Abstract
Introduction
Cutaneous metastases of breast cancer remain a therapeutic challenge. Oxygen flow-assisted topical administration of methotrexate 5% (OFAMTX, 5% methotrexate in a carrier solution) has recently been proven to be an efficacious alternative treatment for extramammary Paget’s disease, which is considered to be an in situ mammary adenocarcinoma of the epidermis.
Case Report
A 51-year-old patient with triple negative breast cancer presenting with biopsy-proven skin metastases on the chest agreed to a treatment with OFAMTX5%. The treatment duration was 2 weeks and consisted of twice-weekly sessions with OFAMTX5% applied to an area of skin of approximately 40 cm2. Skin biopsies were performed before and 2 months after procedure. The tolerance to the treatment was excellent, and no pain sensations were experienced. Two months post-procedure the treated area presented a post-inflammatory hyperpigmentation. No residual metastatic lesions were detectable on the control skin biopsy. Six months post-procedure the patient is still in clinical remission.
Discussion
OFAMTX5% represents an alternative skin-directed, painless, patient-friendly and efficacious adjuvant treatment for superficial metastatic lesions of breast cancer. Larger series are required to evaluate the potential of OFAMTX5% for the treatment of superficial metastatic lesions of breast cancer.
Keywords: Breast cancer, Methotrexate, OFAMTX, Skin-directed therapy, Skin metastases, Topical treatment
Key Summary Points
| Cutaneous metastases of breast cancer (CMOBM) remain a therapeutic challenge. |
| The oxygen flow assisted administration of topical methotrexate 5% (OFAMTX5%) achieved clinical and histological cure in a patient with superficial CMOBM, with no relapse for at least 6 months. |
| OFAMTX5% represents a new treatment option for superficial CMOBM. |
Introduction
Cutaneous metastases occur in up to one-third of all patients with breast cancer and are usually a sign of poor prognosis [1]. Cutaneous metastases of breast cancer (CMOBC) have a major impact on the quality of life of the patient and often represent difficult therapeutic challenges. Therapeutic options are systemic agents and/or skin-directed treatments. Radiotherapy [2], surgical excision [3] or electrochemotherapy [4] are indicated for thicker skin metastases, either as monotherapy or adjuvant treatment. For thinner, more superficial skin metastases, topical treatments may be useful, such as imiquimod 5% cream [3, 5–8], cryotherapy [8], 5-fluorouracil 5% cream (5-FU) [8], photodynamic therapy (PDT) [9–11] and applications of miltefosine 6% solution [2, 12, 13]. All these treatments are either used as monotherapy or as adjuvant treatment.
Oxygen flow assisted topical administration of methotrexate 5% (OFAMTX, 5% methotrexate in a carrier solution) is a recently described technique that facilitates the permeation of the epidermis by medications, enabling penetration of the skin by high-molecular-weight molecules that normally do not do so [14]. This technique has been found to have interesting treatment response rates for superficial basal cell carcinoma, mycosis fungoides and extramammary Paget’s disease (EMPD), a chronically evolving and recurrent intraepidermal adenocarcinoma [14]. As methotrexate is a chemotherapy drug used to treat certain breast cancers [15], we have evaluated the efficacy of OFAMTX5% for treating superficial CMOBC. The patient provided written consent for publication of her clinical images and her case.
Case Report
A 51-year-old woman presented with an indurated mass of her right breast, suggesting a carcinomatous mastitis with axillary lymph node extension. Magnetic resonance imaging revealed a lesion affecting the entire right breast, 8 cm in size, with an infiltration of the nipple and the axillary lymph nodes. A biopsy of the breast lesion revealed an invasive ductal carcinoma, grade III by Bloom grading system, triple negative (estrogen receptor−, progesterone receptor− and HER-2−), with a high proliferation index (Ki67: 63%). Needle aspiration of axillary lymph nodes was also positive. Positron emission tomography (PET) and bone scintigraphy did not reveal distant metastasis. A neo-adjuvant chemotherapy was initiated with four courses of epirubicin and cyclophosphamide (75 mg/m2 and 500 mg/m2, respectively, every 15 days), followed by 12 once-weekly courses of paclitaxel (80 mg/m2). The overall clinical and biological tolerance was very good. One month later, a mastectomy and a sentinel lymph node biopsy were performed. Histological studies revealed multiple scattered lympho-vascular embolizations involving about 20% of the tumor mass. There was infiltration of two of the three lymph nodes, with micrometastases measuring 1.8- and 5-mm. Immunohistochemistry confirmed the triple negative character of the carcinoma. Histological staging was T1cN1cMx.
