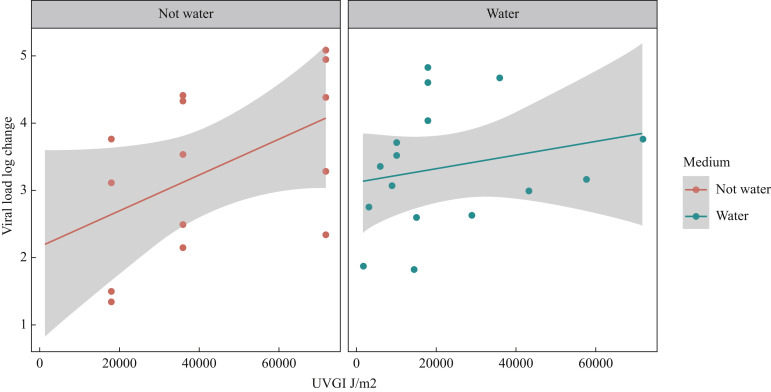
One mask was removed from the viral analysis as an outlier. Fisher et al. reported a log reduction in viral load of 0.1 ± 0.2 for the Cardinal N95-ML in contrast to 2.9 ± 0.2 and >4.8 for the other masks they evaluated [28]. The low log reduction for the Cardinal N95-ML was attributed to a high-shielding outer mask layer that limited the amount of UV-C that reached the filter layers of the mask. Collapsing the viral studies (see Methods) resulted in 30 different UVGI arms, evaluating doses ranging from 1500 to 72,000 m/J2 (Table V ). Fifty-three percent (N = 16) of UVGI arms used a water medium vs 47% (N = 14) who used another medium such as beef extract, 271B, or artificial saliva. All 15 UVGI arms that administered a cumulative dose >20,000 m/J2 observed a ≥2-log reduction in viral load compared with 73% (N = 11) of the 15 UVGI intervention arms that administered a cumulative dose <20,000 m/J2. The average log reduction for the UVGI intervention arms that administered a dose above or below 20,000 J/m2 was 3.61 ± 0.99 and 3.14 ± 1.09, respectively. A ≥3-log reduction was observed in 73% (N = 11) of UVGI arms administering a dose >20,000 J/m2 and 86% (N = 7) of UVGI arms administering a cumulative dose >40,000 J/m2. The average log reduction when using a dose >40,000 J/m2 was 3.74 ± 0.98. The exploratory Figure 3 suggests that the cumulative UVGI dose seems to have a greater effect on viral load in those lab trials where the viral particles were applied to the mask not using water. If mask performance in a clinical practice environment is more similar to the non-water medium (because organic matter and viral particles are found on masks), then the intensity of the UVGI light may affect the decontamination process.
Table V.
Log reduction in viral pathogens following ultraviolet germicidal irradiation in water and non-water mediums
| First author, year | Viral pathogen used in each study | Cumulative dose (J/m2) | Medium | Medium description | Average number of measurements | Log change | Standard error |
|---|---|---|---|---|---|---|---|
| Cumulative dose <20,000 J/m2 | |||||||
| Fisher, 2010 | MS2 | 1500 | Water | Water | 4 | 1.88 | 0.20 |
| Fisher, 2010 | MS2 | 3000 | Water | Water | 4 | 2.75 | 0.30 |
| Fisher, 2010 | MS2 | 6000 | Water | Water | 4 | 3.35 | 0.35 |
| Woo, 2012 | MS2 | 9000 | Water | Water | 3 | 3.07 | 0.42 |
| Mills, 2018 | H1N1 | 10,000 | Not water | AS | 3 | 3.71 | 0.24 |
| Mills, 2018 | H1N1 | 10,000 | Not water | Sebum | 3 | 3.51 | 0.35 |
| Vo, 2009 | MS2 | 14,400 | Water | 271B | 3 | 1.83 | 0.31 |
| Fisher, 2010 | MS2 | 15,000 | Water | Water | 4 | 3.85 | 0.26 |
| Heimbuch, 2011 | H1N1 | 18,000 | Water | Water | 3 | 4.81 | 0.45 |
| Lore, 2012 | H5N1 | 18,000 | Water | Water | 9 | 4.60 | 0.21 |
| Woo, 2012 | MS2 | 18,000 | Water | Water | 3 | 4.04 | 0.40 |
| Woo, 2012 | MS2 | 18,000 | Not water | AS (0.6%) | 3 | 1.50 | 0.32 |
| Woo, 2012 | MS2 | 18,000 | Not water | BE (0.6%) | 3 | 1.35 | 0.31 |
| Woo, 2012 | MS2 | 18,000 | Not water | Mucin-free AS (0.6% & 0.3%) | 3 | 3.76 | 0.30 |
| Woo, 2012 | MS2 | 18,000 | Not water | 0.3% Salt-free AS (0.3% mucin medium) | 3 | 3.12 | 0.31 |
| Cumulative dose >20,000 J/m2 | |||||||
| Vo, 2009 | MS2 | 28,800 | Water | 271B | 3 | 2.64 | 0.31 |
| Woo, 2012 | MS2 | 36,000 | Water | Water | 3 | 4.67 | 0.17 |
| Woo, 2012 | MS2 | 36,000 | Not water | AS (0.6%) | 3 | 2.50 | 0.35 |
| Woo, 2012 | MS2 | 36,000 | Not water | BE (0.3%) | 3 | 2.15 | 0.31 |
