The paralogous COPI coat subunit γ1-COP plays a unique role in promoting neurite outgrowth during the neuronal differentiation of mouse pluripotent cells.
Abstract
Coat protein complex I (COPI)–coated vesicles mediate membrane trafficking between Golgi cisternae as well as retrieval of proteins from the Golgi to the endoplasmic reticulum. There are several flavors of the COPI coat defined by paralogous subunits of the protein complex coatomer. However, whether paralogous COPI proteins have specific functions is currently unknown. Here, we show that the paralogous coatomer subunits γ1-COP and γ2-COP are differentially expressed during the neuronal differentiation of mouse pluripotent cells. Moreover, through a combination of genome editing experiments, we demonstrate that whereas γ-COP paralogs are largely functionally redundant, γ1-COP specifically promotes neurite outgrowth. Our work stresses a role of the COPI pathway in neuronal polarization and provides evidence for distinct functions for coatomer paralogous subunits in this process.
Introduction
In eukaryotes, membrane trafficking of cargo proteins and lipids is crucial to maintain cellular homeostasis and intracellular organelle identity. In the early secretory pathway in mammals, coat protein complex II (COPII) vesicles mediate the export of cargo from the ER to the Golgi apparatus, whereas COPI vesicles promote the retrieval of proteins from the Golgi to the ER and intra-Golgi transport. COPI vesicles are formed at the Golgi through the GTP-dependent recruitment of a coat protein complex, termed coatomer, by the small GTPase ARF1. Once recruited to membranes, coatomer polymerizes to form a lattice that shapes a nascent bud that eventually pinches off as a small-coated vesicle (Béthune & Wieland, 2018). Through sorting signals exposed on their cytoplasmic domains, transmembrane cargo proteins interact with coatomer and are taken up in COPI vesicles (Barlowe & Helenius, 2016). Coatomer is made of seven equimolar COP subunits (α-,β-,β′-,γ-,δ-,ε-, and ζ-COP) that are highly conserved from yeast to human. In mammals, two coatomer subunits come as two paralogs: γ1-COP and γ2-COP that share 80% protein sequence identity and that are encoded by the Copg1 and Copg2 genes (Blagitko et al, 1999), and ζ1-COP and ζ2-COP encoded by the Copz1 and Copz2 genes (Futatsumori et al, 2000). In the COPII system, paralogs of the SEC24 subunit expand the cargo repertoire of COPII vesicles by providing distinct binding sites for specific sorting motifs (Mancias & Goldberg, 2007, 2008; Bonnon et al, 2010; Adolf et al, 2016, 2019). By contrast, proteomics profiling of paralog-specific COPI vesicles generated in vitro from HeLa cells revealed no major difference in their cargo content (Adolf et al, 2019) and to date, no specialized function has been ascribed to the paralogous COP subunits, which until now have thus been considered as functionally redundant.
Whereas the general mechanisms of cargo sorting and formation of COPI vesicles are well described, cell type–specific functions are much less well studied. Several lines of evidence, however, suggest tissue-specific functions of the otherwise essential COPI pathway. Indeed, mutations affecting COP subunits have been associated to diseases, notably neurodegenerative disorders (Xu et al, 2010; Izumi et al, 2016; Bettayeb et al, 2016a, 2016b). Moreover, binding of α-COP to the survival motor neuron protein seems to promote neurite outgrowth in motor neurons (Li et al, 2015). As defects in the COPI pathway lead to specific effects in the nervous system, we decided to analyze the expression profile and function of the γ-COP paralogs during neurogenesis. By examining publicly available mRNA expression profiling data, we found that Copg1, but not Copg2, is up-regulated as mouse embryonic stem cells (mES) differentiate into neurons. We observed the same in murine pluripotent P19 cells, another model cell line for neuronal differentiation. Specific KO of Copg1 or Copg2 in P19 cells revealed that whereas both gene disruptions led to slower cell growth, neither of the two paralogs alone is essential for cell viability. Remarkably, whereas KO of Copg2 did not affect retinoic acid (RA)–mediated P19 cell neuronal differentiation, disruption of Copg1 led to the formation of loose embryoid bodies (EBs) and to reduced neurite outgrowth. Overexpression of γ2-COP or knock-in (KI) of Copg2 in the Copg1 locus revealed that whereas higher expression of γ2-COP can compensate for the loss of γ1-COP for the formation of EBs, γ1-COP is required to promote neurite outgrowth. Altogether, our findings support an essential role of COPI vesicles during neurogenesis and reveal for the first time a paralog-specific function for a COPI protein subunit.
Results
Differential expression of Copg1 and Copg2 during neuronal differentiation
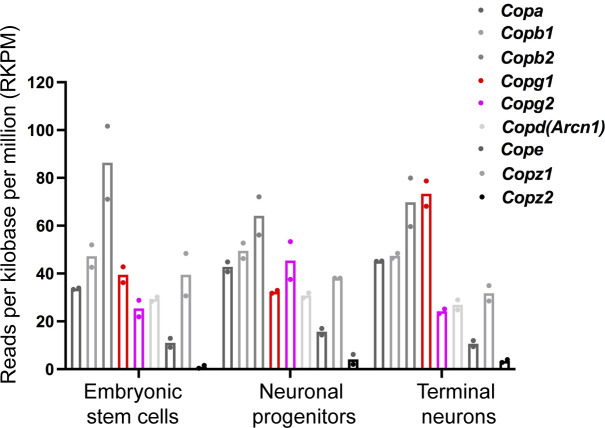
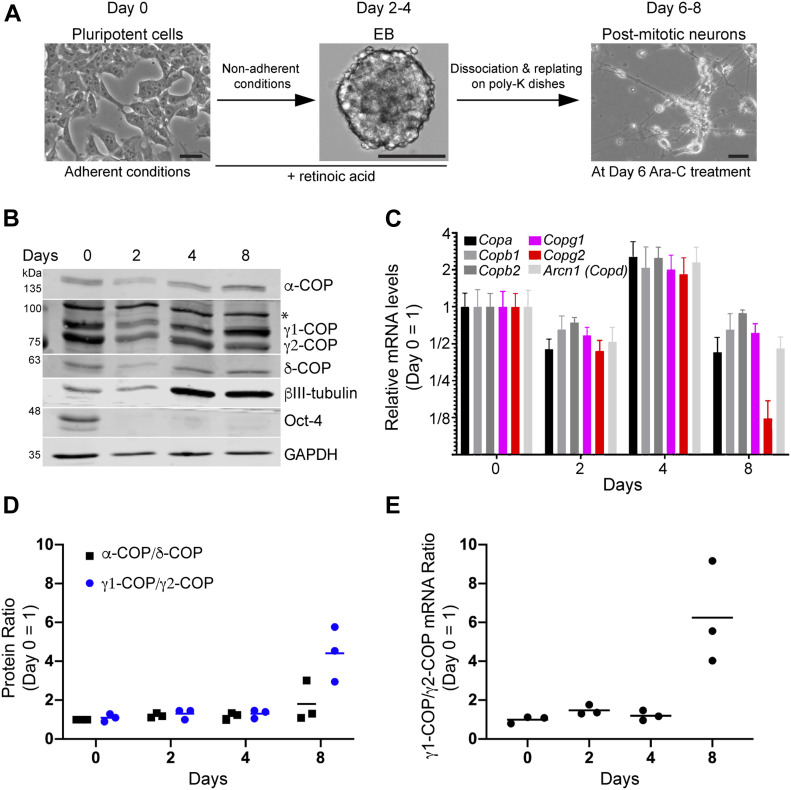
To investigate a potential paralog-specific function of COP subunits during neurogenesis, we first examined publicly available RNAseq expression profiling data performed on mES, their derived neuronal progenitors and terminal neurons (Tippmann et al, 2012). In the three differentiation stages, Copz2 was marginally expressed compared with the other COP subunits with expression levels 10–40 times lower than those of Copz1 (Fig S1). Interestingly, whereas all other COP subunit-coding genes were expressed at similar levels at all three differentiation stages, Copg1 was strongly up-regulated in terminal neuron (Fig S1) suggesting γ1-COP might exert a unique function during the biogenesis of neurons. To examine whether a differential expression of Copg1 and Copg2 is also observed in another neuronal differentiation system we then analyzed mRNA and protein levels of the two γ-COP paralogs in P19 embryonal carcinoma cells. Compared with mES, P19 cells are closer to epiblast-derived stem cells and are a well-established and robust model system for neuronal differentiation (Morassutti et al, 1994; Staines et al, 1994; Kelly & Gatie, 2017). Similar to mES, P19 cells can be differentiated into neurons applying a two-step differentiation protocol (Fig 1A). Cells are first incubated with RA-containing medium under non-adherent conditions to form EBs (day 2–4). EBs are then dissociated and the cells seeded under adherent conditions to form post-mitotic neurons (day 6–8). After this procedure, cell lysates were collected at different stages of differentiation and analyzed by western blot. As expected, the pluripotency marker Oct-4 was only detectable at day 0, whereas the neuron-specific class III beta-tubulin (βIII-tubulin or Tuj-1 antigen) is strongly up-regulated as the cells progress to day 8 (Fig 1B). The expression levels of different COP subunits were also assessed by western blot (Fig 1B) using previously characterized specific antibodies against each of the γ-COP paralog (Moelleken et al, 2007) and by RT-qPCR (Fig 1C). In the western blot analysis, we surveyed α-, γ-, and δ-COP as they belong to each of the three building blocks that make the stable heptameric complex. Indeed, under high salt conditions (Lowe & Kreis, 1995) or upon chemical modification of exposed lysines (Pavel et al, 1998), coatomer can be separated into the subcomplexes α-/β′-/ε-COP, γ-/ζ-COP, and β-/δ-COP. To analyze the relative expression of the two γ-COP paralogs independent of antibody-dependent signal intensities, we considered the ratio of the γ1-COP over γ2-COP signals during differentiation. This ratio was similar in pluripotent cells and EBs; by contrast, there was relatively more γ1-COP in neurons (Fig 1D). This is in contrast to the ratio of α-COP over δ-COP that remained stable over the whole differentiation. This was corroborated at the mRNA levels as Copg2 mRNA levels decreased at day 8 of differentiation compared with the transcripts of all the other COP subunits, including Copg1 (Fig 1C and E). Altogether, the expression of γ1-COP is increased compared with γ2-COP during the late stage of neuronal differentiation of pluripotent cells.
Figure S1. Differential expression of Copg1 and Copg2 mRNAs during neuronal differentiation of mouse embryonic stem cells.
mRNA-seq analysis of cDNAs derived from mouse embryonic stem cells, neuronal progenitors, and terminal neurons from two independent differentiation experiments (Tippmann et al, 2012). Normalized RKPM obtained for the COP subunits are indicated. The dataset used to generate this figure is publicly available on the GEO database (accession number GSE34473).
Figure 1. Differential expression of γ-COP paralogs during neuronal differentiation of P19 cells.
(A) Schematic representation of the two-step differentiation protocol. Scale bar is 50 μm. (B) Western blot analysis of P19 cell lysates at different stages of differentiation. The asterisk (*) marks a nonspecific signal. (C) Relative expression of mRNAs coding for the indicated COP subunits during neuronal differentiation by RT-qPCR. (B, C, D, E) Quantification (n = 3) of the γ1-COP/γ2-COP and α-COP/δ-COP protein and of the γ1-COP/γ2-COP mRNA ratios from (B, C) respectively; bars are means.
Source data are available for this figure.
