Abstract
The COVID-19-, SARS- and MERS-related coronaviruses share many genomic and structural similarities. However, the SARS-CoV-2 is less pathogenic than SARS-CoV and MERS-CoV. Despite some differences in the cytokine patterns, it seems that the cytokine storm plays a crucial role in the pathogenesis of COVID-19-, SARS- and MERS. Monocytes and macrophages may be infected by SARS-CoV-2 through ACE2-dependent and ACE2-independent pathways. SARS-CoV-2 can effectively suppress the anti-viral IFN response in monocytes and macrophages. Since macrophages and dendritic cells (DCs) act as antigen presenting cells (APCs), the infection of these cells by SARS-CoV-2 impairs the adaptive immune responses against the virus. Upon infection, monocytes migrate to the tissues where they become infected resident macrophages, allowing viruses to spread through all organs and tissues. The SARS-CoV-2-infected monocytes and macrophages can produce large amounts of numerous types of pro-inflammatory cytokines and chemokines, which contribute to local tissue inflammation and a dangerous systemic inflammatory response called cytokine storm. Both local tissue inflammation and the cytokine storm play a fundamental role in the development of COVID-19-related complications, such as acute respiratory distress syndrome (ARDS), which is a main cause of death in COVID-19 patients. Here, we describe the monocytes and macrophage responses during severe coronavirus infections, while highlighting potential therapeutic interventions to attenuate macrophage-related inflammatory reactions in possible approaches for COVID-19 treatment.
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ACE2, angiotensin-converting enzyme 2; ADAM17, ADAM metallopeptidase domain 17; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; CD, cluster of differentiation; CRP, C-reactive protein; CTL, cytotoxic T lymphocyte; IFN, interferon; IRF, interferon regulatory factor; MCP-1, monocyte chemoattractant protein-1; MDA5, melanoma differentiation-associated protein 5; MERS-CoV, Middle East respiratory syndrome-related coronavirus; MHC, major histocompatibility complex; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NLRP3, Nod-like receptor protein 3; PAMPs, pathogen-associated molecular patterns; PBMCs, peripheral blood mononuclear cells; pDCs, plasmacytoid dendritic cells; PRRs, pattern recognition receptors; PRRSV, porcine reproductive and respiratory syndrome virus; RIG-I, retinoic acid-inducible gene I; SARS-CoV, severe acute respiratory syndrome-related coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; siRNA, small interfering RNA; STAT, signal transducers and activators of transcription; TMPRSS2, transmembrane serine protease 2; Treg, regulatory T cells; WHO, World Health Organization
Keywords: COVID-19, SARS-CoV-2, Macrophages, Monocytes, Pathogenesis
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-mediated COVID-19 has emerged during the late 2019 and caused a serious public health threat, forcing the WHO to announce the SARS-CoV-2 outbreak as a pandemic [1].
SARS-CoV-2, as a member of the coronavirus family, is an enveloped virus containing a positive-sense single-stranded RNA molecule [2]. SARS-CoV-2 exhibits approximately 80.0% and 50.0% genetic similarity with SARS-CoV and MERS-CoV, respectively [3]. The principal structural proteins of SARS-CoV-2 are spike (S), membrane (M), envelope (E), nucleocapsid (N) proteins and some accessory proteins [2] (Fig. 1 ). The S protein of SARS-CoV-2 plays a central role in the viral entry into host type 2 alveolar cells that express its receptor angiotensin-converting enzyme 2 (ACE2) [4,5]. The SARS-CoV-2-related S protein binds to ACE2 with an affinity 10–20 fold stronger than that of SARS-CoV [6,7]. The SARS-CoV-2-related S protein is also larger than the SARS-CoV-related S protein, and its receptor binding domain is different [3]. However, dipeptidyl peptidase 4 is used by MERS-CoV to enter host cells [8]. SARS-CoV-2 may also utilize antibody-dependent internalization [4] implying antibody-mediated neutralization may not be easily achieved by immunotherapy or prophylactic vaccination strategies. The ACE2 expression has also been reported by the endothelial cells of the blood vessels and by epithelial cells of the lung, intestine, heart, and kidney [9]. The clinical manifestations of COVID-19 appear after an incubation of about 5 days with a range of 2 to 14 days [10]. The duration from the start of COVID-19 symptoms to death varied from 6 to 41 days with a median of 14 days. This duration is affected by the age and immune status of patients [11]. Patients with underlying diseases, including diabetes, hypertension, malignancy, chronic respiratory and cardiovascular problems are more susceptible to COVID-19 [12]. However, milder clinical symptoms were reported in children, when innate immunity is highly effective [12].
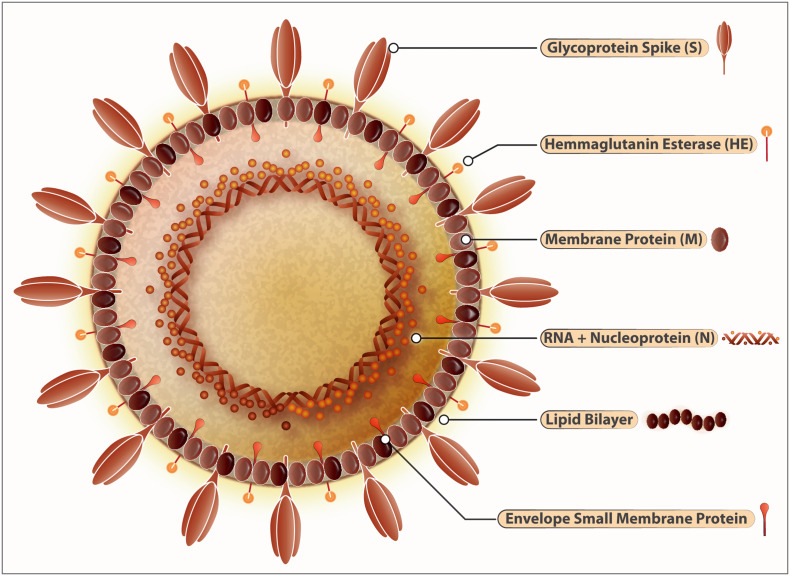
Fig. 1.
Structure of SARS-CoV-2.
SARS-Cov-2 belongs to the family of RNA viruses with other members including SARS-CoV and MERS-CoV. These viruses have characteristic crown-like-protrusions spike proteins (S) used to gain entry into the host cells and thereby inflict the respiratory disease COVID-19. ACE-2 is the host receptor mediating this process of internalization. Protease ACE-2 mediates the cleavage of spike protein which then releases an epitope that allows the subsequent fusion of the virus with the host cells. Inside the host, SARS-CoV-2 thrives in epithelial cells of lungs, kidneys, small intestines, and endothelial cells within arteries and veins. The viral genome is composed of a positive sense (+) RNA (~30 kb). The coronavirus group-2 also has hemagglutinin–acetylesterase (HE) glycoprotein that has an affinity to bind with sugar moieties on the cell membranes. The RNA-dependent-RNA polymerase can switch templates during the replication, a highly error-prone process. The virus envelope protein plays a central role in virus morphogenesis and assembly and its interaction with other viral proteins. The nucleocapsid (N), Spike (S), Envelope (E), and Membrane (M) structural proteins embedded into a lipid bilayer are the characteristic hallmarks of the SARS-CoV-2. For the virus to replicate into the host cells in inserts its RNA into the cells like monocytes and macrophages and takeovers the cellular machinery to produce new virions.
COVID-19 patients exhibit clinical symptoms such as fever, nonproductive cough, dyspnea, myalgia, fatigue, and radiographic reports of pneumonia that resemble the symptoms of SARS-CoV and MERS-CoV infections [11,13]. The pathogenicity of SARS-CoV-2 is low compared to SARS-CoV and MERS-CoV, so that the fatality rate of COVID-19 (about 2.3%) is lower than SARS (about 9.5%) and MERS (about 34.4%) [2,3,8]. Severe cases of COVID-19 have lower numbers of lymphocytes, higher numbers of leukocytes, greater neutrophil-lymphocyte-ratio, as well as smaller proportions of monocytes and eosinophils [1,14]. The COVID-19 also invades the central nervous system to induce neurological abnormalities. Coronaviruses can primarily invade peripheral nerves, but may be transported retrogradely to the CNS [15]. Reportedly, ACE2 expression in the CNS provides a direct route for SARS-CoV-2 to enter the brain [16] establishing a causal relationship with the respiratory failure in COVID-19 patients [15,16]. Certain manifestations- loss of smell, taste, ataxia and convulsions- are associated with neurological deficits in COVID-19 patients [15].
While the COVID-19 pathogenesis remains to be clarified, its similarities with SARS-CoV and MERS-CoV pathogenesis may provide insights into SARS-CoV-2 pathogenesis [6]. The coronavirus-associated severe pneumonia is usually related to rapid replication of the virus, extensive aggregation of inflammatory cells, elevated production of inflammatory mediators and the virus-induced immunopathology contributes to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [17,18]. The inflammatory response may play protective or destructive roles during infection with SARS-CoV-2 [[17], [18], [19]].
Monocytes constituting about 5–9% of the total peripheral leukocytes, remain in the circulation for 1–2 days, following which these cells may differentiate to tissue-resident macrophages [20]. Macrophage as an essential immune cell is widespread throughout the body, including the respiratory system. The primary activities of the macrophage include phagocytosis, antigen presentation, secretion of cytokines, production of antibacterial compounds and releasing enzymes that remodel the extracellular matrix [21]. However, the improper hyperactivation of macrophages may contribute to the development of immuno-pathologic reactions [20,22].
The lung injury of SARS-CoV-infected patients appears to happen directly through viral disruption of alveolar and bronchial epithelial cells and macrophages, and indirectly via triggering inflammatory mediators [23]. Both infected- and uninfected-macrophages exist in large numbers in the lungs of severe SARS patients [24,25] and are causally related to the severity of coronavirus infections. In this review, we discuss possible macrophage-mediated inflammatory responses in SARS-CoV-2 infection and propose potential therapeutic interventions for attenuating macrophage-related inflammatory reactions.
2. Monocyte/macrophage-mediated anti-viral responses
Macrophages express various types of pattern recognition receptors (PRRs). Therefore, they are able to identify viral-related pathogen-associated molecular pattern molecules (PAMPs) [26]. In mucosal pulmonary infections, alveolar macrophages and epithelial cells lining the airways, provide the initial sources of type I IFNs, while plasmacytoid DCs secrete type I IFNs when viruses pass the local barrier and become systemic [26]. The viral-related double-stranded RNA is a potent inducer of IFNs and macrophages serve as a major source of IFN-α and IFN-β in response to viral infections [27]. For RNA viruses such as coronavirus, the viral-related RNAs are detected by either the endosomal TLR3 and TLR7, or the cytosolic viral RNA sensors, such as RIG-I/MDA5 leading to IRF-3-, IRF-7-, and NF-κB-mediated expression of type I IFNs (such as IFN-α and IFN-β), and other pro-inflammatory cytokines [28]. IFN-IFNR interactions trigger the anti-viral activity of IFNs by activating the receptor-associated tyrosine kinases (TYK2 and JAK1) that in turn, phosphorylate the Signal Transducers and Activators of Transcription (STAT) molecules. The STAT phosphorylation triggers their homo- and heterodimerization, and their transportation to the nucleolus. STAT1 and STAT2 are recognized as the principle IFN-stimulated transcription factors. The STAT1-STAT2 heterodimer interacts with IRF-9 to create the IFN-stimulated gene (ISG) factor 3 (ISGF3) that triggers the ISG expression (Fig. 2 ). The ISG products directly exert anti-viral activities by interfering with viral replication and transmission [28]. Although, IFN response can be protective in SARS-CoV and MERS-CoV infections, the type I IFN-mediated anti-viral response is inhibited in both infections [29,30]. SARS-CoV and MERS-CoV use a number of strategies to inactivate signals that cause type I IFN production. The dampening of type I IFN-mediated anti-viral responses strongly relates to disease severity [4,17]. SARS-CoV and MERS-CoV trigger the formation of vesicles that lack PRRs, preventing the dsRNA recognition by host PRRs [31]. At the induction phase of type I IFNs, SARS-CoV and MERS-CoV interfere with the signaling pathways located downstream of cytoplasmic RNA sensors [29]. SARS-CoV inhibits type I IFNs expression by human monocyte-derived DCs and macrophages through IRF-3 inactivation [32,33]. It also interrupts the IL-28 and IL-29 production, which are considered as the members of IFN-λ family [33,34]. Once type I IFN is secreted, both viruses suppress IFN signaling by preventing STAT-1, IRF-3, and IRF-7 activation [30]. Both structural (such as M, N) and non-structural (ORF) proteins of viruses contribute to the suppression of type I IFN response [4,30] (Fig. 2).
