Abstract
Recent advances in stem cell biology and tissue engineering have laid the groundwork for building complex tissues in a dish. We propose that these technologies are ready for a new challenge: recapitulating cardiac morphogenesis in vitro. In development, the heart transforms from a simple linear tube to a four-chambered organ through a complex process called looping. In this perspective, we re-examine heart tube looping through the lens of an engineer and argue that the linear heart tube is an advantageous starting point for tissue engineering. We summarize the structures, signaling pathways, and stresses in the looping heart, and evaluate approaches that could be used to build a linear heart tube and guide it through the process of looping.
Keywords: heart tube looping, tissue engineering, organogenesis, stem cells, biomaterials, mechanobiology
Why Build a Heart Tube?
Significant advances have been made in understanding the mechanisms of embryogenesis with over two centuries of studies in several species (e.g., chicken, mouse, human embryos) [1–4]. Animal models have provided a wealth of biological information on the most primitive structures of the developing heart through cell-fate mapping studies, histological sectioning, and three-dimensional (3D) reconstructions [5,6]. However, given the ethical concerns of studying human embryos, there is a paucity of human data to compare to development in animal models and extrapolate differences. Therefore, there is a need to build human models of heart development that allow for examination of innate cellular behavior and their contribution to cardiac morphogenesis and function. Specifically, we propose that modern tissue engineering approaches and stem-cell derived heart-cell populations can be combined to begin engineering models of the earliest structure in the sequence of embryonic heart development – the linear heart tube. Given its tubular structure, comparatively simpler to the chambered heart associated with later points of development, the linear heart tube would be an approachable starting point to model. By providing a “seed structure” of the primitive heart (i.e. the linear heart tube at stage 10) and promoting its morphogenesis, this approach would provide an opportunity to learn more about developmental biology through reverse engineering. In turn, this would be a critical step for developing more representative models of heart development that can be used for more advanced modeling of heart disease, have applications in regenerative medicine, and enable biological discovery.
What We Know: The Basics of Heart Tube Looping
The primitive heart tube is one of the first structures to form in the developing embryo, just three weeks after fertilization in humans (Figure 1A) [7]. The rightward bending or looping of the heart tube is the first event that breaks developmental symmetry in the embryo and is highly conserved among vertebrates. Furthermore, each of the stages of heart development are highly conserved among vertebrates although the speed and timing of these events are variable (Figure 1B). This intricate and robust process requires timely positioning, migration, proliferation and cell-fate specification of several cell populations in concert with embryonic structural and mechanical cues.
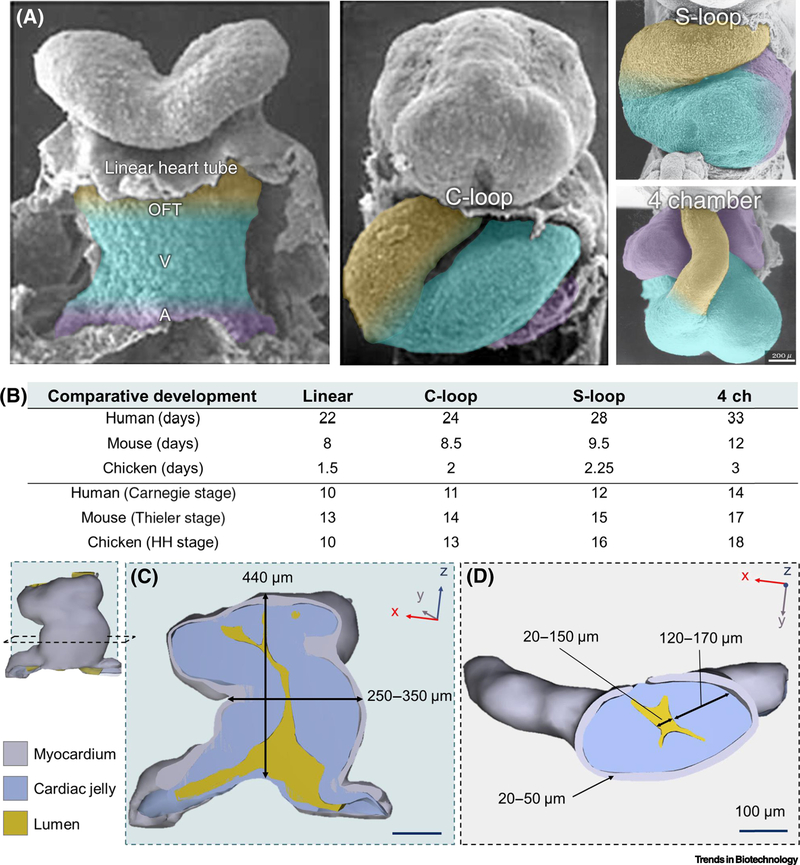
Figure 1. Blueprints of the lopping human heart tube.
