Abstract
Background:
It is unclear whether cadmium (Cd) exposure during fetal brain development is associated with child neurobehavior.
Objective:
To examine the potential associations between Cd exposure during pregnancy and neurobehavior among children.
Methods:
We used data from 276 children in the Health Outcomes and Measures of the Environment (HOME) Study, a well-established prospective pregnancy and birth cohort. We measured maternal urinary Cd concentrations at 26 weeks of gestation. For cognitive function, we assessed Mental Development Index (MDI) and Full-Scale Intelligence Quotient (FSIQ) using the Bayley Scales of Infant Development-II, the Wechsler Preschool and Primary Scales of Intelligence-III, or the Wechsler Intelligence Scales for Children-IV at ages 1, 2, 3, 5, and 8 years. We assessed child behaviors using the Behavior Assessment System for Children-2 at ages 2, 3, 4, 5, and 8 years, yielding four composite measures: Externalizing Problems, Internalizing Problems, Behavioral Symptoms Index, and Adaptive Skills. We used linear mixed models with covariate adjustment to estimate the associations between maternal urinary Cd concentrations and child neurobehavior.
Results:
We categorized study participants into three groups based on maternal urinary Cd concentrations (Group 1: < limit of detection (LOD), Group 2: 0.06–0.22 μg/g creatinine, Group 3: >0.22 μg/g creatinine). In linear mixed models adjusting for maternal and child characteristics, maternal urinary Cd levels were not significantly associated with cognitive function at ages 1, 2, 3, 5, and 8 years or with behavioral composite measures at 2, 3, 4, 5, and 8 years.
Conclusions:
No significant associations were observed between maternal urinary Cd and cognitive or behavioral measures in children at 1 to 8 years of age in this study.
Keywords: Cadmium, Prenatal exposure, Child development, Neurobehavior
Introduction
Heavy metals are particularly concerning to children’s health considering the relatively high probability of exposure and the potential adverse effects on development and neurobehavior (Claus Henn et al., 2014). Cadmium (Cd), which is an industrial and environmental pollutant, is present in our environment via anthropogenic activities and natural processes (Espart et al., 2018; Luparello et al., 2011). The main route of exposure in the general population is ingestion of contaminated food or water (Vacchi-Suzzi et al., 2016). Tobacco is also a major source of Cd for active smokers and individuals who are passively exposed to second hand smoke (Faroon et al., 2012). Smoking can double the lifetime Cd burden in non-occupationally exposed populations (Klaassen et al., 2013). The renal, respiratory, skeletal, and cardiovascular systems are the most common targets of long-term Cd exposure in adults (Vacchi-Suzzi et al., 2016; Luparello et al., 2011). Cd can induce oxidative stress, apoptosis in embryonic cells, and DNA methylation modifications (Espart et al., 2018; Luparello et al., 2011; McAleer and Tuan, 2001). Due to its particularly long half-life in the body and toxicity, concerns over Cd’s adverse health effects are increasing.
Although Cd is a known carcinogen, it is understudied as a developmental toxicant. Cd can partially cross the placental barrier from mother to fetus (Sanders et al., 2015), and Cd levels during pregnancy have been reported to be associated with adverse outcomes in children. Specifically, an increase in cord blood Cd was found to be associated with decreased height, weight, and head circumference in children up to 3 years of age. (Lin et al., 2011; Rollin et al., 2015; Sanders et al., 2014). Evidence from experimental animal studies indicates that Cd may be a developmental neurotoxicant. Chow et al. reported that prenatal Cd exposure reduced neuronal differentiation and axonogenesis in zebrafish embryonic brain (Chow et al., 2008). Gestational Cd exposure (50 ppm of Cd as Cadmium Chloride in the drinking water ) was additionally observed to alter crucial brain enzyme activities in important regions of the brain of rat offspring (Stolakis et al., 2013). A recent study reported that maternal exposure to Cd (1 and 5 mg/kg body weight) could impair cognitive development of male offspring through the Coronin-1a signaling pathway (Feng et al., 2019).
However, evidence regarding maternal Cd exposure and its relationship with neurological development from population studies is limited. Some epidemiologic studies have examined neurotoxicity and developmental toxicity of Cd, but the results were inconsistent. Cohort studies in different regions found that Cd exposure during gestation was negatively associated with intelligence scores in childhood (Forns et al., 2014; Jeong et al., 2015; Kippler et al., 2012; Tian et al., 2009; Wang et al., 2016). Yet, a recent study suggested that the associations between prenatal exposure to Cd and neurodevelopment were less clear and needed further investigation (Freire et al., 2018).
The objective of this study was to investigate the association of Cd exposure quantified in maternal urine at 26-week gestation, which reflects the amount of Cd stored in the body (Ciesielski et al., 2012; Lauwerys et al., 1994), and neurobehavior, assessed repeatedly from infancy to school-age, in mother-child dyads from a United States (US) cohort.