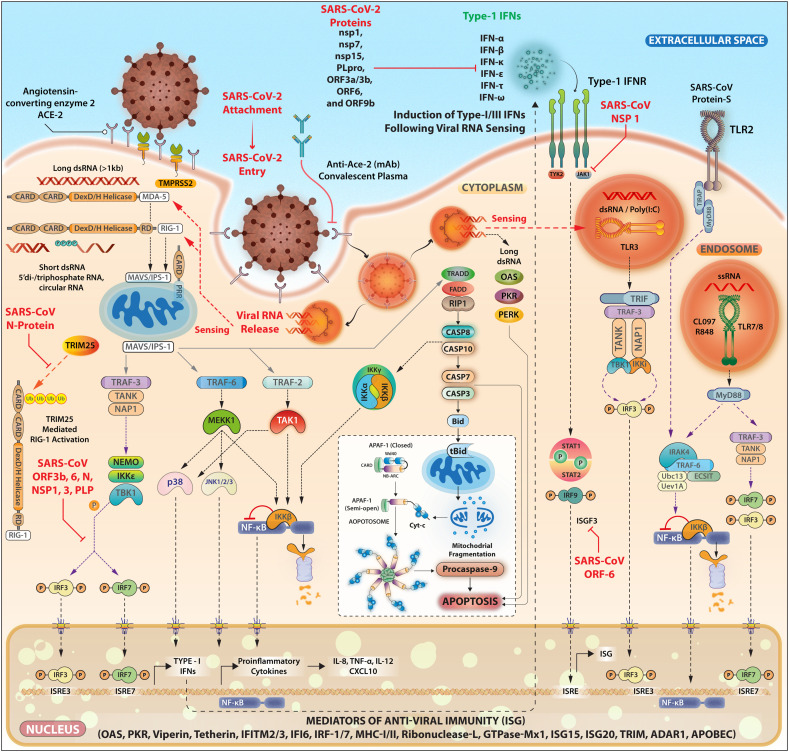
Fig. 2.
SARS-CoV-2 intracellular signaling and potential targets for immunoevasion and host cells particularly macrophages elicit a variety of antiviral immunity genes shown above.
The induction of TLR2 (via S protein), TLR3, TLR7, TLR8 (via virus-derived RNA following internalization process) leads to the expression of the pro-inflammatory mediators and IFNs through induction of the transcription factors NF-κB, IRF3, and IRF7. Viral-derived RNAs activate PKR- and OAS-related anti-viral pathways. The cytoplasmic sensors such as MAD-5 and RIG-1 also recognize various types of virus-derived RNAs and signal through adaptor MAVS/ISP-1 (located on our membrane of mitochondria). SARS-CoV-2 affects the cells of the innate immune system in particular monocytes, macrophages, and dendritic cells. These cells of the innate system play a crucial role in curbing the viral replication through the induction of Type-I IFNs assisted with the complement proteins and natural immunoglobulins against viral epitopes. These cellular responses (induction of proinflammatory mediators and IFNs Type-I, III) are tightly regulated by a series of intracellular signaling pathways elicited by surface receptors like TLRs, DC-SIGN, FcRs, and ACE-2 and TMPRSS2. Upon the viral entry into the host cells (via the clathrin-dependent internalization or ACE-2 mediated internalization) the spontaneous unloading of viral RNA is a subsequent step that follows. The viral RNA triggers the activation of intracellular RNA sensors like RIG-1 and MDA-5 each operating with distinct RNA conformations. RNA sensors then interact with MAVS that initiates Type-1 IFN signaling by activating the nuclear translocation of NF-κB and IRF3. The oligomeric RIG-1-CARD assembly and the polymeric formation of MAVS act as a signalosome for conducting the viral sensing signals further, which bifurcates into the activation of TRAF-2/6 to activate IKK complex and NF-κB activation. The other branch signals through TRAF-3 and activates the TANK/IKKγ/IKKε/TBK1 complex that acts as activators of IRF-3/7. Altogether the IRF-3/7 activation along with NF-κB drives the IFN and proinflammatory gene expression with the help of CREB-binding protein/p300 and transcription factors c-Jun and ATF-2. The IFN synthesized and secreted this way acts on the distant cells (paracrine) mode for spreading anti-viral immunity and in autocrine modes to fortify the intracellular viral clearance. SARS-CoV-2, however, attenuates these signaling pathways at various interception nodes. SARS-CoV proteins like ORF-9b may attenuate this antiviral response through targeting of MAVS by seizing poly (C)-binding protein 2 (PCBP2) and the HECT domain E3 ligase AIP4 to trigger the degradation of MAVS (not shown) along with TRAF-3 and TRAF-6. While ORF6 is reported to antagonize the STAT-1 function by sequestering its nuclear import factors. SARS-ORF-3b, ORF-6, and nucleocapsid protein function to antagonize interferon production. Besides this, the host mRNA destabilizing functions of NSP-1 are also reported. However, Nsp1 protein suppresses IFN-β mRNA accumulation without inhibiting IRF3 dimerization. Similarly, SARS-CoV NSP-15 inhibits MAVS-induced apoptosis sustaining the intracellular viral presence. SARS-CoV N protein can activate AP-1 but not the NF-κB signaling pathway. SARS-CoV proteins have been shown to inhibit the JAK-STAT pathway in the infected cell that responds to the Type-I IFNs secreted from bystander/neighboring cells. STAT-1-STAT-2-heterodimers combines with the IRF-9 to form the ISGF3 complex. This complex is crucial for the activation of genes harboring ISRE in their promoter regions. Viral protein ORF-6 blocks the nuclear import of ISGF3 by reducing the available import factor KPNB1 (Kβ1). Accordingly, various types of the IFNs are secreted from viral-infected cells that may induce various anti-viral restriction factors (such as OAS, PKR, viperin tetherin, IFITM, RNase L, GTPase, TRIM, ADAR1, APOBEC, and others) following binding to their receptors. Coronavirus-derived nonstructural protein also contributes to abrogate IFN expression or IFN-related signaling pathways. SARS-CoV-derived N protein can inactivate anti-virus restriction factor TRIM25 (RING-finger E3 ubiquitin ligase that controls RIG-I ubiquitination and IFN-β production). MAVS/ISP-1-related pathways may cause apoptosis through the induction of the caspases-mitochondria-mediated pathway.
Accessory protein 4a of MERS-CoV interferes with expression of IFNs by preventing the MDA5 activation and blocks IFN-mediated ISG expression by suppressing the protein kinase R-related pathways [29]. ORF4a, ORF4b, ORF5, and M proteins of MERS-CoV prevent IRF-3 and IRF-7 activation and block IFN-β production [29]. The remarkably lower type I IFN response and Th1 cell activity have been reported in a COVID-19 patient with poor outcome than in a recovered patient [35]. SARS-CoV-2 may adopt similar strategies to modulate the IFN anti-viral response [4]. In vitro experiments indicated that SARS-COV-2 exhibits higher vulnerability to type I IFN compared to SARS-COV. SARS-CoV-2 does not interfere with type I IFN-mediated STAT-1 phosphorylation [36,37] SARS-CoV-2 infection induces weak type I- and III IFN responses and reduced ISG expression [38]. Although most SARS-CoV-2- and SARS-CoV-related proteins exhibit more than 90.0% sequence homology, ORF3b, ORF6 and nsp3- all of which prevent IFN response- display relatively small amino acid homology [36]. SARS-CoV-2-derived ORF3b suppresses the expression of T1 IFN more efficient compared to that of SARS-CoV [39]. The identification and targeting of the IFN escape pathways used by SARS-CoV-2 are prominent for effective COVID-19 management [6].
In SARS and MERS mouse models, the delayed type I IFN response impairs the viral control accompanied by hyperinflammation, massive infiltration of neutrophils and macrophages (the main producers of pro-inflammatory cytokines), apoptosis of T lymphocytes, and apoptosis of epithelial and endothelial cells, which eventually leading to ARDS [37]. The mild and moderate SARS-CoV-2 infection has been associated with a powerful type I IFN response, characterized by the potent ISGs expression in the BALF samples of COVID-19 patients [37]. Moreover, type I IFN is detected in plasma samples collected from COVID-19 patients during the first week of disease [37,40]. However, IFN is not produced by about 20% of patients [37]. Furthermore, patients with mild and moderate COVID-19 exhibit greater type I IFN response during 8 to 12 days compared to severe patients [37]. Accordingly, the clinical COVID-19 outcome may be influenced by the time and extent of IFN response. The absence of IFN response in macrophages may allow viral replication to continue leading to severe respiratory infection during the first week of illness. The ensuing adaptive immune response faces the task of clearing a widespread infection, thus exacerbating the disease pathology [33].
Following the Th subset paradigm and based on the cultures of macrophages and in tumors, these cells are categorized into two principle subtypes, including M1 macrophages, which secrete great quantities of proinflammatory cytokines (such as IFN-γ, TNF-α, IL-6, and IL-12), nitric oxide and reactive oxygen species (ROS), whereas M2 macrophages release large concentrations of anti-inflammatory cytokines such as IL-10, TGF-β and IL-1 receptor antagonist [41,42]. M2 macrophages may be more subcategorized to M2a, M2b and M2c types which are induced from non-polarized macrophages via stimulation with IL-4/IL-13, immune complexes plus TLR agonists, and IL-10/TGF-β/glucocorticoids, respectively [43]. The M2d type (mentioned only in mice) can be induced from M1 macrophage via stimulation with adenosine [43]. The microenvironment of tissues under pathological and physiological circumstances can also influence the activity of the macrophages. Instead of a separate bipolar M1/M2 model, a vast range of macrophage activation states has been proposed to exist in resident tissues [44,45]. Therefore, as the monocytes migrate to different tissues and differentiate to macrophages under the local environmental factors, macrophages can widely vary in their phenotypic and functional characteristics. For example, certain lung M1- and M2-like phenotypes with differential markers were identified in COVID-19 patients and healthy individuals [46].
According to experimental investigations using animal models of respiratory syncytial virus (RSV) infection, it has been proposed that the lung macrophage polarization toward an M1-like phenotype contributes to the control of viral replication [44]. In several viral respiratory diseases such as SARS and influenza, the virus infection causes significant depletion of macrophages (mainly M1-like phenotypes) via apoptosis and necrosis that facilitate the viral replication [44]. However, the balanced activation of M2-like phenotype is essential to limit the RSV-mediated immunopathologic reactions [44,47]. Liao et al. indicated that the bronchoalveolar lavage fluid (BALF) from patients with severe COVID-19 infection contained higher frequencies of FCN1+- and FCN1lo SPP1+ macrophages (M1-like macrophages), while BALF from patients with moderate infection and healthy controls contained a higher frequency of FABP4+ macrophages (M2-like macrophages) [46].
In addition to inducing the antiviral response, type I IFNs can control the macrophage polarization [44]. Type I IFNs may cause M1-like type polarization through STAT-1 and STAT-2-related signaling pathways, while they can lead to M2-like macrophage polarization via STAT-3 and STAT-6-related pathways [44,45]. Accordingly, in the presence of type I IFNs, the high expression of STAT-1 and STAT-2 in macrophages leads to their polarization toward M1-like type, while the high expression of STAT-3 and STAT-6 in macrophages causes their polarization toward M2-like type [44,45]. Using an animal model of PRRSV infection, it was reported that STAT1–6 genes are expressed in alveolar macrophages, however, the gene expression of STAT1 and STAT2 was significantly increased (10–200 folds) compared to other STATs after virus infection [44]. It has been proposed that macrophages express differently various ratios of STAT molecules according to their tissue microenvironment and functional status, and IFNs perform a dual role in M1- and M2-like macrophage polarization [44]. However, macrophage-tropic viruses have been equipped with mechanisms to divert macrophage polarization in their favourite direction. For example, HIV can divert macrophage polarization toward M2-like type through the induction of IL-4 and IL-10 production [44]. Several types of DNA viruses induce M2-like macrophage polarization through encoding analogue IL-10 (vIL-10) [48]. Depending on the virus type, the polarization of macrophages toward M2-like type may contribute to the viral infection persistence through dampening effective anti-virus immune responses [44]. Moreover, the overproduction of the pro-inflammatory cytokines or cytokine storm may covert macrophages into an over-inflamed phenotype that can contribute to pathologic reactions [44]. The involvement of multiple factors and their downstream transcription factors in macrophage differentiation suggest that the macrophage subsets strict polarization into M1-type and M2-type is unlikely and that such rigidity would run against the principle of cellular plasticity to optimize the need-based regulation of biological responses. The kinetics of the macrophage polarization during the various stages of COVID-19, its association with pathogenesis and its orientation in the favourite direction need to be evaluated in future studies.
3. SARS-CoV-2 infects monocytes and macrophages
A subgroup of peripheral CD14+ monocytes and macrophages separated from healthy individuals express ACE2 [49] that is used by SARS-CoV to infect these cells despite low or no replication [50]. SARS-CoV also enters into ACE2 non-expressor leukocytes [51,52]. The C-type lectin receptors- liver/lymph node-specific intercellular adhesion molecule-3-grabbing integrin (L-SIGN) or DC-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN) and antibody-mediated internalization can provide further routes for cellular infection by SARS-CoV [53,54]. HCoV-229E effectively infects and kills DCs leading to a delay in the induction of adaptive anti-viral immune response, providing time for viral replicate in the host [55].
The MERS-CoV infects the blood monocyte-derived macrophages (MDMs) and DCs [56]. MERS-CoV exhibits poor replication but strong induction of antiviral responses (IFN-λ1, CXCL10 and Mx) in human MDMs and DCs [57]. MERS-CoV efficiently replicates inside MDMs and overcomes the host innate immunity [58]. The MERS-CoV-infected phagocytes can act as reservoirs and transporters of virus, assisting the viral replication and dissemination [58].
