To the Editor
The coronavirus disease 2019 is a global health pandemic emergency. The disease, named coronavirus disease 2019 (COVID-19, SARS-CoV-2 infection) by the WHO, presents symptoms that range widely from mild to severe and fatal, respiratory and systemic, conditions [1]. In patients with SARS-CoV-2 infection, sustained inflammatory response results in cytokine storm, leading to activation of both coagulation system and lectin plus alternative complement pathways [[2], [3], [4]]. Indeed, in patients with severe SARS-CoV-2 infection, recent studies have shown increased D-dimer, as a poor prognostic marker, associated with thrombocytopenia and mild prolonged prothrombin time (PT) [5]. These data together with the evidence of organ damage involving lung, kidney and heart are very evocative of thrombotic microangiopathy secondary to infection of COVID-19 [3].
Here, we carried out a retrospective study on 50 patients with COVID-19 consecutively hospitalized for respiratory symptoms with a chest X-ray compatible with bilateral interstitial pneumonia. Data on complete blood count, liver function as alanine aminotransferase (ALT) and aspartate aminotransferase (AST), bilirubin, kidney function as creatinine, lactate dehydrogenase (LDH), D-dimer, activated partial thromboplastin time (APTT), PT, fibrinogen, ferritin, and C-reactive protein (CPR) were collected simultaneous measurements at patients' admission to COVID-19 area.
All calculations were performed using the IBM SPSS 23.0 (IBM Inc., Armonk, NY, USA) statistical package. While normal continuous variables were expressed as means ± standard deviations, skewed variables (e.g. CRP, creatinine, ALT, AST, bilirubin, ferritin, LDH, D-dimer, ADAMTS13) were logarithmically transformed and geometric means with 95% confidence intervals (CIs) were reported. The correlations among continuous variables were analyzed by Pearson's correlation test and by linear regression analysis. To assess the independent predictors of D-dimer variability, all the variables showing an association with the D-dimer at univariate analysis were included in a regression model with backward stepwise selection of variables. LDH values across D-dimer quartiles were evaluated by ANOVA with polynomial contrasts for linear trend. A P-value <0.05 was considered significant.
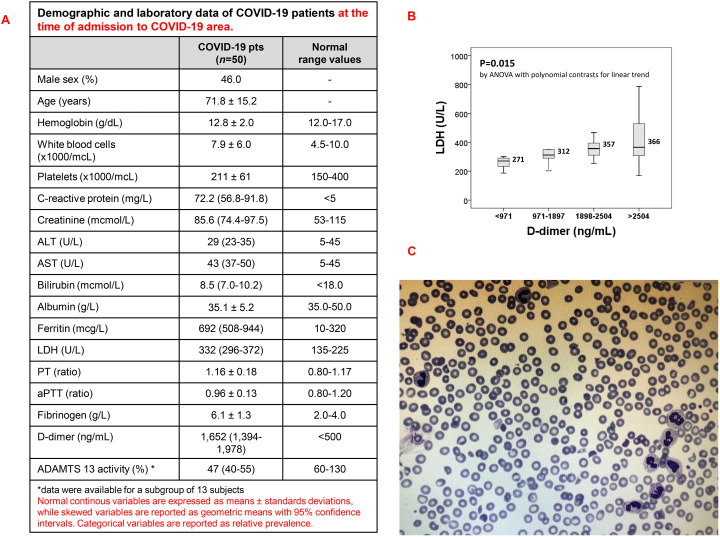
The laboratory characteristics of the study sample are reported in Fig. 1A. Beyond the expected inflammation-related alterations, COVID-19 patients had a pronounced increase of D-dimer plasma concentration. D-dimer values correlated directly with creatinine (R = 0.490, P < 0.001), CRP (R = 0.323, P = 0.024), bilirubin (R = 0.305, P = 0.031), LDH (R = 0.345, P = 0.027), and inversely with albumin (R = −0.295, P = 0.042). Including all these parameters, as well as sex and age, in a regression model with backward stepwise selection of variables, only creatinine (standardized beta-coefficient 0.300, P = 0.035) and LDH (standardized beta-coefficient 0.308, P = 0.028) remained significantly associated with D-dimer values. Stratifying the study population according D-dimer values, LDH progressively increased from the lowest to the highest D-dimer quartile as shown by Fig. 1 (P = 0.015 by ANOVA with polynomial contrasts for linear trend) (Fig. 1B). Abnormal shaped red cells and were evaluated by Sysmex® XN-9000 analyzer (Sysmex Corp.) instrument and the presence of schistocytes was confirmed by optical microscopy. The amount of schistocytes was lower than 5% in all patients (average of schistocytes 2–4%, Fig. 1C). On the basis of this association and taking into account that the presence of schistocytes and the increase LDH characterizes thrombotic microangiopathy (noteworthy, in our study sample LDH showed also an inverse correlation with platelet count, R = −0.344, P = 0.028 but not with Hb), in a subgroup of 13 subjects we performed a further laboratory evaluation of D-dimer and ADAMTS13 activity. The analysis confirmed the increase in D-dimer values (1700 ng/mL with 95%CI 1367–2515, normal range <500 ng/mL) and showed a mild reduction of ADAMTS13 activity (47% with 95%CI 40–55, normal range 60–130%). Although not statistically significant, there was a trend of inverse correlation between D-dimer and ADAMTS13 (R = −0.355, P = 0.233). Our results showing a correlation between D-dimer and LDH, while no association was found with traditional coagulation parameters, appear consistent with thrombotic microangiopathy (TMA) secondary to COVID-19 more than disseminated intravascular