Abstract
Pulse wave analysis enables stroke volume to be estimated from an arterial blood pressure waveform. Multi-beat analysis is a novel pulse wave analysis method. We aimed to investigate cardiac output (CO) estimations using multi-beat analysis of the radial arterial blood pressure waveform in patients undergoing off-pump coronary artery bypass surgery (OPCAB) using intermittent pulmonary artery thermodilution (PATD) as the reference method. This was a prospective clinical method comparison study. In 58 patients, we measured CO using PATD (PATD-CO; reference method) and simultaneously recorded the radial arterial blood pressure waveform that we used for off-line estimation of CO based on multi-beat analysis (MBA-CO; test method) using the Argos CO monitor (Retia Medical; Valhalla, NY, USA). The final analysis was performed using 572 paired CO measurements. We performed Bland–Altman analysis accounting for multiple observations per patient. To describe the ability of the test method to track changes in CO over time we computed four-quadrant plots using a central exclusion zone of 15% and calculated the concordance rate. Mean PATD-CO was 4.13 ± 1.26 L/min and mean MBA-CO was 4.31 ± 1.25 L/min. The mean of the differences between PATD-CO and MBA-CO was − 0.20 L/min with a standard deviation of ± 1.14 L/min and 95% limits of agreement of − 2.48 to + 2.08 L/min. The concordance rate for CO changes between PATD-CO and MBA-CO was 89%. CO estimations using multi-beat analysis (Argos monitor) show reasonable agreement and trending ability compared with PATD-CO as the reference method in adult patients during OPCAB.
Keywords: Pulse wave analysis, Pulse contour analysis, Pulmonary artery catheter, Swan-Ganz catheter, Hemodynamic monitoring, Hemodynamics, Cardiovascular dynamics, Anesthesia
Introduction
Cardiac output (CO) monitoring is recommended in high-risk surgical and critically ill patients [1–3]. Various methods for CO estimation with different physical measurement principles and degrees of invasiveness are available [4, 5]. Uncalibrated pulse wave analysis, the estimation of stroke volume based on the analysis of the arterial blood pressure waveform, constitutes a minimally invasive method and enables CO to be estimated continuously [3, 6–8].
One method to mathematically analyze the radial arterial blood pressure waveform to estimate stroke volume and CO is the so-called multi-beat analysis (MBA) [9, 10]. This method assumes a Windkessel model of the cardiovascular system. When estimating the Windkessel model parameters it attenuates the confounding effects of peripheral pulse wave reflections due to changes in the peripheral vascular resistance. The method works by reverse-engineering the arterial tree impulse response by analyzing the arterial blood pressure waveform over time scales greater than a cardiac cycle (i.e., over multiple heart beats) in order to extract a waveform that would be present in the absence of high frequency wave reflections [9, 10]. An arterial compliance scale factor is then applied according to a proprietary formula that includes the measured blood pressure and patient age, height, weight, and sex. Thus, CO is estimated over several cardiac cycles and stroke volume is calculated knowing the heart rate. No external calibration is needed.
Measurements of CO using multi-beat analysis have been prospectively compared with aortic flow probe-derived CO measurements in swine [10] and retrospectively with thermodilution CO measurements in hemodynamically stable intensive care unit patients (using arterial blood pressure waveforms from the freely accessible Multiparameter Intelligent Monitoring in Intensive Care (MIMIC II) database) [9, 11, 12]. Just recently, a commercially available monitor using MBA for CO estimation from a radial arterial blood pressure signal in adults—the Argos CO monitor (Retia Medical; Valhalla, NY, USA)—became available and was cleared by the US Food and Drug Administration (FDA). The device estimates and displays CO every 5 s using multi-beat analysis over 20 s or 5 min.
To the best of our knowledge, there are no clinical data on CO measurements using multi-beat analysis in patients having cardiac surgery who often show rapid changes in cardiovascular dynamics. We, therefore, investigated CO estimations using multi-beat analysis of the radial arterial blood pressure waveform in a clinical method comparison study in patients having off-pump coronary artery bypass surgery (OPCAB) using intermittent pulmonary artery thermodilution (PATD) as the reference method.