Disruption of Copg1 or Copg2 in P19 cells
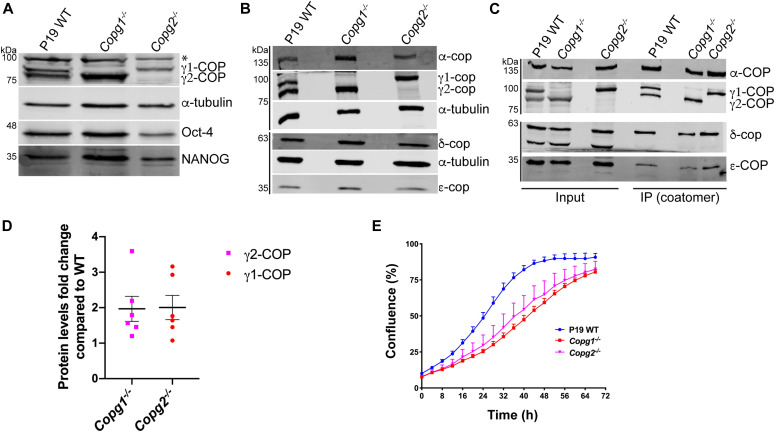
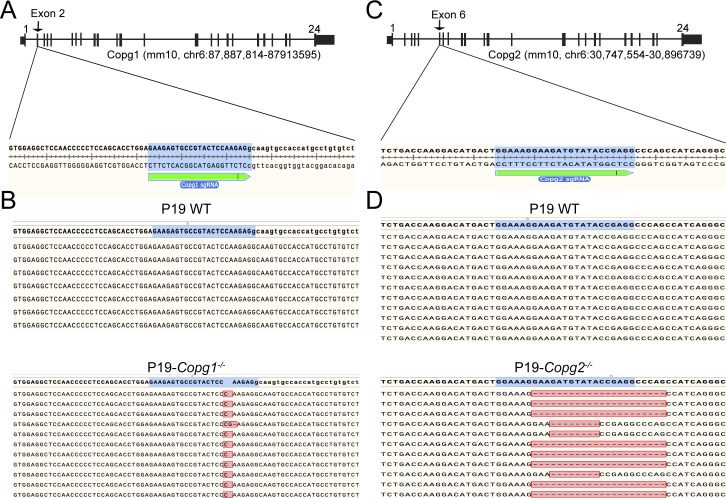
To study potential paralog-specific functions of COPI vesicles, we then generated Copg1 and Copg2 KO P19 cells. To do so, we followed a Cas9-mediated genome editing approach using individual specific single guide RNAs (sgRNAs) directed against either Copg1 or Copg2 (see the Materials and Methods section). After clonal selection, we obtained cell lines that showed absence of γ1-COP (Copg1−/− cells) or γ2-COP (Copg2−/− cells) by western blot analysis (Fig 2A). Moreover, bi-allelic disruption of Copg1 or Copg2 was confirmed by sequencing analysis of the respective genomic loci (Fig S2). Importantly, the KO and clonal selection procedure did not lead to loss of expression of the pluripotency markers Oct-4 and NANOG (Fig 2A), indicating that the KO cell lines are still undifferentiated cells.
Figure 2. Characterization of P19 KO cell lines.
(A) Western blot analysis of the expression levels of γ1-COP and γ2-COP, the pluripotency markers Oct-4 and Nanog, and α-tubulin as a loading control in pluripotent P19 WT and KO cell lysates. The asterisk (*) marks a nonspecific signal. (B) Western blot analysis of various COP subunits in pluripotent P19 WT and KO cell lysates. (C) Immunoprecipitation of native coatomer from P19 WT or KO cell lysates using the CM1 monoclonal antibody. Various COP subunits were analyzed by western blotting as indicated. (D) Ratios of γ2-COP western blot signals (normalized to α-tubulin) in Copg1−/− over WT cells, and of γ1-COP in Copg2−/− over WT cells (bars are means, error bars are SEM, n = 6). (E) Growth curves obtained from a real-time proliferation assay in which the occupied area (% confluence) by P19 WT and KO cells was monitored over 72 h. Curves were generated with the IncuCyte software (n = 5, error bars are SEM).
Source data are available for this figure.
Figure S2. Characterization of P19 Copg1−/− and Copg2−/− cell lines.
(A) Schematic of the Copg1 genomic locus, the sequence targeted by the Copg1 sgRNA is indicated. (B) Sanger sequencing data after PCR amplification of the targeted genomic region, subcloned into a plasmid, and screened for individual clones. In bold is the reference sequence. Sequencing from WT cells (top) indicate an intact sequence, sequencing from Copg1−/− cells (bottom) indicates two mutated alleles (insertion of one or two nt). (A, C) same as in (A) with the Copg2 genomic locus. (C, D) same as in (C) for the Copg2 targeted locus in Copg2−/− cells. Sequencing indicates two mutated alleles (deletion of 8 or 22 nt).
Characterization of Copg1 or Copg2 KO in pluripotent P19 cells
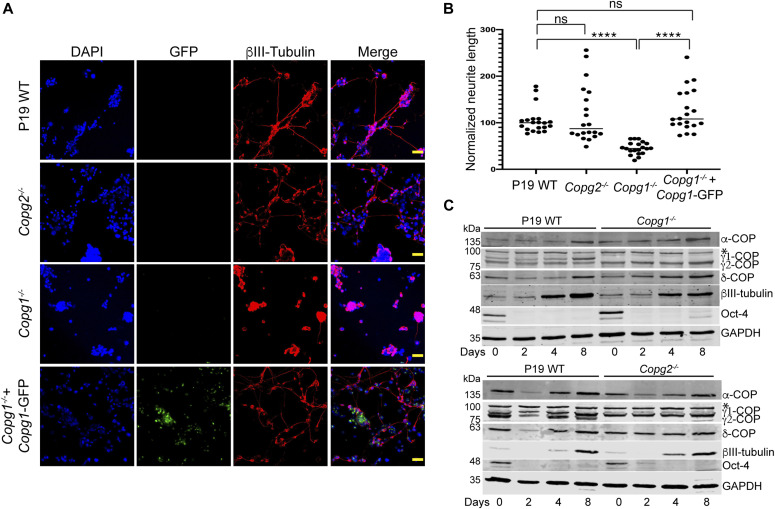
As a functional COPI pathway is essential for viability, obtaining individual Copg1 and Copg2 KO cells demonstrates that both γ-COP paralogs can fulfill essential functions of COPI vesicles and are thus at least partially redundant. It is, hence, expected that depletion of one or the other γ-COP paralog does not strongly affect the global expression levels of COP subunits and the assembly of the coatomer complex. To test these two predictions, we first analyzed the expression of several COP subunits in WT, and Copg1 and Copg2 KO cells. Western blot analysis revealed that expression of α-, δ-, and ε-COP is similar in the three cell lines. As coatomer is an equimolar heptameric complex, the latter observation implies that in the KO cells, the remaining γ-COP paralog is up-regulated. Accordingly, in western blot analyses, γ1-COP expression was reproducibly higher in Copg2−/− cells than in the WT situation, and conversely γ2-COP was up-regulated in Copg1−/− cells compared with WT (Fig 2B–D). Hence, overexpression of one paralog compensates at least partially for the absence of the second one and thus ensures that global coatomer levels are relatively maintained. To verify whether coatomer subunits are correctly incorporated into the complex in the absence of one of the γ-COP paralogs, immunoprecipitation experiments from WT and KO cell lysates were performed using the CM1A10 (CM1) antibody, which specifically recognizes native assembled coatomer (Palmer et al, 1993) probably through its β′-COP subunit (Lowe & Kreis, 1995). As α-, δ-, and ε-COP were precipitated from all three cell lines, this indicates a correct assembly of coatomer in the absence of one specific γ-COP paralog (Fig 2C). Finally, we performed a live cell proliferation assay by measuring the percentage surface occupancy of WT and KO cells in real time as they grow on a cell culture dish under standard conditions. In this assay, both Copg1 and Copg2 KO cells showed significantly slower proliferation rates than WT cells (Fig 2E), suggesting that even though both γ-COP paralogs are individually sufficient to mediate essential COPI functions, paralog-specific nonessential functions exist.
Aberrant Golgi morphology in Copg1 and Copg2 KO cells
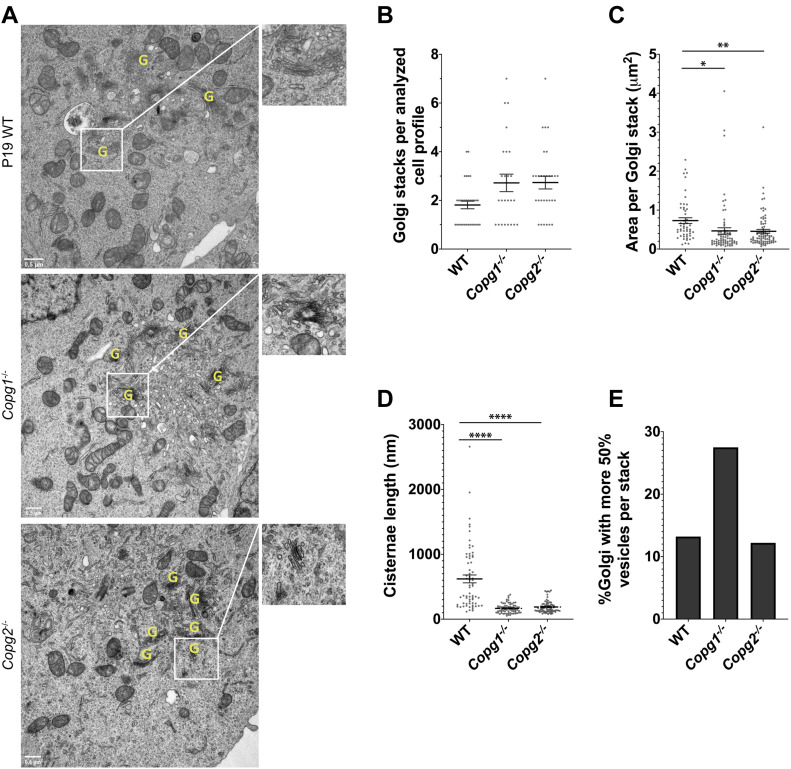
As COPI vesicles are generated at the Golgi, we then analyzed the morphology of that organelle by EM in WT, Copg1−/−, and Copg2−/− cells (Fig 3A). Quantitative assessment of 30 cell profiles per cell line containing at least one Golgi stack revealed an average higher number of Golgi stacks per cell in the absence of either γ1-COP or γ2-COP (2.7 Golgi/cell versus 1.8 in WT, Fig 3B). In addition, the average area per Golgi stack was smaller in both KO cell lines (455–466 × 103 versus 730 × 103 nm2 in WT, Fig 3C). Similarly, the average length per cisternae was shorter in the KO cell lines than in the WT cells (167–188 versus 620 nm in WT, Fig 3D). Specific to the depletion of γ1-COP was the observation of an increased number of Golgi peripheral vesicles (27.5% of observed Golgi for which vesicles accounted for more than 50% of the Golgi area versus 13% and 12% WT and Copg2−/− cells, respectively, Fig 3E) indicative of increased Golgi fragmentation. Altogether, both γ1-COP and γ2-COP are necessary for correct assembly and/or maintenance of the Golgi structure.
Figure 3. Depletion of γ1-COP or γ2-COP affects Golgi morphology.
(A) Electron micrographs of thin sections obtained from P19 WT, Copg1−/−, and Copg2−/− cells as indicated. Identified Golgi stacks are indicated by a yellow G. (B, C, D, E) Quantification of (B) average number of Golgi stacks per cell, (C) average area per Golgi stack, (D) average cisternae length, and (E) percentage of Golgi stacks with more than 50% of vesicles per stack (P-value: * < 0.05, ** < 0.01, **** < 0.0001, n = 30, except for (D): n = 66–72). In (B, C, D) bars are means and error bars are SEM.
