Abstract
Introduction
Diabetes can increase the risk of cardiovascular disease. This study aimed to explore the effect of (-)-epigallocatechin-3-gallate (EGCG) on high glucose (HG)-induced dysfunction and apoptosis of vascular endothelial cells.
Materials and Methods
The viability of human umbilical vein endothelial cells (HUVECs) treated with different concentrations and times of EGCG was detected by CCK-8 assay. The expression levels of ROS, NO and BH4 in HUVECs after treatment were detected by respective ELISA kits. The expression of p-eNOS, eNOS, NOX4, bcl2, bax, cleaved-caspase3, caspase3, p-PI3K, p-AKT, PI3K and AKT in HUVECs was detected by Western blot analysis. The apoptosis of HUVECs after treatment was analyzed by TUNEL assay.
Results
The viability of HUVECs was not obviously changed when treated with different concentrations and times of EGCG. The expression of ROS, NOX4 and eNOS (monomer) was increased, while the expression of NO, p-eNOS, eNOS, BH4 and eNOS (dimer) was decreased in HUVECs of HG group. EGCG could gradually reverse the effect of high glucose on HG-treated HUVECs from 10 μM to 50 μM. The apoptosis of HUVECs was also increased in HG group and EGCG decreased the apoptosis of HUVECs. PI3K/AKT signaling pathway was suppressed in HG-treated HUVECs while activated by EGCG treatment. When the PI3K/AKT signaling pathway was inhibited by LY294002 (AKT inhibitor), the protective effect of EGCG on HG-treated HUVECs was weakened.
Conclusion
EGCG could inhibit eNOS uncoupling and alleviate endothelial dysfunction and apoptosis of HG-treated HUVECs by activating the PI3K/AKT/eNOS pathway.
Keywords: (-)-epigallocatechin-3-gallate, eNOS uncoupling, endothelial dysfunction, apoptosis, human umbilical vein endothelial cells
Introduction
Diabetes, as a chronic metabolic disorder characterized by high blood sugar, poses a serious threat to human life and health. Due to chronic hyperglycemia, diabetes can lead to chronic damage to various tissues, especially the kidney, heart, blood vessels and other complications.1–3 Endothelial dysfunction is one of the common complications of type 1 diabetes. Patients’ insensitivity to insulin can accelerate atherosclerotic lesions and vascular dysfunction, thus increasing the risk of cardiovascular disease.4 Endothelial dysfunction associated with reduced nitric oxide (NO) bioavailability is an important initiator of cardiovascular events in diabetes mellitus.5,6 The decoupling reaction of endothelial nitric oxide synthase (eNOS) induced by hyperglycemia in the endothelial cells of diabetic patients reduces the effectiveness of eNOS, resulting in excessive peroxide anion (O2-), which affects endothelium-dependent relaxation. eNOS is responsible for producing NO. When eNOS is uncoupled, ROS is produced to replace the NO, resulting in endothelial dysfunction and endothelial cell apoptosis.7,8 Therefore, inhibition of eNOS uncoupling and ROS production, and promotion of NO can effectively alleviate endothelial dysfunction and apoptosis of vascular endothelial cells.
(-)-Epigallocatechin-3-gallate (EGCG), as the most effective component in green tea, is recognized as a strong antioxidant, which can effectively scavenge free radicals and reduce the formation of ROS by chelating metal ions to play the role in reducing the level of oxidative stress.9 EGCG has biological activities and pharmacological effects such as oxidation resistance, scavenging free radicals, anti-mutation and anti-tumor formation, particularly prominent role in reducing inflammation and oxidative stress.10,11 Study showed that EGCG could improve vascular re-endothelialization in diabetic rabbits by AKT/eNOS pathway.12 EGCG could inhibit the proliferation of hyperglycemia-induced vascular smooth muscle cells (VSMC) and reduce the inflammatory response of hyperglycemia-induced human umbilical vein endothelial cells (HUVECs).13 EGCG could effectively suppress the apoptosis of H2O2-induced HUVECs.14 However, its role in HG-induced endothelial dysfunction and apoptosis of HUVECs has not been investigated.
Therefore, the purpose of this study was to explore the effect of EGCG on endothelial dysfunction and apoptosis of HG-induced vascular endothelial cells and whether EGCG played its role by PI3K/AKT/eNOS pathway.