(A) SEM images of the developing human heart tube from linear hear tube, C-looped, S-looped, and four-chambered heart (4 ch, bottom right). Future outflow tracts (OFT; yellow), ventricles (V; blue), and atria (A; purple) are color coded to highlight the complex structural transition the heart tube undergoes during morphogenesis. Color coding is a rough anatomical estimation and not exact. Images of the linear and C-loop heart tube stages were adapted from UNSW Embryologyi (https://embryology.med.unsw.edu.au), and S-loop and 4 chamber stages were adapted from [75]. (B) Comparison of the heart’s developmental timeline in different vertebratesii by days post-fertilization and speciesspecific developmental stages for human (Carnegieiii), mouse (Thieleriv [76]), and chick (Hamburger-Hamiltonv (HH) [2]). Comparisons were based on whole embryo developmental stages and do not always reflect the relative stages of cardiac development for each species. (C) 3D reconstruction of the human linear heart tube at stage 10 viewed with a Z-axis or longitudinal cross-section. Measurements of the tube’s height and diameter from the outer-most myocardial walls are overlaid. (D) Y-axis or crosswise section of the linear heart tube and thickness ranges of the myocardial wall (grey), lumen (yellow), and cardiac jelly (blue) layers. In each reconstruction, the surrounding coelomic walls, foregut, or neural tubes are excluded for clarity. All reconstructions and overlaid measurements in (C) and (D) are adapted with permission from the 3D Atlas of Human Embryology (specimen 6330) [77]. Multiple (n=5) measurements of each structure were taken along the z-axis from each cross-sectional view plane in (C) and (D) to create a range of size values. Measurements were taken from a 3D-rendered PDFs using the snap-to-edge tool which is not as accurate as using more precise 3D rendering software but are provided to give an approximate range.
The linear heart tube first consists of two layers of beating heart cells (or cardiomyocytes) encircling a layer of cardiac jelly (see Glossary), which separates the cardiomyocytes from an inner endocardial cell layer that lines the hollow lumen of the heart tube [8,9]. Fate-mapping studies have revealed that certain regions of the linear heart tube are destined to become the different atrial and ventricular chambers of the adult heart (Figure 1A) [10]. The following steps of development act to fold these regions into their anatomical locations within the four-chambered heart [7]. Shortly after the linear heart tube forms it begins to swell in size and rotate, or loop, to the right side to create a C-shaped bend (C-looped heart tube). Subsequently, the section of the heart tube that will become the ventricles moves downward forming the S-looped heart tube. The last stage of looping occurs by nestling the aorta between the developing atria to begin forming a four-chambered organ. These dimensions and basic structural specifications provide several biological design constraints, or “blueprints,” that can be used as a benchmark for developing tissue engineering strategies to model heart morphogenesis in the dish.
The Blueprints: Tissue Engineering the Looping Heart Tube
The heart tube exhibits incredibly complex geometric changes during looping and the identities of the comprising cell populations are also dynamic throughout cardiac morphogenesis (Box 1) [10]. The power of stem cell technology has allowed derivation of specific cardiac cells and cardiac progenitors [11,12]. However, in traditional cell culture, these cells exist in a foreign two-dimensional world compared to the three-dimensional (3D) microenvironments where cells reside in native tissues. To recapitulate heart tube looping in the dish, a 3D environment is almost certainly required. Fortunately, tissue engineering approaches are able to create 3D microenvironments and structural features that resemble native tissue [13]. Currently, no single approach can achieve every design requirement of the heart tube, but with a combination of methodologies and/or further technological advancement this goal could be achieved (Table 1).
Box 1. Cell populations for engineering the heart tube.
The heart begins as two populations of cells within what is called the primitive streak (see Glossary) that migrate upwards toward the anterior or head end of the embryo to form overlapping cardiogenic fields known as the first and second heart fields. These fields migrate toward each other and fuse to form the linear heart tube and begin to provide the first heartbeat. The power of stem cell technology has allowed derivation of cells types from almost every tissue in the body [11], including specific cardiac cells such as cardiac progenitors (first and second heart fields) [11], atrial and ventricular cardiomyocytes [12,71], endocardial cells [72], and endothelial cells [73,74]. Most cardiac tissue engineered platforms utilize more matured or committed cardiomyocytes from stem cell populations as opposed to earlier first and second heart field cell populations. It is worth discussing which cell populations should be utilized to model the developing heart tube in an engineered system. Although a number of cell populations could be incorporated into an engineered model, the cell source should also correlate with the engineering approach taken. For example, if utilizing an organoid approach, starting with an earlier lineage, such as first and second heart field populations, would allow for study of more cell-driven morphogenesis. However, if providing a more constrained or pre-defined environment, such as with bioprinting specific tissue geometries, it might be more useful to incorporate a more committed cardiomyocyte cell population as opposed to a more naïve one. Going forward in building advanced models of heart tube looping and development, one should consider deeply which cell sources are utilized in the context of the engineering approach and the question being explored.
Table 1.