Subjects and methods
Study participants and design
The Health Outcomes and Measures of the Environment (HOME) Study is a prospective pregnancy and birth cohort established in the Greater Cincinnati metropolitan area (Ohio, USA), which included 389 singleton births at delivery and multiple postnatal follow-up visits up to age 8 years. Detailed information on enrollment, inclusion and exclusion criteria, assessments of environmental chemical exposures, and neurobehavioral assessments have been described previously (Braun et al., 2017). Our study included 276 singleton children with maternal urinary Cd concentrations during pregnancy and at least one completed neurobehavioral assessment between 1 and 8 years. The study protocol was approved by the Institutional Review Board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
Maternal urinary Cd measurements
We collected maternal urine samples at 26 weeks of gestation and analyzed them for Cd concentrations following a 10-fold dilution using the Agilent 7700x Inductively Coupled Plasma Mass Spectrometry (ICP-MS) at Dartmouth College. The specimens were stored at −20°C after collection until shipping to the analytical lab. The limit of detection (LOD) was 0.1 μg/L. For instrument calibration, we used urine reference samples from the Center for Toxicology, Quebec and UTAK (Valencia CA) containing total Cd urine levels from 2 – 4.1 μg/L, with an average recovery of 93%, and spiked samples with an average recovery of 98%. Quality control measures further included a composite urine sample to which the laboratory was masked, which was analyzed in replicate in each batch and over multiple batches, with a coefficient of variation (CV) averaging approximately <15% across batches. We measured urinary creatinine concentrations using a kinetic Jaffe reaction (Larsen, 1972). Urinary Cd concentrations were creatinine-standardized (µg/g creatinine) to account for urine dilution by dividing urinary Cd concentrations (µg/L) by urinary creatinine concentrations (mg/dL) and multiplying by 100.
Neurobehavior assessments
Trained and certified HOME Study examiners administered the Bayley Scales of Infant Development-II (BSID-II) to evaluate cognitive (Mental Development Index, MDI) and motor development (Psychomotor Development Index, PDI) of children at ages 1–3 years (Bayley, 1993). We also administered the Wechsler Preschool and Primary Scales of Intelligence-III and the Wechsler Intelligence Scales for Children-IV (WISC-IV) at ages 5 and 8 years, respectively, to assess Full Scale IQ (FSIQ) (Wechsler, 2003; Wechsler, 2004). Both the MDI and FSIQ are measures of cognitive function and are statistically equivalent with a population mean of 100 and a standard deviation of 15.
Parents rated child behavior using the Behavior Assessment System for Children-2 (BASC-2) at ages 2, 3, 4, 5, and 8 years (Reynolds and Kamphaus, 2004). The BASC-2 offers a comprehensive assessment of a child’s adaptive skills and problematic behaviors in community and home settings. It includes four composite scores: Externalizing Problems score (including subscales of aggression, hyperactivity and conduct disorder), Internalizing Problems score (including subscales of anxiety, depression, and somatization), Behavior Symptoms Index (including subscales of aggression, hyperactivity, depression, atypicality, withdrawal, and attention problems), and Adaptive Skills score (including subscales of activities of daily living, adaptability, social skills, and functional communication). A higher score on Adaptive Skills indicates better adaptive skills, while for the other composite scores higher scores indicate more problem behaviors. Neither examiners nor parents were aware of maternal urinary Cd concentrations at the time of neurobehavioral assessment.
Statistical Analysis
Forty four percent of maternal urinary Cd concentrations were below the LOD; 93% were reported as machine reading values as a signal was detected and 7% were imputed as LOD divided by the square root of 2 since there was no signal by the assay (Hornung and Reed, 1990).
We categorized urinary Cd concentrations into three groups. Group 1 was defined as having a urine Cd level <LOD. For individuals with urine Cd concentrations ≥ LOD, we further dichotomized them based on the median concentration after creatinine-adjustment (0.22 μg/g creatinine). Group 2 was defined as having a urine Cd level ≥ LOD, but at or lower than the median (LOD-0.22 μg/g creatinine). Group 3 was defined as having a urine Cd level higher than the median (>0.22 μg/g creatinine) in the study population.
We used linear mixed models with repeated measures to examine the associations between maternal urinary Cd levels and MDI/FSIQ and scores on BASC-2 composites: Externalizing Problems, Internalizing Problems, Behavior Symptoms Index, and Adaptive Skills assessed between 1 and 8 years, accounting for repeated outcome measurements.
We included the following covariates in the final regression models based on results of bivariate analyses examining the relationship with maternal urinary Cd levels and child neurobehavior measurements (p<0.2): maternal age at enrollment, maternal race/ethnicity, marital status, child sex, family income, maternal serum cotinine at 16±3 weeks (ng/mL), maternal blood lead at 16±3 weeks (μg/dL), maternal serum polychlorinated biphenyl (∑PCB) concentrations (PCB congeners-28, 74, 99, 105, 118, 146, 153, 156, 170, 180, 183, 187, 194, 199, and 206), maternal IQ (assessed by the Wechsler Abbreviated Scale of Intelligence) (Wechsler, 1999), maternal depression (assessed by the Beck Depression Inventory II during pregnancy) (Beck et al., 1996), and Home Observation for Measurement of the Environmental (HOME) Inventory score at the 1-year home visit (Caldwell and Bradley, 1984). We also provide a directed acyclic (DAG) graph in supplemental materials (Supplemental Figure 1) to determine the minimal set of covariates needed, which include all the covariates mentioned above except the HOME score at the 1-year home visit. We still adjusted for the HOME score since it is a relatively stable reflection of inherent factors in a family and could act as an effect measure modifier for chemical neurotoxicity. Details regarding the covariates (categorical or continuous) are shown in Tables 1 and 2.
Table 1.