Materials and Methods
Cell Culture
HUVECs were bought from American Type Culture Collection (ATCC, USA). Cells were cultured in M199 containing 20% fetal bovine serum (FBS), 200 µg/mL penicillin, 200 µg/mL streptomycin and 2 mM L-glutamine at 37°C with 5% CO2.15
Cell Treatment
In the preliminary experiment, HUVECs were, respectively, treated with mannitol (25 mM) and high glucose (HG) (25 mM), and osmolarity change caused by 25 mM glucose was demonstrated to have no effect on cell biological activities. HUVECs under routine culture were used as the control group. HUVECs treated with 25 mM glucose were used as the HG group. HUVECs were treated with EGCG at 10 μM, 20 μM and 50 μM. HUVECs were treated with 50 μM EGCG for 6 h, 12 h, 24 h and 48 h. HUVECs were pre-treated with EGCG for 12 h, after which 25 mM glucose was added, and incubation continued for an additional 24 h. HUVECs were also pre-treated with 50 μM EGCG and 10 μM LY294002 (AKT inhibitor) for 12 h and then treated with 25 mM glucose for 24 h.
CCK-8 Assay
HUVECs in the logarithmic phase were seeded into a 96-well plate with a concentration of 5×104 cells/mL and 100 μL cell suspension was added to each well. After EGCG treatment at different concentrations and times, each well of 96-well plate was added with 10 μL CCK-8 solution, which was cultured for 2 h. The optical density value (OD value) was detected at 450 nm with an enzyme micro-plate reader.
Fluorometric ROS Assay
2, 7-dichlorodi-hydrofluoresceindiacetate (DCFH-DA) was diluted with serum-free medium according to the ratio of 1:1000~1:2000. After HUVECs were treated with EGCG and high glucose in a 96-well plate, cell culture medium was discarded and cells were washed with PBS for one time. An appropriate volume of DCFH-DA was added to each well, which could cover the cells adequately. The cells were incubated at 37°C for 20 min in dark and then washed with serum-free cell medium for 3 times to fully remove the DCFH-DA outside the cells. The results were observed by a fluorescence microscope.
ELISA Assay
The expression levels of ROS, NO and BH4 in HUVECs after treatment of EGCG and high glucose were detected by ROS ELISA kit, NO ELISA kit and BH4 ELISA kit following the instructions of kits strictly.
Western Blot Analysis
The RIPA lysate was used to extract the proteins, and the BCA kit was used to measure the protein concentration. The protein concentration was adjusted to be the same and proteins were prepared in a water bath at 100°C for 5 min. Twenty μg proteins were uploaded, separated at a constant pressure of 120 V for 70 min and transferred to PVDF membrane at a constant current of 200 mA for 30 min. Then, PVDF membrane was sealed at room temperature for 1 h, washed with TBST and incubated with p-eNOS (Ser1177) (140 kDa), eNOS (140 kDa), NOX4 (67 kDa), bcl2 (26 kDa), bax (21 kDa), cleaved-caspase3 (17 kDa, 19 kDa), caspase3 (35 kDa), p-PI3K (Tyr458) (85 kDa), p-AKT (Ser473) (60 kDa), PI3K (85 kDa) and AKT (60 kDa) overnight at 4°C. Washed by TBST, PVDF membrane was incubated with goat anti-rabbit IgG-HRP at room temperature for 1 h. After TBST washing, hypersensitive ECL exposure solution was added to the membrane, which was analyzed by gel imaging system.
TUNEL Assay
The experiment was conducted by TUNEL detection kit (Beijing ZhongShan Biotechnology Company) according to its instruction. The main steps were as follows: the sample slide was digested by protease K and then treated with TdT and Biotin-dUTP. Sealed by the sealing liquid, sample slide was orderly treated with streptavidin-HRP working liquid and DAB color reagent. The color was observed and counted under the light microscope.
Statistical Analysis
The experimental data were expressed by mean ± standard deviation (SD). All data were statistically analyzed using SPSS 22.0. One-way ANOVA was used for comparison between multiple groups, and SNK-q test was used for multiple comparisons between groups. P<0.05 was considered statistically different.
Results
The Viability of HUVECs Was Not Affected by the Different Concentrations and Times of EGCG
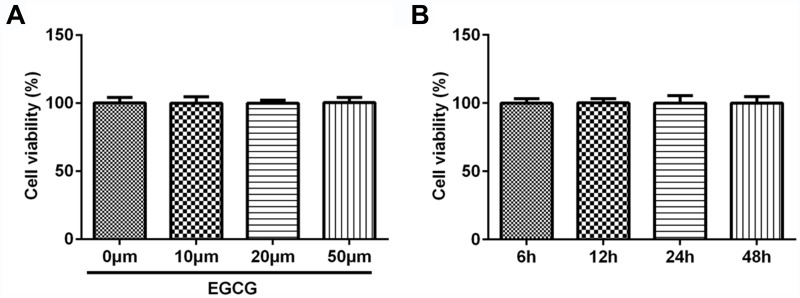
HUVECs were treated with EGCG at 10 μM, 20 μM and 50 μM and the result indicated that the viability of HUVECs was not obviously changed when treated with EGCG from 10 μM to 50 μM (Figure 1A). Therefore, in the subsequent experiment, we study the effect of 50 μM EGCG on the viability of HUVECs at different times. As shown in Figure 1B, the viability of HUVECs was not obviously changed when treated with 50 μM EGCG from 6 h to 48 h.