Depletion of γ1-COP or γ2-COP does not lead to elevated ER stress
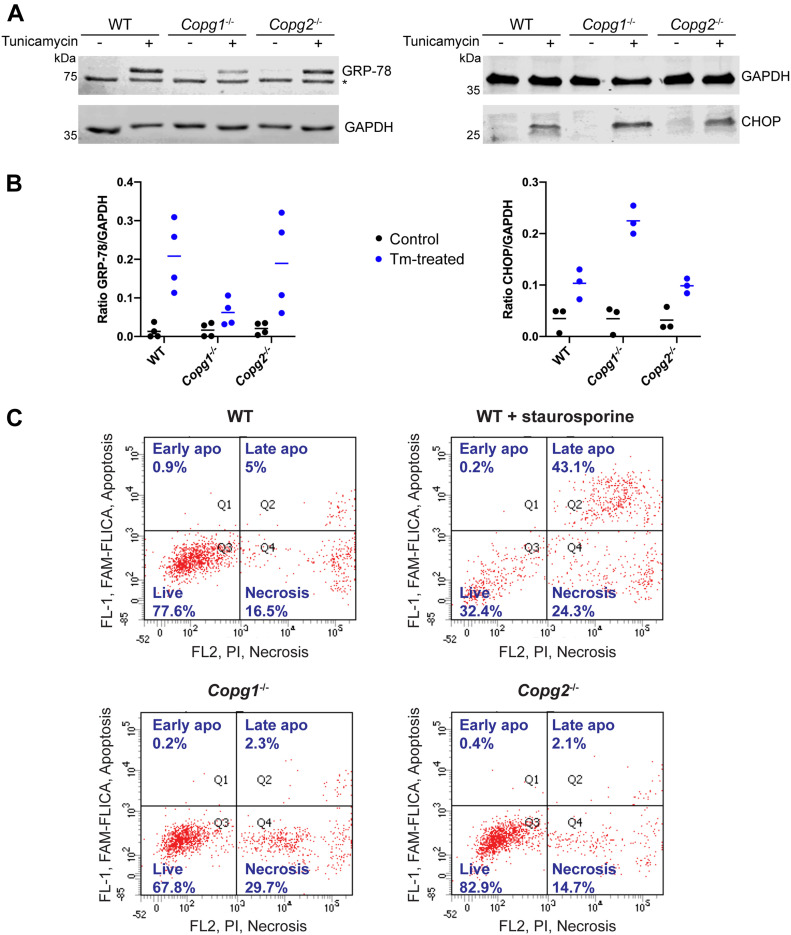
ER stress is known to induce Golgi fragmentation (Nakagomi et al, 2008) and mutations within COP subunits may lead to ER stress (Watkin et al, 2015; Izumi et al, 2016). As ER stress was reported to affect neuronal differentiation of P19 cells (Kawada et al, 2014), we investigated if depletion of γ1-COP or γ2-COP induces ER stress by assessing expression levels of the ER chaperone GRP-78 (alternatively known as Bip) and the transcription factor CHOP, two commonly used ER stress markers (Kennedy et al, 2015). After western blot analysis, we observed similar low expression of GRP-78 and CHOP in WT, Copg1−/−, and Copg2−/− cells (Fig 4A and B). By contrast, induction of GRP-78 and CHOP was clearly visible in cells in which ER stress was induced by tunicamycin, a glycosylation inhibitor that induces the unfolded protein response (Oslowski & Urano, 2011). We note that Copg1−/− cells responded differently to tunicamycin treatment with a lower GRP-78 and a higher CHOP expression when compared with WT and Copg2−/− cells. GRP-78 is a luminal ER protein with a KDEL (Lys-Asp-Glu-Leu) retention signal at its C terminus. The KDEL sequence of escaped ER-resident proteins is recognized at the Golgi by the KDEL receptor which, when complexed with its ligands, is transported back to the ER by COPI vesicles (Jin et al, 2017). CHOP induces the expression of apoptotic genes and is normally up-regulated upon chronic ER stress when the cells cannot cope anymore with the load of misfolded protein in the ER (Oyadomari & Mori, 2004). One possibility that may explain the phenotype of Copg1−/− cells is that in these cells, retrograde transport to the ER might be more affected than in Copg2−/− cells, leading to impaired retention of GRP-78 in the ER. This might render Copg1−/− cells more sensitive to ER stress and thereby lead to a stronger induction of apoptosis via CHOP. At that point, we have not further explored this possibility. In any case, our data suggest that depletion of γ1-COP or γ2-COP per se does not lead to elevated ER stress.
Figure 4. Depletion of γ1-COP or γ2-COP neither induces ER stress nor apoptosis.
(A) Western blot analysis of the expression of the ER stress markers GRP-78 and CHOP and of GAPDH in P19 WT, Copg1−/−, and Copg2−/− cells. When indicated, ER stress was induced with tunicamycin. The asterisk indicates a nonspecific signal. (A, B) Quantification of (A). Tm, tunicamycin. (C) Dual fluorescence flow cytometry assay to detect activated caspase-3/7 (FL1, FAM-FLICA) and membrane damage (FL2, propidium iodide) in P19 WT, Copg1−/−, and Copg2−/− cells. Apoptosis (apo) was induced in WT cells with staurosporine as indicated. The percentage of cells detected in each quadrant is indicated (n = 4,686–7,145 cells).
Source data are available for this figure.
Depletion of γ1-COP or γ2-COP does not lead to increased apoptosis
Golgi fragmentation can also be a consequence of apoptosis (Hicks & Machamer, 2005). Because depletion of γ1-COP or γ2-COP led to reduced proliferation rates (Fig 2E), we asked if both proteins are needed to maintain the survival of P19 cells. To do so, we monitored the activity of caspase-3/-7 using FLICA (Fluorescent Labeled Inhibitors of Caspases) probes (Bedner et al, 2000). To distinguish apoptosis from necrosis, cells were counter-stained with the membrane impermeant DNA dye propidium iodide (PI). Thus, flow cytometry analysis allows distinguishing living (FLICA and PI negative), necrotic (FLICA negative and PI positive), early apoptotic cells (FLICA positive and PI negative), and late apoptotic cells (FLICA and PI positive). By contrast to cells treated with staurosporine, an apoptosis-inducing drug through the activation of caspase-3 (Chae et al, 2000), we did not observe elevated apoptotic activity when comparing Copg1−/− and Copg2−/− to WT cells (Fig 4C). Hence, depletion of γ1-COP or γ2-COP does not lead to increased apoptosis.
Expression of γ1-COP is necessary for the formation of tight embryonic bodies
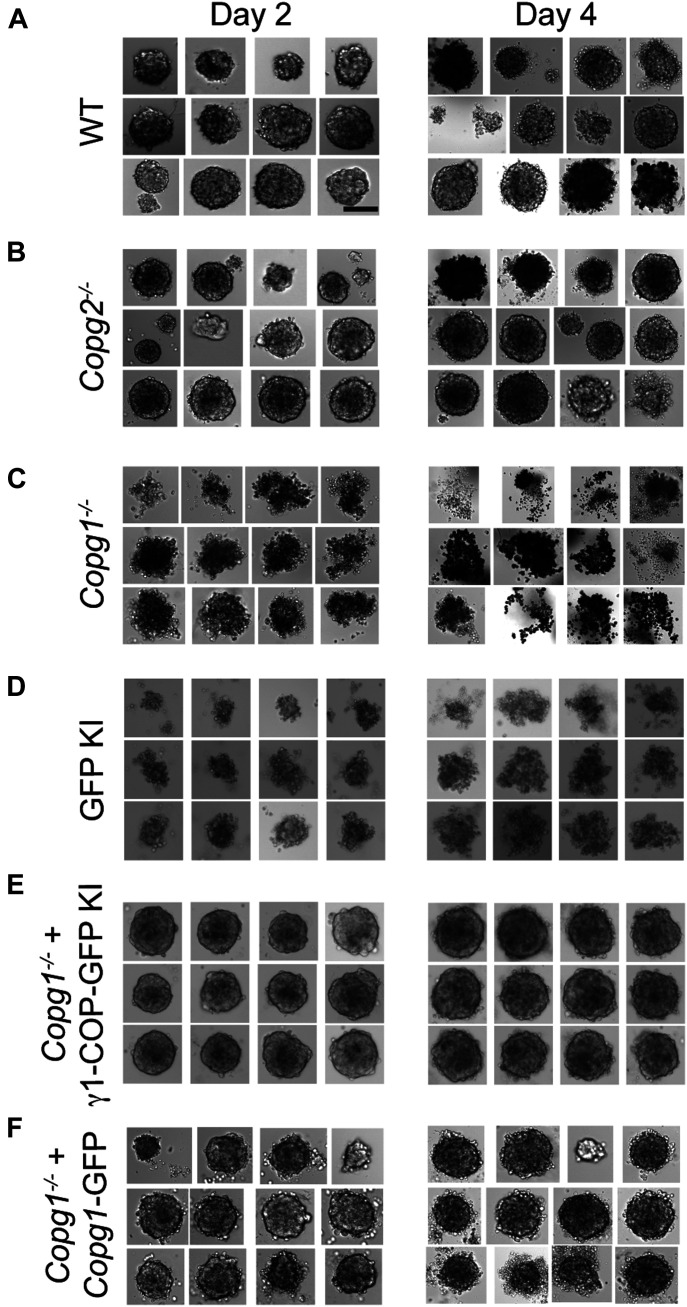
We went on to analyze whether γ-COP paralogs have specific functions during neurogenesis. To address this, P19 WT and KO cells were submitted to the two-step differentiation protocol described in Fig 1A, in which cells are first led to aggregate under non-adherent conditions to form EBs. In this first step, cell aggregation in EBs is essential for the subsequent formation of RA-induced P19 neurons (Jones-Villeneuve et al, 1982; Pachernik et al, 2005). In initial experiments, we performed EB formation in bacterial dishes as described in the original differentiation protocol (Lee et al, 2007). Under these conditions, we noticed that EBs generated from Copg1−/− cells seemed smaller and exhibited a less compact morphology than EBs from WT and Copg2−/− cells. However, as EBs were free floating in the dishes, it was difficult to obtain pictures and to perform quantitative analyses. To investigate EB morphology in a more controlled way, we turned to a hanging drop protocol in which 200 dissociated cells are seeded in hanging drops of 20 μl RA-containing cell culture medium. The drops were then analyzed by light microscopy at day 2 and day 4 of differentiation. Typically, in the WT situation, virtually all cells had aggregated to form a single, sometimes two, spherical EBs with sharp boundaries per drop (Fig 5A). At day 4, EBs appeared slightly larger and denser than at day 2 and also sometimes started to disaggregate. The morphology of EBs obtained from Copg2−/− cells was similar to WT EBs, indicating that γ2-COP is not necessary for EB formation (Fig 5B). In contrast, EBs obtained from Copg1−/− cells had a strikingly distinct morphology with much less well-defined shapes and boundaries, and a general looser and more scattered appearance. At day 4 of differentiation, the difference was even more striking with hardly recognizable tight EBs and many disaggregated cells in the drops (Fig 5C). One possible explanation for this phenotype might be that in Copg1−/− cells, cell adhesion proteins are less efficiently trafficked to the cell surface.
Figure 5. Depletion of γ1-COP affects the formation of embryoid bodies.
(A, B, C, D, E, F) Photographs of embryoid bodies from P19 WT (A), Copg2−/− (B), Copg1−/− (C), GFP KI (D), Copg1−/− + γ1-GFP KI (E), and Copg1−/− + Copg1-GFP (F) cells formed in hanging drops over 2 or 4 d of culture as indicated. (A) Scale bar in (A) is 50 μm and applies to all panels.