Potential tissue engineering approaches for building a heart tube.
| Formation of tube structures | Fine feature resolution (<50 μm) | Multiple cell types | Compatible with diverse matrices | Enables self-assembly | References | |
|---|---|---|---|---|---|---|
| Bioprinting | ••• | • | •• | •• | • | [12–14,27] |
| Organoids | •• | ••• | •• | • | ••• | [9,20–23] |
| Cell sheet engineering | •• | ••• | •• | • | •• | [18,24–25] |
| Tissue casting & molding | ••• | • | •• | •• | • | [11,15–19] |
Has been achieved
Possible with minor modifications
Requires further development
To recapitulate the heart tube’s shape (Figure 1C–D), it would be important to be able to place different cell types (e.g. myocardial vs. endocardial) within 3D space to create a hollow structure. Some technologies are already intrinsically capable of fabricating complex tissue structures that would readily enable the development of a tubular structure at scale. For example, bioprinting [14] offers the ability to spatially control the placement of hydrogels (or bioinks) in three dimensions. It has recently been used to print structures resembling the branching, multiscale architecture of the vascular network [15,16]. These networks contained perfusable vessels as small as 100 μm in diameter, which is close in scale to the heart tube’s inner (~15–150 μm) and outer (~300 μm) diameters (Figure 1C–D). To fabricate the macroscopic structure of the heart tube, tissue casting and molding could provide a compatible approach. Casting and molding approaches have been utilized to create several tissue geometries, such as strips [17,18], patches [19,20], tubes [21], and even heart ventricle shapes [22]. Although molding and casting have not yet been used to fabricate tissue structures on the scale of the heart tube, < 300 μm, pushing this boundary will likely be easiest for simple geometries like a hollow tube.
Bioprinting and tissue casting approaches are useful for recapitulating the heart tube’s macroscopic structure, however they still need further optimization to achieve the finer resolution required to position cells into layers only two cells wide (~20–50 μm), as found in the myocardial layer of the heart tube (Figure 1D) [9]. For example, organoids offer a facile method to create small cell clusters (10’s −1000’s of cells) with higher cellpacking densities and some control over input cell population identity and ratios [23]. The self-assembled structures in organoids often feature repeating units only a few cell layers thick, like those seen in heart tube looping [24,25]. However, to create tubular structures on the scale of the linear heart tube (diameter ~300 μm), organoids currently require further assembly to form large organized structures [26,27]. Another approach to that could achieve higher cellular resolution would be cell-sheet engineering, which has enabled the use of cell monolayers (~10 μm) to engineer thick (~50–200 μm) functional tissues one cell layer at a time [28,29]. Cell sheets have also been wrapped around scaffolding materials to create tubular structures (e.g. inner diameter = 2 mm, cell layer thickness ~ 200 μm, [21]), which could be useful to model the 3D geometry of the heart tube while also recreating its layered nature and cellular density [9]. A challenge to this approach is that sheets do not have the structural integrity to form a free-standing tube without additional scaffolding (e.g. [21]) and would also require down-scaling to achieve < 300 μm macroscopic features.
To enable the heart tube’s dynamic movement during looping in a tissue engineered model, the final engineered structure should be free to move in 3D space. Therefore, the matrix material that encapsulates or comprises that structure must allow for cells to easily remodel it and mimic the environmental stiffnesses that cells would experience in vivo [30]. Bioprinting and tissue molding approaches already use customizable matrices to control the cross-linking properties of hydrogels or maintain a specific shape [31]. Although these biomaterials can be tuned, formulating hydrogels to have extremely soft biological stiffnesses like the cardiac jelly’s [30] can make fabricating free-standing 3D structures nearly impossible. It may be necessary to develop novel bioprinting or casting approaches that incorporate the self-assembling nature of the organoid platforms to allow the cells themselves to drive morphogenesis as they naturally would in vivo. It is possible that by using a combinatorial tissue engineering approach to spatially organize cells into structures that mimic the native heart tube’s geometry and matrix properties, that the C-looping process in the dish could be initiated.
Pulling Levers: Modulating Genes and Signaling Pathways
While biofabrication strategies can produce the structure of the linear heart tube, looping requires orchestrated signaling cues that direct cells to migrate, proliferate, and differentiate throughout normal development [32]. The signaling pathways that govern heart tube looping are associated with specific spatiotemporal patterns of gene expression [33] (Figure 2). For example, in the linear heart tube, cells in areas that eventually form the ventricles and atria up-regulate cardiac differentiation pathways like Nkx2–5 and Foxh-1 [34]. Cells that form the atrioventricular canal, on the other hand, show signs of down-regulation of these pathways as well as up-regulation of many noncardiac genes [35]. There are several factors and pathways that act as drivers of physiological change. Some of them are known to act in a specific region of the looping heart (Figure 2A, Tbx family of proteins [36], BMP2/4 [35], BMP10 [37], Notch [35], Hippo [38], FGF8/10 [39] pathways), while others are believed to act before tube formation, establishing asymmetry that later leads to looping (Nodal [40], Wnt/β-catenin [41]). The path to recreating these events in an engineered model is through dynamic control over both the cells and their environment over time. There are at least three existing approaches that enable these types of patterning: source-sink gradients of soluble factors, functional hydrogels, and optogenetic approaches (Figure 2B).
Figure 2. Spatiotemporal patterns of gene expression for heart looping may be engineered in vitro.