Maternal urine cadmium levels at 26 weeks of gestation and child neurodevelopment test scores at age 8 years by maternal and child characteristics
| Categorical characteristics | Maternal Cd (μg/g creatinine, GM[GSD]) | Group 1 n (%) | Group 2 n (%) | Group 3 n (%) | n with FSIQ at age 8 | FSIQ at age 8 (mean ± SD) | n with Adaptive Skills score at age 8 | Adaptive Skills score at age 8 (mean ± SD) |
|---|---|---|---|---|---|---|---|---|
| All participants | 0.17 (2.29) | 121 (43.8) | 78 (28.2) | 77 (27.9) | 173 | 102.0±15.9 | 179 | 49.5±9.7 |
| Maternal age (years)† | ||||||||
| <25 | 0.13 (2.20) | 19 (35.2) | 26 (48.1) | 9 (16.7) | 42 | 94.3±17.1 | 44 | 49.2±11.1 |
| 25–34 | 0.17 (2.28) | 87 (49.1) | 41 (23.2) | 49 (27.7) | 106 | 102.8±14.3 | 108 | 49.4±9.2 |
| ≥35 | 0.21 (2.30) | 15 (33.3) | 11 (24.4) | 19 (42.3) | 25 | 111.6±14.4 | 27 | 50.1±9.5 |
| Race/ethnicity*,†,‡ | ||||||||
| Non-Hispanic White | 0.18 (2.39) | 100 (52.4) | 36 (18.8) | 55 (28.8) | 110 | 107.8±13.0 | 114 | 50.6±8.3 |
| Non-Hispanic Black and others | 0.14 (2.04) | 21 (24.7) | 42 (49.4) | 22 (25.9) | 63 | 91.9±15.4 | 65 | 47.4±11.6 |
| Marital status† | ||||||||
| Married/living with partner | 0.17 (2.31) | 109 (47.4) | 56 (24.3) | 65 (28.3) | 133 | 105.5±14.2 | 139 | 50.1±9.0 |
| Not married, living alone | 0.13 (2.19) | 12 (26.1) | 22 (47.8) | 12 (26.1) | 40 | 90.6±15.9 | 40 | 47.1±11.6 |
| Child sex‡ | ||||||||
| Male | 0.17 (2.34) | 51 (40.2) | 35 (27.5) | 41 (31.3) | 79 | 101.7±14.7 | 80 | 47.2±9.2 |
| Female | 0.17 (2.25) | 70 (47.0) | 43 (28.9) | 36 (24.1) | 94 | 102.3±16.8 | 99 | 51.3±9.8 |
| Family Income*,†,‡ | ||||||||
| <$40.000 | 0.15 (2.08) | 25 (27.8) | 40 (44.4) | 25 (27.8) | 66 | 93.3±15.9 | 67 | 47.0±11.0 |
| $40,000–79,999 | 0.18 (2.33) | 47 (46.1) | 26 (25.5) | 29 (28.4) | 58 | 104.0±13.2 | 62 | 49.5±9.3 |
| ≥$80,000 | 0.18(2.44) | 49 (58.3) | 12 (14.3) | 23 (27.4) | 49 | 111.4±12.4 | 50 | 52.7±7.2 |
Group 1: < LOD; Group 2: 0.06–0.22 μg/g creatinine; Group 3: >0.22 μg/g creatinine.
FSIQ, Full-Scale IQ; GM, geometric mean; GSD, geometric standard deviation; SD, standard deviation.
p<0.05 for Cd categories.
p<0.05 for FSIQ at age 8 years.
p<0.05 for Adaptative Skills score at age 8 years.
The statistical tests included t-test, analysis of variance and Chi-square test.
Table 2.
Pearson correlation coefficients of continuous characteristics with child neurodevelopmental test score at age 8 years.
| Continuous characteristics | n | Mean ± SD | Pearson r with maternal Cd (log10 μg/g creatinine) | n with FSIQ at age 8 | Mean ± SD | Pearson r with FSIQ at age 8 | n with Adaptive Skills score at age 8 | Mean ± SD | Pearson r with Adaptive Skills score at age 8 |
|---|---|---|---|---|---|---|---|---|---|
| Maternal IQ† | 264 | 107.97±14.22 | 0.10 | 162 | 106.94±15.11 | 0.49 | 168 | 107.20±15.07 | 0.12 |
| Maternal BDI depression (score at baseline)*†,‡ | 274 | 9.35±6.35 | −0.13 | 172 | 9.94±6.64 | −0.32 | 178 | 9.92±6.64 | −0.35 |
| Maternal blood lead at 16w [GM(GSD), μg/dL ]† | 276 | 0.65(1.49) | 0.003 | 173 | 0.64 (1.45) | −0.20 | 179 | 0.64 (1.44) | −0.12 |
| Maternal serum ∑PCBs* [GM (GSD), ng/g lipid]† | 228 | 45.58(1.73) | 0.14 | 150 | 44.98 (1.74) | 0. 20 | 153 | 45.00 (1.73) | 0.10 |
| Maternal cotinine at 16w [GM(GSD), ng/mL]†,‡ | 270 | 0.04(19.88) | −0.003 | 170 | 0.05 (21.94) | −0.32 | 176 | 0.05 (22.74) | −0.16 |
| HOME score at age 1 visit†,‡ | 261 | 39.85±4.87 | 0.11 | 161 | 39.63±4.92 | 0.39 | 167 | 39.69±4.89 | 0.21 |
GM, geometric mean; GSD, geometric standard deviation; BDI, Beck Depression Index; FSIQ, Full-Scale IQ; SD, standard deviation.
p<0.05 for cadmium.
p<0.05 for FSIQ at age 8 years.
p<0.05 for Adaptive Skills score at age 8 years.