The potential mechanisms of SARS-CoV-2 entry into host cells along with its life-cycle are summarized in the Fig. 3A. The SARS-CoV-2 may use same or similar routes as utilized by SARS-CoV and MERS-CoV to infect host MDMs. As mentioned above, a certain population of CD14+ monocytes and macrophages derived from healthy individuals express ACE2 [49]. Thus, the monocytes and macrophages in patients with COVID-19 may be directly infected by SARS-CoV-2 [49]. The monocytes with vacuolated appearance were indicated in COVID-19 patients [49]. The numbers of classical monocytes (CD14++CD16−) decrease, but the numbers of intermediate (CD14++CD16+) and non-classical (CD14+ CD16++) monocytes increase in COVID-19 patients [49]. Upon infection, monocytes alter the cytokine/chemokine secretion patterns, differentiate into macrophages and migrate to the tissues to become infected resident macrophages [21]. Alternatively, infected apoptotic epithelial cells can be phagocytosed by macrophages to get infected. The Trojan horse phenomena and direct cell-to-cell syncytial contacts allow viruses to spread through all organs and tissues [21]. Despite the limited ACE2 expression on alveolar macrophages [59], immunohistochemical staining showed the presence of the SARS-CoV-2 antigens in the alveolar epithelial cells and macrophages [60]. Other receptors such as CD147 may mediate the entry of SARS-CoV-2 into T cells [61], however, the CD147 expression by monocytes and macrophages from COVID-19 needs validation.
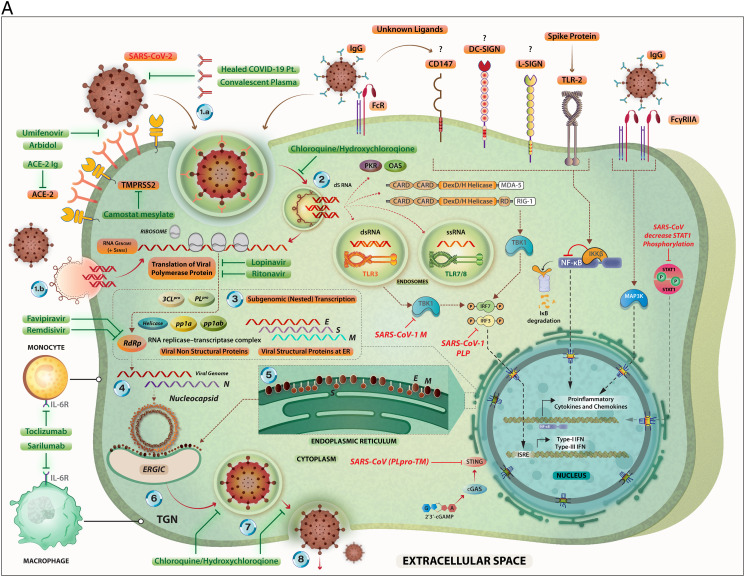
Fig. 3.
A. The steps in the life-cycle of SARS-CoV-2 are summarized along with the potential mechanism of entry into the host cells. Possible interventions using drugs and their targets during the SARS-CoV-2 life cycle are depicted. Specific signaling proteins that are targets of inhibition to suppress the host immunity are also depicted.
The SARS-CoV-2 can infect its target cells through ACE2-dependent and ACE2-independent (including virus binding to cell surface molecules CD147, DC-SIGN, and L-SIGN; endocytosis of virions or virus-containing apoptotic bodies, and attachment of the virus-coated IgG to FcR). The SARS-CoV-2-related RNA molecules are recognized by intra-cellular PRRs including endosomal TLR3, TLR7 and TLR8; and cytoplasmic sensors including MDA5 and RIG-I. These PRR-mediated signaling pathways eventually may lead to the expression of Type I- and Type III interferons. However, coronaviruses interfere with IFN production through inactivating of the IRF-3. The binding of the SARS-CoV-2-related S protein to the surface TLR2 and the attachment of the virus-coated IgG to FcγRIIA lead to the expression of the pro-inflammatory cytokines and chemokines via induction of the NF-κB and MAPK-related pathways, respectively. The RNA transcription, translation, viral protein synthesis, viral assembling, viral budding to ER, viral transportation into Golgi vesicles, and exocytosis of infective virions are key steps in the cycle life of coronaviruses, that may be targeted by therapeutic agents.
Key to the life cycle of SARS-CoV-2 inside the host cells (monocytes and macrophages along with other cell types): 1.a. Virus entry via ACE-2 mediated endocytosis; 1.b. virus entry through membrane fusion (following binding with ACE-2 and TMPRSS2); 2. release of the viral genome; 3. translation of viral polymerase protein; 4. RNA replication; 5. translation of viral structural proteins (S, M, E) via ER bound ribosomes and nucleocapsid (N) in the cytoplasm; 6. virion assembly at ERGIC; 7. formation of mature virion inside Golgi vesicle; 8. release of infective virions via exocytosis.
The drugs (experimental/repurposed) that are currently prescribed the management of COVID-19 are mentioned above in green boxes. Each of these drugs/antibodies have specific intervention points where they either inhibit- the viral proteins or crucial process like viral entry, translation of viral proteins, assembly of new virions and viral budding, etc. thereby suppressing the multiplication of SARS-CoV-2. An extended list of such drugs is presented in Table 1.
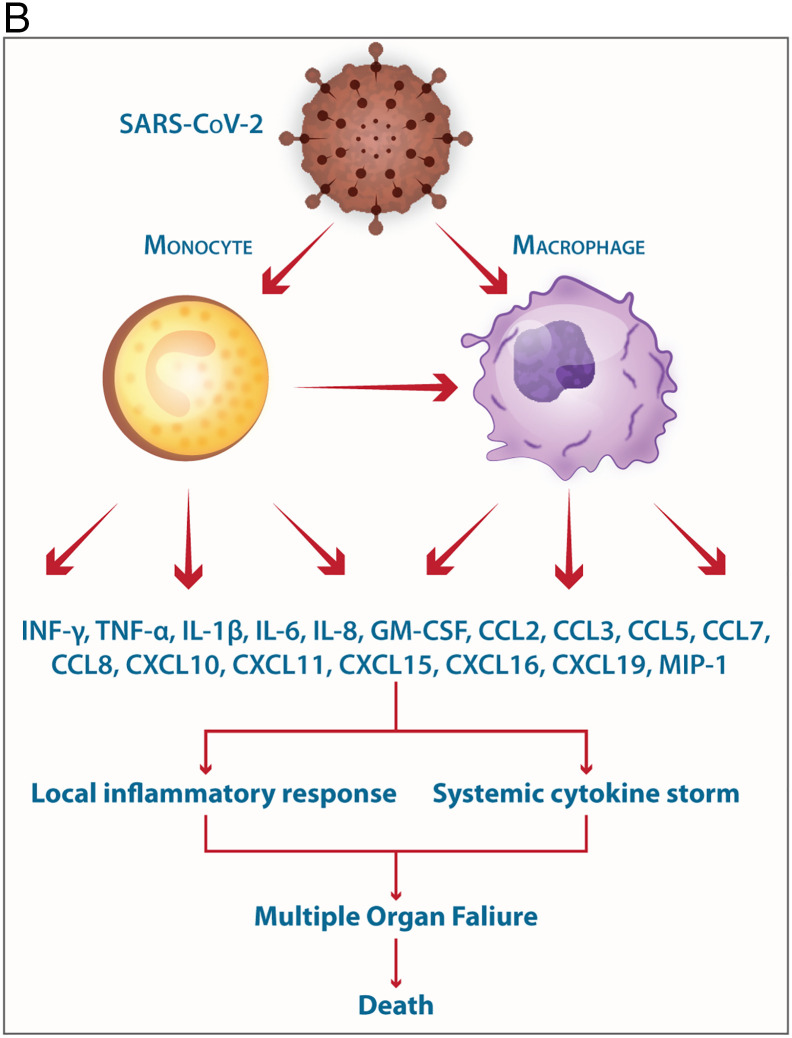
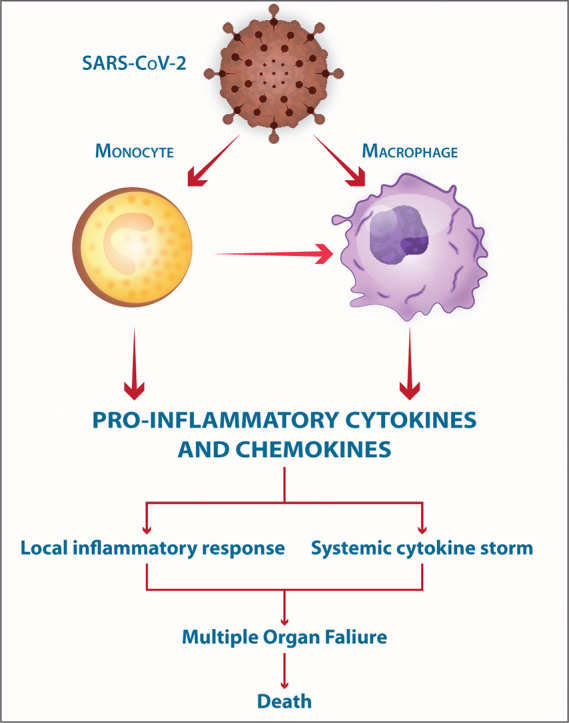
B. Involvement of monocytes/macrophages in the pathogenesis of SARS-CoV-2 the elicitation of mediators of the Cytokine storm is shown.
The mild COVID-19 and moderate COVID-19 were associated with the effective expression of type I IFNs and ISGs in the lungs. Thus, an appropriate local IFN response in the respiratory system can control SARS-CoV-2 infection accompanied by mild and moderate forms of the disease. However, a lower proportion of the SARS-CoV-2-infected patients exhibit severe symptoms. It was proposed, when the viral load is high and the primary local IFN response is failed, the SARS-CoV-2 enters the blood from the lungs and attacks organs expressing high levels of ACE2. The SARS-CoV-2-infected monocytes and macrophages can produce large amounts of numerous types of pro-inflammatory cytokines and chemokines which contribute to the local tissue inflammation and cytokine storm. Both local tissue inflammation and cytokine storm play a key role in the development of COVID-19-related multi-organ failure which causes death in some COVID-19 patients.
It should be mentioned that monocytes may have some limitations to support viral dissemination, such as short lifespan and inability to establish viral replication. The capability of SARS-CoV-2 to overcome these limitations, perhaps through enhancing the monocyte survival and differentiation of infected monocytes into productive long-lived macrophages, needs to be clarified in future studies. After the differentiation of the infected monocytes to tissue macrophages, viral replication can then begin, causing progeny virions that can infect the surrounding cells. Although, the SARS-CoV-2 persistence in the monocytes may be short-term without detectable viral replication, the SARS-CoV-2-infected monocytes can produce large amounts of inflammatory mediators that support COVID-19-associated complications.
4. Antibody-dependent infection of monocytes and macrophages
Antiviral neutralizing antibodies play a key role in viral clearance, but the antibody-dependent enhancement (ADE) happens when anti-viral antibodies fail to neutralize the virus [62,63]. ADE facilitates viral internalization and enhances target cell infection by binding of virus-antibody complex to FcR [62]. Both neutralizing- and non-neutralizing antibodies may trigger ADE [64]. ADE has been demonstrated in infections with certain types of viruses such as dengue viruses, Zika virus, Ebola virus, HIV, and influenza virus [62]. In some coronavirus-related infectious diseases, passive transfer of antibodies or prior immunity, increase the disease severity [65].
In the presence of vaccine-induced antiviral antibodies, SARS-CoV shows a higher tendency toward primary human leukocytes, which do not express the typical receptor for the virus. Anti-coronavirus antibodies can increase viral replication in macrophage cultures [66]. The anti-S antibody promotes the SARS-CoV infection of monocytic and lymphoid immune cell lines and human macrophages [67]. After the initiation of viral gene transcription and viral protein synthesis, the replication process is halted without the generation of the progeny virus [68]. ADE via FcγRIIA (CD32A) was more prominent than with FcγRIIB, as these receptors have an immunoreceptor tyrosine-based activation motif (ITAM) and immunoreceptor tyrosine-based inhibitory motif (ITIM), respectively [68]. As alveolar macrophages express high levels of FcγRIIA, the FcγRIIA-mediated ADE may play a key role in the COVID-19 pathogenesis [64]. The FcγRIIA is expressed by alveolar macrophages and once is ligated by IgG molecules, it signals through ITAM inducing pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-1, and IL-6 [64] prompting the use of tocilizumab (an IL-6 receptor antagonist) in COVID-19-related clinical trials [64]. The targeting of the FcγRIIA-related downstream signaling elements such as the Src family of tyrosine kinases may also attenuate the inflammatory responses [64].
Primary inflammatory responses to SARS-CoV occur prior to the appearance of anti-virus antibodies and are mediated by viral replication, viral-mediated ACE2 downregulation, host anti-viral inflammatory responses and cellular death through apoptosis and/or pyroptosis [69]. Secondary inflammatory responses develop after the generation of anti-virus antibodies limiting viral replication. However, the binding of the virus-Ab complex to FcR can lead to the accumulation of pro-inflammatory M1 macrophages in the lungs escalating lung injury through secretion of inflammatory chemokines MCP-1 and IL-8 [69]. The binding of the virus-anti-S-IgG complex to FcR present on monocytes/macrophages induces pro-inflammatory responses and therefore, FcR blockade reduces the production of inflammatory cytokines [70].