Methods
Study design and setting
The study was reviewed and approved by the local ethics committee (Ethikkommission der Ärztekammer Hamburg, Hamburg, Germany) and all patients gave written informed consent to participate in the study. For this clinical method comparison study we prospectively included patients scheduled for OPCAB in the University Heart Center Hamburg of the University Medical Center Hamburg-Eppendorf (Hamburg, Germany). The study was performed between May 2016 and January 2017. During surgery, we performed intermittent PATD measurements to assess reference CO values and simultaneously recorded the radial arterial blood pressure waveform that we used for off-line estimation of CO by MBA using the Argos CO monitor (test method). All patients were mechanically ventilated in a pressure-controlled mode with a tidal volume of 6–8 mL/kg predicted bodyweight to maintain normocapnia.
Inclusion and exclusion criteria
Adult patients scheduled for OPCAB in whom continuous invasive arterial blood pressure monitoring using a radial arterial catheter and advanced hemodynamic monitoring using a pulmonary artery catheter were planned for clinical indications were eligible for study inclusion. Exclusion criteria were age < 18 years, atrial fibrillation or other high-grade cardiac arrhythmias or missing informed consent.
Study measurements
During surgery, we systematically performed two sets of study CO measurements at six different time points (resulting in a total of 12 study CO measurements). One study CO measurement consisted of intermittent PATD to assess PATD-CO (reference method) and simultaneous recording of the radial arterial blood pressure waveform that we later used to estimate CO using MBA (MBA-CO; test method). In some patients, we were not able to perform all six planned interventions because of a shorter than expected duration of surgery: We performed measurements around six interventions in 22 patients, five interventions in 20 patients, four interventions in 7 patients, three interventions in 8 patients, and two interventions in 1 patient. The time points for study CO measurements were not pre-defined but determined by clinical interventions expected to markedly change the patient’s cardiovascular dynamics (e.g., administration of fluid and vasopressive or inotropic medication). We performed one set of study CO measurements prior to the clinical intervention and one shortly after the intervention.
Intermittent PATD was performed via an ARROW pulmonary artery catheter (Teleflex Medical Europe; Co Westmeath, Ireland). For PATD-CO measurements we performed four consecutive injections of the thermal indicator (10 mL ice-cold sodium chloride) randomly over the respiratory cycle and averaged the CO values resulting in an averaged PATD-CO.
The Argos CO monitor uses multi-beat analysis of the arterial blood pressure waveform to estimate CO and was the test method in this study. During PATD-CO measurements, we simultaneously recorded the arterial blood pressure waveform at a sampling rate of 100 Hz from the radial arterial catheter using a personal computer with dedicated data acquisition software (Dräger Data Grabber; Dräger, Lübeck, Germany). Since a good quality of the arterial blood pressure waveform is a prerequisite for pulse wave analysis we carefully leveled the pressure transducers to the height of the right atrium and zeroed the system to atmospheric pressure. In addition, we confirmed adequate damping properties of the tubing/transducer-system by square wave tests. In an off-line analysis, these blood pressure waveform segments (starting 20 s before the first and stopping 20 s after the last PATD measurement) were retrospectively fed into the Argos CO monitor along with patient age, sex, height, and weight. The Argos CO monitor accepts an analog arterial blood pressure waveform as an input (scaled to 100 mmHg/V). The recorded BP waveform was fed into the Argos CO monitor via a 3.5 mm stereo cable, using an MP150 digital-to-analog converter (BIOPAC System, Inc., Goleta, CA, USA). In this study, we used the Argos CO monitor in the 20-s mode.
Data processing
We performed a total of 665 study CO measurements in 60 patients (convenience sampling according to availability of investigators). We excluded 47 study CO measurements due to the unavailability of BP waveform segments matching the corresponding PATD-CO measurements. We further excluded one segment due to unstable PATD-CO measurements. We further excluded five segments due to instability and two segments due to artifact in the BP waveform. We were interested in paired measurements (before and after interventions) and therefore excluded 38 unpaired data segments. After this process of data exclusion, in 2 patients, no study CO measurement data remained. Final analyses were performed on the remaining 572 paired study CO measurements from 58 patients.