We tested the interaction between maternal Cd and child age on neurobehavioral outcomes by including an interaction term of maternal Cd (categorical variable) and child age in the regression models. We also tested the interaction term of maternal Cd (categorical variable) and child sex on neurobehavioral outcomes. We performed three sensitivity analyses. First, we used generalized additive models to test the non-linearity of maternal urinary Cd concentration as a continuous variable, including machine reading values and imputed values (Wood, 2017). Non-linear relation was not found, so we modeled continuous urinary Cd concentrations (machine reading values and imputed values included) in the linear mixed models. Second, we analyzed a subgroup of children with non-smoking mothers to determine whether results differed from the whole sample. Third, we repeated the analyses after excluding maternal serum cotinine as a covariate in the models, since tobacco is a major source of Cd exposure. We analyzed data using SAS (Version 9.4; SAS Institute Inc., Cary, NC, USA) and the mgcv package (Version 1.8–27) in R (Version 3.5.3) (R Core Team, 2019).
Results
Urinary Cd concentrations of 121 (44%) pregnant women were < LOD and labeled as Group 1 (Table 1). Of the 155 HOME Study mothers with Cd levels ≥LOD, 78 (28%) had concentrations between 0.06 and 0.22 μg/g creatinine (Group 2) and 77 (28%) had urinary Cd concentrations >0.22 μg/g creatinine (Group 3). Mothers who were non-Hispanic white or who had an annual family income >$40,000 had significantly higher percentages of urinary Cd concentrations that were <LOD.
Compared with other groups, children whose mothers were non-Hispanic white, >25 years of age, married or living with a partner, and had a family income >$80,000 had higher FSIQ and Adaptive Skills scores at 8 years (Table 1). Maternal IQ, maternal serum ∑PCB concentrations, and HOME Inventory scores at age 1 year were positively correlated with child FSIQ and Adaptive Skills scores, whereas maternal BDI depression score, maternal blood lead levels, and serum cotinine levels were inversely correlated with FSIQ and Adaptive Skills scores at 8 years (Table 2).
Children whose mothers’ urine Cd concentrations were <LOD had the highest scores for FSIQ (106.1±15.9) and Adaptive Skills (51.3±8.1) at age 8 years compared to children of mothers who had detectable Cd levels (Table 3).
Table 3.
Maternal urine cadmium levels at 26 weeks of gestation and child neurodevelopmental test scores at age 1, 2, 3, 4, 5, and 8 years
| Group 1 |
Group 2 |
Group 3 |
||||
|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| MDI at age 1 | 118 | 94.0 ± 9.8 | 67 | 93.1 ± 9.4 | 73 | 93.9 ±10.6 |
| MDI at age 2 | 103 | 90.4 ±13.7 | 53 | 86.8 ±15.8 | 65 | 89.2 ±14.2 |
| MDI at age 3 | 92 | 95.5 ±13.9 | 49 | 93.5 ±13.4 | 59 | 91.6 ±12.7 |
| FSIQ at age 5 | 77 | 105.2 ±14.5 | 42 | 98.6 ± 14.5 | 38 | 102.2 ±15.5 |
| FSIQ at age 8* | 71 | 106.1 ± 15.9 | 53 | 97.1 ± 15.1 | 49 | 101.5 ± 15.3 |
| Adaptive Skills score at age 2 | 103 | 41.0±6.3 | 56 | 41.0±5.8 | 64 | 41.7±7.3 |
| Adaptive Skills score at age 3 | 98 | 50.8±5.9 | 51 | 50.0±7.2 | 58 | 50.8±8.2 |
| Adaptive Skills score at age 4 | 76 | 49.6±7.1 | 38 | 48.6±9.1 | 38 | 49.6±9.4 |
| Adaptive Skills score at age 5† | 80 | 52.8±7.7 | 42 | 48.2±9.5 | 41 | 52.6±8.8 |
| Adaptive Skills score at age 8 | 76 | 51.3 ± 8.1 | 53 | 47.5 ± 10.5 | 50 | 48.7 ± 10.6 |
Group 1: < LOD; Group 2: 0.06–0.22 μg/g creatinine; Group 3: >0.22 μg/g creatinine.
MDI, Mental Development Index; FSIQ, Full-Scale IQ; AKL, Adaptive Skills score; SD, standard deviation.
p<0.05 for FSIQ at age 8 years.
p<0.05 for Adaptative Skills score at age 5 years.
The statistical test included analysis of variance.
In repeated measurements analysis, maternal urinary Cd concentrations were not significantly associated with MDI/FSIQ or any of the four composite BASC-2 scores. We analyzed the interaction between maternal Cd exposure and child age on neurobehavioral outcomes. For MDI/FSIQ, the p-value for the interaction term was 0.09. Positive associations were observed between maternal Cd levels and MDI at age 1 year with statistical significance. Compared with children in the Group 1 who had prenatal Cd levels <LOD, MDI scores were 3.8 (95% CI: 0.1, 7.5) points higher among children in Group 2, and 4.0 (95% CI: 0.1, 7.8) points higher among those in Group 3. The associations between maternal Cd and cognition assessed in later years were not statistically significant (Figure 1). Similarly, at age 2 years, we observed positive associations between maternal Cd levels and Adaptive Skills. When compared to Group 1, children in Groups 2 and 3 had parent-reported behavioral scores that were 2.9 (95% CI: 0.4, 5.3) and 3.8 (95% CI: 1.3, 6.2) points higher on Adaptive Skills, respectively. At age 3 years or later, the associations were positive but not statistically significant (Figure 2). The interaction term of maternal Cd and child age on Adaptive Skills was not statistically significant (p=0.4). There were no significant associations between maternal Cd exposure and Externalizing Problems, Internalizing Problems, or the Behavior Symptoms Index at any age of assessment (Supplemental Figure 2).
Figure 1.