| Woo, 2012 | MS2 | 36,000 | Not water | Mucin-free AS (0.3%) | 3 | 4.33 | 0.21 |
| Woo, 2012 | MS2 | 36,000 | Not water | Mucin-free AS (0.6%) | 3 | 4.42 | 0.31 |
| Woo, 2012 | MS2 | 36,000 | Not water | 0.3% Salt-free AS (0.3% mucin medium) | 3 | 3.54 | 0.34 |
| Cumulative dose >40,000 J/m2 | |||||||
| Vo, 2009 | MS2 | 43,200 | Water | 271B | 3 | 3.00 | 0.31 |
| Vo, 2009 | MS2 | 57,600 | Water | 271B | 3 | 3.16 | 0.31 |
| Vo, 2009 | MS2 | 72,000 | Water | 271B | 3 | 3.76 | 0.31 |
| Woo, 2012 | MS2 | 72,000 | Not water | AS (0.6%) | 3 | 3.28 | 0.37 |
| Woo, 2012 | MS2 | 72,000 | Not water | BE (0.3%) | 3 | 2.34 | 0.30 |
| Woo, 2012 | MS2 | 72,000 | Not water | Mucin-free AS (0.3%) | 3 | 4.94 | 0.14 |
| Woo, 2012 | MS2 | 72,000 | Not water | Mucin-free AS (0.6%) | 3 | 5.08 | 0.31 |
| Woo, 2012 | MS2 | 72,000 | Not water | 0.3% Salt-free AS (0.3% mucin medium) | 3 | 4.37 | 0.11 |
AS, artificial saliva; BE, beef extract.
Figure 3.
Ultraviolet germicidal irradiation (UVGI) cumulative dose by log change in viral load in trials that used a water versus a non-water medium. Scatter plot showing the relationship between the cumulative UVGI dose (J/m2) and viral load log change post-UVGI. The two horizontal panels represent the medium (or solution) in which the viral particles were applied to the face mask, for these lab trials. Note, this is a descriptive plot and not a meta-regression.
Physical characteristics
Six studies were identified that evaluated some aspect of change in physical appearance and odour [7,9,24,25,27,32] or fit [24,32] (see Supplementary Data). All six studies assessed physical appearance using visual inspection, with one study also performing a manual inspection for changes in texture and feel [9]. Three studies evaluated change in odour by sniffing the FFRs post-UVGI exposure [9,25,32]. Viscusi et al. [32] also determined subjects' perception of odour strength using a visual analogue scale. There were no significant changes in physical appearance, texture or odour to any mask model following a single cycle or three cycles of UVGI exposure.
Only two studies assessed fit. Viscusi et al. performed an eight-exercise standardized fit test pre-UVGI exposure, followed by a multi-donning fit test post-UVGI exposure [32]. A multi-donning fit factor (MDFF10) was calculated as the harmonic mean of the 10 FFs resulting from the replicate multi-donning fit test sessions. FF was the fit factor calculated by the PORTACOUNT Fit Tester as the ratio of the ambient particle concentration outside the respirator compared with the particle concentration inside the respirator. The multi-donning fit factor MDFF10 value following UVGI treatment was favourable. The average MDFF10 value following UVGI treatment was 138.3 ± 28.5 versus 142.7 ± 27.2 for control. None of the six masks showed a statistically significant difference in MDFF10 between arms. The strap of one FFR treated with UVGI broke during the multi-donning fit test, compared with straps from three FFRs in the control group. Bergman et al. [24] used a similar approach but employed one, two and three cycles of UVGI on three different N95 models. Three cycles of UVGI did not cause significant changes in mask fit. The passing rate for the 3M 1860 was 95%, 100% and 100% after one, two and three cycles, respectively. The passing rate for the 3M 1870 was 100% after one, two and three cycles. The passing rate for the KC PFR95-270 (46767) was 95%, 95% and 90% after one, two and three cycles, respectively.