Statistical analysis
For statistical analyses we used R version 3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria). Demographic, biometric, and clinical data are presented as absolute and relative frequencies (percentages) or mean ± standard deviation (SD). The mean CO values ± SD were separately calculated for PATD-CO and MBA-CO.
The correlation between PATD-CO and MBA-CO was computed within subjects taking repeated observations into account and removing the between subject variability (as required for method comparison studies) [13].
To assess the agreement between PATD-CO and MBA-CO we performed Bland–Altman analysis accounting for multiple observations per patient [14] and calculated the mean and SD of the differences, and the 95% limits of agreement (= mean difference ± 1.96 × SD of the difference), and the percentage error [15]. To describe the ability of the multi-beat analysis method to track changes in CO over time we computed four-quadrant plots showing relative CO changes using a central exclusion zone of 15% and calculated the concordance rate [16, 17].
Results
The demographic, biometric, and clinical data of the patients are shown in Table 1. Mean PATD-CO was 4.13 ± 1.26 L/min and mean MBA-CO was 4.31 ± 1.25 L/min. In Fig. 1, the relation and within-subject correlation of PATD-CO and of MBA-CO are shown. The mean of the differences between PATD-CO and MBA-CO was − 0.20 L/min with a SD of ± 1.14 L/min and 95% limits of agreement of − 2.48 to + 2.08 L/min (Fig. 2). The percentage error was 50.8%. The ability of MBA-CO to track changes in PATD-CO is illustrated in Fig. 3. The concordance rate for CO changes between PATD-CO and MBA-CO was 89%.
Table 1.
Baseline patient characteristics
| Number of included patients, n | 58 |
| Age (years) | 70 ± 10 |
| Sex, female [n (%)] | 10 (17) |
| Height (m) | 1.74 ± 0.09 |
| Weight (kg) | 81 ± 15 |
| BMI (kg m−2) | 26.8 ± 4.2 |
Data are displayed as absolute and relative frequencies or mean and standard deviation
Fig. 1.
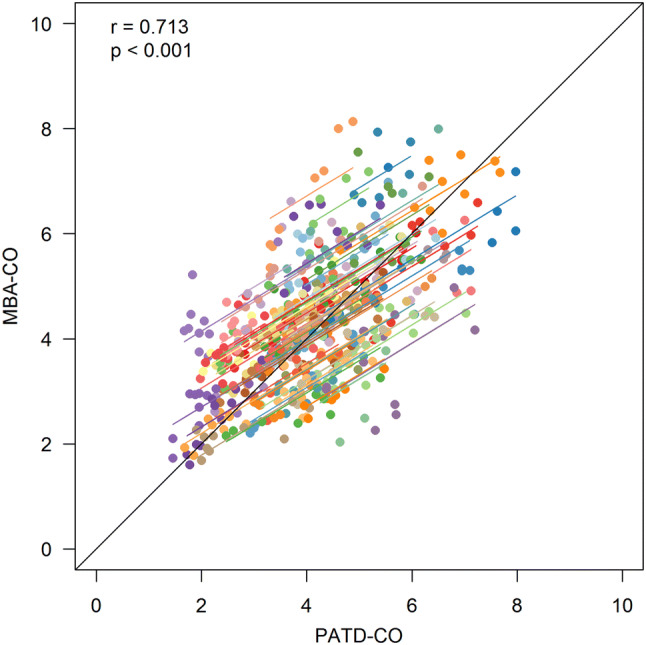
Scatter plot illustrating the relation of cardiac output estimations obtained with multi-beat analysis (MBA-CO; test method) and cardiac output measurements with pulmonary artery thermodilution (PATD-CO; reference method). The within-subject correlation coefficient (r) is presented along a respective illustration of the intra-individual linear trend. Patients are distinguished by color
Fig. 2.