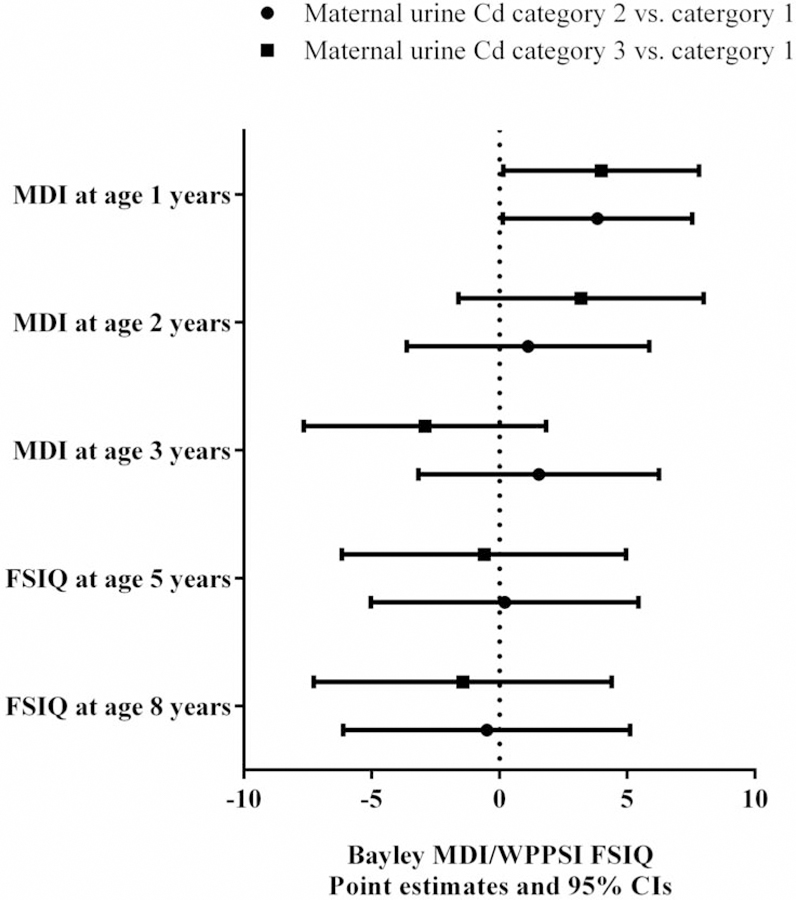
Estimated score differences and 95% CIs in MDI/FSIQ at ages 1–8 years by maternal urinary Cd concentrations. Covariates adjusted for include maternal age at enrollment, race, household income, marital status, child sex, maternal IQ, maternal depression, maternal blood lead level, maternal serum ∑PCBs, maternal serum cotinine level, and HOME Score.
Figure 2.
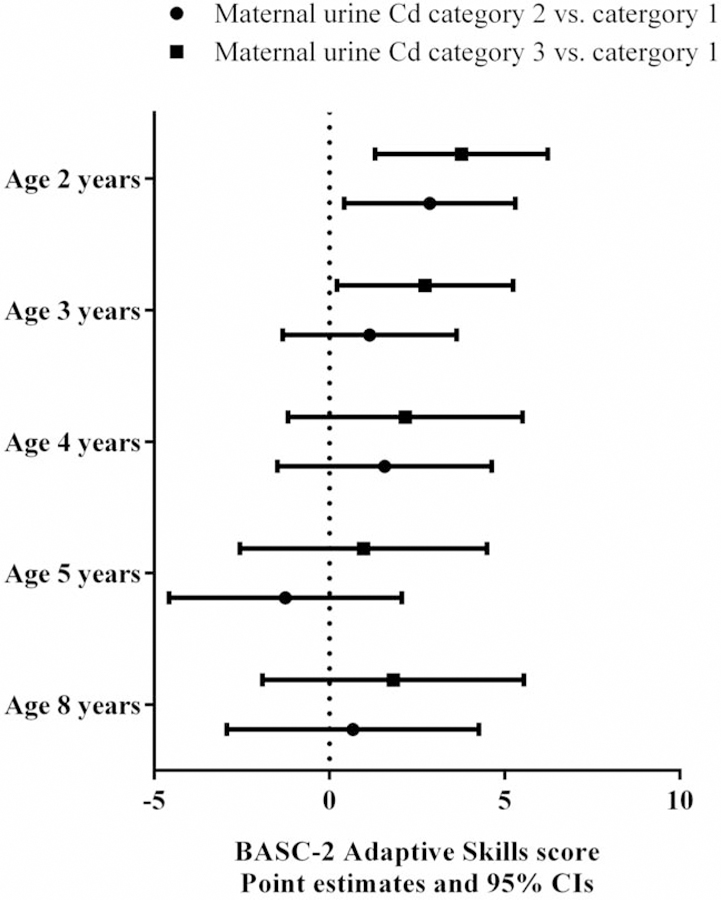
Estimated score differences and 95% CIs in BASC-2 Adaptive Skills score at ages 2–8 years by maternal urinary Cd concentrations. Covariates adjusted include maternal age at enrollment, race, household income, marital status, child sex, maternal IQ, maternal depression, maternal blood lead level, maternal serum ∑PCBs, maternal serum cotinine level, and HOME Score.
We additionally examined for potential interaction between child sex and Cd exposure. However, the interaction term was not significant (p>0.2). We also tested maternal urinary Cd as a continuous variable with adjustment for creatinine in linear mixed models and the results were similar (data not shown). For our sensitivity analyses of cotinine, we excluded 37 mothers who reported actively smoking tobacco during pregnancy. The analysis of nonsmokers yielded similar conclusions regarding associations between maternal Cd levels and MDI/FSIQ as well as for the four BASC-2 composite scores (data not shown). Findings from another sensitivity analysis that did not adjust for maternal serum cotinine levels were in accordance with the results of the fully adjusted models (data not shown).