Other evaluations
Lindsley et al. [22] evaluated the strength of FFR straps following UV-C exposure. The breaking strength of the respirator straps decreased after UVGI exposure compared with paired controls by 10–21% (UV–C dose 590 J/cm2) to 20–51% (UV–C dose 2360 J/cm2). Viscusi et al. [32] evaluated subject experiences on perceived donning ease and FFR comfort following UVGI exposure using a visual analogue scale, as well as responses to an open-ended statement: “Tell us something about this respirator”. Donning ease and FFR comfort was not affected by UVGI, and no distinct patterns were identified in the open-ended comments.
Risk of bias
Studies were assessed for risk of bias. All of the study designs included an untreated or control arm for comparison. Six studies [9,22,24,25,27,32] reported that the N95 FFRs all came from the same lot, and nine studies [[7], [8], [9],24,26,27,29,31,32] stated that laboratory conditions were identical between intervention arms. No studies reported that outcome assessors were blinded, however, the subjects participating in the fit testing in Viscusi et al. [32] were blinded to whether they were evaluating an FFR from the control or intervention arm. There was uncertainty regarding the risk of bias for the germicidal outcomes, as it was not clear whether the individuals performing the evaluations were blinded to treatment arm and/or UVGI dose. The outcome measures related to mask function were evaluated objectively (i.e. aerosol penetration, airflow resistance measured using a machine) (see Supplementary Data).
Discussion
This is the first systematic review to synthesize the existing evidence on decontamination of N95 FFRs using UVGI. We found that a single cycle of UVGI with UV-C light does not affect N95 FFR performance, and was able to decontaminate mask surfaces exposed to viruses in laboratory conditions without significant changes in FFR appearance or odour. We observed that level of decontamination was associated with cumulative UV dose and the conditions used to simulate viral spread, specifically the addition of salts and biological particulate (saliva and protein). The limited body of evidence evaluating UVGI impact on physical characteristics and fit did not present evidence of negative effects.
This systematic review identified five studies that reported on changes in aerosol penetration following UVGI. NIOSH has established a 95% filter efficiency standard (i.e. a filter penetration of <5%) for N95 FFR [14]. All five studies that reported on aerosol penetration adhered to NIOSH testing standards. Results showed minimal change in filter efficiency following the application of multiple different UVGI protocols on a variety of FFR models, and all FFRs evaluated maintained the standard filter efficiency of ≥95%. A recent report from N95 FFR manufacturer 3M on N95 decontamination and reuse emphasizes the critical importance of ensuring the decontamination method does not compromise filter performance [16]. The report also includes the results of an internal study which found that 3M N95 FFRs maintained a filter efficiency of 95% following repeated UVGI exposure (five to 10 UV-C cycles), however they did not provide sufficient information to calculate a cumulative UV-C dose. In addition to standards for filter efficiency, NIOSH has also established standards for airflow resistance of N95 FFRs. Testing is performed using a filter tester at 85 L/min of constant airflow, and to meet certification requirements, N95 FFRs must demonstrate a peak average inhalation of 35 mm (343.2 Pa) and an exhalation resistance to airflow 25 mm (245.1 Pa) H2O pressure [33]. This systematic review found three studies that evaluated airflow filtration using standardized testing protocols following UVGI. None of the seven FFRs evaluated across the three studies demonstrated significant changes in airflow resistance following UVGI, and all FFRs maintained the NIOSH airflow standards.
Mask fit is another important consideration, as improper fit results in an inadequate seal of the mask against the wearer's face, reducing the mask's ability to prevent particle penetration [34]. Fit testing is performed according to the Occupational Safety and Health Administration (OSHA) respiratory protection regulation using a PORTACOUNT Fit Tester to determine the volume of test substance that is leaking into the mask. Only two papers were identified that evaluated FFR fit following UVGI exposure. While the results from Viscusi et al. [32] and Bergman et al. [24] showed no significant change in mask fit following UVGI, they both used a modified version of the OSHA fit-testing protocol following UVGI decontamination. Further, the fit testing was not performed in real-world conditions. Therefore, the evidence regarding the effects of UVGI on FFR fit is limited. Further investigation using additional UVGI protocols and mask models in a clinical setting is required in order to confirm whether or not UVGI alters the fit of N95 respirators.