(A) Select signaling pathways and factors involved in the development of the looping heart tube that can potentially be controlled using existing techniques. The looping heart tube can be broadly divided into four areas based on what part of the heart they will form: outflow tract, future ventricles, future atria, and future atrioventricular canal. Cells in each of those areas have unique gene and protein expression profiles that allow them to specialize in performing their specific tasks. While previously published reviews described these expression profiles in detail [6,34–36,38– 41], here we have identified the few that can potentially be controlled using currently existing techniques (specific reference numbers provided in figure image). (B) Techniques for controlling heart tube looping-associated pathways. We have identified three main existing approaches to modulating signaling pathways in engineered or explanted native tissues: controlled diffusion chambers for creating gradients of soluble factors, functionalized hydrogels, and optogenetic methods. Diffusion-based techniques rely on controlling incubation chamber geometry to prevent convective flows, controlling medium flow in a microfluidic chamber, or both. Functional hydrogels allow for photopatterning and photorelease of a variety of factors with micron-level resolution. Additionally, they allow for control of the mechanical properties of local cell environment via photomediated gel cross-linking or degradation. Finally, optogenetic methods rely on genetic modifications of the cells allowing for spatiotemporal up- and downregulation of specific expression factors in those cells using light.
Controlled concentration gradients of soluble proteins and small molecules can be created using source-sink diffusion chambers to modulate signaling pathways in engineered tissues [42]. For example, Tabata and colleagues created concentration gradients of leukemia inhibitory factor in 3D hydrogels to spatially control differentiation of mouse embryonic stem cells [43]. These methods do not require chemical modification and are therefore compatible with virtually all commercially available proteins and small molecules. However, such techniques have geometric limitations because they require restriction of convective flow so that the protein gradient can be controlled by diffusion only. In engineering a heart tube, these methods could be used to create linear gradients along the length of a tube or radial gradients across a tube wall to mimic the spatial distribution of soluble factors in the heart tube.
Functional hydrogels have recently emerged as a useful tool for spatiotemporal patterning of signaling molecules inside engineered tissues [44,45]. They allow patterning of soluble factors, trans-membrane proteins, and extracellular matrix (ECM) proteins to mimic native cell-cell and cell-ECM interactions. These hydrogels can be patterned with micron-level resolution and the patterned molecules can later be released using a pre-determined stimulus, such as light, pH, matrix metalloproteinases, or a combination thereof [44,46]. For example, Shadish and colleagues were able to achieve dynamic control of proliferation and cell signaling by photo-patterning and photo-releasing growth factors in hydrogels with micrometer resolution [47]. While these techniques often require modification of the patterned molecule that may change its activity, the resolution and versatility of functional hydrogels enables biomimicry and signaling engineering at the scale of the human heart tube.
Optogenetic approaches have been used to regulate cell pathways in engineered systems [48,49]. They allow for direct activation of intracellular signaling pathways using light activated proteins, but they require genetic engineering of specific cell lines. For example, Arrenberg and colleagues created genetically encoded, light-sensitive cardiomyocytes in zebrafish whose contractions could be controlled by light, allowing them to study cardiac arrhythmias in living organisms [48]. Optogenetic control of cardiomyocyte contraction could be used for simulating the electrophysiology of the developing heart tube [50]. Optogenetic approaches could be used to control signaling pathway activation on an individual cell basis to mimic the signaling observed in heart tube looping [51]. The resolution of optogenetic techniques is similar to photosensitive hydrogels and is limited by the resolution of the light source, but can achieve control at subcellular levels [52].
Stress Testing: Loads on the Developing Heart Tube
The changes in cellular composition and the activation of signaling pathways are coupled with structural and functional changes in and around the heart tube. From a mechanical perspective these changes manifest as forces in several broad categories (Figure 3). Reaction forces arise at the connections between the heart tube and the developing vasculature and where other organs impinge on the heart [53,54]. These can change throughout development as other organs grow and physically exert their influence on the heart. Forces internal to the heart tube arise from growth and proliferation of cells and the remodeling of different matrix components [55]. The contraction of myocardial cells and formation of a patent lumen lead to the development and strengthening of hemodynamic forces, which play a key role in the continued development of the heart [56]. Experimental modification of the forces applied to developing animal hearts is associated with dysfunction and can be used to model congenital heart defects [57]. Once the structure of the heart tube has been fabricated, these dynamic loads on the developing heart must be replicated.
Figure 3. Mechanobiological cues are critical in heart tube morphogenesis.
(A) Forces acting on the heart tube can arise from (1) boundary conditions and reaction forces at the inflow at outflow tracts, (2) cellular forces from the growth, proliferation, and contraction of the cells of the heart tube, (3) hemodynamic forces from the flow of blood through the heart tube, and (4) forces from the growth and extension of the embryo and organs that border the heart tube. (B) The result of these loads on the heart tube are deformations that contribute to C-looping, including extension and bending at the heart-foregut interface, bending due to cell differential cell growth and contraction, and distention of the heart tube lumen due to hemodynamic pressure changes. Color in (B) represents strain given the applied tissue deformation.