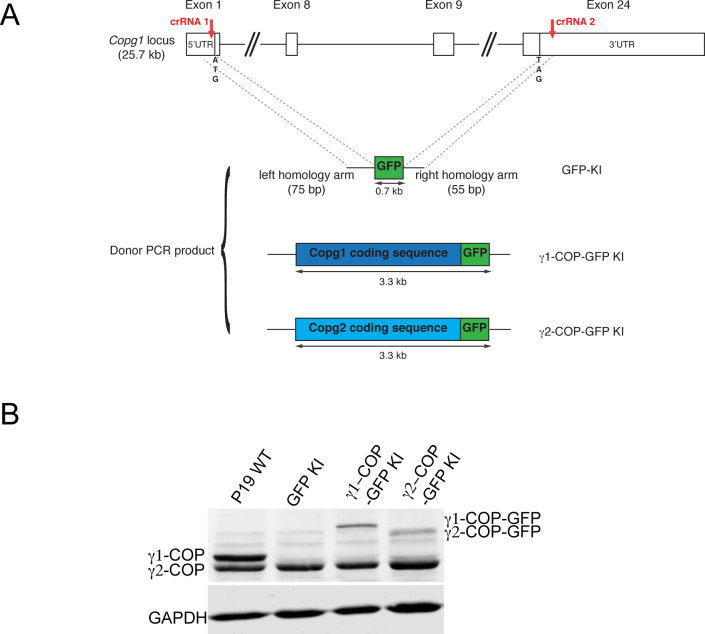
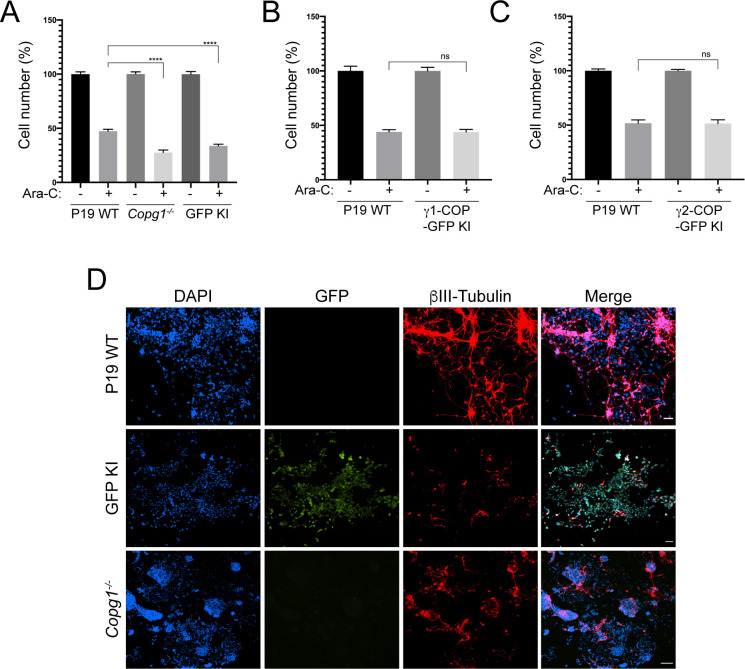
These observations suggest that expression of γ1-COP is necessary for EB formation. However, as the cell line used in these experiments was obtained from a clonal selection after a Cas9-induced double-strand break within the Copg1 gene, we decided to perform additional experiments aimed at addressing potential off-target effects of the sgRNA or clone-specific phenotypes. First, we constructed another Copg1 KO cell line using a different strategy. Instead of inducing the formation of indels within the Copg1 gene, the whole Copg1 locus was removed by two single cuts induced by the Cas12a enzyme guided by two CRISPR RNAs (crRNAs) and replaced by the coding sequence of either GFP (P19 GFP KI cell line) or of γ1-COP-GFP (P19 γ1-COP-GFP KI cell line) as a control (Fig S3A and B, and see the Materials and Methods section). In these KI cell lines, disruption of the Copg1 locus (GFP KI) also led to strongly impaired EB formation, whereas replacement of the Copg1 locus by the coding sequence of γ1-COP-GFP had no effect (Fig 5D and E). The fact that both the Copg1−/− and GFP KI cell lines show similar phenotypes but are two different clones derived from two different genome editing strategies strongly suggests that the absence of γ1-COP is the cause of defective EB formation.
Figure S3. Generation and characterization of P19 knock-in (KI) cell lines.
(A) General strategy to obtain KI cell lines. Schematic of the Copg1 genomic locus, the sites targeted by the crRNAs to guide two Cas12a-induced cleavages are indicated. The three donor PCR products with short homology arms that direct homology-directed repair are also represented. (B) Western blot analysis of the expression levels of γ1-COP and γ2-COP, and GAPDH as a loading control in pluripotent P19 WT, GFP KI, γ1-COP-GFP KI, and γ2-COP-GFP KI cell lysates.
Source data are available for this figure.
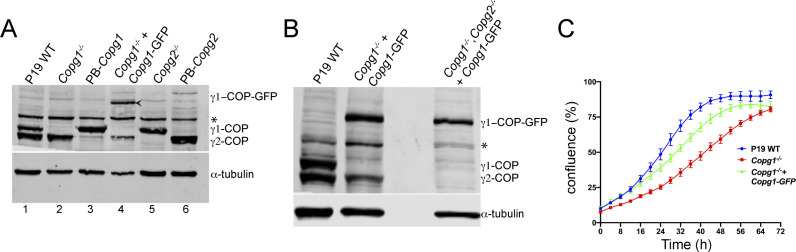
As a further specificity control, we followed a rescue approach in which γ1-COP was reintroduced into Copg1−/− cells. We first constructed rescued cell lines through the transfection of Copg1 and Copg2 KO cells with a PiggyBac (PB) transposon vector driving the constitutive expression of γ1-COP and γ2-COP, respectively, through a strong CAG promoter (resulting in Copg1−/−-PB-Copg1 and Copg2−/−-PB-Copg2 cell lines). However, western blot analysis revealed that strong overexpression of one paralog led to a much weaker expression of the other paralog (Fig S4A). In fact, Copg1−/−-PB-Copg1 cells appeared like a mimic of Copg2 KO cells, whereas Copg2−/−-PB-Copg2 cells looked like a mimic of Copg1 KO cells (Fig S4A, compare lanes 3–5 and 6–2). Previous studies showed that, except for ζ-COP, individual COP subunits are not detectable as monomers in the cytoplasm and only exist as part of the heptameric coatomer complex (Kuge et al, 1993; Lowe & Kreis, 1996). We therefore assume that the overexpressed γ-COP efficiently competes with its endogenous counterpart for assembly in the coatomer complex and that the excessive non-assembled protein gets rapidly degraded. As our goal was to obtain a rescue cell line with both γ-COP paralogs expressed close to their endogenous concentrations, we then turned to another strategy in which we used a bacterial artificial chromosome (BAC) that carries the whole Copg1 gene locus under the control of its native promoter. Through recombineering, the Copg1 gene was fused at the 3′ end to the localization and affinity purification tag that comprises a GFP sequence (Cheeseman & Desai, 2005). This strategy ensures that the reintroduced transgene is expressed at levels similar to the endogenous gene and under the control of its native regulatory elements (Poser et al, 2008). Western blot analysis of lysates of the rescue cell line (termed P19 Copg1−/− + Copg1-GFP) revealed that γ1-COP-GFP was indeed expressed at similar levels to endogenous γ1-COP in the WT cell line (Fig S4A, lane 4). Moreover, by disrupting Copg2 in this rescued cell line, we successfully obtained P19 cells that solely express γ1-COP-GFP in a double Copg1−/−, Copg2−/− background (Fig S4B). As the COPI pathway is essential, this demonstrates that GFP-tagged γ1-COP is functional. Accordingly, the P19 Copg1−/− + Copg1–GFP–rescued cell line showed faster proliferation rates than Copg1−/− cells (Fig S4C) and formation of tight EBs (Fig 5F). Hence, altogether, our data show that it is indeed the absence of the γ1-COP protein that prevents proper formation of EBs during neuronal differentiation of Copg1 KO cells.
Figure S4. Characterization of rescued P19 cell lines.
(A) Western blot analysis of the expression levels of γ1-COP and γ2-COP, and α-tubulin as a loading control in pluripotent P19 WT, Copg1−/−, PB-Copg1 (Copg1−/− cells rescued with a PiggyBac transposon vector), Copg1−/− + Copg1-GFP (Copg1−/− cells rescued with a bacterial artificial chromosome), and Copg2−/−, PB-Copg2 cell lysates. The asterisk (*) marks a nonspecific signal. The arrow head indicates γ1-COP-GFP in lane 4. (A, B) Same as in (A) for P19 WT, Copg1−/− + Copg1-GFP, and Copg1−/−, Copg2−/− + Copg1-GFP cells. (C) Growth curves obtained from a real-time proliferation assay in which the occupied area (% confluence) by P19 WT, Copg1−/−, and Copg1−/− + Copg1-GFP cells was monitored over 72 h. Curves were generated with the IncuCyte software (n = 5, error bars are SEM).
Source data are available for this figure.
Expression of γ1-COP promotes neurite outgrowth
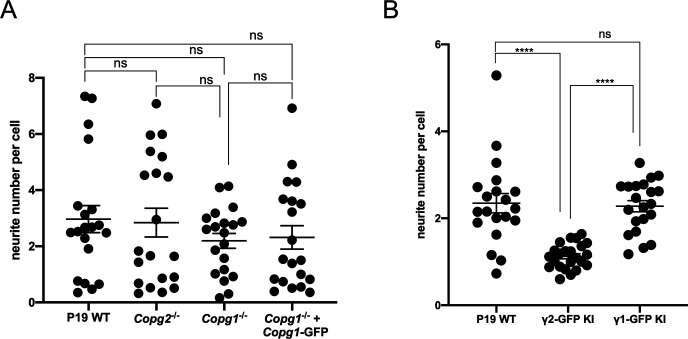
We then analyzed WT and KO cells during the second step of neuronal differentiation, in which EB cells are dissociated, seeded on poly-lysine–coated plates (day 5 of differentiation) and grown for an additional 4 d (until day 8 of differentiation). 2 d after plating, cytosine arabinoside (ara-C) was added to poison dividing cells and hence enrich for post-mitotic neurons. During the second stage, P19 WT cells efficiently differentiated into neurons with the strong up-regulation of βIII-tubulin and the extension of long neurites (Fig 6A, top row). P19 Copg2−/− cells showed the same hallmarks of neuronal differentiation as WT cells with increased βIII-tubulin expression and long neurites (Fig 6A, second row from top). By contrast, Copg1−/− cells hardly showed any long neurites at day 8 of differentiation even though the cells expressed βIII-tubulin (Fig 6A, third row from top). The P19 Copg1−/− + Copg1–GFP–rescued cell line showed similar neurite extensions to WT and Copg2−/− cells (Fig 6A, bottom row). Further demonstrating the specificity of the Copg1−/− cell phenotype, the GFP KI cell line also hardly showed any extended neurites, whereas the γ1-COP-GFP KI cell line behaved similar to the WT cells (Fig S5). Semi-supervised automated measurements of neurite lengths from WT, KO, and rescued cells were performed using the NeuriteQuant software (Dehmelt et al, 2011). The quantification corroborated the qualitative observations with a similar higher average neurite length per cell for WT, Copg2−/−, and rescued Copg1−/− cells than Copg1−/− cells (Fig 6B). We did not measure a significant difference of the number of neurites per Tuj-1–positive cells between the four cell lines (Fig S6A); however, with cells such as P19 that grow at relatively high densities, the measurement of this parameter might be less reliable because the assignment of unique neurite attachment points is challenging.
Figure 6. Depletion of γ1-COP leads to impaired neurite outgrowth.
(A) Representative fluorescence microscopy images of P19 WT, Copg2−/−, Copg1−/−, and Copg1−/− + Copg1-GFP cells as indicated at day 8 of differentiation to analyze the expression of the neuronal marker βIII-tubulin (indirect immunofluorescence) and GFP (direct fluorescence). (B) Quantification of the normalized neurite length (number of pixel/cell). (A) Each dot represents the value obtained for one picture (usually corresponding to ca. 100 cells) as in (A). 20 random images (a total of ca. 2,000 cells) were taken for the analysis. The horizontal bars represent the median value (set to 100 for WT) obtained for each cell line. **** indicates P-value < 0.0001, n = 20. Scale bar is 60 μm. (C) Western blot analysis of various COP subunits, βIII-tubulin, Oct-4, and GAPDH in P19 WT, Copg1−/−, and Copg2−/− cells at different time point of differentiation as indicated. The asterisk (*) marks a nonspecific signal.
Source data are available for this figure.
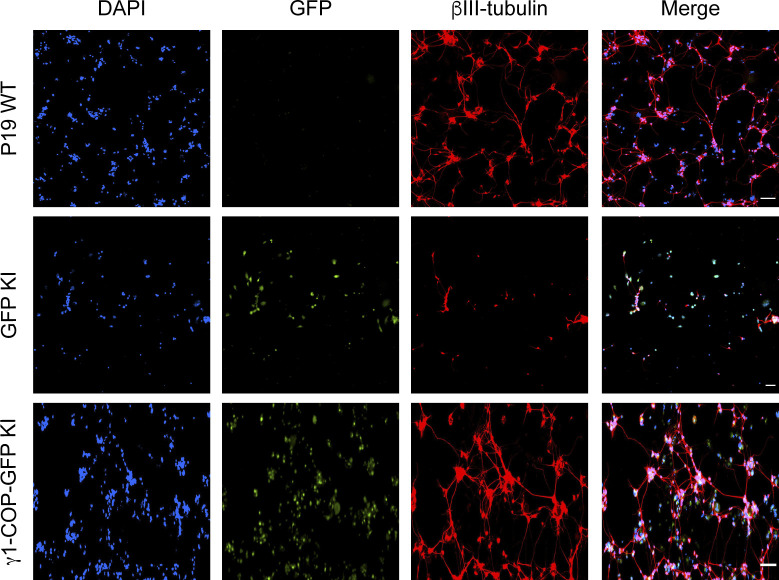
Figure S5. Inhibition of neurite outgrowth in P19 GFP knock-in (KI) cells.