Figure 1.
The viability of HUVECs was not affected by the different concentrations and times of EGCG. (A) The cell viability of HUVECs treated with different concentrations of EGCG was detected by CCK-8 assay. (B) The cell viability of HUVECs treated with 50 μM EGCG at different times was detected by CCK-8 assay.
EGCG Alleviates Endothelial Dysfunction of Hyperglycemia-Induced Vascular Endothelial Cells
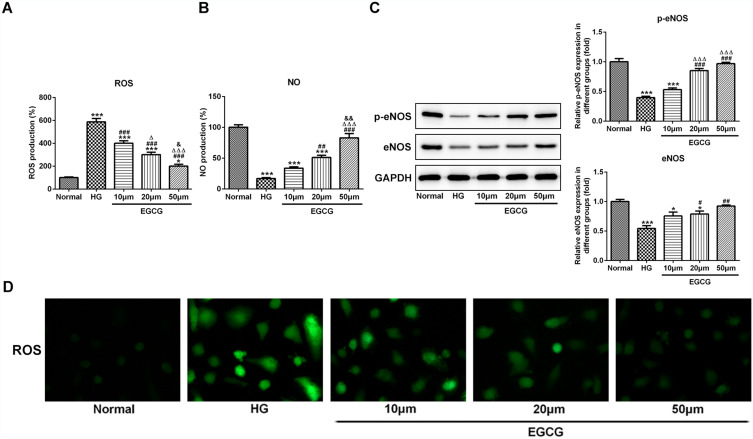
The expression level of ROS was increased in HG group and EGCG gradually decreased the expression level of ROS as the concentration changing from 10 μM to 50 μM (Figure 2A and D). The expression level of NO was increased in HG group and EGCG gradually increased the expression level of NO as the concentration changing from 10 μM to 50 μM (Figure 2B). The expression of p-eNOS and eNOS was decreased in HG group. With the concentration changing from 10 μM to 50 μM, the expression of p-eNOS and eNOS was gradually increased (Figure 2C).
Figure 2.
EGCG alleviates endothelial dysfunction of hyperglycemia-induced vascular endothelial cells. (A) The ROS levels in HG-treated HUVECs affected by EGCG was detected by ROS ELISA assay. *P<0.05 and ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆P<0.05 and ∆∆∆P<0.001 vs EGCG10μM group. &P<0.05 vs EGCG20μM group. (B) The NO levels in HG-treated HUVECs affected by EGCG was detected by ROS ELISA assay. ***P<0.001 vs Normal group. ##P<0.01 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG10μM group. &&P<0.01 vs EGCG20μM group. (C) The expression of p-eNOS and eNOS in HG-treated HUVECs affected by EGCG was detected by Western blot analysis. *P<0.05 and ***P<0.001 vs Normal group. #P<0.05, ##P<0.01 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG10μM group. (D) The ROS levels in HG-treated HUVECs affected by EGCG was detected by fluorometric ROS assay.
EGCG Increases the BH4 Expression and Inhibits the NOX4 Expression and eNOS Uncoupling
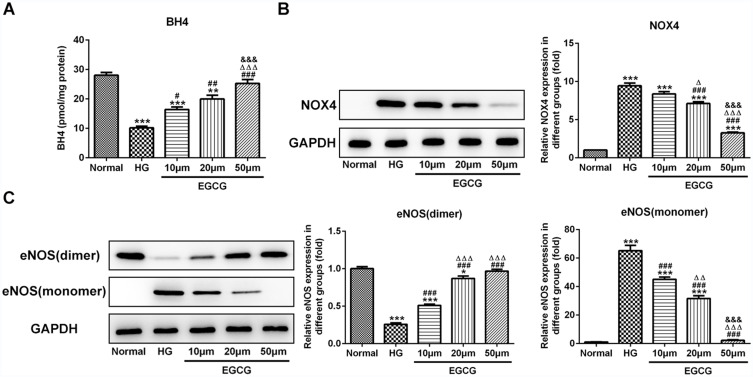
When HUVECs were treated with high glucose, BH4 expression was decreased. EGCG could improve the BH4 expression in HG-treated HUVECs from 10 μM to 50 μM (Figure 3A). As shown in Figure 3B, high glucose up-regulated the BH4 expression in HUVECs while down-regulated by EGCG from 10 μM to 50 μM. As shown in Figure 3C, high glucose decreased the eNOS (dimer) expression and increased the eNOS (monomer) expression while EGCG reversed the expression of eNOS (dimer) and eNOS (monomer) in HG-treated HUVECs from 10 μM to 50 μM.