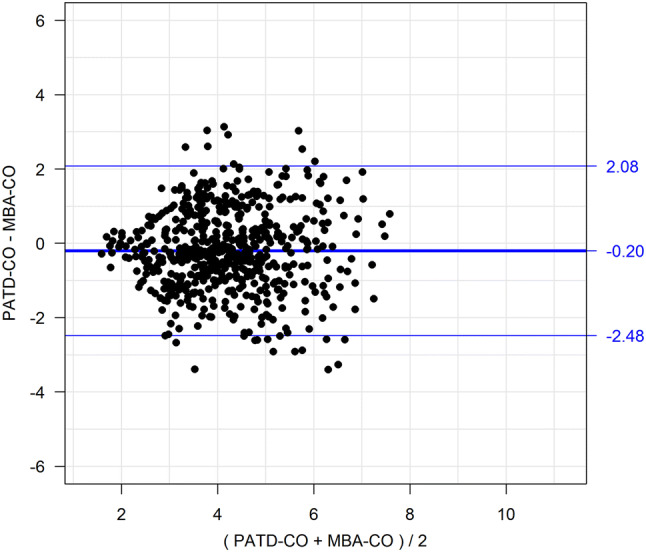
Bland–Altman plot showing the agreement between cardiac output estimations obtained with multi-beat analysis (MBA-CO; test method) and cardiac output measurements with pulmonary artery thermodilution (PATD-CO; reference method). The mean of the differences between MBA-CO and PATD-CO (bold horizontal line) and the upper and lower 95% limits of agreement (thin horizontal lines) are shown
Fig. 3.
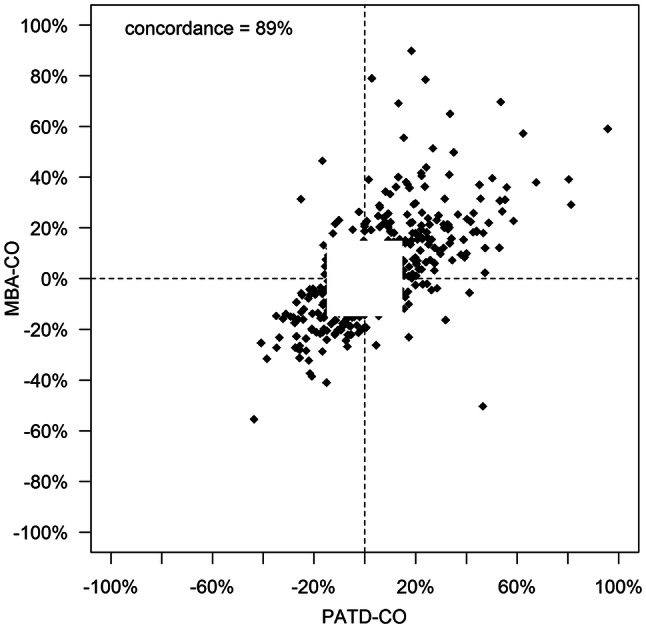
The capability of the multi-beat analysis method to track relative changes in cardiac output is shown in a four-quadrant plot with an exclusion zone of 15%. MBA-CO, cardiac output estimations obtained with multi-beat analysis (test method); PATD-CO, cardiac output measurements with pulmonary artery thermodilution (reference method)
Discussion
This was a method comparison study investigating CO estimations using multi-beat analysis of the radial arterial blood pressure waveform (Argos monitor) in patients having OPCAB using intermittent PATD as the reference method. CO estimations using multi-beat analysis showed reasonable agreement and trending ability compared with PATD-CO.
The arterial system is a branching tree of elastic arteries; therefore, the mathematical analysis of the arterial blood pressure waveform is complex because various cardiovascular factors—including the contractility of the heart, vascular compliance, aortic impedance, and peripheral vascular resistance—influence blood flow wave propagation and reflection and thus the shape of the waveform [18]. Different mathematical models have been proposed to describe the blood flow through the arterial system [18, 19]. “Windkessel models” explain the shape of the arterial blood pressure waveform by the interactions between the blood flow (stroke volume) and the arterial compliance (i.e., the elastic properties and the buffering capacity of the aorta and the large arteries) and the peripheral resistance generated by the small arteries and arterioles [6, 18–21]. The addition of other parameters, the characteristic aortic impedance [22] and the inertia of the blood [23, 24], results in the more complex three-element and four-element Windkessel models [18, 19, 22].