The six studies that evaluate changes in mask appearance following UVGI did not report any significant changes in physical appearance or odour following a single cycle of UVGI exposure [7,9,27,32], a single continuous exposure equivalent to three cycles of UVGI [25] or three cycles of UVGI [24]. This is in contrast to the report by 3M, who observed physical degradation of their FFRs following five to 10 cycles of UVGI. As a result, 3M still does not recommend decontamination and reuse of N95 FFRs at this time [16]. It is likely that the physical observation observed by 3M was due to the number of UVGI cycles employed. Therefore, there may be a limit on the number of UVGI cycles that can be applied to a given FFR before the mask begins to break down. Further, there is evidence that mask fit deteriorates through repeated donning and doffing [35] but with careful donning, five safe reuses appear possible [36]. The number of decontamination and re-use cycles that can be applied to an FFR will be limited by breakdown imposed by both UVGI and donning and doffing.
While maintaining the function and fit of the FFR is critical, an equally important metric for evaluating UVGI protocols is their ability to eradicate infectious material from the mask surface. The seven studies that reported on decontamination demonstrated that exposure to UV-C light can significantly reduce the number of viable viral pathogens from N95 FFR, with cumulative doses of >20,000 J/m2 and >40,000 J/m2 consistently resulting in log reductions of ≥2 and ≥3, respectively. However, it is important to note that these evaluations were all performed in a laboratory setting and do not represent real-world conditions. There is a rationale to suggest that the decontamination effect of UVGI could actually be more effective in the real-world setting. Mills et al. overloaded the mask surface with more virus than would be observed following a real-life contamination event, yet still observed ≥3-log reduction in viral load on 12 of the 15 masks evaluated [29]. Of note, the fact that a significant reduction was not observed in all 15 masks suggests that mask model and material should be considered as factors that may influence the success of UVGI decontamination. For example, Fisher et al. reported a log reduction of 0.1 ± 0.2 for the Cardinal N95-ML in contrast to 2.9 ± 0.2 and >4.8 for the other masks evaluated [28], which was attributed to a high-shielding outer mask layer that limited the amount of UV-C that reached the filter layers of the mask.
Additional evidence that decontamination may be more effective in the real-world setting comes from the time elapsed between UVGI and measurement of pathogens in the studies examined. The majority of authors measured pathogen levels immediately following UVGI decontamination. The one study by Lin et al. that measured bacterial levels immediately after, and 24 h after, UVGI found that bacterial levels were further reduced by 24 h [8]. This is despite the fact that the mask material was stored at worst-case temperature (37°C) and humidity (95% relative humidity) conditions for the 24-h period. It is well established that bacteria and virus levels on surfaces decrease over time [37,38]. In the real-world setting, FFRs undergoing UVGI decontamination could be allowed to sit for an extended period of time (e.g., 24 h) prior to re-use, in order to further enhance the decontamination process. Regardless of the UVGI protocol ultimately selected, a recent study of SARS-CoV-2 persistence on a variety of surfaces showing, at minimum, a 1-log decline in infectivity every 24 h suggests that, where possible, a 1-week holding period for respirators following decontamination will materially decrease risk of viral persistence [39].
Our exploratory analysis showed that when FFRs were inoculated with viruses in a non-water medium, a higher cumulative dose of UV-C was required to achieve a 3-log reduction in viral pathogens. In the clinical setting, FFRs can become soiled during wear (make-up, facial oil, saliva) and this may limit the decontamination effect of UVGI. Therefore, it is recommended that masks that are visibly soiled are discarded rather than decontaminated and reused.
Overall, the evidence demonstrating that N95 FFR performance is maintained following a single-cycle of UVGI can be classified as strong. Findings were consistent across multiple studies, aerosol penetration and airflow resistance are measured objectively, and all studies that reported on FFR function used a control arm in their study design. The evidence regarding the effectiveness of UVGI to decontaminate mask material is less strong. Although findings were also consistent across studies and a control group was always incorporated into study design, outcome assessors were not blinded. Further, the existing evidence is all laboratory based, and is not reflective of real-world conditions. Data regarding the effect of UVGI on FFR fit is limited, thus it is not possible to conclude whether this method of decontamination alters the fit of N95 masks.