In Chinese macaques vaccinated with SARS-CoV-derived S protein, the acute lung injury was more severe than in unvaccinated control animals [70]. In SARS-CoV infected macaques, the development of anti-S IgG prior to viral clearance promotes the MCP-1 and IL-8 production in the lungs and enhances the recruitment of pro-inflammatory alveolar macrophages, and abrogates the wound-healing responses that result in serious lung injury [69,70]. The adoptive transfer of the anti-S IgG to macaques reduces viral loads after subsequent challenge with SARS-CoV but caused significant alveolar damage than the control animals [70]. Similar observations concerning the SARS-CoV vaccine-induced lung damages have also been reported in mice and African green monkeys [71,72]. The greater lung infiltration of eosinophils, and elevated levels of IL-5 and IL-13 have been also reported in MERS-CoV-vaccinated mice following challenge infection [73].
The patients who died from SARS exhibited similar inflammatory responses, lack of wound healing-related macrophages, and quicker production of anti-virus antibody. Their sera increased the SARS-CoV-mediated IL-8 and MCP-1 secretion by human wound healing-related macrophages [70]. Indeed, in SARS-CoV infected patients, the development of ARDS accompanied with antiviral IgG seroconversion in 80% of patients [74] and the patients who developed the anti-S antibody faster had a higher chance of dying from the disease [75]. Collectively, the coronavirus-specific antibody may increase the uptake of viruses by macrophages through interaction with FcR, resulting in the activation of macrophages and the secretion of chemokines and other cytokines, which contribute to the immunopathogenesis and disease enhancement.
5. Monocytes/macrophages-mediated inflammatory responses in COVID-19
5.1. Monocytes/macrophages-mediated local tissue inflammation
Although, SARS-CoV is unable to induce potent IFN-α and INF-β response in human macrophages, it induces CXCL10, CCL2 CCL3 (MIP- 1α), CCL7 (MCP-3), and CCL8 (MCP-2) in macrophages contributing to the pathogenesis of SARS [50,76]. Furthermore, in non-productive infection of DCs, SARS-CoV induces the expression of CXCL10, CCL2, CCL3, CCL5, and TNF-α [50,76].
SARS-CoV-infected monocytes exhibit inflammatory responses and changes in the expression of immune-related genes [77]. Pro-inflammatory cytokines, including GM-CSF, IFN-α, IL-6, and TNF-α trigger the monocytes differentiation to macrophages [77]. Treatment of peripheral blood monocytes with S protein increases the expression of CXCL10, CXCL11, CCL15, CCL16 and CCL19. The expression of CCR5 (the receptor for CCL5, CCL4, CCL3), XCR1, Lymphotoxin-β receptor, IL-10RA and IL17R was also increased [77,78]. The viral S protein-treated monocytes increase TLR2-activated NF-κB-mediated secretion of MIP-1β, IL-1β, IL-8, IL-6 and TNFα and attract neutrophils, monocytes, natural killer (NK)-, T-, and B cells to the infection site to instruct the initial adaptive immunity [78]. A truncated S protein induces the TNF-α and IL-6 expression in a murine macrophage cell line [79]. Higher blood levels of CXCL10 and CCL2- the chemotactic factor for monocytes/macrophages- have been detected in patients with SARS [33,80]. These cells could be M2 macrophages promoting SARS-CoV-associated lung pathology [81]. The SARS-CoV-mediated chemokine production induces a cycle of monocyte/macrophage recruitment in a synergistic manner, that leads to the immunopathology [33]. Both CCL2 [82] and CXCL10 [83] can suppress hematopoietic progenitor cell growth, which may contribute to lymphopenia, a prominent event in SARS [33]. Similarly, CCL2 and CXCL10 may contribute to the SARS-CoV-2-associated lymphopenia, that is observed in severe cases of COVID-19 [14].
MERS-CoV-infected macrophages contribute significantly to the development of MERS symptoms [58]. MERS-CoV replication in the macrophages results in extreme cytotoxicity and induces the expression of pro-inflammatory mediators which may promote MERS-related complications [84]. MERS-CoV-infected macrophages secrete pro-inflammatory chemokines and cytokines such as IL-1β, IL-6 and CXCL8 [56]. MERS-CoV infection in MDMs and DCs leads to the release of IL-2, IL-3, CCL2, CCL3 and RANTES in humans [56] or CXCL10, CCL5, IL-12, and IFN-γ, although no IFN-β and low IFN-α levels were measured in mouse [85,86]. MERS-CoV-infected human plasmacytoid DCs, produce large amounts of type I and III IFNs, in particular IFN-α, although the viral replication was abortive [87]. The expression of chemokines such as CXCL10, CCL2, CCL3, CCL5 and CXCL8 is also upregulated by abortively MERS-CoV-infected DCs and macrophages that may contribute to the influx of monocytes/macrophages into the infected tissues [86] causing tissue damage via promoting leukocyte aggregation in the lower parts of the respiratory tract [58]. The infiltration of macrophages and neutrophils in the lung tissues of the MERS-CoV-infected rhesus macaques [88] and the progression of pneumonia severity and respiratory dysfunction in MERS patients are attributed to the cytokine/chemokine induction [89].
Excessive upregulation of the neutrophil-attractant chemokines (CXCL1, CXCL2, CXCL8, CXCL10, CCL2 and CCL7) and monocyte-attractant chemokines (such as CCL2, CCL3, CCL4, CCL7, CCL8, CCL20, CXCL6 and CXCL11) has been reported in BALF samples from COVID-19 patients [37]. These chemokines can perform a crucial role in the development of pulmonary dysfunction by recruiting leukocytes in the lungs [37].In COVID-19, the infiltrated leukocytes in alveoli were mainly macrophages and monocytes [60]. There were also intermediate numbers of multinucleated giant cells with lower numbers of eosinophils, neutrophils and lymphocytes [60]. The alveolar septum-related blood vessels were congested, edematous and widened, with a modest aggregation of monocytes and lymphocytes [60]. Liao et al. indicated that the severe and moderate COVID-19 patients had higher frequency of macrophages (M1-like macrophages) in BALF and higher CXCL9, CXCL10 and CXCL11 concentrations compared to healthy individuals. However, CXCL16 concentrations were greater in moderate patients than in severe infection [46]. Thus, lung macrophages in severe COVID-19 infection may play a more important role in promoting local inflammation by recruiting inflammatory cells.
Macrophages can often crosstalk with the ACE2-expressing cells, in several organs such as the lung, liver and stomach. The CD74 is expressed on the macrophages and serves as a receptor for MIF (migration inhibitory factor), which participates in inflammatory responses [90]. SARS-CoV-2-infected cells can interact with macrophages in the lung, stomach and liver. Macrophages attracted by SARS-CoV-2-infected cells through CD74-MIF interaction and other pathways may play destructive or protective functions [90]. Therefore, the targeting of SARS-CoV-2-induced chemokines in the monocytes, macrophages, and DCs may attenuate the inflammatory responses and prevent organ failure (Fig. 3B).
5.2. Monocytes and macrophages potentiate cytokine storm
Sudden and quickly advancing clinical exacerbation has frequently been reported in the late stages (about 7–10 days) of COVID-19 which is associated with the cytokine storm [37]. Despite some differences in cytokine patterns between patients with COVID-19, SARS and MERS, it seems that the cytokine storm plays a key role in the development of SARS-CoV-2-related complications such as ARDS [4,6,91]. Elevated circulating quantities of IL-1β, IL-2, IL-7, IL-9, IL-10, IL-17, G-CSF, GM-CSF, IFN-γ, TNF-α, CXCL8, CXCL10, MCP1, MIP1A and MIP1B have been detected in the COVID-19 patients, especially in those needing ICU facilities [92]. Many of these cytokines and chemokines from monocytes/macrophages can escalate the pathogenesis. In COVID-19 patients, the total number of monocytes/macrophages in peripheral blood may not vary between SARS-CoV-2-infected patients and healthy individuals, but there is a greater proportion of activated monocyte/macrophage in patients. The severely affected COVID-19 patients display a significantly higher proportion of larger monocytes [49]. These monocytes exhibit the CD14, CD11b, and CD16 expression, along with elements related with both M1 and M2 polarization, that secrete IL-6, IL-10, and TNF-α [49]. There is also a higher proportion of CD14+ CD16+ inflammatory monocytes in COVID-19 patients compared to healthy individuals [93,94]. The proportion of CD14+CD16+ monocytes is much higher in more serious pulmonary complications [49]. The proportion of GM-CSF and IL-6-producing monocytes is higher in severe patients, representing as association with cytokine storm [49]. The Th1 cell-derived GM-CSF can activate the monocyte/macrophage leading to the development of the IL-6-secreting CD14+CD16+ monocytes, which may migrate to the lungs and induce subsequent lung damage along with the cytokine storm [49]. SARS-CoV-2 infection may also cause pyroptosis in macrophages and lymphocytes [95]. In SARS-CoV infection, viroporin-3a activates the NLRP3 inflammasome and induces IL-1β secretion by macrophages, indicating the possible induction of pyroptosis [96], which can cause the release of large amounts of pro-inflammatory factors.
Collectively, the SARS-CoV-2-mediated local tissue inflammation accompanied by systemic cytokine storm can lead to multiple organ failure, leading to death in some COVID-19 patients (Fig. 3B).
6. Angiotensin induces inflammatory responses in monocytes and macrophages
ACE2 is a type I transmembrane protein, whose enzymatic domain placed on the outer surface of cells where angiotensin II is converted to angiotensin 1–7 [97]. Angiotensin 1–7 is a vasodilator and acts as a modulator of the renin- angiotensin system (RAS) [97]. ACE2 reduces the angiotensin II levels, which have directly pro-inflammatory and pro-oxidant properties. ACE2 thus plays a pivotal role in the modulation of excessive inflammatory responses [97,98]. ACE2 exerts beneficial effects in a number of pathological situations, including SARS-CoV-2 infection, as it directly protects the lungs against ARDS [99]. The ACE2-mediated virus internalization is facilitated by the host membrane cell TMPRSS2 protease, which primes the viral S protein to allow the virus to enter the cell [97,99]. Virus-mediated ACE2 downregulation can diminish its activity, attenuate its anti-inflammatory properties, and reinforce the angiotensin II impacts in the susceptible patients [97]. Under inflammatory conditions, the transmembrane disintegrin ADAM17 can also cleave the membrane-linked ACE2, which releases ACE2 into circulation or interstitium [98,100].
ACE2 expression by monocytes/macrophages from COVID-19 patients is significantly lower than healthy individuals [49]. SARS-CoV S-protein downregulates ACE2 and induces the shedding of catalytically active ACE2 ectodomain [101]. SARS-CoV infection, as well as inflammatory cytokines such as IL-1β and TNF-α, can enhance ACE2 shedding [101]. The soluble ACE2 may be directly contributed to the inflammatory reactions of SARS-CoV, and perhaps SARS-CoV-2 [98]. The reduced activity of pulmonary ACE2 was related to the ALI/ARDS [98,99]. The reduced ACE2 expression enhances vascular permeability, increases lung edema, increases neutrophil accumulation, and diminishes lung function [102].
7. Monocytes and macrophages as targets for therapeutic interventions
As mentioned above, monocyte- and macrophage-derived cytokines and chemokines can play a crucial role in the COVID-19 pathogenesis. Resident alveolar macrophages play a protective role during the early phase of SARS-CoV-2 infection [26]. However, large infiltration of monocytes, putative precursors of alveolar macrophages, may cause severe lung inflammation [24,25]. Interfering with upstream signals causing cytokine production can effectively dampen the occurrence of the cytokine storm [103]. The TLR- and inflammasome-mediated signaling inhibitors can induce monocytes and macrophages to secrete pro-inflammatory mediators. The targeting of the TLR-related pathways (such as using IRAK inhibitors) and targeting the inflammasomes may have beneficial therapeutic impacts [104,105].
Interfering with cytokine-mediated signaling pathways could also significantly reduce hyperinflammation in patients with severe COVID-19. Inflammatory responses could be mitigated by targeting pro-inflammatory cytokines or their receptors. Several trials using IL-1 inhibitors, IL-6 inhibitors, TNF-α inhibitors and JAK inhibitors are ongoing [103].
Interfering with massive monocyte infiltration can also attenuate local tissue inflammation. It seems that pathological macrophages mainly derive from circulating monocytes that massively infiltrate the lungs and other organs rather than from tissue-resident macrophage populations. The targeting of monocyte/macrophage-attracting chemokines using small-molecule antagonists, neutralizing monoclonal antibodies and siRNA can modulate the monocyte/macrophage recruitment to the inflamed organ and prevent tissue injury [106]. The circulating CD14+ monocytes accumulate in inflamed tissues using the chemokine receptor CCR2 [107]. CCR2 blockade could potentially help to reduce the accumulation of pathological monocytes in inflamed tissues, although other CCR2-independent mechanisms may also contribute to the monocyte accumulation in tissues during severe inflammation. Treatment with anti-CCL2 neutralizing antibodies may interfere with the recruitment of monocytes and subsequent macrophage accumulation. For example, in a murine model of hepatocellular carcinoma, it has been demonstrated that the blocking of the CCL2/CCR2 axis using a CCR2 antagonist inhibits the recruitment of inflammatory monocytes, infiltration of the tumor-associated macrophages (TAMs) and M2 macrophage polarization, which support anti-tumor immune response [108]. Trial targeting CCR5, another chemokine receptor that regulates monocyte and T cell migration, have been initiated in patients with COVID-19 (NCT04343651) [103]. The re-programming of pro-inflammatory macrophages to anti-inflammatory macrophages may be also considered in COVID-19. For example, GM-CSF is known as an inducer of M1 macrophage polarization and the therapeutic agents inhibiting GM-CSF are in phase I and II clinical trials in patients with rheumatoid arthritis [109].