The analysis of the arterial blood pressure waveform, referred to as pulse wave analysis, can be used to continuously estimate stroke volume and CO [3, 6–8]; various different methods and mathematical algorithms have been proposed for pulse wave analysis [7, 25, 26]. Assuming a linear relationship between pulse pressure and stroke volume and deriving arterial compliance from biometric factors and waveform characteristics, pulse pressure can be used as a surrogate for stroke volume [6, 26]. Other methods use the systolic part of the area under the arterial blood pressure curve to estimate stroke volume or perform a so-called “pulse power analysis” by converting the arterial blood pressure waveform into a sine wave and analyzing it using the root mean square method [6, 26]. However, because of wave phenomena and transmission effects within the arterial tree the shape of an individual arterial blood pressure waveform is not solely a function of stroke volume, but a complex function of cardiac ejection, wave propagation, and wave reflections.
The MBA method investigated in our study analyzes the waveform over longer time scales that include multiple heart beats, estimates the contribution of the different heart beats within this sequence, attenuates the confounding effects of complex wave reflections, and estimates a theoretical arterial blood pressure waveform that would be generated by one single isolated heart beat [9, 10, 27]. The analysis of this theoretical arterial blood pressure waveform enables peripheral vascular resistance to be estimated (assuming that arterial compliance is constant) and CO to be calculated using mean arterial pressure and peripheral vascular resistance [9, 10, 27].
In our study, CO estimations using multi-beat analysis (Argos monitor) showed reasonable agreement and trending ability compared with PATD-CO as the reference method in adult patients during OPCAB. Pulse wave analysis might especially be useful to indicate changes in CO. We revealed a concordance rate between changes in PATD-CO and changes in MBA-CO of 89%. In comparison to our study, the concordance rate was lower in two recent studies investigating the trending capabilities of an established pulse wave analysis algorithm (FloTrac; Edwards Lifesciences, Irvine; CA, USA) compared with PATD in patients having cardiac surgery using cardiopulmonary bypass [28, 29].
We solely included patients having OPCAB. We chose this patient collective for several reasons. First, patients having OPCAB in our institution are routinely monitored with a radial arterial catheter and a pulmonary artery catheter. This allows performing studies on pulse wave analysis in comparison with the clinical gold standard method (i.e., intermittent PATD) [4, 30]. Further, patients having OPCAB are highly complex with regard to alterations and changes in cardiovascular dynamics. Rapid changes in the hemodynamic status of the patients during OPCAB made it possible to assess the ability of multi-beat analysis to track rapid changes in CO. However, on the other hand, marked alterations and rapid changes in vasomotor tone are a well-described limitation of pulse wave analysis methods and may have influenced the measurement performance of multi-beat analysis in our study [6–8]. In addition, patients with coronary artery disease may have altered arterial compliance. Therefore, our results should not indiscriminately be transferred to other groups of surgical or critically ill patients, especially patients who are hemodynamically more stable. In addition, this was a single center study performed in a tertiary care medical university center. Therefore, our results might also not be generalizable to other clinical settings. Further, the blood pressure waveform segments were retrospectively fed into the Argos CO monitor for CO estimation (off-line analysis). Thus, further studies using the Argos CO monitor at the bedside in less complex patients and other clinical settings are needed.
Conclusions
CO estimations using multi-beat analysis (Argos monitor) show reasonable agreement and trending ability compared with PATD-CO as the reference method in adult patients during OPCAB.
Acknowledgements
We thank Ricardo Toledo-Crow, PhD (Advanced Science Research Center, City University of New York, New York, NY, USA) for his support with off-line analysis, i.e., feeding arterial blood pressure waveforms into the Argos CO monitor (Retia Medical) for CO estimation.
Funding
The study was funded with an institutional restricted research grant by Retia Medical (Valhalla, NY, USA).