Figure 3.
EGCG increases the BH4 expression and inhibits the NOX4 expression and eNOS uncoupling. (A) The BH4 levels in HG-treated HUVECs affected by EGCG was determined by BH4 ELISA assay. **P<0.01 and ***P<0.001 vs Normal group. #P<0.05, ##P<0.01 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG10μM group. &&&P<0.001 vs EGCG20μM group. (B) The NOX4 expression in HG-treated HUVECs affected by EGCG was detected by Western blot analysis. ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆P<0.05 and ∆∆∆P<0.001 vs EGCG10μM group. &&&P<0.001 vs EGCG20μM group. (C) The expression of eNOS (dimer) and eNOS (monomer) in HG-treated HUVECs affected by EGCG was detected by Western blot analysis. *P<0.05 and ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆∆P<0.01 and ∆∆∆P<0.001 vs EGCG10μM group. &&&P<0.001 vs EGCG20μM group.
EGCG Alleviates the Apoptosis of Hyperglycemia-Induced Vascular Endothelial Cells
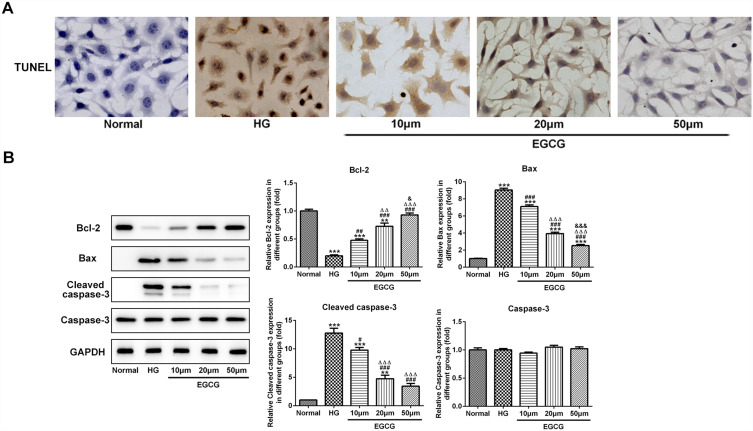
High glucose effectively increased the apoptosis of HUVECs, which was suppressed by EGCG from 10 μM to 50 μM (Figure 4A). As shown in Figure 4B, the bcl2 expression was decreased and expression of bax and cleaved-caspase3 was increased in HG-treated HUVECs while EGCG reversed the expression of bcl2, bax and cleaved-caspase3 in HG-treated HUVECs from 10 μM to 50 μM.
Figure 4.
EGCG alleviates the apoptosis of hyperglycemia-induced vascular endothelial cells. (A) The apoptosis of HG-treated HUVECs affected by EGCG was analyzed by TUNEL assay. (B) The expression of bcl2, bax, cleaved-caspase3 and caspase3 in HG-treated HUVECs affected by EGCG was detected by Western blot analysis. **P<0.01 and ***P<0.001 vs Normal group. #P<0.05, ##P<0.01 and ###P<0.001 vs HG group. ∆∆P<0.01 and ∆∆∆P<0.001 vs EGCG10μM group. &P<0.05 and &&&P<0.001 vs EGCG20μM group.
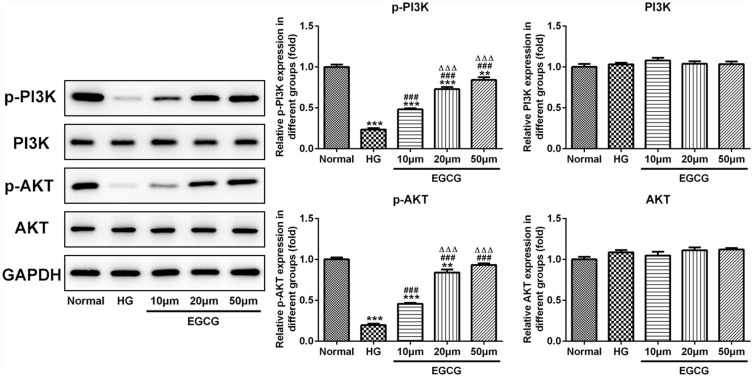
EGCG Activates the PI3K/AKT Pathway in Hyperglycemia-Induced Vascular Endothelial Cells
As shown in Figure 5, the expression of P-PI3K and P-AKT was decreased when HUVECs were treated with high glucose. Moreover, EGCG effectively increased the expression of P-PI3K and P-AKT in HG-treated HUVECs from 10 μM to 50 μM. The expression of PI3K and AKT in five groups were not significantly changed.