The development of the heart is synchronized with the growth of other organs in the embryo. Contact with these growing structures, including the developing foregut and the rapidly remodeling vasculature, induce additional forces on the heart tube. As a result of this positioning the stresses and strains applied by the endoderm appear to help elongate the heart tube before and during looping [53,58]. Abstracting these conditions establishes targets for external forces that should be applied to surface boundaries of the heart tube. Computational models and studies in model organisms can provide estimates for applied forces and strains on the early stage heart [58]. The supports at either end of the engineered heart tube should mimic those found in vivo, with appropriate degrees of freedom and programmed changes in position and fixation. A variety of approaches could be used to accomplish this goal. For example, engineered constructs mounted on motorized supports could be stretched [59] to mimic the extension of the heart tube. More complex actuation could be achieved using motorized micromanipulators that can grab and twist tissues, but it may be cumbersome to apply multiple forces simultaneously due to space constraints [60]. Micromanipulation strategies that apply forces remotely through magnetic or acoustic fields also have resolution at the heart tube scale. Magnetic particles incorporated into the tissue could be actuated by external magnetic fields to apply controlled forces, although these particles can have biological effects themselves [60,61]. Acoustic tweezers which use sound waves to induce localized forces could be used without incorporating additional components into the tissue itself [62]. These field effect methods often have limited range and strength, which may not scale well as the tissue grows larger. Another approach might utilize programmable shape-memory polymers adhered to the engineered tissue that could replicate the normal bending and twisting of the heart tube, but the temperatures needed to fully actuate some of these polymers may not be compatible with cell culture [63].
The cells of the heart tube also undergo dynamic changes that drive structural morphological changes. Hyperplasia and hypertrophy contribute to the greater curvature of the heart tube with such signals absent on the contralateral side [55]. Inducing cells to generate these forces in engineered tissues will require the use of previously introduced signaling strategies that both drive and inhibit localized growth and expansion. Sandwiched between the myocardial and endocardial layers of the heart tube is the cardiac jelly, a very soft gel (E ≈ 20 Pa) that plays an important role in cardiac morphogenesis [64]. Although not strictly required for the initial phase of C-looping [8], the cardiac jelly is a structural component that enables the heart tube to pump more efficiently in its earliest stage [65]. As looping advances the cardiac jelly remains an important component whose remodeling contributes to trabeculation as well as the formation of the heart valves. To mimic the biomechanical and biochemical changes of the cardiac jelly during looping smart biomaterials could be essential tools [66]. These materials could be engineered to tune the local mechanical (e.g. stiffness) and biochemical properties [67] of an engineered cardiac jelly over time in response to light, temperature, or other signals. Replicating the complex composition and soft mechanics of the cardiac jelly, however, will be challenging.
Facilitated by an unevenly distributed cardiac jelly, the valve-less heart tube’s beating leads to productive flow as early as the endocardial tubes fuse [5]. This fusion brings hemodynamic forces with it as shear forces stimulate endocardial-myocardial signaling and the pressure of blood flow and the remodeling cardiac jelly alters myocardial loading [68]. Estimates of these forces can draw from a large body of literature in model organisms where intracardiac pressure (3 – 30 mmHg), blood velocity, and beating frequency (1.5 – 3 Hz) are documented at the earliest stages of heart development [69].
These hemodynamic loads on the developing heart tube are some of the most straightforward forces to manipulate using syringe pumps and pressure controllers which can dynamically regulate the flow waveform when coupled with flow and pressure sensors.
Future Perspectives: New Hearts, New Hope
The transformation of the heart from a thin-walled linear tubular structure to a densely vascularized four-chambered organ is a process that has fascinated scientists for more than a century. Tremendous insight into this process of heart tube looping has come from animal models. However, subtle differences in heart development exist between species [70], so extrapolating to a human heart may be misleading. Additional answers to open questions on heart development could come through engineering a human model of it in the dish. Here, we argue for starting on the journey towards this goal. Advances in biomaterials, tissue and cell engineering, along with decades of accumulated knowledge of the mechanisms involved in cardiac development can provide the foundation necessary for taking the first steps toward a grand challenge of fabricating a complete human heart. The first of many milestones on this journey will be to re-create the process of C-looping using a complex combination of existing bioengineering approaches (see Outstanding Questions).
Outstanding Questions.
What are the right cells? A major challenge in engineering the heart tube is selecting the appropriate starting cell populations. While stem cell derived cardiomyocytes are less mature than adult cardiomyocytes, there is not enough evidence to suggest they are equivalent to cardiomyocytes that form the linear heart tube. Are the best cells for engineering the heart tube terminally differentiated cardiomyocytes? Or cells like those found in the first or second heart fields? Or something in between?
What are the right cues? Development is guided by complex spatiotemporal cues acting on a wide variety of signaling pathways. Despite considerable progress in our understanding of these pathways, the details of which cues are present in what locations, at what time, and in what concentrations are incomplete. What is the interaction between different spatial and temporal cues in heart tube looping? Are the responses to these cues a linear combination (i.e. superposition), or is there a combinatorial effect? What is the tolerance on the timing and localization of their presentation? Answers to these questions will establish new constraints on engineering the heart tube in a dish.
When do we let biology take over? The approaches in this article outline complex methods of dictating both the structure of a tissue and its interactions with the environment. The unknowns in this design space are vast and it is not clear what combination of prescribed (i.e. engineered or fabricated) and self-assembled structures and signaling will lead to replicating the process of development in a dish. How many degrees of freedom does the heart tube need (both mechanically and biochemically) to twist and turn into a four chambered heart? Can these structural changes be engineered in a way that is not representative of normal biology? Is looping purely a genetic program that will run its proper course given the right initial conditions? Or, can the process be nurtured along by careful design and advanced technology?