Clinical trials using type I and type III IFNs have been initiated in COVID-19 patients to interfere with viral replication. The receptor of type III IFNs (IFN-λs) mainly expressed by epithelial cells and restricted types of leukocytes [110]. As receptor of type III IFNs has lower distribution compared to receptor of type I IFNs, thus IFN-λs can induce powerful local antiviral responses without triggering systemic harmful inflammatory responses. Therefore, the clinical use of IFN-λ in COVID-19 may be very promising, and clinical trials are undertaken (NCT04331899 and NCT04343976) [110].
Although, IFNs may be protective during the early stages of the disease, the extended IFN-γ production could eventually cause macrophage hyperactivation. Trials have been initiated using IFN-γ inhibitors in COVID-19 patients suffering from respiratory distress and hyperinflammation (NCT04324021) [103].
Identification of the exact organ-specific macrophages-related markers can provide unique determinants for targeting different macrophage subsets in certain organs. Novel drug delivery using nanoparticles could deliver therapeutic elements to tissue or specific macrophage subsets and exert manipulations with high specificity and low toxicity [111]. The monitoring of macrophage recruitment and responsiveness to drugs using noninvasive imaging methods like positron emission tomography is a great approach for promoting the delivery of drugs to macrophages [111]. Development of strategies to target infected monocytes and macrophages may also be considered. Mannosylated nanoparticles can selectively target macrophages via the mannose receptor (CD206) in vitro [112]. The beneficial effects of macrophage deletion using monoclonal antibody against M-CSF receptor have been also demonstrated in mouse models of colorectal adenocarcinoma and fibrosarcoma [113]. The safety, pharmacokinetics, pharmacodynamics, and anti-tumor activity of a human antibody against M-CSF receptor (AMG 820) in a human's phase I study of solid tumors were evaluated [114]. However, the singular treatment with AMG 820 exhibited weak antitumor activity. The manipulation of the macrophages using nanotechnology-based system exhibited promising beneficial effects in mouse models of tumors and inflammatory diseases [115].
Melatonin may also have the potentials to limit the COVID-19-related complications due to its anti-inflammation, anti-oxidative and immune-promoting properties [116]. Melatonin suppresses NF-κB, inhibits the production of the pro-inflammatory cytokines, and exerts anti-inflammatory effects through inducing the sirtuin-1 which downregulates the macrophage polarization toward the pro-inflammatory form that may prevent ARDS development [116].
A list of potential therapeutic agents with anti-viral, anti-bacterial, anti-parasitic, anti-proliferative-, anti-inflammatory-, immunosuppressive and immunomodulatory properties (experimental/repurposed) currently considered as potential candidates/employed for ongoing trials of COVID-19 management has been provided in Table 1 . The mechanism of action of the therapeutic agents has also been mentioned in Table 1. Anti-viral agents have specific intervention points in crucial stages like viral entry, viral replication, translation of viral proteins, assembling of new virions and viral budding, etc. thereby they can suppress the multiplication of SARS-CoV-2 (Table 1 and Fig. 3A). Many therapeutic agents suggested for repurposing in the COVID-19 treatment are commercially available and their dosage, safety and toxicity in humans is well documented, due to years of clinical use. This can allow their rapid assessment in phase II- and III clinical trials [117].
Table 1.
Therapeutic agents that are currently considered as potential candidates/employed for ongoing trials and the treatment of COVID-19 disease.
| Drug | Class/small molecules | Mechanism | Indication | Status for COVID-19/Clinical Trials-identifier |
|---|---|---|---|---|
| Abacavir | NRTI | RNaseH | HIV-1 infection | Abacavir + Lamivudine. |
| Acalabrutinib | Kinase inhibitors | Tyrosine kinase BTK | Mantle cell lymphoma | Repurposing, Clinical Trial (NCT04380688). |
| ACEIs (Captopril) | ACE inhibitor | ACE | Renovascular hypertension | Repurposing, Clinical Trial (NCT04345406). |
| Almitrine | Diphenylmethylpiperazine derivative | ATP1A1 | Chronic obstructive pulmonary disease | Repurposing, Clinical Trial (NCT04357457). |
| Amoxicillin | Penicillin-like antibiotics | Penicillin-binding protein | Bacterial infections | Azithromycin + amoxicillin/clavulanate Repurposing, Clinical Trial (NCT04363060). |
| Anakinra | Recombinant protein | IL-1Ra antagonist | NOMID, RA | Repurposing, Clinical Trial (NCT04362111). |
| Angiotensin (1–7) | Heptapeptide | – | Peripheral blood cell abnormalities | Repurposing, Clinical Trial (NCT04332666). |
| Anti-Corona VS2 immunoglobulin | Purified hyper Ig | – | Convalescent COVID-19 hyper immunoglobulin | Repurposing, Clinical Trial (NCT04383548). |
| Artemisinin | Sesquiterpene lactone | – | Anti-malarial | Repurposing, Clinical Trial (NCT04387240). |
| ASC09F | Protease inhibitor | HIV-1 protease | HIV type-1 infections | Combinatorial ASC09F + Oseltamivir Clinical Trial (NCT04261270). |
| Ascorbic acid | Vitamin-c | – | Numerousa | Repurposing, Clinical Trial (NCT04323514). |
| Aspirin | NSAID, small molecule | COX-1, COX-2 | Pain, fever, and inflammation Combination therapy of COVID |
(Aspirin+ Clopidogrel + Rivaroxaban + Atorvastatin + Omeprazole) Clinical Trial (NCT04333407). |
| Atazanavir | Protease inhibitors | HIV-1 protease | HIV-1 infection | Predicted/repurposing, CT (NCT00084019) |
| Atorvastatin | Statins | HMG-CoA reductase | Dyslipidemias | Repurposing, Clinical Trial (NCT04380402). |
| Atovaquone | Small molecule | Cytochrome b | Antimalarial, antipneumocystic | Repurposing, Clinical Trial (NCT04339426). |
| Avdoralimab | mAb | Anti-C5aR | Checkpoint-immunotherapies | Repurposing, Clinical Trial (NCT04371367). |
| Aviptadil | Vasoactive intestinal polypeptide (VIP) | – | ARDS, ALI, Dyspnea, COVID-19 | Repurposing, Clinical Trial (NCT04360096). |
| AVM0703 | Small molecule | – | Lymphoma, immunostimulation | Experimental, Clinical Trial (NCT04366115). |
| Azithromycin | Macrolide antibiotics | Bacterial 23S rRNA (50S) | SARS-CoV-2 infection | Clinical Trials (NCT04338698)/(NCT04324463). |
| Azoximer bromide | Immunomodulator | – | Damage of the immune system | Repurposing, Clinical Trial (NCT04381377). |
| Baricitinib | Immunosuppressants | JAK-1/2 | Rheumatoid arthritis | Repurposing, Clinical Trial (NCT04358614). |
| Bevacizumab | mAb | VEGF-A | Cancer-Chemo, ALI, ARDS | Repurposing, Clinical Trial (NCT04305106). |
| Bicalutamide | Anti-androgens | Androgen receptor | Prostate cancer | Repurposing, Clinical Trial (NCT04374279). |
| BLD-2660 | Small molecule | Calpain inhibitors | Fibrosis | Repurposing, Clinical Trial (NCT04334460). |
| Bortezomib | Proteasome inhibitors | 26S proteasome | Multiple myeloma (MM) | Patients with MM and COVID-19 together, Clinical Trial (NCT00872521). |
| Bromhexine | Mucolytic agent | – | Respiratory disorders | Experimental, Clinical Trial (NCT04355026). |
| Calcifediol | Vitamin D3 metabolite | Vitamin D3 receptor | Refractory rickets, hypocalcemia | Combinatorial BAT + Calcifediol Clinical Trial (NCT04366908). |
| Camostat Mesilate | Small molecule | TM protease serine 2 | Chronic pancreatitis | Clinical Trial (NCT04321096)/(NCT04353284). |
| Canakinumab | mAb | Anti-human-IL-1β | CAPS | Repurposing, Clinical Trial (NCT04362813). |
| Carfilzomib | Proteasome inhibitors | Proteasome (β5 and β5i) | Antineoplastic agent | Predicted/repurposing. |
| Carmofur | Pyrimidine analogue | FAAH, SARS-CoV-2 Mpro | Antineoplastic agent | Predicted via X-ray crystal structure studies. |
| Carrimycin | Macrolide antibiotic | – | Gram-positive bacteria, Mtb | Repurposing, Clinical Trial (NCT04286503). |
| CD24Fc | Immunomodulator | – | GVHD | Repurposing, Clinical Trial (NCT04317040). |
| Chalcone | Ketone | Numerousa | Broad activity spectrum | Currently undetermined for COVID-19. |
| Chloroquine | Small molecule | ACE2, TLR9, GST-1 | SARS-CoV-2 infection | Repurposing/experimental, numerous CTs Clinical Trial (NCT04333628)/(NCT04349371). |
| Chlorpromazine | Phenothiazine antipsychotics | Dopamine receptors | Antipsychotic agent, anti-emetic | Repurposing, Clinical Trial (NCT04366739). |
| Ciclesonide | Corticosteroids | Glucocorticoid receptor | Perennial allergic rhinitis | Repurposing, Clinical Trial (NCT04381364). |
| Cinanserin | 5-HT2CR-antagonist | 3C-like proteinase | SARS-CoV/HCoV-229E | Experimental in vivo evidence. |
| Clazakizumab | mAb | Anti-IL-6 | Psoriatic arthritis | Repurposing, Clinical Trial (NCT04348500). |
| Clevudine | Small molecule | HBV polymerase | Hepatitis B | Repurposing, Clinical Trial (NCT04347915). |
| Clopidogrel | Thienopyridine | P2Y12 ADP platelet receptors | Myocardial infarction | Repurposing, Clinical Trial (NCT04368377). |
| CM4620 | Small molecule | CRAC channels | Pancreatitis; pneumonia | CM4620-injectable emulsion, CT (NCT04345614). |
| Cobicistat | CYP3A inhibitors | CYP3A | HIV-1 infection | Combinatorial Darunavir + Cobicistat, Clinical Trial (NCT04252274). |
| Colchicine | Anti-gout agents | Microtubule inhibitor | Gout management | Repurposing, Clinical Trial (NCT04326790). |
| Cyclosporin A | Immunosuppressant | Calcineurin | Transplant; COVID-19 | Repurposing, Clinical Trial (NCT04341038). |
| Danoprevir | NS3/4A protease inhibitor | Genome polyprotein | HCV infection | Combinatorial Danoprevir + Ritonavir, Clinical Trial (NCT04345276). |
| Dapagliflozin | SGLT2 inhibitors | Na/glucose cotransporter 2 | Type 2 diabetes mellitus | Repurposing, Clinical Trial (NCT04350593). |
| Darunavir | Protease inhibitor | Gag-Pol proteins | HIV-1 infection | Clinical Trial (NCT04252274)/(NCT04304053). |
| DAS-181 | Recombinant proteins | Sialic acid | Influenza virus | Repurposing, Clinical Trial (NCT04324489). |
| Deferoxamine | Chelating agents | Fe2+ chelating agent | Iron or aluminum toxicity | Repurposing, Clinical Trial (NCT04333550). |
| Defibrotide | ss-Oligos | Adenosine receptor A1 | Sinusoidal obstruction syndrome | Repurposing, Clinical Trial (NCT04348383). |
| Dexamethasone | Immunosuppressant | Glucocorticoid receptor | Bacterial infections | Repurposing, Clinical Trial (NCT04325061). |
| Dexmedetomidine | Small molecule | α2-Adrenergic agonist | For sedation of ICU patients | Repurposing, Clinical Trial (NCT04350086). |
| DFV890 | Small molecule | – | Multiple indications | Repurposing, Clinical Trial (NCT04382053). |
| Disulfiram | Small molecule | ADH/MERS-CoV PLpr | Chronic alcoholism | Experimental inhibition of SARS-CoV. |
| Dornase alfa | R-deoxyribonuclease I | DNA | Cystic fibrosis | Repurposing, Clinical Trial (NCT04359654). |
| Doxycycline | Tetracycline antibiotics | 16S ribosomal RNA | Bacterial infections | Repurposing, Clinical Trial (NCT04371952). |
| Duvelisib | PI3-Kinase inhibitor | PI-3-K γ/δ | Chronic lymphocytic leukemia | Repurposing, Clinical Trial (NCT04372602). |
| Ebselen | Organoselenium drug | EPHX2/COVID-19 Mpro | – | Computer-aided drug design. |
| Eculizumab | mAb | Complement C5 | Paroxysmal nocturnal hemoglobinuria (PNH) | Repurposing, Clinical Trial (NCT04346797). |
| Eicosapentaenoic acid (EPA-FFA) | PUFA | Prostaglandin G/H synthase 2 | Hyperglyceridemic subjects | Repurposing, Clinical Trial (NCT04335032). |
| EIDD-2801 | Isopropylester prodrug | Viral error catastrophe | SARS-CoV-2, MERS-CoV | Experimental. |
| Elbasvir | HCV NS5A inhibitors | HCV NSP5A | Hepatitis C | Computational/repurposing. |
| Elvitegravir | Integrase inhibitor | INSTI | HIV-1 infection | Undetermined. |
| Emodin | Trihydroxyanthraquinone | CKIIα | Polycystic kidney | Experimental for blocking S protein and ACE2 interaction/repurposing. |
| Emtricitabine | NRTIs | HIV-1 reverse transcriptase/RNaseH | HIV-1 Infection | Combinatorial Emtricitabine/tenofovir disoproxil Clinical Trial (NCT04334928). |
| Enoxaparin | LMWH | Antithrombin-III | Deep vein thrombosis | Repurposing, Clinical Trial (NCT04366960). |
| Enzaplatovir | Fusion inhibitor | F protein | Respiratory syncytial virus | Undetermined. |
| Escin | Saponins (triterpenoid) | – | Experimental anti-cancer | Repurposing, Clinical Trial (NCT04322344). |