Figure 5.
EGCG activates the PI3K/AKT pathway in hyperglycemia-induced vascular endothelial cells. The expression of P-PI3K, P-AKT, PI3K and AKT in HG-treated HUVECs affected by EGCG was detected by Western blot analysis. **P<0.01 and ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG10μM group.
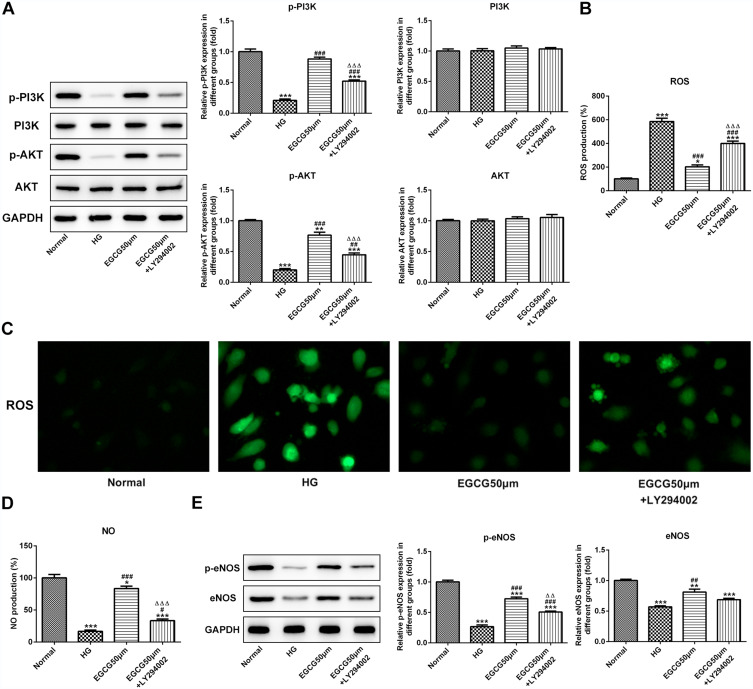
EGCG Alleviates Endothelial Dysfunction of Hyperglycemia-Induced Vascular Endothelial Cells by Activating the PI3K/AKT Pathway
As shown in Figure 6A, the expression of p-PI3K and p-AKT in HG group was decreased, which was improved by 50 μM EGCG. LY294002 suppressed the promotion effect of 50 μM EGCG on the expression of p-PI3K and p-AKT. The expression of PI3K and AKT in four groups were not changed obviously. The ROS expression level was increased in HG-treated HUVECs and 50 μM EGCG could decrease the ROS expression level in HG-treated HUVECs. LY294002 increased the ROS expression level in EGCG50μM +LY294002 group (Figure 6B and C). High glucose suppressed the NO expression level and 50 μM EGCG increased the NO expression level in HG-treated HUVECs. LY294002 inhibited the role of EGCG in improving the NO expression level (Figure 6D). The expression of p-eNOS and eNOS in HG group was decreased while 50 μM EGCG increased the expression of p-eNOS and eNOS in HG-treated HUVECs. LY294002 suppressed the improvement of EGCG for the expression of p-eNOS and eNOS in HG-treated HUVECs (Figure 6E).
Figure 6.
EGCG alleviates endothelial dysfunction of hyperglycemia-induced vascular endothelial cells by activating the PI3K/AKT pathway. (A) The expression of P-PI3K, P-AKT, PI3K and AKT in HG-treated HUVECs affected by EGCG and LY294002 was determined by Western blot analysis. **P<0.01 and ***P<0.001 vs Normal group. ##P<0.01 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG50μM group. (B) The ROS levels in HG-treated HUVECs affected by EGCG and LY294002 was detected by ROS ELISA assay. *P<0.05 and ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG50μM group. (C) The ROS levels in HG-treated HUVECs affected by EGCG and LY294002 was detected by fluorometric ROS assay. (D) The NO levels in HG-treated HUVECs affected by EGCG and LY294002 was analyzed by ROS ELISA assay. *P<0.05 and ***P<0.001 vs Normal group. #P<0.05 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG50μM group. (E) The expression of p-eNOS and eNOS in HG-treated HUVECs affected by EGCG and LY294002 was determined by Western blot analysis. **P<0.01 and ***P<0.001 vs Normal group. ##P<0.01 and ###P<0.001 vs HG group. ∆∆P<0.01 vs EGCG50μM group.