Based on the available evidence, we recommend a cumulative dose of no less than 20,000 and ideally 40,000 J/m2 be used for clinical application of UVGI and/or further investigation. The 40,000 J/m2 cumulative dose consistently resulted in ≥3-log reduction in viral pathogens. Modelling derived by Fisher et al. for influenza contamination of FFRs from aerosol sources showed full decontamination would require a log reduction of 3 [40]. In addition, this dose has been shown to not alter FFR performance (particle penetration, airflow resistance). Data from one study that evaluated mask fit following a comparable dose of UVGI (32,400 J/m2) showed no change in mask fit, but additional investigation would be prudent. Only two of the included studies evaluated more than one cycle of UVGI exposure and reported that aerosol penetration and airflow resistance standards [25], and mask fit [24] were maintained following exposure to three cycles of UVGI. This limited data suggests that three cycles of UVGI decontamination may be possible. However, in the real-world setting, mask breakdown due to wear and due to donning and doffing need to be accounted for in addition to any changes imposed on the FFR by repeated cycles of UVGI. While Bergman et al. [24] included multi-donning and doffing in their evaluation of mask fit, masks were not worn in a clinical setting in between fit evaluations. Assessment of aerosol penetration and airflow resistance following three UV cycles did not account for the impact of wear, or donning and doffing [25]. Thus, based on the existing evidence, it is not possible to comment on the maximum number of UVGI cycles that can safely be applied to a N95 FFR.
Limitations
Although this systematic review provides valuable information regarding the possibility of UVGI decontamination for the safe reuse of FFR, a number of limitations must be acknowledged. Each study used a different combination of mask types. In order to address this, we aggregated across mask types within each study, treating the pooled replicates across mask types as our statistically independent sampling unit. This is appropriate for our research question aimed at performance of FFRs in general (where we assume little difference between mask types). If there are large differences between mask types, our approach might artificially inflate our sample size; if this were the case it would unlikely change our findings, due to the consistency of these studies conclusions and low heterogeneity.
In conclusion, the function of N95 masks, based on aerosol penetration and airflow filtration, is maintained following a single cycle of UVGI. Decontamination using UV light in the laboratory setting suggests that this can be a successful method of removing infectious pathogens from FFRs. Future studies should use a cumulative UV-C dose of 40,000 J/m2 and focus on validating the effectiveness of UVGI decontamination in the real-world setting, and on determining the impact of UVGI on mask fit as well as the maximum number of UVGI cycles that can be safely applied to an N95 FFR.
Acknowledgements
The authors are grateful to Nafisa Neault for her assistance in verifying the information in Table I, Table II, Table III, Table IV, Table V.
Table IV.
Ultraviolet germicidal irradiation (UVGI) interventions and N95 filtering facemask respirators used to evaluate UVGI decontamination on viral or bacterial load
| Author and year | Wavelength (nm) | Watts (W) | Duration (min)a | Dose (J/m2) | Intensity (mW/cm2) | Distance (cm)b | UVGI study arms | N95 masks evaluated |
|---|---|---|---|---|---|---|---|---|
| Fisher, 2010c | NR | 40 | 1–300 | 1500–5000 | 2.5 | NR | Model A-1: 10 min, 15000 J/m2 Model C 1: 1 min, 1500 J/m2 Model C-2: 2 min, 3000 J/m2 Model C-3: 4 min, 6000 J/m2 Model C-4: 10 min, 15,000 J/m2 Model F 1: 1 min, 1500 J/m2 Model F-2: 2 min, 3000 J/m2 Model F-3: 4 min, 6000 J/m2 Model F-4: 10 min, 15,000 J/m2 |
Cardinal N95-ML (A) 3M 8210 (C) 3M 1870 (F) |
| Heimbuch, 2011d | 254 | 80 | 15 | 18,000 | 1.6–2.2 | 25 | Single UVGI arm | 3M 8000 3M 8210 Moldex 1500 3M 1860 3M 1870 Kimberly Clark PFR |
| Lin, 2018e | 254 | 6 | 1–20 | 11,340 to 226,800 | 18.9 | 10 | Arm 1: UVC 245 nm, 1 min, 11,340 J/m2 Arm 2: UVC 245 nm, 2 min, 22,680 J/m2 Arm 3: UVC 245 nm, 5 min, 45,360 J/m2 Arm 4: UVC 245 nm, 10 min, 113,400 J/m2 Arm 5: UVC 245 nm, 20 min, 226,800 J/m2 |