Two weeks following surgery, isolated macular and slightly infiltrated erythematous skin lesions were evidenced in the near vicinity of the mastectomy scar. A skin biopsy revealed a dermal infiltration with breast carcinoma cells, triple negative, with a Ki67 of 80%. Capecitabine chemotherapy (1250 mg/m2, twice-daily, 2 weeks every 3 weeks) was initiated and adjuvant radiotherapy was administered (50 Gy, 25 sessions of 2 Gy) to the right chest wall and the supraclavicular region, followed by a boost on the scar and skin metastases (16 Gy, 8 sessions of 2 Gy). Five months later, new cutaneous metastases appeared, presenting as Velpeau’s nodules on the edge of the lower fields of the radiotherapy area (Fig. 1a). As the PET scanner did not reveal any internal and/or lymph node metastases, a skin-directed treatment with OFAMTX5% was applied (twice weekly for 2 weeks) to the lesions using the Dermadrop® device (MEDDROP GMBH, Hamburg, Germany) (Fig. 2a). The patient provided written informed consent for the off-label use of this technique (Eudract number 2017-000615-16).
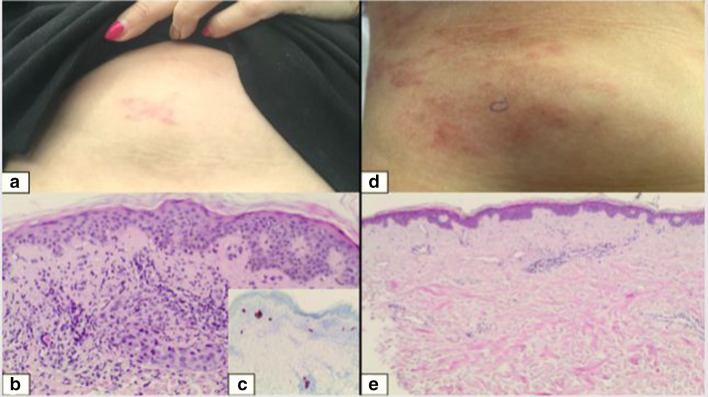
Fig. 1.
a Clinical aspect of breast cancer skin metastases before treatment, b histology image showing superficial tumoral infiltrate in the dermis, c immunohistochemistry image showing cytokeratin-7 positive tumor cells, d clinical aspect 2 months post-oxygen flow assisted topical administration of methotrexate 5% (OFAMTX; 5% methotrexate in a carrier solution) procedure, e control biopsy revealing no residual tumor nests
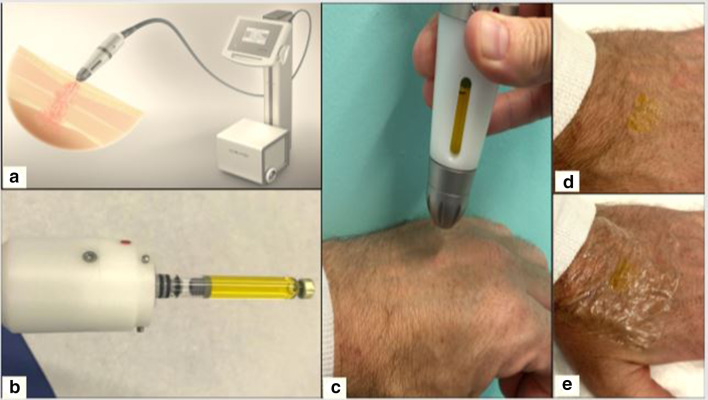
Fig. 2.
a The Dermadrop® device, b the handpiece containing a methotrexate 5% (yellow color) cartridge, c handpiece applying methotrexate in the carrier solution to the skin, d saturation point: the methotrexate solution is no longer absorbed by the skin, e the treated site is covered with a plastic film for 24 h
In this procedure, a methotrexate 5% cartridge is inserted into the handpiece (Fig. 2b), following which the oxygen flow is started, projecting the methotrexate with the carrier solution through a fine needle onto the skin (Fig. 2c). The application continues for 5–10 min until a full saturation of the treated area is obtained, i.e. a yellowish liquid film remains on the skin (Fig. 2d). A plastic dressing is then applied for 24 h (Fig. 2e).
A biopsy performed just prior to the OFAMTX5% treatment revealed once again a superficial and deep dermal infiltration of neoplastic mammary cells (Fig. 1b) with cytokeratin-7 immunohistochemical expression (Fig. 1c). The treatment sessions were well tolerated and the patient did not experience any procedure-related discomfort during or after the sessions. Two months following the procedure, a post-inflammatory pigmentation was observed (Fig. 1d) but there was no clinical evidence of recurring infiltrated lesions. A control skin biopsy was performed and revealed a total remission (Fig. 1e). At the control visits 3 and 6 months after treatment, the post-inflammatory hyperpigmentation had faded and there was still no clinical evidence of a local recurrence of CMOBC.