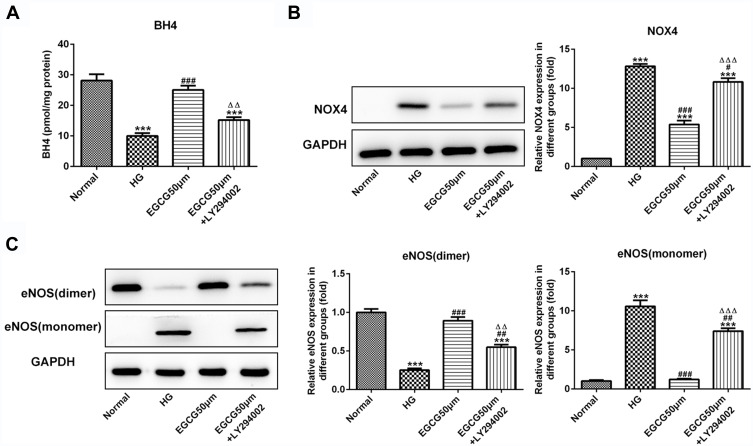
EGCG Increases the BH4 Expression and Inhibits the NOX4 Expression and eNOS Uncoupling by Activating the PI3K/AKT Pathway
BH4 expression in HG-treated HUVECs was decreased while increased by the treatment of 50 μM EGCG. The effect of EGCG on the BH4 expression was inhibited by LY294002 (Figure 7A). NOX4 expression was increased in HUVECs of HG group and decreased in EGCG50μM group. NOX4 expression was increased in HUVECs of EGCG50μM +LY294002 group compared with EGCG50μM group (Figure 7B). As shown in Figure 7C, the expression of eNOS (dimer) was decreased and eNOS (monomer) was increased in HUVECs of HG group. EGCG effectively increased the expression of eNOS (dimer) and decreased the expression of eNOS (monomer). The regulatory effects of EGCG on the expression of eNOS (dimer) and eNOS (monomer) was reversed by LY294002.
Figure 7.
EGCG increases the BH4 expression and inhibits the NOX4 expression and eNOS uncoupling by activating the PI3K/AKT pathway. (A) The BH4 levels in HG-treated HUVECs affected by EGCG and LY294002 was detected by BH4 ELISA assay. ***P<0.001 vs Normal group. ###P<0.001 vs HG group. ∆∆P<0.01 vs EGCG50μM group. (B) The NOX4 expression in HG-treated HUVECs affected by EGCG and LY294002 was determined by Western blot analysis. ***P<0.001 vs Normal group. #P<0.05 and ###P<0.001 vs HG group. ∆∆∆P<0.001 vs EGCG50μM group. (C) The expression of eNOS (dimer) and eNOS (monomer) in HG-treated HUVECs affected by EGCG and LY294002 was determined by Western blot analysis. ***P<0.001 vs Normal group. ##P<0.01 and ###P<0.001 vs HG group. ∆∆P<0.01 and ∆∆∆P<0.001 vs EGCG50μM group.
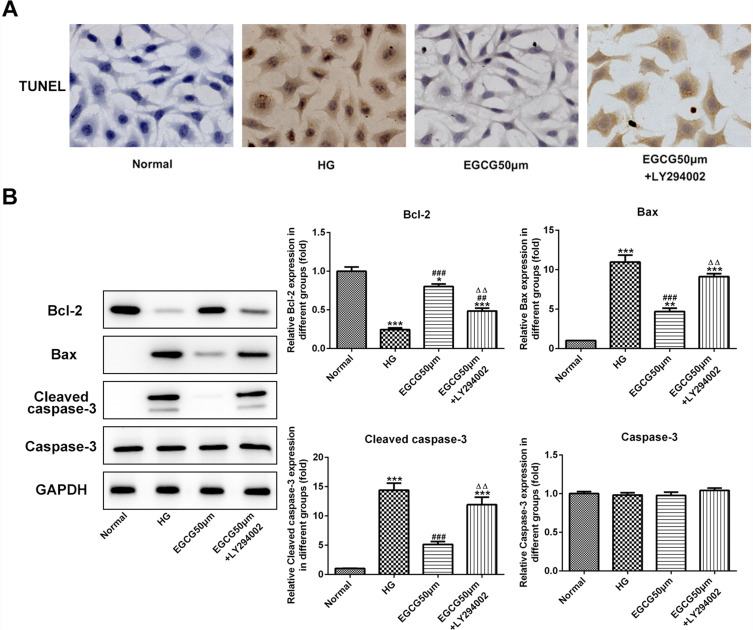
EGCG Alleviates the Apoptosis of Hyperglycemia-Induced Vascular Endothelial Cells by Activating the PI3K/AKT Pathway
The apoptosis of HUVECs was increased when HUVECs were treated with high glucose. EGCG decreased the apoptosis of HG-treated HUVECs and LY294002 increased the apoptosis of HUVECs treated with EGCG and high glucose (Figure 8A). The bcl2 expression was decreased and the expression of bax and cleaved-caspase3 was increased in HG-treated HUVECs, which was reversed by EGCG treatment. LY294002 down-regulated the bcl2 expression and up-regulated the expression of bax and cleaved-caspase3 in HUVECs treated with EGCG and high glucose (Figure 8B).