| Etoposide | Plant alkaloids | DNA topoisomerase 2-α | Testicular-tumor, SCLC | Repurposing, Clinical Trial (NCT04356690). |
| Famotidine | H2 blockers | Histamine H2 receptor | Active gastric ulcer | Combinatorial HCQ + Famotidine, CT (NCT04370262). |
| Fingolimod | S1PR modulators | S1PR | SARS-CoV-2 virus, multiple sclerosis | SARS-CoV-2 virus infection in MS patients. Clinical Trial (NCT04280588). |
| Fluoxetine | SSRIs | SLC6A4 | Depressive disorder, OCD | Repurposing, Clinical Trial (NCT04377308). |
| Fluvoxamine | SSRIs | SLC6A4 | Obsessive-compulsive disorder | Repurposing, Clinical Trial (NCT04342663). |
| Fosamprenavir | Protease inhibitors | HIV-1 protease | HIV-1 infection | Candidate for 3CL-protease. CT (NCT00094523). |
| FT516 | hnCD16 Fc receptor | ADCC induction | Cancer immunotherapy | Experimental, Clinical Trial (NCT04363346). |
| Galidesivir | Pyrrolopyrimidines | RDRP disruption | Zaire Ebolavirus | Repurposing, Clinical Trial (NCT03891420). |
| Gimsilumab | mAb | GM-CSF | RA, ARDS | Repurposing, Clinical Trial (NCT04351243). |
| Human rsACE2 | Recombinant protein | Inhibits virus attachment | SARS-CoV-2 | Repurposing, Clinical Trial (NCT04287686). |
| Hydrocortisone | Glucocorticoids | Glucocorticoid receptor | Reducing inflammation | Repurposing, Clinical Trial (NCT04348305). |
| Hydroxychloroquine | Small molecule | ACE-2, TLR7, TLR9 | Malaria, RA, SLE | In vitro SARS-CoV-2 inhibition, effective in COVID-19 patients. Numerous ongoing global Clinical Trials (NCT04334928) / (NCT04353271). |
| Ibrutinib | Kinase inhibitors | Tyrosine kinase BTK | B-cell non-Hodgkin lymphoma | Repurposing, Clinical Trial (NCT04375397). |
| Ifenprodil | Small molecule | NMDA1; GIRK channels | Cerebral vasodilator | Repurposing, Clinical Trial (NCT04382924). |
| IFX-1 | mAb | C5a | – | Repurposing, Clinical Trial (NCT04333420). |
| IMU-838 | Small molecule | DHODH | Crohn's disease; MS | Repurposing, Clinical Trial (NCT04379271). |
| Indinavir | Protease inhibitor | HIV-1 protease | HIV-1 infection | Prediction via in silico molecular docking. |
| Indomethacin | NSAID | Prostaglandin G/H synthase 1 | RA, ankylosing spondylitis | Combinatorial HCQ + Zithromax oral product Clinical Trial (NCT04344457). |
| Interferon-β1a | Interferons | JAK/STAT activation | MS, SARS-CoV-2, MERS | Repurposing, Clinical Trial (NCT04343768). |
| Isotretinoin | Retinoids | Retinoic acid receptor γ/α | Recalcitrant nodular acne | Repurposing, Clinical Trial (NCT04361422). |
| Ivermectin | Small molecule | Gly-R-α3, GABA-Rβ3 | Intestinal strongyloidiasis | Repurposing, Clinical Trial (NCT04360356). |
| Leflunomide | Disease-modifying antirheumatic drugs | DHODH | Rheumatoid arthritis | Repurposing, Clinical Trial (NCT04361214). |
| Lenalidomide | Immunomodulatory drugs | Protein cereblon | Multiple myeloma | Repurposing, Clinical Trial (NCT04361643). |
| Lenzilumab | Anti-hu GM-CSF mAb | GM-CSF | Chronic myelomonocytic leukemia | Repurposing, Clinical Trial (NCT04351152). |
| Leronlimab | mAb | CCR5 | Anti-HIV, COVID-19 | Clinical Trials (NCT04343651)/(NCT04347239). |
| Levamisole | Antihelmintic | nAChRα3 | Dukes' stage C colon cancer, melanoma, and head/neck cancer | Combinatorial Formoterol + Budesonide, Clinical Trial (NCT04331470). |
| Lidocaine | Anesthetics | Sodium channels | Local anesthesia | Intubation and extubation I patients with COVID-19. |
| Linagliptin | DPP-4 inhibitors | Dipeptidyl peptidase 4 | Type II diabetes | Repurposing, Clinical Trial (NCT04371978). |
| Lopinavir | Protease inhibitor | HIV-1 protease | SARS-CoV-2 infection | IFN-β1b + lopinavir-ritonavir combination for COVID-19. Clinical Trial (NCT04307693). |
| Losartan | Angiotensin receptor blockers (ARBs) | Angiotensin-II-R (Type-1) | Hypertension | Repurposing, Clinical Trial (NCT04335123). |
| Low-dose interleukin-2 | Interleukin | IL-2R | Treg induced protection from SARS-CoV2-ARDS | Repurposing, Clinical Trial (NCT04357444). |
| LY3127804 | mAb | Angiopoietin 2 | ARDS | Repurposing, Clinical Trial (NCT04342897). |
| Maribavir | Benzimidazole ribosides | Protein kinase UL97 | Human cytomegalovirus | Undetermined. |
| Mefloquine | Antimalarials | Fe(II)-protoporphyrin IX | Moderate acute malaria | Combinatorial Mefloquine + azithromycin ± tocilizumab Clinical Trial (NCT04347031). |
| Melatonin | Biogenic amine | Melatonin receptor type 1A | Insomnia | Repurposing, Clinical Trial (NCT04353128). |
| Melphalan | Alkylating agent | DNA | MM, ovarian cancer, melanoma, and amyloidosis | Repurposing, Clinical Trial (NCT04380376). |
| Meplazumab | Anti-CD147-hu-mAb | Interleukin-5 | Severe eosinophilic asthma | Repurposing, Clinical Trial (NCT04275245). |
| Metenkefalin | Opioid growth factor | – | Experimental anti-tumors | Combinatorial metenkefalin + tridecactide, Clinical Trial (NCT04374032). |
| Methylprednisolone | Glucocorticoids | Glucocorticoid-R | COVID-19 pneumonia | Repurposing, Clinical Trial (NCT04273321). |
| Montelukast | LTRAs | CLR1 | Anti-asthma | Undetermined. |
| MRx-4DP0004 | Small molecule | – | Immunomodulators, asthma | Repurposing, Clinical Trial (NCT04363372). |
| N4-Hydroxycytidine | Ribonucleoside analogue | Viral error catastrophe | SARS-CoV-2, MERS-CoV | Experimental inhibition. |
| N-803 | Small molecule | IL-15 receptor agonist | Anti-cancerous | Repurposing, Clinical Trial (NCT04385849). |
| N-acetylcysteine | Mucolytic agent | – | Paracetamol overdose, CF,COPD | Repurposing, Clinical Trial (NCT04374461). |
| Nafamostat Mesilate | Serine protease inhibitor | Serine protease | Liver transplant | Repurposing, Clinical Trial (NCT04352400). |
| Naltrexone | Opiate antagonists | Delta-type opioid receptor | Managing opiate dependence | Repurposing, Clinical Trial (NCT04365985). |
| Naproxen | NSAIDs | PTGS1 | Rheumatoid arthritis | Repurposing, Clinical Trial (NCT04325633). |
| Nintedanib | Kinase inhibitors | Receptor tyrosine kinases | Idiopathic pulmonary fibrosis | Repurposing, Clinical Trial (NCT04338802). |
| Nitazoxanide | Small molecule | Pyruvate-flavodoxin oxidoreductase | Diarrhea, antiprotozoal | Combinatorial CHQ + Nitazoxanide, Clinical Trial (NCT04351347). |
| Nitric oxide | Small molecule | Soluble guanylate cyclase | Hypoxic respiratory failure | Repurposing, Clinical Trial (NCT04338828). |
| Nivolumab | mAb | PD-1 | Immune checkpoint therapy | Repurposing, Clinical Trial (NCT04343144). |
| NT-17, IL-7 | Interleukin | Interleukin-7 receptor | Immunosenescence/stimulation | Clinical Trial (NCT04380948). |
| Olokizumab | mAb | IL-6 | Rheumatoid arthritis | Combinatorial with RPH-104, Clinical Trial (NCT04380519). |
| Oseltamivir | Neuraminidase inhibitors | Neuraminidase | Influenza A and B | Combinatorial, Clinical Trial (NCT04338698). |
| Otilimab | mAb | – | Rheumatoid arthritis | Repurposing, Clinical Trial (NCT04376684). |
| Oxytocin | Hormone | Oxytocin receptor | Labor induction | Repurposing, Clinical Trial (NCT04386447). |
| Pegylated IFN-λ | IFNs | IFN-λR | Viral infections/anti-cancerous | Repurposing, Clinical Trial (NCT04343976). |
| Plitidepsin | Didemnins | Putative eEF1A2 | Anti-neoplastic | Repurposing, Clinical Trial (NCT04382066). |
| Poractant alfa | Surfactant | – | Respiratory distress syndrome | Repurposing, Clinical Trial (NCT04384731). |
| Povidone | Synthetic polymer | – | Antiseptic | Repurposing, Clinical Trial (NCT04347954). |
| Prazosin | Small molecule | α1-Adrenergic receptor | Hypertension, High BP | Repurposing, Clinical Trial (NCT04365257). |
| Presatovir | 1-Benzoylpiperidines | F protein | Respiratory syncytial virus | Undetermined for treatment of COVID-19. |
| Progesterone | Hormone | Progesterone receptor | Endometrial hyperplasia | Repurposing, Clinical Trial (NCT04365127). |
| Px12 | Small molecule | TrxR1 | Anti-cancer | Experimental/computational. |
| Pyridostigmine bromide | Acetylcholinesterase inhibitor | Acetylcholinesterase | Myasthenia gravis | Repurposing, Clinical Trial (NCT04343963). |
| Raltegravir | Integrase inhibitors | HIV integrase | HIV-1 infection | Undetermined. |
| Ramipril | ACE inhibitor | ACE | Hypertension | Repurposing, Clinical Trial (NCT04366050). |
| Ravulizumab | mAb | Complement 5 inhibitor | Paroxysmal nocturnal hemoglobinuria | Repurposing, Clinical Trial (NCT04369469). |
| RBT-9 (Stannous Protoporphyrin) | Metalloporphyrins | – | Serum bilirubin quencher | Clinical Trial (NCT04364763). |
| Recombinant TPA | Recombinant protein | Plasminogen | Acute myocardial infarction | Repurposing, Clinical Trial (NCT04356833). |
| Remdesivir | Small molecule | Viral RNA polymerases | SARS-CoV-2 infection | Clinical improvement in COVID-19 patients. Clinical Trial (NCT04292899). |
| Remestemcel-L | Stem cells | – | GVHD | Repurposing, Clinical Trial (NCT04366830). |
| Resiniferatoxin | Capsaicin analogue | TrpV1 | Interstitial cystitis | Undetermined. |
| Resistant Starch | – | – | – | Repurposing, Clinical Trial (NCT04342689). |
| Resveratrol | Phytoalexin | Nucleocapsid (N) protein | MERS-CoV | Experimental MERS-CoV inhibition. |
| Ribavirin | Nucleoside analogues | IMPDH inhibitor/RdRP | Hep C, RSV, SARS-CoV-2 | Repurposing. Clinical Trial (NCT04276688). |
| Ringer's acetate | Isotonic crystalloid | – | Low blood volume or low BP | Repurposing, Clinical Trial (NCT02765191). |
| Ritonavir | Protease inhibitor | HIV-1 protease | HIV-1 infection | Repurposing, Clinical Trial (NCT04321174) |
| Ruxolitinib | Kinase inhibitors | Tyrosine kinase JAK1 | Myelofibrosis | Repurposing, Clinical Trial (NCT04355793). |
| Saquinavir | Protease inhibitor | HIV-1 protease | HIV-1 infection | Computationally predicted. |
| Sargramostim | rHu GM-CSF | GMCSF-Rα | Immunostimulator | Repurposing, Clinical Trial (NCT04326920). |
| Sarilumab | mAb | Human anti-IL-6R | Severe reactive RA | Repurposing, Clinical Trial (NCT04359901). |
| Selinexor | Selective Inhibitor of Nuclear Export (SINE) | XPO1 | Refractory multiple myeloma | Repurposing, Clinical Trial (NCT04349098). |
| Sevoflurane | General anesthetics | GABA-AR1 | General anesthesia | Repurposing, Clinical Trial (NCT04359862). |
| Sildenafil | Phosphodiesterase (PDE) inhibitors | cGMP-specific 3′,5′-cyclic phosphodiesterase | Erectile dysfunction, pulmonary hypertension | Repurposing, Clinical Trial (NCT04304313). |
| Simvastatin | Statins | HMG-CoA reductase | Hyperlipidemia | Combinatorial Ruxolitinib + Simvastatin, Clinical Trial (NCT04348695). |
| Sirolimus | Immunosuppressants | mTOR | Organ transplantation | Repurposing, Clinical Trial (NCT04341675). |
| Sirukumab | mAb | IL-6 | Rheumatoid arthritis | Repurposing, Clinical Trial (NCT04380961). |
| Spironolactone | Potassium-sparing diuretics | Mineralocorticoid receptor | Class III-IV heart failure | Repurposing, Clinical Trial (NCT04345887). |
| TAK-981 | Small molecule | SUMO inhibitor | Solid tumors or lymphomas | Repurposing, Clinical Trial (NCT03648372). |
| TD-0903 | Kinase inhibitors | JAK | Acute lung injury | Repurposing, Clinical Trial (NCT04350736). |
| TDZD-8 | Thiadiazolidine derivative | GSK-3β inhibitor | – | Undetermined. |
| Telmisartan | Angiotensin receptor blockers (ARBs) | AT1 receptor | Hypertension, type-2 DM | Repurposing, Clinical Trial (NCT04355936). |
| Tetrandrine | Bis-benzylisoquinoline alkaloid | P-glycoprotein 1 | Experimental anti-cancer agent | Repurposing, Clinical Trial (NCT04308317). |
| Thalidomide | Immunomodulatory drugs | Protein cereblon, TNF | Erythema nodosum leprosum | Repurposing, Clinical Trial (NCT04273529). |
| Tideglusib | Thiadiazolidinone | GSK-3β inhibitor | Alzheimer disease | Undetermined. |
| Tinzaparin | LMWH | Antithrombin-III | Pulmonary embolism | Repurposing, Clinical Trial (NCT04344756). |
| Tipranavir | Protease inhibitor | HIV-1 protease | HIV-1 infection | Predicted/molecular docking. |
| Tirofiban | Antiplatelet drug | Integrin α-IIb | Acute coronary syndrome | Repurposing, Clinical Trial (NCT04368377). |
| TJ003234 | Anti-GM-CSF mAb | GM-CSF | Cytokine storm | Clinical Trial (NCT04341116). |
| TMC-310911 | Small molecule | HIV-1 protease | SARS-CoV2, HIV-1 | Investigative treatment. |
| Tocilizumab | mAb | IL-6Rα | Anti-inflammatory | Cytokine storm in COVID-19 patients, Clinical Trial (NCT04317092). |
| Tradipitant | Aryl-phenylketones | Neurokinin 1 (NK1) receptor antagonist | Eczema, pruritus, gastroparesis | Repurposing, Clinical Trial (NCT04326426). |
| Tranexamic acid | Antifibrinolytics | Plasminogen | Hemophilia | Repurposing, Clinical Trial (NCT04338126). |