Representative fluorescence microscopy images of P19 WT, GFP KI, and γ1-COP-GFP KI as indicated at day 8 of differentiation to analyze the expression of the neuronal marker βIII-tubulin (indirect immunofluorescence) and GFP (direct fluorescence). Scale bar is 100 μm.
Figure S6. Quantification of neurite number per cell.
(A) Quantification of the number of neurite per cell (number of neurite attachment point/Tuj-1 positive nucleus). Each dot represents the value obtained for one picture (usually corresponding to ca. 100 cells) as in Fig 6A. 20 random images (a total of ca. 2,000 cells) were taken for the analysis. The horizontal bars represent the median value obtained for each cell line. (A, B) Same as (A) for the cell lines analyzed in Fig 8B. A two-tailed unpaired t test was performed for the statistical significance analysis (**** indicates P-value < 0.0001, ns, nonsignificant, n = 20).
When performing these assays, we reproducibly observed fewer remaining cells on the dishes at day 8 of differentiation when using Copg1−/− or GFP KI cells, suggesting that these cells differentiate less efficiently. Indeed, if the γ1-COP–lacking cells yielded fewer post-mitotic neurons, they would be more sensitive to the Ara-C treatment, which is toxic to dividing cells. To explore this possibility, we performed the differentiation protocol in the presence or absence of Ara-C for P19 WT, Copg1−/−, and GFP KI cell lines. For each cell line, we then analyzed the percentage of recovered cells after Ara-C treatment (Fig S7A). We found that both γ1-COP–lacking cell lines were more sensitive to Ara-C than WT cells, indirectly suggesting that they differentiate less efficiently into post-mitotic neurons. In support to this hypothesis, immunofluorescence microscopy analysis at day 8 of differentiation showed much less cells positive for the neuronal marker Tuj-1 in the absence of Ara-C treatment for Copg1−/− and GFP KI cells than in the WT situation (Fig S7D). Importantly, Copg1−/− and GFP KI cells did not completely fail to differentiate. Indeed, similar to WT and Copg2−/− cells, Copg1−/− cells rapidly lost expression of Oct-4 (Fig 6C), indicating loss of pluripotency. Moreover, the remaining Copg1−/− and GFP KI cells after the Ara-C treatment showed expression of βIII-tubulin (Figs 6A and C and S5), implying a correctly implemented neuronal differentiation program.
Figure S7. Reduced survival after Ara-C treatment and differentiation efficiency in Copg1 KO cells.
(A, B, C) Quantification of cell survival upon Ara-C treatment. Cells from dissociated embryoid bodies were seeded at the same density in a cell culture multi-well plate. Recovered cell numbers after 2 d of culture with or without Ara-C were calculated. The numbers obtained in the non-treated samples were set to 100%. (A) Comparison between P19 WT, Copg1−/−, and GFP KI cells. (B) Comparison between P19 WT and γ1-COP-GFP KI cells. (C) Comparison between P19 WT and γ2-COP-GFP KI cells. A two-tailed unpaired t test was performed for the statistical significance analysis (n = 3, **** indicates P-value < 0.0001, ns, nonsignificant, error bars are SEM). (D) Representative fluorescence microscopy images of P19 WT, GFP KI, and Copg1−/− cells as indicated at day 8 of differentiation without Ara-C treatment to analyze the expression of the neuronal marker βIII-tubulin (indirect immunofluorescence) and GFP (direct fluorescence). Scale bar is 100 μm.
Altogether, the data suggest that expression of γ1-COP regulates the efficiency of neuronal differentiation and promotes neurite outgrowth.
Generation of P19 cells expressing γ2-COP from the Copg1 locus
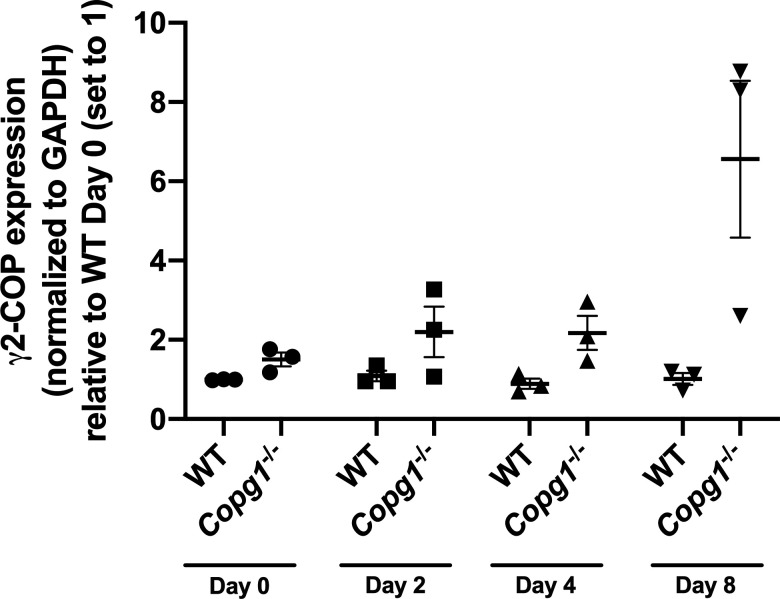
In non-differentiated Copg1−/− cells, the absence of γ1-COP is at least partly compensated by an increased expression of γ2-COP that seems to maintain global coatomer levels (Fig 2A–D). However, as we observed an increase in γ1-COP expression at the expense of γ2-COP in neurons derived from P19 WT cells (Fig 1), it is possible that Copg1−/− cells do not express enough γ2-COP to compensate at the neuronal stage, thereby restricting the amount of assembled coatomer, and possibly leading to the impaired neurite outgrowth phenotype.
Quantification by western blot of γ2-COP expression in Copg1−/− cells at the non-differentiated, EB, and neuron stages indicates that in contrast to WT cells, Copg1−/− cells do show an increased expression of γ2-COP at the neuron stage (Figs 6C and S8). However, as previous estimations found that there is about twice as much γ1-COP than γ2-COP in various cell lines (Wegmann et al, 2004; Moelleken et al, 2007), it is possible that the increased expression of γ2-COP in Copg1−/− cells is not sufficient to maintain physiological levels of γ-COP in P19 cells throughout differentiation. In that case, we cannot exclude that in Copg1−/− cells, a substantial portion of the cellular coatomer simply misses the γ-COP subunit, as was previously suggested in β-COP–depleted cells (Styers et al, 2008). Our immunoprecipitation data (Fig 2C) cannot address this possibility as we do not know how the western blot signals for γ1- and γ2-COP correlate with the actual concentrations of the proteins. Hence, to characterize the capacity of γ2-COP to replace γ1-COP in a more defined way, we decided to substitute the γ2-COP coding sequence for γ1-COP at the endogenous Copg1 locus. We thus generated a P19 γ2-COP-GFP KI cell line following the same Cas12a-mediated genome editing strategy that was used to generate the P19 GFP KI and P19 γ1-COP-GFP KI cell lines (Fig S3A and B).
Figure S8. Quantification of γ2-COP expression in WT and Copg1−/− cells during differentiation.
Western blot signals for γ2-COP and GAPDH as on Fig 6C were quantified by densitometry. The ratios of γ2-COP over GAPDH signal measured from lysates obtained at the indicate days of differentiation are reported (n = 3).
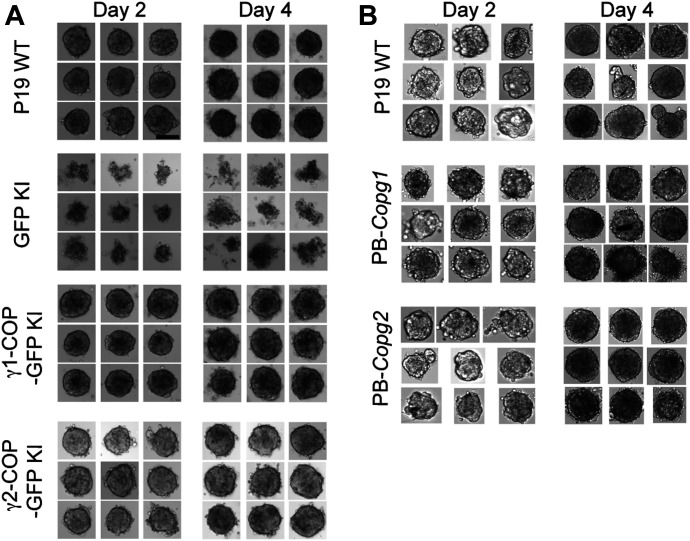
Expression of γ2-COP from the Copg1 locus or overexpression of γ2-COP rescues the formation of tight EBs
We next assessed if EB formation specifically requires the γ1-COP protein or just the expression of enough γ-COP irrespective of the paralog identity. To do so, P19 WT, GFP KI, γ1-COP-GFP KI, and γ2-COP-GFP KI cell lines were used to form EBs in hanging drops. As shown above, whereas WT and γ1-COP-GFP KI formed tight EBs, GFP KI cells showed scattered EBs (Fig 7A). Strikingly, γ2-COP-GFP KI cells were able to form tight EBs similar to WT cells (Fig 7A, bottom rows). Because these cells do not express γ1-COP, this demonstrates that the phenotype of Copg1−/− and GFP KI cells is not due to a missing specific function mediated by γ1-COP. It rather appears that the absence of γ1-COP is not fully compensated by the up-regulation of endogenous γ2-COP in Copg1−/− cells, but that otherwise both paralogs can support the formation of EBs. To further explore this possibility, we turned to the PB-rescued cell lines we initially generated to rescue Copg1 and Copg2 KO cells. Indeed, in these two cell lines, overexpression of one or the other γ-COP paralog leads to essentially one population of coatomer complex that contains the overexpressed protein (Fig S4A). Strikingly, both rescue cell lines showed EB formation comparable with WT cells (Fig 7B). Whereas this was expected for Copg1−/−-PB-Copg1 cells as they mimic Copg2 KO cells, the phenotype of Copg2−/−-PB-Copg2 cells suggests that when enough γ2-COP is expressed, EB formation can proceed normally even in the absence of γ1-COP. Thus, our data show that rather than the presence of a specific γ-COP paralog, it is a sufficient expression level of γ-COP that is essential for the formation of EBs in P19 cells.
Figure 7. Overexpression of γ2-COP rescues the formation of tight embryoid bodies.
(A) Photographs of embryoid bodies from P19 WT, GFP-KI, Copg1−/− + γ1-COP-GFP KI, and Copg1−/− + γ2-COP-GFP cells formed in hanging drops over 2 or 4 d of culture as indicated. (A, B) Same as in (A) with P19 WT, PB-Copg1, and PB-Copg2 cells. (A) Scale bar in (A) is 50 μm and applies to all panels.