Discussion
In this study, we examined prospective associations between prenatal exposures to Cd and child neurobehavior. We found that prenatal exposure to Cd, assessed from maternal urine measured at ~26 weeks of gestation, was not associated with neurobehavioral outcomes at ages 1 to 8 years, including MDI/FSIQ and four BASC-2 composite scores in the HOME Study children. While we observed positive associations between maternal urinary Cd concentrations and MDI/FSIQ as well as Adaptive Skills scores in early childhood (age 1–2 years), no associations were present in later childhood.
Compared with the adult central nervous system (CNS), which is protected from toxic agents by the blood-brain barrier, the developing CNS is more vulnerable to perturbations from toxicants (Rodier, 1995). Cd is regarded as a potential neurotoxicant with a long biological half-life and the ability to partially cross the placenta and blood-brain barrier (Geng and Wang, 2019; Wang and Du Y, 2013). Therefore, continuous exposure, even at low levels, may plausibly lead to adverse neurobehavioral effects. However, epidemiological evidence to support a relationship between gestational exposure to Cd and children’s neurobehavior is limited.
Only a small number of prospective studies have examined the relationship between prenatal exposure to Cd and neurobehavior. Findings from these studies have been equivocal. Previously, cohort studies in mainland China, Rural Bangladesh, and Korea have reported an inverse relationship between prenatal Cd levels and IQ test scores in pre-school children. High levels of Cd in cord blood were negatively associated with intelligence scores after controlling for lead exposure (Tian et al., 2009). In a population-based pregnancy and birth cohort in rural Bangladesh, Kippler et al. reported that early-life low-level Cd exposure was associated with lower child IQ scores at age 5 years (Kippler et al., 2012). A Korean cohort study concluded that maternal blood Cd concentration during early pregnancy was inversely associated with performance IQ but not with cognitive IQ at 60 months of month (Jeong et al., 2015). Another prospective birth cohort study found that maternal blood Cd concentration was inversely associated with infants’ developmental quotients in mainland China at age 12 months (Wang et al., 2016).
By contrast, in the Environmental and Childhood (INMA) Project, researchers did not observe an association between placental Cd concentrations and cognition at ages 4–5 years (Freire et al., 2018). Moreover, they found that Cd exposure was positively associated with perceptual-performance scores, indicating improvements. Similar null associations were also reported by Forns et al. in their examination of seven metal exposures (cobalt, copper, arsenic, Cd, antimony, thallium, and lead) in two different periods of pregnancy and neuropsychological development in preschool children (Forns et al., 2014). The heterogeneity of the conclusions could be due to sample size, study design, different biological samples of exposure (i.e. blood, urine, placenta etc.), age difference for neurobehavioral evaluation and different measures used, lack of adjustment for potential confounders, and other methodological constraints.
Cd can cause oxidative stress, interfere with neuronal differentiation and neurotransmitters, and disrupt fetal brain development (Andersson et al., 1997; Gulisano et al., 2009; Kippler et al., 2012; Mates et al., 2010). In our study, we observed that maternal urinary Cd levels were positively associated with MDI at age 1 year and with Adaptive Skills score at age 2 years. However, the positive relationship was no longer statistically significant after adjustment for essential covariates and potential confounders. In our data, over 40% had maternal urinary Cd concentrations below the LOD. The maternal urinary Cd concentrations were lower in our study than a previous Spanish study with null findings, in which over 80% were above LOD in the 1st and 3rd trimesters with a median of 0.55 μg/g creatinine and 0.53 μg/g creatinine, respectively (Forns et al., 2014). Importantly, the ratio between metal concentrations in cord blood and maternal blood was lower for Cd (close to 0.1) than lead (close to 0.8), indicating that Cd does not transfer as readily into fetal circulation (Kim et al., 2019). Moreover, Kim et al. suggested that low levels of Cd could have a protective effect against lead, a well-known neurotoxicant (Kim et al., 2013). Other biological evidence also shows that Cd could stimulate DNA synthesis, cell proliferation, and ovarian progesterone biosynthesis at low levels, while causing cell necrosis, chromosomal aberrations in different cell lines, and inhibiting progesterone biosynthesis at high levels (Henson and Chedrese, 2004; Templeton and Liu, 2010). Finally, the possibility of chance findings cannot be excluded.
Strengths and limitations
A major strength of our study is that we followed study participants with low levels of maternal urinary Cd concentrations and conducted standardized neurobehavioral measurements in a prospective birth cohort with repeated follow-ups between ages 1–8 years. The follow-up rates were 83%, 70%, 65%, 48%, 54%, and 58% at 1–8 years of age, respectively (Braun et al., 2017). We measured Cd concentrations in maternal urine instead of blood, which is more representative of long-term exposure. We collected an extensive set of covariates and were able to adjust for a variety of potential confounders, including maternal depression, maternal IQ, sociodemographic factors, and exposure to tobacco, lead, ∑PCBs. But the possibility of unmeasured confounding factors, such as the effects of genetic influences or dietary habits, cannot be completely ruled out. Also, the relatively high LOD in our assay may influence the precision of measurements, especially for the lower exposure levels. Due to the physiological changes during pregnancy, the renal handling of creatinine may alter, leading to variations of correcting for the dilution of urinary biomarker concentrations (MacPherson et al., 2018). A further limitation is that postnatal Cd exposures were not considered in our analysis, and other studies have indicated that postnatal exposure may increase the risk of adverse neurobehavioral outcomes (Ciesielski et al., 2012; Rodriguez-Barranco et al., 2014). Further, toxicological studies indicate Cd may have an antagonistic relationship with Pb (Kim et al., 2013). This documented interplay with Pb may be only one of many unknown interactions between other environmental contaminants. As such, epidemiological studies examining Cd may benefit from examining this exposure as part of a mixture, which is more representative of a real-life exposure scenario. We were further limited by our modest sample size, particularly in the exploration of potential sex differences. Additional studies using multiple prospective measurements with a larger sample size will have more sufficient power to comprehensively examine the role of maternal Cd exposure in children’s neurobehavior.