| Triazavirin | NNRTIs | GN analogue | SARS-CoV-2 and H5N1 | Investigative treatment. |
| Triiodothyronine | Hormone | THR-α/β | Hypothyroidism | Repurposing, Clinical Trial (NCT04348513). |
| Umifenovir | Fusion inhibitors | Viral proteins and lipids | Influenza, SARS-CoV2 infection | Adjunctive treatment of COVID-19. Repurposing, Clinical Trial (NCT04350684) |
| Valsartan | Angiotensin receptor blockers (ARBs) | AT2 receptor | Myocardial infarction | Repurposing, Clinical Trial (NCT04335786). |
| Verapamil | Ca2+ channel blockers | L-type calcium channels | Prinzmetal's angina | Repurposing, Verapamil, and Amiodarone, Clinical Trial (NCT04351763). |
| Xiyanping | Andrographolide sulfonate | – | Anti-inflammatory and antiviral | Cotreatment with Lopinavir/ritonavir, Clinical Trial (NCT04295551). |
| Zanubrutinib | Immune checkpoint inhibitors | Tyrosine kinase BTK | Mantle cell lymphoma | Repurposing, Clinical Trial (NCT04382586). |
| Zilucoplan | Macrocyclic peptide inhibitor | Complement component 5 | IMNM, MG, ALS | Repurposing, Clinical Trial (NCT04382755). |
Sources: Table compiled through information derived from PUBMED; https://www.cdc.gov/;https://www.who.int/;https://www.drugbank.ca/ and https://clinicaltrials.gov.
8. Conclusion
In mucosal respiratory infections, alveolar macrophages serve as the first anti-viral defense through production type I IFNs. Monocytes/macrophages are the principal leukocytes attracted to the alveolar space in the initial response to respiratory viral infection. Monocytes and macrophages may be directly infected by SARS-CoV-2 through ACE2-dependent process or indirectly infected via ACE2-independent pathways using L-SIGN, DC-SIGN, CD147, ADE, and phagocytosis of virus-containing apoptotic bodies. SARS-CoV-2 can effectively suppress the anti-viral IFN response in monocytes and macrophages. As DCs, monocytes, and macrophages can act as APCs, the SARS-CoV-2 infection of these cells impairs the anti-viral adaptive immune responses. Upon infection, monocytes migrate to tissues where they become infected resident macrophages, allowing viruses to spread through all organs and tissues. Both infected- and uninfected macrophages can be found in the lungs of patients with COVID-19. Monocytes and macrophages can communicate with other cell types via direct cell-cell contacts, leading to the virus dissemination. The SARS-CoV-2-infected monocytes and macrophages can produce large amounts of numerous types of pro-inflammatory cytokines and chemokines, which contribute to the local tissue inflammation and dangerous systemic inflammatory response as named cytokine storm. Low expression of ACE2 by monocytes/macrophages of COVID-19 patients may also promote pathological reactions due to pro-inflammatory properties of angiotensin II and dysfunction of the renin-angiotensin system (RAS). Both local tissue inflammation and cytokine storm play a fundamental role in the development of COVID-19-related complications, such as ARDS, which is the main cause of death in SARS-CoV-2-infected patients (Fig. 3B).
Although the modulation of macrophage activation may be considered as a promising therapeutic approach for COVID-19, a better understanding of macrophage polarization and heterogeneity during COVID-19 is required. Moreover, the patterns of the macrophage polarization may vary during the different stages of the COVID-19 and need to be clarified in future researches. Various types of macrophages can perform a decisive role in the outcome of the COVID-19. Since macrophage polarization is a reversible process, it is necessary to clarify the factors affecting macrophage plasticity during COVID-19 and how to manipulate macrophage plasticity in a favourable direction. Moreover, macrophages from different organs may express different markers. The better understanding of which subsets of monocytes/macrophages drive disease pathology is important for the development of proper therapeutic interventions [103].
Declaration of competing interest
Authors declare that they do not have any conflicts of interest.
References
- 1.Kermali M., Khalsa R.K., Pillai K., Ismail Z., Harky A. The role of biomarkers in diagnosis of COVID-19 - a systematic review. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S., Padya B.S., Fernandes G., Mutalik S., Prassl R. Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabaan A.A., Al-Ahmed S.H., Haque S., Sah R., Tiwari R., Malik Y.S., Dhama K., Yatoo M.I., Bonilla-Aldana D.K., Rodriguez-Morales A.J. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Le infezioni in medicina. 2020;28(2):174–184. [PubMed] [Google Scholar]
- 4.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., Wang D.Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International journal of oral science. 2020;12(1):8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher D., Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18(1):57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38(2):337–342. [PubMed] [Google Scholar]
- 14.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. pii: ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P., Cho S.G., Kumar N.S., Subramaniam M.D. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 17.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Elia R.V., Harrison K., Oyston P.C., Lukaszewski R.A., Clark G.C. Targeting the “cytokine storm” for therapeutic benefit. Clin. Vaccine Immunol. 2013;20(3):319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdanpanah F., Hamblin M.R., Rezaei N. The immune system and COVID-19: friend or foe? Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hume D.A., Irvine K.M., Pridans C. The mononuclear phagocyte system: the relationship between monocytes and macrophages. Trends Immunol. 2019;40(2):98–112. doi: 10.1016/j.it.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Nikitina E., Larionova I., Choinzonov E., Kzhyshkowska J. Monocytes and macrophages as viral targets and reservoirs. Int. J. Mol. Sci. 2018;19(9) doi: 10.3390/ijms19092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafarzadeh A., Larussa T., Nemati M., Jalapour S. T cell subsets play an important role in the determination of the clinical outcome of Helicobacter pylori infection. Microb. Pathog. 2018;116:227–236. doi: 10.1016/j.micpath.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lung pathology of fatal severe acute respiratory syndrome. Lancet (London, England) 2003;361(9371):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., Selbs E., McEvoy C.P., Hayden C.D., Fukuoka J., Taubenberger J.K., Travis W.D. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiecki M., Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Current opinion in virology. 2011;1(6):463–475. doi: 10.1016/j.coviro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumagai Y., Takeuchi O., Kato H., Kumar H., Matsui K., Morii E., Aozasa K., Kawai T., Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27(2):240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shokri S., Mahmoudvand S., Taherkhani R., Farshadpour F. Modulation of the immune response by Middle East respiratory syndrome coronavirus. J. Cell. Physiol. 2019;234(3):2143–2151. doi: 10.1002/jcp.27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kindler E., Thiel V., Weber F. Interaction of SARS and MERS coronaviruses with the antiviral interferon response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80(12):5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel M., Pichlmair A., Martinez-Sobrido L., Cros J., Garcia-Sastre A., Haller O., Weber F. Inhibition of Beta interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79(4):2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung C.Y., Poon L.L., Ng I.H., Luk W., Sia S.F., Wu M.H., Chan K.H., Yuen K.Y., Gordon S., Guan Y., Peiris J.S. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79(12):7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreakos E., Salagianni M., Galani I.E., Koltsida O. Interferon-lambdas: front-line guardians of immunity and homeostasis in the respiratory tract. Front. Immunol. 2017;8:1232. doi: 10.3389/fimmu.2017.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faure E., Poissy J., Goffard A., Fournier C., Kipnis E., Titecat M., Bortolotti P., Martinez L., Dubucquoi S., Dessein R., Gosset P., Mathieu D., Guery B. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lokugamage K.G., Hage A., Schindewolf C., Rajsbaum R., Menachery V.D. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv. 2020 doi: 10.1101/2020.03.07.982264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., Francois B., Seve P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;19(7) doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Moller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konno Y., Kimura I., Uriu K., Fukushi M., Irie T., Koyanagi Y., Nakagawa S., Sato K. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is further increased by a naturally occurring elongation variant. bioRxiv. 2020 doi: 10.1101/2020.05.11.088179. (2020.05.11.088179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A. Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 2020;146(1):206–208. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jafarzadeh A., Nemati M. Therapeutic potentials of ginger for treatment of multiple sclerosis: a review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties. J. Neuroimmunol. 2018;324:54–75. doi: 10.1016/j.jneuroim.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Nemati M., Malla N., Yadav M., Khorramdelazad H., Jafarzadeh A. Humoral and T cell-mediated immune response against trichomoniasis. Parasite Immunol. 2018;40(3) doi: 10.1111/pim.12510. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Smith W., Hao D., He B., Kong L. M1 and M2 macrophage polarization and potentially therapeutic naturally occurring compounds. Int. Immunopharmacol. 2019;70:459–466. doi: 10.1016/j.intimp.2019.02.050. [DOI] [PubMed] [Google Scholar]
- 44.Sang Y., Miller L.C., Blecha F. Macrophage polarization in virus-host interactions. Journal of clinical & cellular immunology. 2015;6(2) doi: 10.4172/2155-9899.1000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drareni K., Gautier J.F., Venteclef N., Alzaid F. Transcriptional control of macrophage polarisation in type 2 diabetes. Semin. Immunopathol. 2019;41(4):515–529. doi: 10.1007/s00281-019-00748-1. [DOI] [PubMed] [Google Scholar]
- 46.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 47.Shirey K.A., Lai W., Pletneva L.M., Karp C.L., Divanovic S., Blanco J.C., Vogel S.N. Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal Immunol. 2014;7(3):549–557. doi: 10.1038/mi.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas J.M., Avia M., Martin V., Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017;2017 doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D., Guo R., Lei L., Liu H., Wang Y., Wang Y., Dai T., Zhang T., Lai Y., Wang J., Liu Z., He A., O’Dwyer M., Hu J. COVID-19 infection induces readily detectable morphological and inflammation-related phenotypic changes in peripheral blood monocytes, the severity of which correlate with patient outcome. medRxiv. 2020 doi: 10.1101/2020.03.24.20042655. (2020.03.24.20042655) [DOI] [Google Scholar]