A looping engineered heart tube would be a powerful testbed to explore the fundamental mechanisms that drive heart morphogenesis. Although all the techniques and methods discussed above could be employed to accomplish this goal, further research into heart development is still required. Key insights need to be made into which signals or genes regulate looping and when they turn on or off in the process. Approaches that perturb these pathways in a model system like an engineered heart tube could be a way to learn how they are connected and how they orchestrate the process of cardiac development. However, it is important to be cautious of overengineering a system because though it might be possible to incur looping in the dish, the final process may not be representative of the true biology. Thus, it would be important to validate the observations made from these models with those from normal human development, but the data is sparse due to technical limitations and ethical issues.
Further morphogenesis beyond this first stage toward a four-chambered structure would require additional technological advances, including the integration of a supporting vasculature, the scale-up of applied mechanical cues, spatial accommodations for hypertrophic growth, and accuracy in manipulating a contractile tissue that is moving. Overcoming these challenges will have far-reaching impact in biomedical research, enabling a deeper understanding of cardiac development and providing researchers with a platform to methodically explore interventions for congenital heart defects. This knowledge could further be combined with advances in regenerative engineering approaches to address long-standing needs for more mature and functional in vitro cardiac tissues, with applications as therapeutics themselves or in the process of drug discovery.
Highlights Box.
There is high potential for current technologies to be used to recapitulate heart development in vitro by focusing on its earliest stage of morphogenesis: the embryonic heart tube.
Recent advances in stem cell biology have enabled the production of cardiac progenitor lineages for bioengineering approaches.
Biofabrication techniques can create structures and features of the heart tube at the appropriate scale.
Biochemical cues that govern cardiac morphogenesis can be controlled and studied through techniques like engineered diffusion gradients, functionalized hydrogels, and optogenetics.
Mechanical cues that influence cardiac growth and remodeling can be applied to engineered heart tubes using hemodynamic flow and direct or field-based micromanipulation techniques.
Acknowledgements
This work was supported by a generous donation from Gree Real Estate, as well as additional awards: NIH 1F31HL145809-01A1 (to N.P.W.), NIH F32HL143851 (to D.E.), NIH HL141187 and HL142624 (to J.D.), NIH R01HL146436 and UG3 EB028094 (to D.H.K.), NSF CAREER Award DMR 1652141 (to C.A.D.), NIH R01HL141570 (to Y.Z.), NIH DP2HL137188 (to K.R.S.), NSF CBET-1509106 & CMMI-1661730, NIH EB028094 (to N.J.S.). We also acknowledge the De Snoo – van ‘t Hoogerhuijs Foundation for supporting this work. We thank Jaco Hagoort for assisting in the creation of the 3D-rendered images of the human heart tube used throughout this manuscript.
Glossary
- Acoustic tweezers
A generic term for contactless technologies that use sound waves to precisely manipulate and exert forces on biological particles
- Atrioventricular canal
The region of the developing heart that eventually gives rise to the septum and valves between the atria and ventricles in the 4 chambered heart
- Bioprinting
An approach in which biomaterials and/or cells are assembled using one of several techniques that may involve extrusion or light to control the structure of the engineered tissue
- Cardiac jelly
A soft biomaterial that fills the space between the outer contractile layer of the heart tube and the inner lumen where blood flows
- Carnegie, Hamburger-Hamilton (HH), and Thieler stages
standardized systems used to characterize vertebrate embryological development based on gestational stage rather than time
- Cell-sheet engineering
An approach that involves the formation and assembly of individual cell sheets into multilayered structures that recompile
- Extracellular matrix
The network of extracellular biomolecules that provides structural and biochemical support to cells
- First and second heart fields (FHF/SHF)
two cardiogenic cell populations that eventually give rise to the heart’s structures, including the ventricles (from FHF), atria (SHF/FHF), and inflow and outflow tracts (SHF)
- Hemodynamic forces
The mechanical loads induced by blood flow, including pressure and shear stress
- Hydrogel
Water-swollen networks derived from natural and/or synthetic polymers commonly exploited in 3D cell culture
- Optogenetics
A genetic engineering approach that modifies cells to express light sensitive proteins that enable the control of certain biological functions
- Organoid
A tissue generated from the self-assembly of stem or progenitor cells into structures that replicate organ-specific functions
- Primitive streak
a linear band of thickened tissue that forms as the first structure in gastrulation of the early embryo that begins to form the different germ layers (mesoderm, endoderm, and ectoderm)
- Shape-memory polymers
Materials which can revert from a deformed shape to a pre-programmed permanent state based on environmental conditions
- Smart biomaterials
Materials that have been designed to respond to external stimuli by changing one or more of its physical or chemical properties
- Spatiotemporal patterning
The patterning of biochemical and biomechanical cues in an engineered tissue with controlled presentation in 3D space and time
- Tissue casting and molding
Utilizes pre-fabricated scaffolds or negative-space molds that are filled with a mixture of cells and a polymerizing matrix material which casts the cells into desired tissue shape
- Trabeculation
Refers to the process in which clusters of myocardial cells invade into the luminal space of the developing ventricular chambers to form thin muscular projections called trabeculae that are essential for proper cardiac development