In summary, we did not find that prenatal exposure to Cd was adversely associated with MDI/FSIQ or behavior measurements in children. Future studies are warranted to consider both pre- and postnatal exposure to Cd.
Supplementary Material
Supplemental Figure 1. A directed acyclic graph (DAG) to determine minimal sufficient adjustment sets for estimating the total effect of maternal Cd on child neurodevelopment. The minimal sufficient adjustments sets include child sex, family income, marital status, maternal BDI, maternal IQ, maternal PCB, maternal age at enrollment, maternal blood lead, maternal race, and maternal serum cotinine.
Supplemental Figure 2. Estimated score differences and 95% CIs in BASC-2 composite measures of Externalizing Problems, Internalizing Problems, and Behavior Symptoms Index at ages 2–8 years by maternal urinary Cd concentrations. Covariates adjusted for include maternal age at enrollment, race, household income, marital status, child sex, maternal IQ, maternal depression, maternal blood lead level, maternal serum ∑PCBs, maternal serum cotinine level, and HOME Score.
Highlights:
Low levels of prenatal cadmium exposure were found in maternal urine
Repeated neurobehavioral assessments were conducted between age 1–8 years
Maternal urinary cadmium was not adversely associated with child cognitive function
Prenatal cadmium exposure was not adversely associated with behavioral measurements
Funding
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES024381, R01 ES025214, R01 ES014575, R00 ES020346, P30ES006096; EPA P01 R829389).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study protocol was approved by the Institutional Review Board (IRB) at the Cincinnati Children’s Hospital Medical Center (CCHMC). The Centers for Disease Control and Prevention (CDC) deferred to the CCHMC IRB as the IRB of record.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Andersson H, et al. , 1997. Low-level cadmium exposure of lactating rats causes alterations in brain serotonin levels in the offspring. Neurotoxicol Teratol 19, 105–15. DOI: 10.1016/s0892-0362(96)00218-8 [DOI] [PubMed] [Google Scholar]
- Bayley N, 1993. Bayley scales of infant development: Manual. Psychological Corporation. [Google Scholar]
- Beck AT, et al. , 1996. Beck depression inventory-II. San Antonio: 78, 490–498. [Google Scholar]
- Braun JM, et al. , 2017. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 46, 24 DOI: 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH, 1984. Home observation for measurement of the environment. University of Arkansas at Little Rock Little Rock. [Google Scholar]
- Chow ES, et al. , 2008. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat Toxicol 87, 157–69. DOI: 10.1016/j.aquatox.2008.01.019 [DOI] [PubMed] [Google Scholar]
- Ciesielski T, et al. , 2012. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ Health Perspect 120, 758–63. DOI: 10.1289/ehp.1104152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, et al. , 2014. Chemical mixtures and childrenʼs health. Current Opinion in Pediatrics. 26, 223–229. DOI: 10.1097/MOP.0000000000000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espart A, et al. , 2018. Cadmium exposure during pregnancy and lactation: materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics 10, 1359–1367. DOI: 10.1039/c8mt00174j [DOI] [PubMed] [Google Scholar]
- Faroon O, et al. , 2012. Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry (US), Atlanta (GA). [PubMed] [Google Scholar]
- Feng J, et al. , 2019. Maternal exposure to cadmium impairs cognitive development of offspring by targeting the Coronin-1a signaling pathway. Chemosphere. 225, 765–774. DOI: 10.1016/j.chemosphere.2019.03.094 [DOI] [PubMed] [Google Scholar]
- Forns J, et al. , 2014. Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology. 40, 16–22. DOI: 10.1016/j.neuro.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Freire C, et al. , 2018. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: The Environment and Childhood (INMA) Project. Sci Total Environ 621, 340–351. DOI: 10.1016/j.scitotenv.2017.11.273 [DOI] [PubMed] [Google Scholar]
- Geng HX, Wang L, 2019. Cadmium: Toxic effects on placental and embryonic development. Environ Toxicol Pharmacol 67, 102–107. DOI: 10.1016/j.etap.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Gulisano M, et al. , 2009. Cadmium modulates proliferation and differentiation of human neuroblasts. J Neurosci Res 87, 228–37. DOI: 10.1002/jnr.21830 [DOI] [PubMed] [Google Scholar]
- Henson MC, Chedrese PJ, 2004. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med (Maywood). 229, 383–92. DOI: 10.1177/153537020422900506 [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 5, 46–51. [Google Scholar]
- Jeong KS, et al. , 2015. Performance IQ in children is associated with blood cadmium concentration in early pregnancy. Journal of Trace Elements in Medicine and Biology. 30, 107–111. DOI: 10.1016/j.jtemb.2014.11.007 [DOI] [PubMed] [Google Scholar]
- Kim S, et al. , 2019. Metal concentrations in pregnant women and neonates from informal electronic waste recycling. J Expo Sci Environ Epidemiol 29, 406–415. DOI: 10.1038/s41370-018-0054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, et al. , 2013. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: The Mothers and Children’s Environmental Health (MOCEH) study. NeuroToxicology. 35, 15–22. DOI: 10.1016/j.neuro.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Kippler M, et al. , 2012. Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Environ Health Perspect. 120, 1462–8. DOI: 10.1289/ehp.1104431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CD, et al. , 2013. Casarett and Doull’s toxicology: the basic science of poisons. McGraw-Hill Education, New York. [Google Scholar]