Compliance with ethical standards
Conflicts of interest
BS has received institutional restricted research grants from Retia Medical (Valhalla, NY, USA). BS collaborates with Pulsion Medical Systems (Feldkirchen, Germany) as a member of the medical advisory board and has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from Pulsion Medical Systems. BS has received research support and honoraria for giving lectures from Edwards Lifesciences (Irvine, CA, USA). BS has received institutional restricted research grants, honoraria for giving lectures, and refunds of travel expenses from CNSystems Medizintechnik (Graz, Austria). BS has received institutional restricted research grants, honoraria for consulting, and refunds of travel expenses from Tensys Medical (San Diego, CA, USA). BS has received honoraria for giving lectures from Philips Medizin Systeme Böblingen (Böblingen, Germany). SR received honoraria for giving lectures from Baxter (Rome, Italy), BBraun (Melsungen, Germany), Orion Pharma (Espoo, Finland), Medigas (Assago, Italy), and Vygon (Écouen, France). SR has received honoraria for consulting from Vygon and Medtronic (Minneapolis, MN, USA). SR has received institutional restricted research grants from Pall International (Milano, Italy) and Baxter (Rome, Italy). GG has received travel expenses from Vygon. For all other authors there is no conflict of interest to declare.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals
This method comparison study was reviewed and approved by the ethics committee (Ethikkomission der Ärztekammer Hamburg, Hamburg, Germany). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bernd Saugel and Julia Heeschen contributed equally to the study.
References
- 1.Vincent JL, Pelosi P, Pearse R, Payen D, Perel A, Hoeft A, Romagnoli S, Ranieri VM, Ichai C, Forget P, Della Rocca G, Rhodes A. Perioperative cardiovascular monitoring of high-risk patients: a consensus of 12. Crit Care. 2015;19:224. doi: 10.1186/s13054-015-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Rhodes A, Perel A, Martin GS, Della Rocca G, Vallet B, Pinsky MR, Hofer CK, Teboul JL, de Boode WP, Scolletta S, Vieillard-Baron A, De Backer D, Walley KR, Maggiorini M, Singer M. Clinical review: update on hemodynamic monitoring-a consensus of 16. Crit Care. 2011;15:229. doi: 10.1186/cc10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saugel B, Vincent JL. Cardiac output monitoring: how to choose the optimal method for the individual patient. Curr Opin Crit Care. 2018;24:165–172. doi: 10.1097/mcc.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 4.De Backer D, Bakker J, Cecconi M, Hajjar L, Liu DW, Lobo S, Monnet X, Morelli A, Myatra SN, Perel A, Pinsky MR, Saugel B, Teboul JL, Vieillard-Baron A, Vincent JL. Alternatives to the Swan-Ganz catheter. Intensive Care Med. 2018;44:730–741. doi: 10.1007/s00134-018-5187-8. [DOI] [PubMed] [Google Scholar]
- 5.Teboul JL, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, Perel A, Pinsky MR, Reuter DA, Rhodes A, Squara P, Vincent JL, Scheeren TW. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42:1350–1359. doi: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 6.Thiele RH, Durieux ME. Arterial waveform analysis for the anesthesiologist: past, present, and future concepts. Anesth Analg. 2011;113:766–776. doi: 10.1213/ANE.0b013e31822773ec. [DOI] [PubMed] [Google Scholar]
- 7.Esper SA, Pinsky MR. Arterial waveform analysis. Best Pract Res Clin Anaesthesiol. 2014;28(4):363–380. doi: 10.1016/j.bpa.2014.08.0027. [DOI] [PubMed] [Google Scholar]
- 8.Jozwiak M, Monnet X, Teboul JL. Pressure waveform analysis. Anesth Analg. 2017;126(6):1930–1933. doi: 10.1213/ane.0000000000002527. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z, Mukkamala R. Continuous cardiac output monitoring in humans by invasive and noninvasive peripheral blood pressure waveform analysis. J Appl Physiol. 2006;101(2):598–608. doi: 10.1152/japplphysiol.01488.2005. [DOI] [PubMed] [Google Scholar]
- 10.Mukkamala R, Reisner AT, Hojman HM, Mark RG, Cohen RJ. Continuous cardiac output monitoring by peripheral blood pressure waveform analysis. IEEE Trans Biomed Eng. 2006;53(3):459–467. doi: 10.1109/tbme.2005.869780. [DOI] [PubMed] [Google Scholar]