- 50.Yilla M., Harcourt B.H., Hickman C.J., McGrew M., Tamin A., Goldsmith C.S., Bellini W.J., Anderson L.J. SARS-coronavirus replication in human peripheral monocytes/macrophages. Virus Res. 2005;107(1):93–101. doi: 10.1016/j.virusres.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 53.Jeffers S.A., Tusell S.M., Gillim-Ross L., Hemmila E.M., Achenbach J.E., Babcock G.J., Thomas W.D., Jr., Thackray L.B., Young M.D., Mason R.J., Ambrosino D.M., Wentworth D.E., Demartini J.C., Holmes K.V. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(44):15748–15753. doi: 10.1073/pnas.0403812101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mesel-Lemoine M., Millet J., Vidalain P.O., Law H., Vabret A., Lorin V., Escriou N., Albert M.L., Nal B., Tangy F. A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes. J. Virol. 2012;86(14):7577–7587. doi: 10.1128/JVI.00269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau S.K.P., Lau C.C.Y., Chan K.H., Li C.P.Y., Chen H., Jin D.Y., Chan J.F.W., Woo P.C.Y., Yuen K.Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. The Journal of general virology. 2013;94(Pt 12):2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 57.Tynell J., Westenius V., Ronkko E., Munster V.J., Melen K., Osterlund P., Julkunen I. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. The Journal of general virology. 2016;97(2):344–355. doi: 10.1099/jgv.0.000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J., Chu H., Li C., Wong B.H., Cheng Z.S., Poon V.K., Sun T., Lau C.C., Wong K.K., Chan J.Y., Chan J.F., To K.K., Chan K.H., Zheng B.J., Yuen K.Y. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clinical immunology (Orlando, Fla.) 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y., Li Q.R., Huang X., Cui Y., Li X.Z., Tang W., Pan P.F., Huang X.Q., Ding Y.Q., Bian X.W. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi = Chinese journal of pathology. 2020;49(0):E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 61.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P., Gong L., Zhang Y., Cui H.-Y., Geng J.-J., Wang B., Sun X.-X., Wang C.-F., Yang X., Lin P., Deng Y.-Q., Wei D., Yang X.-M., Zhu Y.-M., Zhang K., Zheng Z.-H., Miao J.-L., Guo T., Shi Y., Zhang J., Fu L., Wang Q.-Y., Bian H., Zhu P., Chen Z.-N. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. (2020.03.14.988345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jafarzadeh A., Bagheri-Jamebozorgi M., Nemati M., Golsaz-Shirazi F., Shokri F. Human leukocyte antigens influence the antibody response to hepatitis B vaccine. Iranian journal of allergy, asthma, and immunology. 2015;14(3):233–245. [PubMed] [Google Scholar]
- 64.Kadkhoda K. COVID-19: an immunopathological view. mSphere. 2020;5(2) doi: 10.1128/mSphere.00344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss R.C., Scott F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 1981;4(2):175–189. doi: 10.1016/0147-9571(81)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hohdatsu T., Yamada M., Tominaga R., Makino K., Kida K., Koyama H. Antibody-dependent enhancement of feline infectious peritonitis virus infection in feline alveolar macrophages and human monocyte cell line U937 by serum of cats experimentally or naturally infected with feline coronavirus. J. Vet. Med. Sci. 1998;60(1):49–55. doi: 10.1292/jvms.60.49. [DOI] [PubMed] [Google Scholar]
- 67.Jaume M., Yip M.S., Cheung C.Y., Leung H.L., Li P.H., Kien F., Dutry I., Callendret B., Escriou N., Altmeyer R., Nal B., Daeron M., Bruzzone R., Peiris J.S. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcgammaR pathway. J. Virol. 2011;85(20):10582–10597. doi: 10.1128/JVI.00671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yip M.S., Leung N.H., Cheung C.Y., Li P.H., Lee H.H., Daeron M., Peiris J.S., Bruzzone R., Jaume M. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol. J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu Y., Cheng Y., Wu Y. 2020. Understanding SARS-CoV-2-mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., Wu T., Cheung K.W., Chan K.H., Alvarez X., Qin C., Lackner A., Perlman S., Yuen K.Y., Chen Z. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI insight. 2019;4(4) doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tseng C.T., Sbrana E., Iwata-Yoshikawa N., Newman P.C., Garron T., Atmar R.L., Peters C.J., Couch R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J. Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Agrawal A.S., Tao X., Algaissi A., Garron T., Narayanan K., Peng B.H., Couch R.B., Tseng C.T. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Human vaccines & immunotherapeutics. 2016;12(9):2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet (London, England) 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L., Zhang F., Yu W., He T., Yu J., Yi C.E., Ba L., Li W., Farzan M., Chen Z., Yuen K.Y., Ho D. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J. Med. Virol. 2006;78(1):1–8. doi: 10.1002/jmv.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziegler T., Matikainen S., Ronkko E., Osterlund P., Sillanpaa M., Siren J., Fagerlund R., Immonen M., Melen K., Julkunen I. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J. Virol. 2005;79(21):13800–13805. doi: 10.1128/JVI.79.21.13800-13805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu W., Yen Y.T., Singh S., Kao C.L., Wu-Hsieh B.A. SARS-CoV regulates immune function-related gene expression in human monocytic cells. Viral Immunol. 2012;25(4):277–288. doi: 10.1089/vim.2011.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142(1–2):19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., Kong L., Fang X., Zheng H., Wu Z., She Y. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128(1–2):1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Page C., Goicochea L., Matthews K., Zhang Y., Klover P., Holtzman M.J., Hennighausen L., Frieman M. Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection. J. Virol. 2012;86(24):13334–13349. doi: 10.1128/JVI.01689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Broxmeyer H.E., Sherry B., Cooper S., Lu L., Maze R., Beckmann M.P., Cerami A., Ralph P. Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. Interacting effects involving suppression, synergistic suppression, and blocking of suppression. Journal of immunology (Baltimore, Md.: 1950) 1993;150(8 Pt 1):3448–3458. [PubMed] [Google Scholar]
- 83.Sarris A.H., Broxmeyer H.E., Wirthmueller U., Karasavvas N., Cooper S., Lu L., Krueger J., Ravetch J.V. Human interferon-inducible protein 10: expression and purification of recombinant protein demonstrate inhibition of early human hematopoietic progenitors. J. Exp. Med. 1993;178(3):1127–1132. doi: 10.1084/jem.178.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choudhry H., Bakhrebah M.A., Abdulaal W.H., Zamzami M.A., Baothman O.A., Hassan M.A., Zeyadi M., Helmi N., Alzahrani F., Ali A., Zakaria M.K., Kamal M.A., Warsi M.K., Ahmed F., Rasool M., Jamal M.S. Middle East respiratory syndrome: pathogenesis and therapeutic developments. Futur. Virol. 2019;14(4):237–246. doi: 10.2217/fvl-2018-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chu H., Zhou J., Wong B.H., Li C., Cheng Z.S., Lin X., Poon V.K., Sun T., Lau C.C., Chan J.F., To K.K., Chan K.H., Lu L., Zheng B.J., Yuen K.Y. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014;454-455:197–205. doi: 10.1016/j.virol.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou J., Chu H., Chan J.F., Yuen K.Y. Middle East respiratory syndrome coronavirus infection: virus-host cell interactions and implications on pathogenesis. Virol. J. 2015;12:218. doi: 10.1186/s12985-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheuplein V.A., Seifried J., Malczyk A.H., Miller L., Hocker L., Vergara-Alert J., Dolnik O., Zielecki F., Becker B., Spreitzer I., Konig R., Becker S., Waibler Z., Muhlebach M.D. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. J. Virol. 2015;89(7):3859–3869. doi: 10.1128/JVI.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munster V.J., de Wit E., Feldmann H. Pneumonia from human coronavirus in a macaque model. N. Engl. J. Med. 2013;368(16):1560–1562. doi: 10.1056/NEJMc1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drosten C., Seilmaier M., Corman V.M., Hartmann W., Scheible G., Sack S., Guggemos W., Kallies R., Muth D., Junglen S., Muller M.A., Haas W., Guberina H., Rohnisch T., Schmid-Wendtner M., Aldabbagh S., Dittmer U., Gold H., Graf P., Bonin F., Rambaut A., Wendtner C.M. Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect. Dis. 2013;13(9):745–751. doi: 10.1016/S1473-3099(13)70154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inandiklioglu N., Akkoc T. Immune responses to SARS-CoV, MERS-CoV and SARS-CoV-2. Adv. Exp. Med. Biol. 2020 doi: 10.1007/5584_2020_549. [DOI] [PubMed] [Google Scholar]
- 92.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y., Sun R., Tian Z., Xu X., Wei H. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020 doi: 10.1101/2020.02.12.945576. (2020.02.12.945576) [DOI] [Google Scholar]
- 94.Zhou Y., Fu B., Zheng X., Wang D., Zhao C., qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020 doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen I.Y., Moriyama M., Chang M.F., Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 98.Banu N., Panikar S.S., Leal L.R., Leal A.R. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to Macrophage Activation Syndrome: therapeutic implications. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan T., Xiao R., Lin G. Angiotensin-converting enzyme 2 in severe acute respiratory syndrome coronavirus and SARS-CoV-2: a double-edged sword? FASEB J. 2020;34(5):6017–6026. doi: 10.1096/fj.202000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pedersen K.B., Chodavarapu H., Porretta C., Robinson L.K., Lazartigues E. Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology. 2015;156(12):4411–4425. doi: 10.1210/en.2015-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., Hooper N.M., Turner A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280(34):30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93(5):543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nemati M., Larussa T., Khorramdelazad H., Mahmoodi M., Jafarzadeh A. Toll-like receptor 2: an important immunomodulatory molecule during Helicobacter pylori infection. Life Sci. 2017;178:17–29. doi: 10.1016/j.lfs.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 105.Deng J., Yu X.Q., Wang P.H. Inflammasome activation and Th17 responses. Mol. Immunol. 2019;107:142–164. doi: 10.1016/j.molimm.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 106.Jafarzadeh A., Nemati M., Jafarzadeh S. The important role played by chemokines influence the clinical outcome of Helicobacter pylori infection. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.116688. [DOI] [PubMed] [Google Scholar]
- 107.Rana A.K., Li Y., Dang Q., Yang F. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018;65:348–359. doi: 10.1016/j.intimp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 108.Li X., Yao W., Yuan Y., Chen P., Li B., Li J., Chu R., Song H., Xie D., Jiang X., Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66(1):157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 109.Ardura J.A., Rackov G., Izquierdo E., Alonso V., Gortazar A.R., Escribese M.M. Targeting macrophages: friends or foes in disease? Front. Pharmacol. 2019;10:1255. doi: 10.3389/fphar.2019.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R., Kotenko S.V., Lazear H.M., O’Brien T.R., Odendall C., Onabajo O.O., Piontkivska H., Santer D.M., Reich N.C., Wack A., Zanoni I. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 2020;217(5) doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.He W., Kapate N., Shields C.W.t., Mitragotri S. Drug delivery to macrophages: a review of targeting drugs and drug carriers to macrophages for inflammatory diseases. Adv. Drug Deliv. Rev. 2019 doi: 10.1016/j.addr.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 112.Ortega R.A., Barham W., Sharman K., Tikhomirov O., Giorgio T.D., Yull F.E. Manipulating the NF-kappaB pathway in macrophages using mannosylated, siRNA-delivering nanoparticles can induce immunostimulatory and tumor cytotoxic functions. Int. J. Nanomedicine. 2016;11:2163–2177. doi: 10.2147/IJN.S93483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I., Jones T., Jucknischke U., Scheiblich S., Kaluza K., Gorr I.H., Walz A., Abiraj K., Cassier P.A., Sica A., Gomez-Roca C., de Visser K.E., Italiano A., Le Tourneau C., Delord J.P., Levitsky H., Blay J.Y., Ruttinger D. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 114.Papadopoulos K.P., Gluck L., Martin L.P., Olszanski A.J., Tolcher A.W., Ngarmchamnanrith G., Rasmussen E., Amore B.M., Nagorsen D., Hill J.S., Stephenson J., Jr. First-in-human study of AMG 820, a monoclonal anti-colony-stimulating factor 1 receptor antibody, in patients with advanced solid tumors. Clin. Cancer Res. 2017;23(19):5703–5710. doi: 10.1158/1078-0432.CCR-16-3261. [DOI] [PubMed] [Google Scholar]
- 115.Ponzoni M., Pastorino F., Di Paolo D., Perri P., Brignole C. Targeting macrophages as a potential therapeutic intervention: impact on inflammatory diseases and cancer. Int. J. Mol. Sci. 2018;19(7) doi: 10.3390/ijms19071953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ciliberto G., Mancini R., Paggi M.G. Drug repurposing against COVID-19: focus on anticancer agents. J. Exp. Clin. Cancer Res. 2020;39(1):86. doi: 10.1186/s13046-020-01590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]