Figure 8.
EGCG alleviates the apoptosis of hyperglycemia-induced vascular endothelial cells by activating the PI3K/AKT pathway. (A) The apoptosis of HG-treated HUVECs affected by EGCG and LY294002 was analyzed by TUNEL assay. (B) The expression of bcl2, bax, cleaved-caspase3 and caspase3 in HG-treated HUVECs affected by EGCG and LY294002 was detected by Western blot analysis. *P<0.05, **P<0.01 and ***P<0.001 vs Normal group. ##P<0.01 and ###P<0.001 vs HG group. ∆∆P<0.01 vs EGCG50μM group.
Discussion
Type 2 diabetes is a common endocrine and metabolic disease and a major risk factor for the occurrence and development of cardiovascular diseases such as myocardial infarction and stroke.16 The study showed that hyperglycemia was a risk factor for cardiovascular diseases such as coronary heart disease. Hyperglycemia related advanced glycation end-products (AGEs) were toxins that interacted with receptors on endothelial cells to stimulate the inflammatory response and further promote the generation and development of inflammation.17
In the environment of insulin resistance, endothelial dysfunction could lead to cardiovascular disease including hypertension atherosclerosis and coronary artery disease.18 NO is considered to be the most effective endogenous vasodilator in vivo, and the decreased bioavailability of NO is a marker of endothelial dysfunction. NO participated in vascular wall homeostasis through platelet aggregation, leukocyte adhesion inhibition and anti-inflammatory effects.19 In the state of insulin resistance, NO synthesis was selectively impaired, and compensatory hyperinsulinemia might be activated the MAPK pathway, leading to enhanced vasoconstriction and stimulation of the inflammatory response.20 In addition, insulin resistance led to the increase of prothrombin factors, pro-inflammatory markers and ROS, which resulted in the increase of intracellular levels of adhesion molecule 1(ICAM1) and vascular cell adhesion molecule 1(VCAM-1).21 The relationship between endothelial function and insulin metabolism was important. The association between insulin resistance and endothelial signaling disorders led to inflammation, disrupted the balance between endothelial vasodilator and vasoconstrictor, and increased cardiovascular risk.22 In this study, the ROS expression was increased and NO expression was decreased in HUVECs when treated with high glucose. The expression of p-eNOS and eNOS was also decreased in HG-treated HUVECs, which could not produce the NO. EGCG could alleviate the endothelial dysfunction by reversing the expression changes of ROS, NO, p-eNOS and eNOS in HG-treated HUVECs.
Tetrahydrobiopterin (BH4) is an important cofactor for eNOS (dimer) making the NADPH oxidized to NO.23 When diabetic vascular complications occurred, the activation of NAPDH oxidase (NOX) was considered to play an important role in ROS-induced oxidative stress. NOX4 was the most abundant nitrogen oxide subtype in the vascular system and reported as a major source of ROS regeneration.24–26 The present study indicated that the expression of NOX4 and eNOS (monomer) was increased and the expression of BH4 and eNOS (dimer) was decreased in HG-treated HUVECs. EGCG could reverse the expression changes of NOX4, eNOS (monomer), BH4 and eNOS (dimer) in HG-treated HUVECs.
Vascular endothelial cells could secrete a variety of vasoactive substances, such as ET and NO, and participated in IRS-1/PI3K/AKT/NO and IRS-1/RAS/MAPK/ET-1 signaling pathways, thereby regulating endothelial function.27 Endothelial dysfunction in type 2 diabetes was associated with inhibition of the PI3K/AKT/eNOS signaling pathway. Rosiglitazone was associated with the enhancement of eNOS activity and up-regulation of the PI3K/AKT pathway. This study indicated that EGCG protected against HG-treated HUVECs by increasing eNOS expression and activating the PI3K/AKT pathway.
In conclusion, EGCG could inhibit eNOS uncoupling and alleviates endothelial dysfunction and apoptosis of HG-treated HUVECs by activating the PI3K/AKT/eNOS pathway. The present study provided a new direction for the treatment of cardiovascular disease caused by diabetes.