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallingford JB (2019) The 200-year effort to see the embryo. Science 365, 758–759 [DOI] [PubMed] [Google Scholar]
- 2.Hamburger V and Hamilton HL (1992) A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. Off. Publ. Am. Assoc. Anat 195, 231–272 [DOI] [PubMed] [Google Scholar]
- 3.Becker JR et al. (2011) Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Dis. Model. Mech 4, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchardt T and Braun T (2007) Cardiovascular regeneration in non-mammalian model systems: what are the differences between newts and man? Thromb. Haemost 98, 311–318 [PubMed] [Google Scholar]
- 5.Ivanovitch K et al. (2017) Live imaging of heart tube development in mouse reveals alternating phases of cardiac differentiation and morphogenesis. eLife 6, e30668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Günthel M et al. (2018) Development, Proliferation, and Growth of the Mammalian Heart. Mol. Ther. J. Am. Soc. Gene Ther 26, 1599–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorman A et al. (2003) Development of the heart: (1) formation of the cardiac chambers and arterial trunks. Heart Br. Card. Soc 89, 806–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Männer J and Yelbuz TM (2019) Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos. J. Cardiovasc. Dev. Dis 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sizarov A et al. (2011) Formation of the Building Plan of the Human Heart: Morphogenesis, Growth, and Differentiation. Circulation 123, 1125–1135 [DOI] [PubMed] [Google Scholar]
- 10.Van Vliet P et al. (2012) Early cardiac development: A view from stem cells to embryos. Cardiovasc. Res 96, 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen P et al. (2018) Precardiac organoids form two heart fields via Bmp/Wnt signaling. Nat. Commun 9, 3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH et al. (2017) Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 21, 179–194.e4 [DOI] [PubMed] [Google Scholar]
- 13.Hughes AJ et al. (2018) Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Dev. Cell 44, 165–178.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan B (2017) State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann. Biomed. Eng 45, 195–209 [DOI] [PubMed] [Google Scholar]
- 15.Lee A et al. (2019) 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 [DOI] [PubMed] [Google Scholar]
- 16.Grigoryan B et al. (2019) Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bielawski KS et al. (2016) Real-Time Force and Frequency Analysis of Engineered Human Heart Tissue Derived from Induced Pluripotent Stem Cells Using Magnetic Sensing. Tissue Eng. - Part C Methods 22, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronaldson-Bouchard K et al. (2018) Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redd MA et al. (2019) Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat. Commun 10, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shadrin IY et al. (2017) Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun 8, 1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuruyama S et al. (2019) Pulsatile tubular cardiac tissues fabricated by wrapping human iPS cells-derived cardiomyocyte sheets. Regen. Ther 11, 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacQueen LA et al. (2018) A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng DOI: 10.1038/s41551-018-0271-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fatehullah A et al. (2016) Organoids as an in vitro model of human development and disease. Nat. Cell Biol 18, 246–254 [DOI] [PubMed] [Google Scholar]
- 24.Freedman BS et al. (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat. Commun 6, 8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancaster MA et al. (2017) Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol 35, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skylar-Scott MA et al. (2019) Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv 5, eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wimmer RA et al. (2019) Human blood vessel organoids as a model of diabetic vasculopathy. Nature 565, 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine H et al. (2013) In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiao A et al. (2018) Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell-secreted extracellular matrix. J. Biomed. Mater. Res. A 106, 1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao J et al. (2012) Viscoelastic material properties of the myocardium and cardiac jelly in the looping chick heart. J. Biomech. Eng 134, 024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miri AK et al. (2019) Multiscale bioprinting of vascularized models. Biomaterials 198, 204–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner M and Siddiqui M. a. Q. (2007) Signal transduction in early heart development (I): cardiogenic induction and heart tube formation. Exp. Biol. Med. Maywood NJ 232, 852–865 [PubMed] [Google Scholar]
- 33.Paige SL et al. (2015) Molecular regulation of cardiomyocyte differentiation. Circ. Res 116, 341–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava D (2006) Making or breaking the heart: from lineage determination to morphogenesis. Cell 126, 1037–1048 [DOI] [PubMed] [Google Scholar]
- 35.Rutenberg JB et al. (2006) Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Dev. Camb. Engl 133, 4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greulich F et al. (2011) Mechanisms of T-box gene function in the developing heart. Cardiovasc. Res 91, 212–222 [DOI] [PubMed] [Google Scholar]
- 37.Wilsbacher L and McNally EM (2016) Genetics of Cardiac Developmental Disorders: Cardiomyocyte Proliferation and Growth and Relevance to Heart Failure. Annu. Rev. Pathol 11, 395–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J et al. (2018) The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol 15, 672–684 [DOI] [PubMed] [Google Scholar]