Discussion
This case demonstrates the possibility of treating superficial CMOBC with OFAMTX5% in a painless manner, while continuing chemotherapy. OFAMTX5% can be considered to be an alternative topical treatment option to other topical therapies, such as miltefosine [2, 12, 13], imiquimod [3, 5–7], 5-FU [8] and methylaminolevulinate [11] or aminolevulinate-PDT. These latter topical therapies are associated with grade 1–3 cutaneous toxicities, sometimes requiring dose tapering or treatment interruption. A major advantage of topical treatments is the general absence of systemic adverse events, with the exception of a flu-like syndrome observed in some patients receiving imiquimod. In addition, topical therapies are beneficial for treating superficial lesions regardless of the size due to their increased tolerability. Treatments are usually of long duration and recurrences are frequent; consequently, the tolerability of topical treatments is of utmost importance to maintain patient comfort and compliance with the treatment. Using topical treatments as adjuvant therapy with systemic agents likely increases the overall efficacy of the chosen treatment on cutaneous metastases. Combining topical therapies with different action mechanisms, i.e. immunostimulatory (imiquimod) and antimetabolic (methotrexate, 5-FU, miltefosine), probably further enhances effectiveness. However, it remains difficult to directly compare study results and adverse effect profiles due to the different action mechanisms, treatment durations, assessment methods, among others. A comparative overview of the topical treatment options for superficial breast cancer skin metastases is given in Table 1.
Table 1.
Topical treatment options for superficial breast cancer metastases
| Treatment mechanism | Type of study | Administration regimen | Number of patients | Assessment | Adverse events | References |
|---|---|---|---|---|---|---|
|
Mitlefosine 6% solution Antimetabolite |
Open label |
2 drops/10 cm2 1–2 treatment courses/day for 2–164 weeks (median 14 weeks) |
25 |
Treatment-induced effect: 36% of the patients: 1 complete, 2 partial and 6 minor responses. Stable disease: 44% Progressive disease: 20% |
Cutaneous toxicities: Grade 1: 28%; Grade 3: 16% | [12] |
| Double-blind, placebo-controlled |
2 drops/10 cm2 1 treatment course/day for 1 week, then 2 treatment courses/day until treatment failure |
52 | Time to treatment failure: miltefosine arm, 56 days; placebo arm, 21 days | Well tolerated | [13] | |
|
Imiquimod 5% cream Induces endogenous production of interferon |
Case report | 3–5 treatment courses/week, every 3 weeks with cryotherapy | 1 | Initial shrinking of the lesion | [8] | |
| Prospective | 5 treatment courses/weer for 8 weeks | 10 | Partial response: 20% | Grade 1–2 transient local and systemic adverse effects | [5] | |
| Case report | 5 treatment courses/week for 8 weeks | 1 | Size reduction | No reported adverse effects | [3] | |
| Single-arm phase 2 | 4 days of treatment courses for 12 weeks, with paclitaxel treatment 100 mg/m2 | 15 |
5 patients: complete response 5 patients: partial response Overall response rate: 72% (10/14) |
Cutaneous toxicities: Grades 1,2: 92% | [6] | |
|
5-Fluorouracil 5% cream Antimetabolite |
Case series | 2 treatment courses/dday (with cryotherapy) for 4 months | 2 | Good but transient clinical responses | Acceptable local adverse events | [8] |
|
Photodynamic therapy Induces selective apoptosis |
Case series | Intravenous photofrin II-PDT | 37 | 5 complete, 13 partial and 19 non-responders | Well tolerated | [9] |
| Case series | Topical meso-tetra-porphon-PDT | 9 | 3 complete, 4 partial and 2 non-responders | No adverse effects | [10] | |
| Case series | MAL-PDT | 2 |
Superficial clearing at 4 weeks Persistence of lesions in subcutis |
Well tolerated | [11] | |
|
OFAMTX5% Antimetabolite |
Case report | 2 treatment courses/week for 2 weeks | 1 |
Complete histological response at 2 months No recurrence at 6 months |
Well tolerated No topical or systemic adverse effects |
[14] |
MAL Methylaminolevulinate, OFAMTX5% 5% methotrexate in a carrier solution, PDT photodynamic therapy
In conclusion, OFAMTX5% is an effective and well-tolerated treatment for superficial CMOBC. More cases will have to be treated in order to determine optimal treatment regimens and its place in the armamentarium against CMOBC.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Gaëlle Jouret, Elodie Gonne, Pascale Quatresooz, Marie-Annick Reginster, Patrick Collins, Eve Lebas, Guy Jerusalem and Arjen F. Nikkels all participated in the design of the study and of the interpretation of results as well as in the discussion.
Prior Presentation
This work was previously published as a preprint: https://www.researchsquare.com/article/rs-14091/v1.