Expression of γ2-COP from the Copg1 locus or overexpression of γ2-COP does not compensate for the absence of γ1-COP during neurite outgrowth
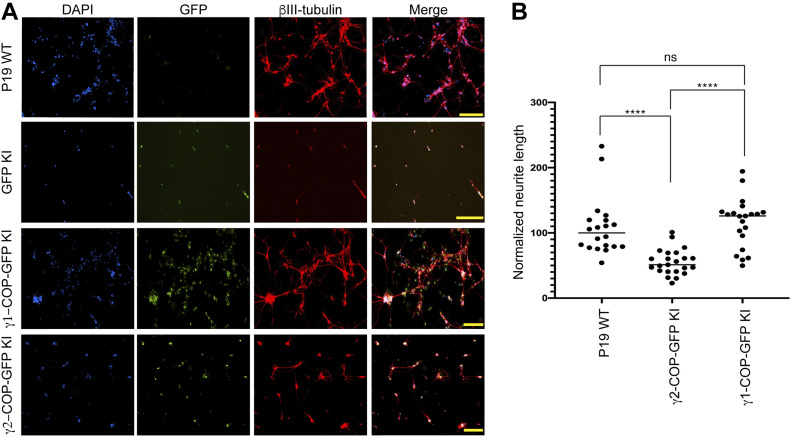
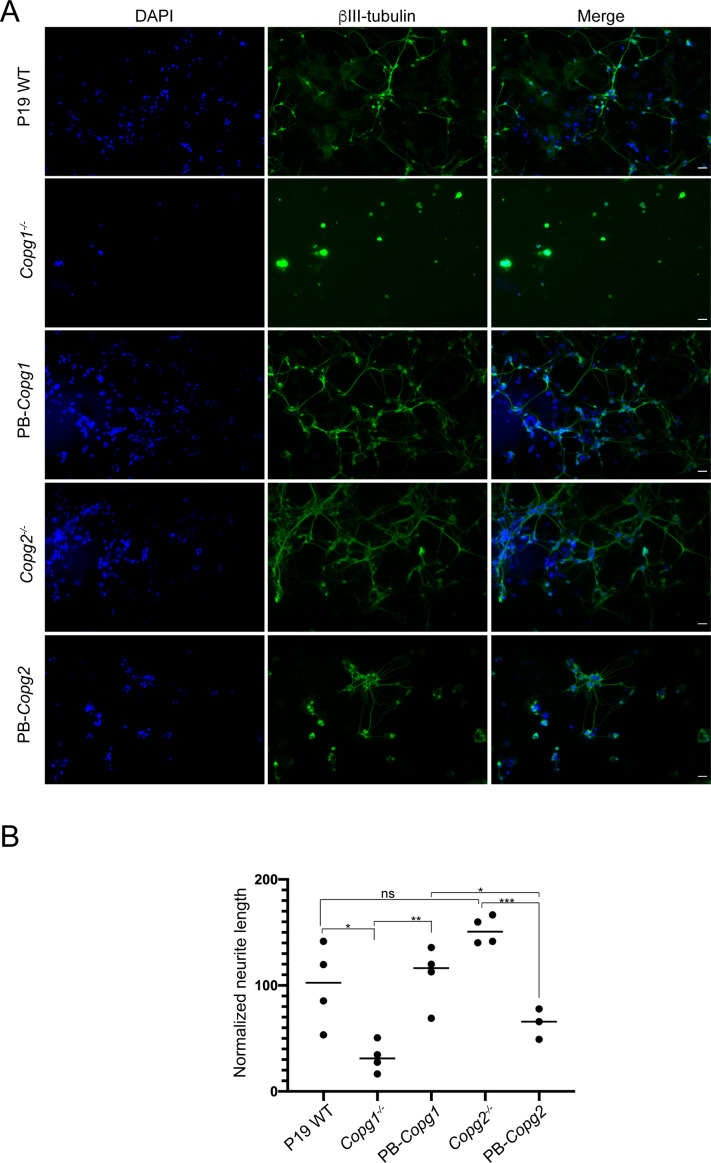
We next analyzed if the absence of γ1-COP can also be compensated by overexpression of γ2-COP during the second step of in vitro neuronal differentiation during which neurite outgrowth occurs. As shown above (Fig S5), microscopic analysis revealed that, whereas P19 GFP KI cells fail to extend long neurites, P19 γ1-COP-GFP KI cells show a network of long neurites comparable with WT cells (Fig 8A). In addition, P19 γ1-COP-GFP KI cells are more resistant to Ara-C treatment than P19 GFP KI cells (Fig S7B). Interestingly, expressing γ2-COP-GFP from the Copg1 locus also resulted in a resistance to Ara-C comparable with WT cells (Fig S7C), suggesting an increased differentiation efficiency compared with γ1-COP–lacking cells. Moreover, P19 γ2-COP-GFP KI cells could extend longer neurites than γ1-COP–lacking cell lines; however, their lengths and amount were not comparable with those of WT cells (Figs 8 and S6B). These observations were corroborated by the software-mediated quantification that showed a significantly lower average neurite length per cell for the γ2-COP-GFP KI cells when compared with WT and γ1-COP-GFP KI cells (Fig 8B). Hence, in contrast to the EB formation step, it appears that γ1-COP has a specific role during the formation of extended neurites. This was corroborated by the PB-rescued cell lines. Indeed, overexpression of γ1-COP in Copg1−/− cells (PB-Copg1 cells) resulted in the formation of neurons with long neurites comparable with the WT situation. In contrast, overexpression of γ2-COP in Copg2−/− cells (PB-Copg2 cells) led to the formation of neurons, but with much shorter neurites (Fig S9A and B). Hence, replacement of γ1-COP by an excess of γ2-COP induces a phenotype comparable with the disruption of the Copg1 gene, suggesting that γ1-COP has a unique role during neuronal differentiation that cannot be taken over by γ2-COP. Of note, as Copg2−/−-PB-Copg2 cells form normal EBs but fail to extend long neurites (Fig 7B); the defective neurite outgrowth observed in Copg1 KO cells is unlikely to be a mere consequence of their incapacity to form proper EBs.
Figure 8. Overexpression of γ2-COP does not compensate the absence of γ1-COP during neurite outgrowth.
(A) Representative fluorescence microscopy images of P19 WT, GFP KI, γ1-COP-GFP KI, and γ2-COP-GFP KI cells as indicated at day 8 of differentiation to analyze the expression of the neuronal marker βIII-tubulin (indirect immunofluorescence) and GFP (direct fluorescence). Scale bar is 200 μm. (B) Quantification of the normalized neurite length (number of pixel/cell). (A) Each dot represents the value obtained for one picture (usually corresponding to ca. 100 cells) as in (A). 20 random images (a total of ca. 2,000 cells) were taken for the analysis. The horizontal bars represent the median value (set to 100 for WT) obtained for each cell line. **** indicates P-value < 0.0001, ns, nonsignificant, n = 20.
Figure S9. Overexpression of γ2-COP does not compensate the absence of γ1-COP during neurite outgrowth in PiggyBac rescued cells.
(A) Representative fluorescence microscopy images of P19 WT, Copg1−/−, PB-Copg1, Copg2−/−, and PB-Copg2 cells as indicated at day 8 of differentiation to analyze the expression of the neuronal marker βIII-tubulin (indirect immunofluorescence). (B) Quantification of the normalized neurite length (number of pixel/cell). (A) Each dot represents the value obtained for one picture (usually corresponding to ca. 100 cells) as in (A). Three to four random images (a total of ca. 400 cells) were taken for the analysis. The horizontal bars represent the median value (set to 100 for WT) obtained for each cell line. A two-tailed unpaired t test was performed for the statistical significance analysis (n = 3 or 4, P-value: * < 0.05, ** < 0.01, *** < 0.001, ns, nonsignificant). Scale bar is 30 μm.
Altogether, our data reveal a paralog-specific function of the COPI pathway during the neuronal differentiation of P19 cells. In this context, γ1-COP promotes efficient neurite outgrowth in differentiated neurons.
Discussion
The Copg2 and Copz2 genes, paralogous to Copg1 and Copz1, were discovered two decades ago (Blagitko et al, 1999; Futatsumori et al, 2000). Since then, it has been an outstanding question whether unique functions can be ascribed to COPI paralogous proteins (Béthune et al, 2006; Arakel & Schwappach, 2018; Luo & Boyce, 2019). Recombinant coatomer complexes that contain either γ1-COP or γ2-COP are equally efficient in producing COPI vesicles from purified Golgi membranes (Sahlmuller et al, 2011) or permeabilized cells (Adolf et al, 2019). Moreover, the proteomes of COPI vesicles prepared with γ1-COP– or γ2-COP–containing coatomer and using permeabilized HeLa cells as donor membranes were virtually indistinguishable (Adolf et al, 2019). Hence, these previous in vitro data suggested that γ1-COP and γ2-COP are functionally redundant. Our present study performed with living cells revisits this conclusion by showing that whereas γ1-COP and γ2-COP are indeed partially functionally redundant, a paralog-specific function of γ1-COP promotes the extension of neurites in pluripotent cell-derived neurons.
The COPI pathway is essential for life, and depletion of non-paralogous COP subunits is lethal in mammalian cells (Hobbie et al, 1994; Shtutman et al, 2011). As we obtained cell lines that lack either γ1-COP or γ2-COP, our data demonstrate that both proteins can support the essential functions of the COPI pathway and that none of the two γ-COP paralogs is essential. However, both Copg1 and Copg2 KO cell lines had slower proliferation rates than WT cells, indicating that γ1-COP and γ2-COP have some nonessential unique functions. Supporting this hypothesis, whereas disruption of Copg1 or Copg2 did not lead to a complete loss of the Golgi as reported upon the depletion of the non-paralogous subunits α- or β-COP (Razi et al, 2009), depletion of either γ1-COP or γ2-COP led to smaller and more numerous Golgi stacks.
Rapid evolution of dosage sharing is a main driving force to maintain gene duplicates in mammals (Lan & Pritchard, 2016). Hence, a difficulty when assessing individual KO of paralogous gene pairs is to disentangle whether the cause of a phenotype is a unique function that can be assigned to one paralog or an aberrant combined expression level of two paralogous genes with essentially overlapping functions. Indeed, whereas our initial data suggested a role of γ1-COP in promoting the formation of tight EBs, KI, and overexpression experiments clearly showed that γ2-COP can take over γ1-COP’s role when expressed in sufficient quantity. This suggests that in this context, expression of γ1-COP and γ2-COP is regulated by dosage balance to achieve a suitable combined expression of γ-COP compatible with a functional COPI pathway. By contrast, the same combination of experiments supports a unique role for γ1-COP that cannot be taken over by γ2-COP in promoting efficient neurite extension at a later stage of P19 cell neuronal differentiation. This finding correlates with the initial observation that in WT cells, expression of γ1-COP is up-regulated at the expense of γ2-COP only at later stages of differentiation, when neurite outgrowth occurs.
What could be the molecular mechanism underlying the role of γ1-COP in promoting neurite outgrowth? Previous studies already hinted at an important role of ER/Golgi trafficking during neuronal polarization. For example, overexpression of the GTP-locked Q71L-ARF1 variant or treatment with the coatomer complex inhibitor 1,3-cyclohexanebis(methylamine) (Hu et al, 1999) leads to impaired dendritic growth in cultured hippocampal neurons (Horton et al, 2005). Moreover, mutations in the COPII components Sec23 and Sar1 also lead to reduced dendritic growth in flies (Ye et al, 2007). In these examples, the outcome of the mutations or treatment is a virtually complete block of ER/Golgi trafficking. This is, however, not expected in the absence of γ1-COP, especially with the concomitant overexpression of γ2-COP, as γ1-COP–depleted cells are viable and γ2-COP–containing coatomer is as efficient in forming COPI vesicles as γ1-COP–containing coatomer in vitro (Sahlmuller et al, 2011; Adolf et al, 2019). One possibility is that γ1-COP acts as a specific cargo receptor for proteins that are important for neuronal polarization. In the absence of γ1-COP, such hypothetical cargo proteins may be mislocalized, leading to the phenotype of Copg1 KO cells. The proteomes of COPI vesicles generated with γ1-COP– or γ2-COP–containing coatomer were reported as virtually identical (Adolf et al, 2019). However, these vesicles were generated from HeLa cells and putative γ1-COP–specific cargo proteins might be neuron specific. The γ-COP paralogs show a differential Golgi localization with γ1-COP being found preferentially at the cis-Golgi and γ2-COP more at the trans-Golgi (Moelleken et al, 2007). This suggests that γ-COP paralogs help maintaining protein concentration gradients across the Golgi stack, with γ1-COP being more active at the ER/cis-Golgi interface. Proteomic analysis of COPI vesicles generated from various cell lines revealed that they mainly contain membrane trafficking regulating proteins, such as SNAREs, and glycosylation enzymes (Adolf et al, 2019). The absence of one γ-COP paralog may then affect the distribution or trafficking kinetics of such proteins across the Golgi, which might explain the phenotype of Copg1 KO cells. Indeed, mutations in both SNAREs (Ulloa et al, 2018) and glycosylation enzymes (Moll et al, 2019) are linked to defective neurite outgrowth and neurological disorders. Notably, within the SNARE protein Membrin, pathogenic mutations that lead to less effective fusion of ER-derived vesicles with the Golgi, result in strongly impaired dendritic growth but do not significantly affect non-neuronal cells (Praschberger et al, 2017). This phenotype, which is similar to the depletion of γ1-COP, highlights the requirement of an intact early secretory pathway for neurite outgrowth. As we observed that depletion of γ1-COP results in a more drastic Golgi fragmentation phenotype than depletion of γ2-COP, the two γ-COP paralogs might affect the distribution of Golgi proteins differently, which might explain why only γ1-COP is required for neurite outgrowth.