3M 8210 |
| Lore, 2012 | 254 | 15 | 15 | 18,000 | 1.6–2.2 | 25 | Single UVGI arm | 3M 1860 3M 1870 |
| Mills, 2018 | 254 | NR | 1 | 1000 | 0.39 | 100 cm | Single UVGI arm | 3M 1860 3M 1870 3M VFlex 1805 Alpha Protech 695 Gerson 1730 Cup Kimberly-ClarkPFR Moldex 1512 Cup Moldex 1712 Flat-fold Moldex EZ-22 Precept 65–3395 Prestige Ameritech RP88020 Sperian HC-NB095 Sperian HC-NB295F U.S. Safety AD2N95A U.S. Safety AD4N95 |
| Vo, 2009 | 254 | 40 | 60–300 | 14,400–72,000 | 0.4 | NR | Arm 1: 1 hr (14,400 J/m2) Arm 2: 2 hr (28,800 J/m2) Arm 3: 3 hr (43,200 J/m2) Arm 4: 4 hr (57,600 J/m2) Arm 5: 5 hr (72,000 J/m2) |
N1105 |
| Woo, 2012 | 254 | 4 | 15–120 | 9,000–72,000 | 1.0 | 10 | Arm 1: 15 min, 9,000 J/m2 Arm 2: 30 min, 18,000 J/m2 Arm 3: 60 min, 36,000 J/m2 Arm 4: 120 min, 72,000 J/m2 |
3M 1870 |
NR, not reported.
Refers to the duration for which the N95 filtering facemask respirator (FFR) models were exposed to ultraviolet.
Refers to the distance from the ultraviolet light to the N95 FFR model(s).
Fisher et al. evaluated 22 different UVGI protocols and six different masks, however, germicidal results were only reported for nine arms and three masks.
Mask models for the following studies obtained through private correspondence: Heimbuch (2011).
Lin et al. also evaluated five UVGI arms using 365-nm ultraviolet-A light.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.07.014.
Conflict of interest statement
None declared.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Bałazy A., Toivola M., Adhikari A., Sivasubramani S., T R., Grinshpun S. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:49–56. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 2.Tan N., Goh L., Lee S. Family physicians' experiences, behaviour, and use of personal protection equipment during the SARS outbreak in Singapore: do they fit the Becker Health Belief Model? Asia Pac J Public Health. 2006;18:49–56. doi: 10.1177/10105395060180030901. [DOI] [PubMed] [Google Scholar]
- 3.Centre for Disease Control and Prevention. Strategies for Optimizing the Supply of N95 Respirators: Conventional Capacity Strategies. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/conventional-capacity-strategies.html [last accessed March 2020].
- 4.Patel A., D'Alessandro M.M., Ireland K.J., Burel W.G., Wencil E.B., Rasmussen S.A. Personal protective equipment supply chain: lessons learned from recent public health emergency responses. Health Secur. 2017;15:244–252. doi: 10.1089/hs.2016.0129. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan A., Jernign D.B., Liedtke L., Strausbaugh L. Hospital preparedness for severe acute respiratory syndrome in the United States: views from a national survey of infectious diseases consultants. Clin Infect Dis. 2004;39:272–274. doi: 10.1086/421777. [DOI] [PubMed] [Google Scholar]
- 6.Bauchner H., Fontanarosa P.B., Livingston E.H. Conserving supply of personal protective equipment—a call for ideas. JAMA. 2020 doi: 10.1001/jama.2020.4770. [DOI] [PubMed] [Google Scholar]
- 7.Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C.Y., Woo M.H., et al. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39:e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin T.H., Tang F.C., Hung P.C., Hua Z.C., Lai C.Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air. 2018;31:31. doi: 10.1111/ina.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S.K., Dahal S., Bhumika T.V., Nair N.S. Evaluating sanitization of toothbrushes using various decontamination methods: a meta-analysis. J Nepal Health Res Counc. 2019;16:364–371. [PubMed] [Google Scholar]
- 12.Napolitani M., Bezzini D., Moirano F., Bedogni C., Messina G. Methods of disinfecting stethoscopes: systematic review. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed I., Fang Y., Lu M., Yan Q., El-Hussein A., Hamblin M.R., et al. Recent patents on light-based anti-infective approaches. Recent Pat Antiinfect Drug Discov. 2018;13:70–88. doi: 10.2174/1872213X11666171108104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Occupational Safety and Health . 1996. NIOSH guide to the selection and use of particulate respirators.https://www.cdc.gov/niosh/docs/96-101/default.html Available at: [last accessed March 2020] [Google Scholar]