- 39.Sirbu IO et al. (2008) Retinoic acid controls heart anteroposterior patterning by downregulating Isl1 through the Fgf8 pathway. Dev. Dyn. Off. Publ. Am. Assoc. Anat 237, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ocaña OH et al. (2017) A right-handed signalling pathway drives heart looping in vertebrates. Nature 549, 86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merks AM et al. (2018) Planar cell polarity signalling coordinates heart tube remodelling through tissue-scale polarisation of actomyosin activity. Nat. Commun 9, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regier MC et al. (2019) User-defined morphogen patterning for directing human cell fate stratification. Sci. Rep 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tabata Y and Lutolf MP (2017) Multiscale microenvironmental perturbation of pluripotent stem cell fate and self-organization. Sci. Rep 7, 44711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruskowitz ER and DeForest CA (2018) Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater 3, 17087 [Google Scholar]
- 45.Brown TE and Anseth KS (2017) Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev 46, 6532–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam RY et al. (2017) Engineering Cellular Microenvironments with Photo- and Enzymatically Responsive Hydrogels: Toward Biomimetic 3D Cell Culture Models. Acc. Chem. Res 50, 703–713 [DOI] [PubMed] [Google Scholar]
- 47.Shadish JA et al. (2019) Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater 18, 1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrenberg AB et al. (2010) Optogenetic control of cardiac function. Science 330, 971–974 [DOI] [PubMed] [Google Scholar]
- 49.Ambrosi CM et al. (2014) Cardiac applications of optogenetics. Prog. Biophys. Mol. Biol 115, 294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe M et al. (2016) Probing the Electrophysiology of the Developing Heart. J. Cardiovasc. Dev. Dis 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polesskaya O et al. (2018) Optogenetic regulation of transcription. BMC Neurosci. 19, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neill PR and Gautam N (2014) Subcellular optogenetic inhibition of G proteins generates signaling gradients and cell migration. Mol. Biol. Cell 25, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kidokoro H et al. (2018) The heart tube forms and elongates through dynamic cell rearrangement coordinated with foregut extension. Development 145, dev152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voronov DA et al. (2004) The role of mechanical forces in dextral rotation during cardiac looping in the chick embryo. Dev. Biol 272, 339–350 [DOI] [PubMed] [Google Scholar]
- 55.Shi Y et al. (2014) Bending of the Looping Heart: Differential Growth Revisited. J. Biomech. Eng 136, 081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poelmann RE and Gittenberger-de Groot AC (2018) Hemodynamics in Cardiac Development. J. Cardiovasc. Dev. Dis 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowalski WJ et al. (2014) Left atrial ligation alters intracardiac flow patterns and the biomechanical landscape in the chick embryo. Dev. Dyn. Off. Publ. Am. Assoc. Anat 243, 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosseini HS et al. (2017) A new hypothesis for foregut and heart tube formation based on differential growth and actomyosin contraction. Dev. Camb. Engl 144, 2381–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fink C et al. (2000) Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 14, 669–679 [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z et al. (2019) Robotic Micromanipulation: Fundamentals and Applications. Annu. Rev. Control Robot. Auton. Syst 2, 181–203 [Google Scholar]
- 61.Hoskins C et al. (2012) The cytotoxicity of polycationic iron oxide nanoparticles: Common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J. Nanobiotechnology 10, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozcelik A et al. (2018) Acoustic tweezers for the life sciences. Nat. Methods 15, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lendlein A and Gould OEC (2019) Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater 4, 116–133 [Google Scholar]
- 64.Zamir EA and Taber LA (2004) Material properties and residual stress in the stage 12 chick heart during cardiac looping. J. Biomech. Eng 126, 823–830 [DOI] [PubMed] [Google Scholar]
- 65.Boselli F et al. (2015) Blood flow mechanics in cardiovascular development. Cell. Mol. Life Sci 72, 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uto K et al. (2017) Dynamically tunable cell culture platforms for tissue engineering and mechanobiology. Prog. Polym. Sci 65, 53–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kloxin AM et al. (2009) Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Granados-Riveron JT and Brook JD (2012) The impact of mechanical forces in heart morphogenesis. Circ. Cardiovasc. Genet 5, 132–142 [DOI] [PubMed] [Google Scholar]
- 69.Lindsey SE et al. (2014) Mechanical regulation of cardiac development. Front. Physiol 5, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meyers EN and Martin GR (1999) Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 285, 403–406 [DOI] [PubMed] [Google Scholar]
- 71.Lian X et al. (2012) Cozzarelli Prize Winner: Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. 109, E1848–E1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bao X et al. (2017) Human pluripotent stem cell-derived epicardial progenitors can differentiate to endocardial-like endothelial cells. Bioeng. Transl. Med 2, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giacomelli E et al. (2017) Co-Differentiation of Human Pluripotent Stem Cells-Derived Cardiomyocytes and Endothelial Cells from Cardiac Mesoderm Provides a Three-Dimensional Model of Cardiac Microtissue. Curr. Protoc. Hum. Genet DOI: 10.1002/cphg.46 [DOI] [PubMed] [Google Scholar]
- 74.Palpant NJ et al. (2017) Generating high-purity cardiac and endothelial derivatives from patterned mesoderm using human pluripotent stem cells. Nat. Protoc 12, 15–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oostra R-J et al. , eds. (2007) Outlines of external development In Steding’s and Virágh’s Scanning Electron Microscopy Atlas of the Developing Human Heart pp. 5–47, Springer [Google Scholar]
- 76.Theiler K (1989) The House Mouse: Atlas of Embryonic Development, Springer-Verlag. [Google Scholar]
- 77.de Bakker BS et al. (2016) An interactive three-dimensional digital atlas and quantitative database of human development. Science 354, aag0053. [DOI] [PubMed] [Google Scholar]