Finding the proteins that are mislocalized upon depletion of γ1-COP will be key to understanding the molecular mechanisms underlying γ1-COP’s function during neuronal polarization. This is at present not possible with a direct proteomic analysis of isolated COPI vesicles from neurons, considering the amount of starting material needed and the limit of detection of the assay (Adolf et al, 2019). More promising might be more global approaches such as dynamic organellar maps (Itzhak et al, 2016) or Hyperplexed Localisation of Organelle Proteins by Isotope Tagging (hyperLOPIT) (Christoforou et al, 2016) in which changes in protein localization upon γ1-COP depletion may be analyzed at the proteome level.
Altogether, we found that whereas both γ-COP paralogs can fulfill essential coatomer functions, specialized paralog-specific functions exist. We reveal here a role for γ1-COP in promoting neurite outgrowth, and it is likely that in other cellular contexts, additional paralog-specific functions of COP subunits await to be found. Of note, many cancer cell lines are sensitive to a down-regulation of ζ1-COP because they barely express its paralog ζ2-COP (Shtutman et al, 2011; Anania et al, 2015, 2017). Potential drugs that would target ζ1-COP have thus been proposed as attractive therapeutic options with supposedly limited side effects because ζ2-COP is expressed in normal cells (Shtutman & Roninson, 2011; Anania et al, 2017). Our study calls for caution when following such approaches as ζ1-COP, like γ1-COP, may have important functions that cannot be taken over by its paralog in specific cellular contexts.
Materials and Methods
Antibodies and reagents
Reagents, plasmids, antibodies, and primers used in this study are listed in Tables S1–S5 (materials used in this study, list of sgRNAs/crRNAs, plasmids used in this study, antibodies used in this study, list of primers used in qPCR experiments). Quantification of western blots was performed with the LI-COR Image Studio software according to the distributor’s instructions.
Table S1 Materials used in this study. (9.2KB, xlsx)
Table S2 List of sgRNAs/crRNAs. (9KB, xlsx)
Table S3 Plasmids used in this study. (9.4KB, xlsx)
Table S4 Antibodies used in this study. (9.5KB, xlsx)
Table S5 List of primers used in qPCR experiments. (9.3KB, xlsx)
Cell culture
The P19 cell line was obtained from Sigma-Aldrich and kept at 37°C with 5% CO2 in a humidified incubator. The growth medium was Minimum Essential Medium Eagle (α-MEM) supplemented with 10% FBS (S181B; Biowest), 2 mM glutamine, and a mixture of penicillin/streptomycin. All the cell lines were regularly checked for mycoplasma contamination.
P19 differentiation
P19 cells were differentiated into neurons using a two-step protocol essentially as previously described (Lee et al, 2007). First, 106 low passage P19 cells were used to seed a 10-cm bacterial dish containing 10 ml of P19 growth medium supplemented with 0.1 μM RA. These non-adherent growth conditions promote cell aggregation and the formation of EBs. After 48 h of aggregation, the medium was replaced with fresh RA-supplemented P19 growth medium. After 4 d of aggregation, EBs were centrifuged at 1,000g for 5 min and then washed once with serum-free media. Then EBs were dissociated using 2 ml of a trypsin (0.05%)–EDTA (0.02%) solution (Cat. no. T3924; Sigma-Aldrich) + 50 μg/ml DNAseI and incubated for 10 min in a 37°C incubator. Next, 4 ml of P19 growth medium were added to stop the trypsin activity and the cells are collected at 1,000g for 5 min. Cell pellets were then resuspended in 5 ml growth medium and passed through a cell strainer (352235; Falcon). Dissociated cells were used to seed poly-L/D-lysine–coated six-well plates (7.5 × 105 cells in 2 ml per well) or, for fluorescence microscopy experiments, to seed poly-D-lysine–coated eight-well chambered coverslip (80826; Ibidi) with 40,000 cells per well in 300 μl P19 growth medium. 48 h after plating (at day 6 of differentiation), the medium was exchanged for P19 growth medium supplemented with 10 μM Ara-C to poison dividing cells. A list of chemicals and their suppliers is provided in Table S1.
Generation of the P19 Copg1−/− and Copg2−/− KO cell lines
P19 Copg1−/− and Copg2−/− cells were generated by following a CRISPR-Cas9 gene editing strategy. sgRNAs designed to cut in early exons of the Copg1 and Copg2 genes (selected with the CRISPR.MIT tool, see Table S2) were cloned into the pSPCas9(BB)-2TA-GFP vector between the BbsI restriction sites (Ran et al, 2013). P19 cells were transfected with these vectors using Lipofectamine 3000 (Thermo Fisher Scientific) following the manufacturer’s instructions. 72 h after transfection, single GFP-positive cells were sorted and transferred into a 96-well plate using a FACS at the CellNetworks/ZMBH-Flow Cytometry & FACS Core Facility. Clonal cell lines were grown and transferred into larger cell culture dishes and then screened by western blot. Clones that showed absence of γ1-COP or γ2-COP were verified by pCR-Blunt cloning/Sanger sequencing of the target locus.
Generation of P19 Copg1−/−-PB-Copg1 and Copg2−/−-PB-Copg2 cell lines
The P19 Copg1−/−-PB-Copg1 and Copg2−/−-PB-Copg2 cell lines were generated using the PB transposon system (Wang et al, 2008). P19 Copg1−/− and Copg2−/− cells seeded in a six-well plate were transfected with an equal amount (1.5 μg each) of pPbase plasmid (coding for the PB transposase) and pCyl50-Copg1 or pCyl50-Copg2 plasmid (coding for the gene to be inserted and the hygromycin resistance gene). In addition, a third control cell line was generated by co-transfection of pPbase and a pCyl50-GFP plasmid. 24 h after transfection, the cells were transferred to a 10-cm plate. After 3 d of incubation, hygromycin was added to the growth medium (at 150 μg/ml), and the medium was replenished every second–third day. After 15 d, all GFP-transfected control cells were green as judged by fluorescence microscopy. Transfected PB-Copg1 and PB-Copg2 cells were kept on selection media for another 5 d and then analyzed by western blot to check expression of γ1-COP or γ2-COP, respectively. Table S3 lists the plasmids used in this study.
Generation of the P19 Copg1−/−-Copg1-GFP cell line
BACs harboring the murine Copg1 locus were GFP tagged by recombineering as described previously (Poser et al, 2008). BAC DNA was isolated from Escherichia coli DH10 cells using a BAC prep kit (Macherey-Nagel). P19 Copg1−/− cells were transfected with 1 μg BAC DNA using Effectene (QIAGEN), and stable clonal cell lines were obtained after selection with 500 μg/ml geneticin (Gibco) and FACS sorting.
Generation of P19 KI cells
P19 KI cells were generated by following a CRISPR-Cas12a/Cpf1 gene editing strategy including crRNAs arrays, a donor template for homology-directed repair (HDR) and a recombination reporter plasmid for selection. Two crRNAs (see Table S2) targeting the Copg1 gene within the 5′UTR and 3′UTR coding sequencing were selected with the CHOPCHOP tool (Labun et al, 2019) and cloned as a dual array between the BsmbI sites of pY109 (lenti-LbCpf1) plasmid (Zetsche et al, 2017) derivative (pY109_2) in which the Puro-selection cassette was removed. The donor template cassettes were PCR products containing homology arms (left 75 bp and right 55 bp for HDR) to the target locus flanking the coding sequence for either GFP (GFP KI cells), γ1-COP-GFP (γ1-COP-GFP KI cells), or γ2-COP-GFP (γ2-COP-GFP KI cells). The donor cassettes were assembled using the NEBuilder HiFi DNA Assembly Master Mix (NEB) and were designed to leave the 5′UTR and 3′UTR of the Copg1 gene intact. The recombination reporter plasmid (pMB1610_pRR-Puro) allows a split puromycin resistance approach for selecting cells in which Cas12a cut and HDR occurred (Flemr & Buhler, 2015). The target sequence for crRNA2 was cloned between the two puromycin fragments cassettes within pMB1610. When the Cas12a-induced DNA strand break in the plasmid is repaired via HDR this results in a functional puromycin resistance. P19 cells were seeded at 200,000 cells per well of a six-well plate. On the next day they were transfected with a mixture of 1.5 μg of modified pY109-crRNA array plasmid, 1 μg of PCR donor cassette and 0.5 μg of pMB1610 reporter plasmid using the JetPrime reagent (Polyplus-transfection) following the manufacturer’s instructions. Puromycin was added 24 h later to 4 μg mL−1 in the growth medium and the cells incubated for another 48 h. Surviving cells were then transferred to a 10 cm dish and allowed to grow to 70–80% confluence. A pool of GFP-positive cells was then selected by FACS and transferred to a 10 cm dish. The cells were allowed to grow to 70–80% confluence and then single GFP-positive cells were selected and transferred to 96-well plates by FACS. Clonal cell lines were grown and transferred to larger cell culture dishes. Cells that showed a Golgi-like GFP signal by fluorescence microscopy were kept for further screening. Suitable clones were selected after western blot analysis of γ1-COP and γ2-COP expression (absence of endogenous γ1-COP and presence of either γ1-COP-GFP or γ2-COP-GFP). The molecular nature of all genome-edited alleles was verified by PCR (outside homology arms to verify a correct junction of the homology arms with the transgenes, and between exon 8 and 9 of Copg1 to verify the absence of the endogenous Copg1 locus) and by pCR-blunt/Sanger sequencing of the complete modified target locus. With these controls we made sure that all three KI cell lines had a bi-allelic insertion of the transgenes.
Hanging drop assay
P19 cells were diluted to 10,000 cells/ml in RA-containing P19 growth medium. 30 drops of 20 μl volume (containing 200 cells) were then deposited inside the lid of a 10-cm bacterial dish filled with 10 ml PBS to avoid evaporation. On the second and fourth day of EB formation, images of individual drops were taken on a Nikon TS100F microscope fitted with a CMOS camera (Imaging source, DMK 23UX174) using 10× air objective.
Immunostaining
For immunostaining, cells were washed with preheated (37°C) PBS, then fixed with PBS supplemented with 4% formaldehyde for 20 min at 37°C. Fixed cells were washed twice with PBS, and then permeabilized with PBS + 0.25% Triton X-100 at RT for 10 min. Cells were then washed twice with PBS + 2% BSA and blocked with PBS + 10% BSA for 30 min at RT. Incubation with primary antibodies diluted in PBS + 2% BSA was then performed either for 1 h at RT or overnight at 4°C. Next, cells were washed three times with PBS + 2% BSA and incubated with the relevant secondary antibody diluted in PBS + 2% BSA for 30 min at RT in the dark. Cells were then washed twice with PBS + 2% BSA and incubated with DAPI (4′,6-diamidino-2-phenylindole diluted at 0.1 μg/ml in PBS) for 10 min at RT in the dark. After three brief and gentle washes, the first two with PBS +2% BSA, the last one with PBS, cells were mounted with Ibidi mounting medium. Images were acquired with a Nikon ECLIPSE Ti2 microscope equipped with an Andor Clara DR or Nikon DS-Qi2 camera. Neurite length was assessed after βIII-tubulin staining by analysis with the NeuriteQuant software (Dehmelt et al, 2011). For each image, total neurite length was normalized to the number of nuclei counted with the Fiji software. A list of antibodies and their working dilutions is given in Table S4.
Chemical treatment experiments
To induce apoptosis, P19 cells were treated with staurosporine (1 μM) for 20 h. Thereafter, immunostaining was performed (see above). To induce ER stress, P19 cells were treated with tunicamycin (2.5 μg mL−1) for 24 h. Thereafter, western blot or FACS analysis was performed.
Apoptosis flow cytometry assay
For each cell line, 300 × 105 cells were used to perform a FAM FLICA Caspase-3/7 assay (Cat. no. ICT093; Bio-Rad) according to the manufacturer’s instructions. Measurements were performed on a BD FACSCanto flow cytometer. The recommended control samples were included to set PMT voltages and adjust fluorescent compensation.
Real-time cell proliferation assay
To assess cell proliferation kinetics, 100,000 cells were seeded per well of a six-well plate and incubated in an IncuCyte Zoom live-cell analysis system (Essen Bioscience) within a 37°C incubator. Images were acquired in every 4 h for a period of 72 h. Data analysis was performed with the IncuCyte ZOOM Software.