Disclosures
Gaëlle Jouret, Elodie Gonne, Pascale Quatresooz, Marie-Annick Reginster, Patrick Collins, Eve Lebas and Guy Jerusalem have nothing to disclose. Arjen F. Nikkels is a member of the journal’s Editorial Board.
Compliance with Ethics Guidelines
The patient was informed on all the procedures and consented to participate. The patient provided written consent for publication of her clinical images and her case.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12241058.
References
- 1.Strickley JD, Jenson AB, Jung JY. Cutaneous metastasis. Hematol Oncol Clin North Am. 2019;33:173–197. doi: 10.1016/j.hoc.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Varol U, Yildiz I, Alacacioglu A, Uslu R. Anticancer therapy for breast cancer patients with skin metastases refractory to conventional treatments. Asian Pac J Cancer Prev. 2014;15:1885–1887. doi: 10.7314/APJCP.2014.15.4.1885. [DOI] [PubMed] [Google Scholar]
- 3.Henriques L, Palumbo M, Guay MP, et al. Imiquimod in the treatment of breast cancer skin metastasis. J Clin Oncol. 2014;32:e22–e25. doi: 10.1200/JCO.2012.46.4883. [DOI] [PubMed] [Google Scholar]
- 4.Wichtowski M, Murawa D, Czarnecki R, Piechocki J, Nowecki Z, Witkiewicz W. Electrochemotherapy in the treatment of breast cancer metastasis to the skin and subcutaneous tissue—multicenter experience. Oncol Res Treat. 2019;42:47–51. doi: 10.1159/000494093. [DOI] [PubMed] [Google Scholar]
- 5.Adams S, Kozhaya L, Martiniuk F, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–6757. doi: 10.1158/1078-0432.CCR-12-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar LG, Lu H, Reichow JL, et al. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol. 2017;3:969–973. doi: 10.1001/jamaoncol.2016.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rozenblit M, Hendrickx W, Heguy A, et al. Transcriptomic profiles conducive to immune-mediated tumor rejection in human breast cancer skin metastases treated with Imiquimod. Sci Rep. 2019;9:8572. doi: 10.1038/s41598-019-42784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnasamy SR, Almazan TH, Suero-Abreu GA, Jung JY. Successful treatment of cutaneous metastatic breast cancer with topical treatments that potentially synergize with systemic therapy: a case series. JAAD Case Rep. 2018;4:711–715. doi: 10.1016/j.jdcr.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan SA, Dougherty TJ, Mang TS. An evaluation of photodynamic therapy in the management of cutaneous metastases of breast cancer. Eur J Cancer. 1993;29A:1686–1690. doi: 10.1016/0959-8049(93)90105-O. [DOI] [PubMed] [Google Scholar]
- 10.Lapes M, Petera J, Jirsa M. Photodynamic therapy of cutaneous metastases of breast cancer after local application of meso-tetra-(para-sulphophenyl)-porphin (TPPS4) J Photochem Photobiol B. 1996;36:205–207. doi: 10.1016/S1011-1344(96)07373-3. [DOI] [PubMed] [Google Scholar]
- 11.Wauters O, Caucanas M, Richert B, Dezfoulian B, Nikkels AF. The clinical relevance of off-label photodynamic therapy in onco-dermatology. Am J Clin Dermatol 2010;1:1–11. https://hdl.handle.net/2268/161519
- 12.Clive S, Gardiner J, Leonard RC. Miltefosine as a topical treatment for cutaneous metastases in breast carcinoma. Cancer Chemother Pharmacol. 1999;44(Suppl):S29–30. doi: 10.1007/s002800051114. [DOI] [PubMed] [Google Scholar]
- 13.Leonard R, Hardy J, van Tienhoven G, et al. Randomized, double-blind, placebo-controlled, multicenter trial of 6% miltefosine solution, a topical chemotherapy in cutaneous metastases from breast cancer. J Clin Oncol. 2001;19:4150–4159. doi: 10.1200/JCO.2001.19.21.4150. [DOI] [PubMed] [Google Scholar]
- 14.Lebas E, Chapelier C, Quatresooz P, Seidel L, Nikkels AF. Exploratory assessment of oxygen flow-assisted cutaneous administration of methotrexate for superficial basal cell carcinoma, mycosis fungoides, and extramammary Paget disease. J Invest Dermatol. 2019;140(3):583–592. doi: 10.1016/j.jid.2019.08.443. [DOI] [PubMed] [Google Scholar]
- 15.Orlando L, Schiavone P, Calvani N, Fedele P, Goldhirsch A, Cinieri S. Response of extensive breast cancer skin metastases to rechallenge with trastuzumab together with low-dose chemotherapy and insulin. Tumori. 2016;102(Suppl. 2):S26–S28. doi: 10.5301/tj.5000488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.