- 15.Lowe J.J., Paladino K.D., Farke J.D., Boulter K., Cawcutt K., Emodi M., et al. Nebraska Medicine; 2020. N95 filtering facemask receptor ultraviolet germicidal irradiation (UVGI) process for decontamination and reuse. [Google Scholar]
- 16.3M . 2020. Disinfection of filtering facepiece respirators.https://multimedia.3m.com/mws/media/1816576O/disinfection-of-disposable-respirators-technical-bulletin.pdf Available at: [last accessed March 2020] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2012. R: a language and environment for statistical computing.http://www.R-project.org/ ISBN 3-900051-07-0. Available at: [last accessed June 2020] [Google Scholar]
- 21.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsley W.G., Martin S.B., Jr., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R., et al. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinadatha C., Simmons S., Dale C., Ganachari-Mallappa N., Villamaria F.C., Goulding N., et al. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. Am J Infect Control. 2015;43:412–414. doi: 10.1016/j.ajic.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Bergman M.S., Viscusi D.J., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J Int Soc Respir Prot. 2011;28:48–59. [Google Scholar]
- 25.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R. DigitalCommons@University of Nebraska – Lincoln; 2010. Evaluation of multiple (3-Cycle) decontamination processing for filtering facepiece respirators. [Google Scholar]
- 26.Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., Hinrichs S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 27.Viscusi D.J., King W.P., Shaffer R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J J Int Soc Respir Prot. 2007;24:93–106. [Google Scholar]
- 28.Fisher E.M., Shaffer R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J Appl Microbiol. 2011;110:287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills D., Harnish D.A., Lawrence C. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vo E., Rengasamy S., Shaffer R. Development of a test system to evaluate procedures for decontamination of respirators containing viral droplets. Appl Environ Microbiol. 2009;75:7303–7309. doi: 10.1128/AEM.00799-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo M.H., Grippin A., Smith T., Wu C.Y., Wander J.D. Effects of relative humidity and spraying medium on ultraviolet (UV) decontamination of filters loaded with viral aerosols. Appl Environ Microbiol. 2012;78:5781–5787. doi: 10.1128/AEM.00465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A., Powell J., et al. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 33.2. Code of Federal Regulations (42 CFR 84.180). Airflow resistance tests. Available at: https://www.govinfo.gov/app/details/CFR-2000-title42-vol1/CFR-2000-title42-vol1-sec84-180 [last accessed March 2020].
- 34.Reponen T., Lee S.A., Grinshpun S.A., Johnson E., McKay R. Effect of fit testing on the protection offered by n95 filtering facepiece respirators against fine particles in a laboratory setting. Ann Occup Hyg. 2011;55:264–271. doi: 10.1093/annhyg/meq085. [DOI] [PubMed] [Google Scholar]
- 35.Vuma C.D., Manganyi J., Wilson K., Rees D. The effect on fit of multiple consecutive donning and doffing of N95 filtering facepiece respirators. Ann Work Expo Health. 2019;63:930–936. doi: 10.1093/annweh/wxz060. [DOI] [PubMed] [Google Scholar]
- 36.Bergman M.S., Viscusi D.J., Zhuang Z., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J Infect Control. 2012;40:375–380. doi: 10.1016/j.ajic.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Bean B., Moore B.M., Sterner B., Peterson L.R., Gerding D.N., Balfour H.H., Jr. Survival of influenza viruses on environmental surfaces. J Infect Dis. 1982;146:47–51. doi: 10.1093/infdis/146.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Yeargin T., Buckley D., Fraser A., Jiang X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am J Infect Control. 2016;44:1365–1373. doi: 10.1016/j.ajic.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 39.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher E.M., Noti J.D., Lindsley W.G., Blachere F.M., Shaffer R.E. Validation and application of models to predict facemask influenza contamination in healthcare settings. Risk Anal. 2014;34:1423–1434. doi: 10.1111/risa.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.