- Larsen K, 1972. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clinica chimica acta; international journal of clinical chemistry. 38, 475 DOI: 10.1016/0009-8981(72)90146-5 [DOI] [PubMed] [Google Scholar]
- Lauwerys RR, et al. , 1994. Cadmium: exposure markers as predictors of nephrotoxic effects. Clin Chem 40, 1391–4. [PubMed] [Google Scholar]
- Lin CM, et al. , 2011. Does prenatal cadmium exposure affect fetal and child growth? Occup Environ Med 68, 641–6. DOI: 10.1136/oem.2010.059758 [DOI] [PubMed] [Google Scholar]
- Luparello C, et al. , 2011. Cadmium as a transcriptional modulator in human cells. Crit Rev Toxicol 41, 75–82. DOI: 10.3109/10408444.2010.529104 [DOI] [PubMed] [Google Scholar]
- MacPherson S, et al. , 2018. Adjusting urinary chemical biomarkers for hydration status during pregnancy. J Expo Sci Environ Epidemiol 28, 481–493. DOI: 10.1038/s41370-018-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates JM, et al. , 2010. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med 49, 1328–41. DOI: 10.1016/j.freeradbiomed.2010.07.028 [DOI] [PubMed] [Google Scholar]
- McAleer MF, Tuan RS, 2001. Metallothionein overexpression in human trophoblastic cells protects against cadmium-induced apoptosis. In Vitr Mol Toxicol 14, 25–42. DOI: 10.1089/109793301316882522 [DOI] [PubMed] [Google Scholar]
- R Core Team, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2019. [Google Scholar]
- Reynolds CR, Kamphaus RW, 2004. Behavior assessment system for children, (BASC-2). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Rodier PM, 1995. Developing Brain as a Target of Toxicity. Environmental Health Perspectives. 103, 73–76. DOI: 10.1289/ehp.95103s673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barranco M, et al. , 2014. Cadmium exposure and neuropsychological development in school children in southwestern Spain. Environ Res 134, 66–73. DOI: 10.1016/j.envres.2014.06.026 [DOI] [PubMed] [Google Scholar]
- Rollin HB, et al. , 2015. Prenatal Exposure to Cadmium, Placental Permeability and Birth Outcomes in Coastal Populations of South Africa. PLoS One. 10, e0142455 DOI: 10.1371/journal.pone.0142455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2014. Cadmium exposure and the epigenome: Exposure-associated patterns of DNA methylation in leukocytes from mother-baby pairs. Epigenetics 9, 212–21. DOI: 10.4161/epi.26798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AP, et al. , 2015. Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Current Environmental Health Reports. 2, 284–294. DOI: 10.1007/s40572-015-0058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolakis V, et al. , 2013. Developmental neurotoxicity of cadmium on enzyme activities of crucial offspring rat brain regions. Biometals 26, 1013–21. DOI: 10.1007/s10534-013-9678-3 [DOI] [PubMed] [Google Scholar]
- Templeton DM, Liu Y, 2010. Multiple roles of cadmium in cell death and survival. Chem Biol Interact 188, 267–75. DOI: 10.1016/j.cbi.2010.03.040 [DOI] [PubMed] [Google Scholar]
- Tian LL, et al. , 2009. Effects of gestational cadmium exposure on pregnancy outcome and development in the offspring at age 4.5 years. Biol Trace Elem Res 132, 51–9. DOI: 10.1007/s12011-009-8391-0 [DOI] [PubMed] [Google Scholar]
- Vacchi-Suzzi C, et al. , 2016. Is urinary cadmium a biomarker of long-term exposure in humans? A review. Current environmental health reports. 3, 450–458. DOI: 10.1007/s40572-016-0107-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Du Y, 2013. Cadmium and its neurotoxic effects. Oxid Med Cell Longev 2013, 898034 DOI: 10.1155/2013/898034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. , 2016. Effects of prenatal exposure to cadmium on neurodevelopment of infants in Shandong, China. Environ Pollut 211, 67–73. DOI: 10.1016/j.envpol.2015.12.038 [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1999. Abbreviated scale of intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D, 2003. Wechsler intelligence scale for children–Fourth Edition (WISC-IV). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D, 2004. Wechsler preschool and primary scale of intelligence—third edition. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wood SN, 2017. Generalized additive models: An introduction with R, second edition.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. A directed acyclic graph (DAG) to determine minimal sufficient adjustment sets for estimating the total effect of maternal Cd on child neurodevelopment. The minimal sufficient adjustments sets include child sex, family income, marital status, maternal BDI, maternal IQ, maternal PCB, maternal age at enrollment, maternal blood lead, maternal race, and maternal serum cotinine.
Supplemental Figure 2. Estimated score differences and 95% CIs in BASC-2 composite measures of Externalizing Problems, Internalizing Problems, and Behavior Symptoms Index at ages 2–8 years by maternal urinary Cd concentrations. Covariates adjusted for include maternal age at enrollment, race, household income, marital status, child sex, maternal IQ, maternal depression, maternal blood lead level, maternal serum ∑PCBs, maternal serum cotinine level, and HOME Score.