Funding Statement
Jiangsu Provincial Key Research and Development Program (BE2018611).
Disclosure
The authors declare they have no competing interests.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease - A statement for healthcare professionals from the American heart association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.CIR.100.10.1134 [DOI] [PubMed] [Google Scholar]
- 2.Matheus ASD, Tannus LRM, Cobas RA, Palma CCS, Negrato CA, Gomes MD. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:1–15. doi: 10.1155/2013/653789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Favard C, Ortega N, Bayard F, Plouet J. Vascular endothelial growth factor and retinal neovascularisation: a new therapeutic approach for diabetic retinopathy. Diabetes Metab. 1996;22:268–273. [PubMed] [Google Scholar]
- 4.Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye. 2006;20(5):546–552. doi: 10.1038/sj.eye.6701920 [DOI] [PubMed] [Google Scholar]
- 5.Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes the role of reparatory mechanisms. Diabetes Care. 2011;34:S285–S290. doi: 10.2337/dc11-s239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park KH, Park WJ. Endothelial dysfunction: clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30(9):1213–1225. doi: 10.3346/jkms.2015.30.9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su J, An XR, Li Q, Li XX, Cong XD, Xu M. Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci Rep. 2018;8:12620. doi: 10.1038/s41598-018-30386-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin W, Ren B, Wang S, et al. Apigenin and naringenin ameliorate PKCβII-associated endothelial dysfunction via regulating ROS/caspase-3 and NO pathway in endothelial cells exposed to high glucose. Vascul Pharmacol. 2016;85:39–49. doi: 10.1016/j.vph.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 9.Sang S, Lambert JD, Ho C-T, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacol Res. 2011;64(2):87–99. doi: 10.1016/j.phrs.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 10.Beecher GR, Warden BA, Merken H. Analysis of tea polyphenols. Proc Soc Exp Biol Med. 1999;220(4):267–270. doi: 10.1046/j.1525-1373.1999.d01-47.x [DOI] [PubMed] [Google Scholar]
- 11.Frei B, Higdon J. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133(10):3275S–3284S. doi: 10.1093/jn/133.10.3275S [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Jin CY, Bi XK, et al. Green Tea Polyphenol Epigallocatechin-3-Gallate Promotes Reendothelialization in Carotid Artery of Diabetic Rabbits by Reactivating Akt/eNOS Pathway. Front Pharmacol. 2018;9:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Han Y, Chen C, et al. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-κB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92(10):589–597. doi: 10.1016/j.lfs.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 14.Meng J, Chen YH, Wang JZ, et al. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann Transl Med. 2020;8(5):200. doi: 10.21037/atm.2020.01.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52(11):2745–2756. doi: 10.1172/JCI107470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 18.Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: insights from therapeutic interventions. J Cent South Univ. 2006;113(15):1888–1904. [PubMed] [Google Scholar]
- 19.Tousoulis D, Simopoulou C, Papageorgiou N, et al. Endothelial dysfunction in conduit arteries and in microcirculation. Novel therapeutic approaches. Pharmacol Ther. 2014;144(3):253–267. doi: 10.1016/j.pharmthera.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 20.Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28(3):527–535. doi: 10.1097/HJH.0b013e3283340da8 [DOI] [PubMed] [Google Scholar]
- 21.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 22.Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. 2016;2016:1–10. doi: 10.1155/2016/3634948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alp N, Channon K. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6 [DOI] [PubMed] [Google Scholar]
- 24.Inoguchi T, Li P, Umeda F, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C–dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49(11):1939–1945. doi: 10.2337/diabetes.49.11.1939 [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Qian L-H, Liu Z-M, Zhao Y, Le Y. Resveratrol protects vascular endothelial cells from high glucose–induced apoptosis through inhibition of NADPH oxidase activation–driven oxidative stress. CNS Neurosci Ther. 2013;19:675–681. doi: 10.1111/cns.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu H, Goettsch C, Xia N, et al. Differential roles of PKCα and PKCɛ in controlling the gene expression of Nox4 in human endothelial cells. Free Radic Biol Med. 2008;44(8):1656–1667. doi: 10.1016/j.freeradbiomed.2008.01.023 [DOI] [PubMed] [Google Scholar]
- 27.Celinski K, Dworzanski T, Fornal R, Korolczuk A, Madro A, Slomka M. Comparison of the anti-inflammatory and therapeutic actions of PPAR-gamma agonists rosiglitazone and troglitazone in experimental colitis. J Physiol Pharmacol. 2012;63(6):631–640. [PubMed] [Google Scholar]