- 11.Mukkamala R, Kuiper J, Ahmad S, Lu Z. Cardiac output monitoring in intensive care patients by radial artery pressure waveform analysis. In: Proceedings of the 26th annual international conference of the IEEE EMBS, vol. 5, pp. 3712–3715; 2004. 10.1109/iembs.2004.1404042. [DOI] [PubMed]
- 12.Zhang G, Mukkamala R. Continuous and minimally invasive cardiac output monitoring by long time interval analysis of a radial arterial pressure waveform: assessment using a large, public intensive care unit patient database. Br J Anaesth. 2012;109(3):339–344. doi: 10.1093/bja/aes099. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–582. doi: 10.1080/105434007013294229. [DOI] [PubMed] [Google Scholar]
- 15.Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Cumput. 1999;15(2):85–91. doi: 10.1023/A:1009982611386. [DOI] [PubMed] [Google Scholar]
- 16.Saugel B, Grothe O, Wagner JY. Tracking changes in cardiac output: statistical considerations on the 4-quadrant plot and the polar plot methodology. Anesth Analg. 2015;121(2):514–524. doi: 10.1213/ane.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 17.Montenij LJ, Buhre WF, Jansen JR, Kruitwagen CL, de Waal EE. Methodology of method comparison studies evaluating the validity of cardiac output monitors: a stepwise approach and checklist. Br J Anaesth. 2016;116(6):750–758. doi: 10.1093/bja/aew094. [DOI] [PubMed] [Google Scholar]
- 18.Segers P, Rietzschel ER, De Buyzere ML, Stergiopulos N, Westerhof N, Van Bortel LM, Gillebert T, Verdonck PR. Three- and four-element Windkessel models: assessment of their fitting performance in a large cohort of healthy middle-aged individuals. Proc Inst Mech Eng Part H J Eng Med. 2008;222(4):417–428. doi: 10.1243/09544119jeim287. [DOI] [PubMed] [Google Scholar]
- 19.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 20.Frank O. The basic shape of the arterial pulse. First treatise: mathematical analysis. 1899. J Mol Cell Cardiol. 1990;22(3):255–277. doi: 10.1016/0022-2828(90)91460-O. [DOI] [PubMed] [Google Scholar]
- 21.Parker KH. A brief history of arterial wave mechanics. Med Biol Eng Comput. 2009;47(2):111–118. doi: 10.1007/s11517-009-0440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerhof N, Elzinga G, Sipkema P. An artificial arterial system for pumping hearts. J Appl Physiol. 1971;31(5):776–781. doi: 10.1152/jappl.1971.31.5.776. [DOI] [PubMed] [Google Scholar]
- 23.Burattini R, Gnudi G. Computer identification of models for the arterial tree input impedance: comparison between two new simple models and first experimental results. Med Biol Eng Comput. 1982;20(2):134–144. doi: 10.1007/BF02441348. [DOI] [PubMed] [Google Scholar]
- 24.Stergiopulos N, Westerhof BE, Westerhof N. Total arterial inertance as the fourth element of the windkessel model. Am J Physiol. 1999;276(1 Pt 2):H81–H88. doi: 10.1152/ajpheart.1999.276.1.H81. [DOI] [PubMed] [Google Scholar]
- 25.Sun JX, Reisner AT, Saeed M, Heldt T, Mark RG. The cardiac output from blood pressure algorithms trial. Crit Care Med. 2009;37(1):72–80. doi: 10.1097/CCM.0b013e3181930174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangkum L, Liu GL, Yu L, Yan H, Kaye AD, Liu H. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth. 2016;30(3):461–480. doi: 10.1007/s00540-016-2154-9. [DOI] [PubMed] [Google Scholar]
- 27.Reisner AT, Xu D, Ryan KL, Convertino VA, Rickards CA, Mukkamala R. Monitoring non-invasive cardiac output and stroke volume during experimental human hypovolaemia and resuscitation. Br J Anaesth. 2011;106(1):23–30. doi: 10.1093/bja/aeq295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SY, Chou AH, Tsai YF, Chang SW, Yang MW, Ting PC, Chen CY. Evaluation of the use of the fourth version FloTrac system in cardiac output measurement before and after cardiopulmonary bypass. J Clin Monit Comput. 2018;32(5):807–815. doi: 10.1007/s10877-017-0071-6. [DOI] [PubMed] [Google Scholar]
- 29.Kusaka Y, Ohchi F, Minami T. Evaluation of the fourth-generation FloTrac/Vigileo system in comparison with the intermittent bolus thermodilution method in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(4):953–960. doi: 10.1053/j.jvca.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev. 2013;2:CD003408. doi: 10.1002/14651858.CD003408.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]