coagulation or infection induced coagulopathy [3]. Moreover, to the best of our knowledge, our data are the first suggesting a relative decrease of ADAMTS13 activity in patients with SARS-CoV2 severe infection, thereby addressing for a potential role for ADAMTS13 impairment in COVID-19 [6]. Relative ADAMTS13 deficiency has been previously reported in severe inflammation or infection, as poor prognosis marker [7]. Endothelial dysfunction plays a key role in microvascular thrombosis secondary to amplified inflammatory response. Among the different mechanisms involved in relative deficiency ADAMTS13, the reduction in either cleavage activity of ADAMTS13 or degradation of ADAMTS13 related to severe inflammation might be invocated in patients with SARS-CoV-2 infection, who show increased interleukin 6 [8]. Indeed, increased plasma von Willebrand factor has been reported in patients with SARS-CoV-2 infection [9,10]. Noteworthy, amplified and sustained inflammatory response in TMA promotes the activation of alternative complement pathway. In animal models of SARS-CoV-2 infection, recent reports indicate the activation of both lectin and alternative complement pathways. The beneficial therapeutic effects of either C5 or C3 blockers described in patients with SARS-CoV-2 infection further support the clinical importance of TMA secondary to COVID-19 [7]. Taken together these findings further support a possible link between SARS-CoV-2 infection and secondary TMA. On the other hand, our data are hypothesis-generating so far and further studies are needed to address the hypothesis of a COVID-19 disease-specific reduction of ADAMTS13 activity, not only and merely related to general inflammatory processes and/or critical illness conditions [11]. Nonetheless, it is worthy to note that clinical management of secondary TMA is based on infusion/exchange of plasma and complement pathway blockers, as well as recent collection of cases on the use of low volume of plasma or plasma exchange highlight the importance to identify the COVID19 patients who might benefit of either complement inhibition or plasma supplementation [3]. The determination of ADAMTS13 activity in COVID19 patients with higher D-Dimer might be useful in select patients for intensive treatment of secondary TMA.
Fig. 1.
A. Demographic and laboratory data of COVID-19 patients. B. Box plot showing LDH values across D-dimer quartiles in the study sample of COVID-19 patients. Median values are also reported. P value was calculated by ANOVA with polynomial contrasts for linear trend. C. Representative images of schistocytes from COVID 19 patients. May-Grünwald-Giemsa–stained smears were imaged under oil at 100× magnification using a PanFluor objective with 1.30 numeric aperture on a Nikon Eclipse DS-5M camera and processed with Nikon Digital Slide (DS-L1).
Declaration of competing interest
The authors have nothing to disclose.
References
- 1.Wu P., Hao X., Lau E.H.Y., Wong J.Y., Leung K.S.M., Wu J.T., Cowling B.J., Leung G.M. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3) doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir. Med. 2020;8:e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success doesn't come easily. Br. J. Haematol. 2020;186:e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 4.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020 doi: 10.1111/ijlh.13244. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas P., Csuka D., Mikes B., Sinkovits G., Reti M., Nemeth E., Racz K., Madach K., Gergely M., Demeter J., Prohaszka Z. Complement activation, inflammation and relative ADAMTS13 deficiency in secondary thrombotic microangiopathies. Immunobiology. 2017;222(2):119–127. doi: 10.1016/j.imbio.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Schwameis M., Schorgenhofer C., Assinger A., Steiner M.M., Jilma B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb. Haemost. 2015;113(4):708–718. doi: 10.1160/TH14-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah U., Nair S.C., Goel A., Balasubramanian K.A., Mackie I., Elias E., Eapen C.E. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: consider low volume plasma exchange and low dose steroid. Thromb. Res. 2020;192:2. doi: 10.1016/j.thromres.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer Hovinga J.A., Zeerleder S., Kessler P., Romani de Wit T., van Mourik J.A., Hack C.E., ten Cate H., Reitsma P.H., Wuillemin W.A., Lämmle B. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J. Thromb. Haemost. 2007;5:2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]