RNA isolation/cDNA preparation/RT-qPCR
RNA isolation, cDNA preparation, and RT-qPCR were performed exactly as described in Amaya Ramirez et al (2018). Primers used for qPCR are listed in Table S5.
Cytosol preparation from adherent cells
For cytosol preparation, two confluent 15-cm cell culture dishes were used. Cells were washed twice with PBS and collected by scrapping in 0.5 ml of lysis buffer (25 mM Tris, pH 7.4, +150 mM NaCl +1 mM EDTA + Protease inhibitor cocktail). Scrapped cells were lysed by passing them 20 times (10 strokes) through a 21-gauge needle and then 20 times through a 27-gauge needle on ice. Cell lysates were first cleared at 4°C, 800g, 5 min and then at 4°C, 100,000g, 1 h. Protein concentration was estimated with a Bradford assay. Typically, a yield of ca. 1.5 mg protein per 15-cm plate was obtained.
Coatomer immunoprecipitation from cytosolic preparations
Per IP, 10 μl Protein G magnetic beads (S1430S; NEB) were used. Beads were washed twice with IP buffer (25 mM Tris, pH 7.4 + 150 mM NaCl + 1 mM EDTA + 0.025% [vol/vol] tween 20). After the second wash, 100 μl of CM1 antibody supernatant was added per 10 μl Protein G beads, the tube was then filled to ca. 1.4 ml with IP buffer and incubated for 1 h at RT on a rotating wheel. Then, the beads were washed once with IP buffer (1 ml). Freshly prepared cytosol corresponding to 500 μg protein was then added to the beads, and if needed, the tubes were filled to ca. 1.4 ml with IP buffer containing protease inhibitors. Cytosol and beads were incubated for 1 h at RT on a rotating wheel. The beads were then washed three times with IP buffer to remove unbound proteins. Bound proteins were eluted by incubating the beads with 20 μl 3× SDS loading buffer at 70°C for 10 min. Beads were then separated from the eluted material on a magnetic rack and the eluted material transferred to a fresh tube.
Electron Microscopy
Cells in a six-well plate were fixed at RT for 30 min by adding the fixative solution (2.5% glutaraldehyde and 2% sucrose in 50 mM KCl, 2.6 mM MgCl2, 2.6 mM CaCl2, and 50 mM cacodylate buffer, pH 7.2). The cells were then rinsed five times for 2 min at RT with 0.1M cacodylate buffer, pH 7.2, and incubated at 4°C for 40 min with Contrast I solution (2% OsO4 in 50 mM cacodylate buffer). The cells were then rinsed five times for 1 min at 4°C and then twice for 5 min at RT with mQ water. Thereafter, the cells were incubated at RT for 30 min in the dark with Contrast II solution (0.5% uranyl acetate in mQ water). Finally, the cells were dehydrated with increasing amounts of ethanol and embedded in epon epoxy resin (Polysciences). Ultrathin sections of 60 nm were contrasted with uranyl acetate and lead citrate using an AC20 automatic contrasting system (Leica) and examined with a T12 electron microscope (Thermo Fisher Scientific). Images were taken from WT, Copg1−/−, and Copg2−/− cell lines. Images were collected from three different grids per condition. Random pictures of 30 cell profiles containing a Golgi stack were collected for quantification. All the measurements were done using ImageJ. For quantification of the area of individual Golgi stacks, the smallest possible ellipse was drawn around each Golgi stacks and the enclosed area was calculated. Cisternae lengths were measured by drawing a segmented line with ImageJ through cisternae for which clear beginnings and ends could be observed. Finally, the surface area of Golgi stacks versus Golgi-associated vesicles was assessed and the percentage of Golgi’s that contained more than 50% vesicles was calculated. Average values and SEM were computed with the Prism 8 software.
Statistical analysis
Statistical significances were calculated using a two-tailed unpaired Student’s parametric t test with Welsh’s correction with the Prism 8 software (GraphPad).
Supplementary Material
Acknowledgements
We thank Felix Wieland (Heidelberg) for the kind gifts of plasmids and antibodies (see Supplemental Data). We thank Ina Poser (Dresden) for BAC tagging and Menna Ahmed for her help in handling the IncuCyte device. We thank the CellNetworks EM, FACS, and Nikon imaging center facilities for their expert support. We thank F Wieland and Mandy Jeske for their critical comments on the manuscript. This work was supported by the excellence initiative of the German federal and state governments (DFG-EXC81).
Author Contributions
M Goyal: formal analysis and investigation.
X Zhao: formal analysis and investigation.
M Bozhinova: investigation.
K Andrade-López: investigation.
C de Heus: formal analysis and investigation.
S Schulze-Dramac: investigation.
M Müller-McNicoll: formal analysis, supervision, funding acquisition, investigation, and writing—review and editing.
J Klumperman: formal analysis, supervision, funding acquisition, investigation, and writing—review and editing.
J Béthune: conceptualization, formal analysis, supervision, funding acquisition, investigation, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Adolf F, Rhiel M, Hessling B, Gao Q, Hellwig A, Béthune J, Wieland FT (2019) Proteomic profiling of mammalian COPII and COPI vesicles. Cell Rep 26: 250–265.e5. 10.1016/j.celrep.2018.12.041f [DOI] [PubMed] [Google Scholar]
- Adolf F, Rhiel M, Reckmann I, Wieland FT (2016) Sec24C/D-isoform-specific sorting of the preassembled ER-Golgi Q-SNARE complex. Mol Biol Cell 27: 2697–2707. 10.1091/mbc.e16-04-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya Ramirez CC, Hubbe P, Mandel N, Bethune J (2018) 4EHP-independent repression of endogenous mRNAs by the RNA-binding protein GIGYF2. Nucleic Acids Res 46: 5792–5808. 10.1093/nar/gky198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anania M, Gasparri F, Cetti E, Fraietta I, Todoerti K, Miranda C, Mazzoni M, Re C, Colombo R, Ukmar G, et al. (2015) Identification of thyroid tumor cell vulnerabilities through a siRNA-based functional screening. Oncotarget 6: 34629–34648. 10.18632/oncotarget.5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anania MC, Cetti E, Lecis D, Todoerti K, Gulino A, Mauro G, Di Marco T, Cleris L, Pagliardini S, Manenti G, et al. (2017) Targeting COPZ1 non-oncogene addiction counteracts the viability of thyroid tumor cells. Cancer Lett 410: 201–211. 10.1016/j.canlet.2017.09.024 [DOI] [PubMed] [Google Scholar]
- Arakel EC, Schwappach B (2018) Formation of COPI-coated vesicles at a glance. J Cell Sci 131: jcs209890 10.1242/jcs.218347 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Helenius A (2016) Cargo capture and bulk flow in the early secretory pathway. Annu Rev Cell Dev Biol 32: 197–222. 10.1146/annurev-cellbio-111315-125016 [DOI] [PubMed] [Google Scholar]
- Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z (2000) Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): Correlation with DNA fragmentation. Exp Cell Res 259: 308–313. 10.1006/excr.2000.4955 [DOI] [PubMed] [Google Scholar]
- Béthune J, Wieland F, Moelleken J (2006) COPI-mediated transport. J Membr Biol 211: 65–79. 10.1007/s00232-006-0859-7 [DOI] [PubMed] [Google Scholar]
- Béthune J, Wieland FT (2018) Assembly of COPI and COPII vesicular coat proteins on membranes. Annu Rev Biophys 47: 63–83. 10.1146/annurev-biophys-070317-033259 [DOI] [PubMed] [Google Scholar]
- Bettayeb K, Chang JC, Luo W, Aryal S, Varotsis D, Randolph L, Netzer WJ, Greengard P, Flajolet M (2016a) delta-COP modulates Abeta peptide formation via retrograde trafficking of APP. Proc Natl Acad Sci U S A 113: 5412–5417. 10.1073/pnas.1604156113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettayeb K, Hooli BV, Parrado AR, Randolph L, Varotsis D, Aryal S, Gresack J, Tanzi RE, Greengard P, Flajolet M (2016b) Relevance of the COPI complex for Alzheimer’s disease progression in vivo. Proc Natl Acad Sci U S A 113: 5418–5423. 10.1073/pnas.1604176113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagitko N, Schulz U, Schinzel AA, Ropers HH, Kalscheuer VM (1999) gamma2-COP, a novel imprinted gene on chromosome 7q32, defines a new imprinting cluster in the human genome. Hum Mol Genet 8: 2387–2396. 10.1093/hmg/8.13.2387 [DOI] [PubMed] [Google Scholar]
- Bonnon C, Wendeler MW, Paccaud JP, Hauri HP (2010) Selective export of human GPI-anchored proteins from the endoplasmic reticulum. J Cell Sci 123: 1705–1715. 10.1242/jcs.062950 [DOI] [PubMed] [Google Scholar]
- Chae HJ, Kang JS, Byun JO, Han KS, Kim DU, Oh SM, Kim HM, Chae SW, Kim HR (2000) Molecular mechanism of staurosporine-induced apoptosis in osteoblasts. Pharmacol Res 42: 373–381. 10.1006/phrs.2000.0700 [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Desai A (2005) A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci STKE 2005: pl1 10.1126/stke.2662005pl1 [DOI] [PubMed] [Google Scholar]
- Christoforou A, Mulvey CM, Breckels LM, Geladaki A, Hurrell T, Hayward PC, Naake T, Gatto L, Viner R, Martinez Arias A, et al. (2016) A draft map of the mouse pluripotent stem cell spatial proteome. Nat Commun 7: 8992 10.1038/ncomms9992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Poplawski G, Hwang E, Halpain S (2011) NeuriteQuant: An open source toolkit for high content screens of neuronal morphogenesis. BMC Neurosci 12: 100 10.1186/1471-2202-12-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemr M, Buhler M (2015) Single-step generation of conditional knockout mouse embryonic stem cells. Cell Rep 12: 709–716. 10.1016/j.celrep.2015.06.051 [DOI] [PubMed] [Google Scholar]
- Futatsumori M, Kasai K, Takatsu H, Shin HW, Nakayama K (2000) Identification and characterization of novel isoforms of COP I subunits. J Biochem 128: 793–801. 10.1093/oxfordjournals.jbchem.a022817 [DOI] [PubMed] [Google Scholar]
- Hicks SW, Machamer CE (2005) Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta 1744: 406–414. 10.1016/j.bbamcr.2005.03.002 [DOI] [PubMed] [Google Scholar]
- Hobbie L, Fisher AS, Lee S, Flint A, Krieger M (1994) Isolation of three classes of conditional lethal Chinese hamster ovary cell mutants with temperature-dependent defects in low density lipoprotein receptor stability and intracellular membrane transport. J Biol Chem 269: 20958–20970. https://www.jbc.org/content/269/33/20958.abstract [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD (2005) Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron 48: 757–771. 10.1016/j.neuron.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Hu T, Kao CY, Hudson RT, Chen A, Draper RK (1999) Inhibition of secretion by 1,3-Cyclohexanebis(methylamine), a dibasic compound that interferes with coatomer function. Mol Biol Cell 10: 921–933. 10.1091/mbc.10.4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak DN, Tyanova S, Cox J, Borner GH (2016) Global, quantitative and dynamic mapping of protein subcellular localization. Elife 5: e16950 10.7554/elife.16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Brett M, Nishi E, Drunat S, Tan ES, Fujiki K, Lebon S, Cham B, Masuda K, Arakawa M, et al. (2016) ARCN1 mutations cause a recognizable craniofacial syndrome due to COPI-mediated transport defects. Am J Hum Genet 99: 451–459. 10.1016/j.ajhg.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Komita M, Aoe T (2017) The role of BiP retrieval by the KDEL receptor in the early secretory pathway and its effect on protein quality control and neurodegeneration. Front Mol Neurosci 10: 222 10.3389/fnmol.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve EM, McBurney MW, Rogers KA, Kalnins VI (1982) Retinoic acid induces embryonal carcinoma cells to differentiate into neurons and glial cells. J Cell Biol 94: 253–262. 10.1083/jcb.94.2.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada K, Iekumo T, Saito R, Kaneko M, Mimori S, Nomura Y, Okuma Y (2014) Aberrant neuronal differentiation and inhibition of dendrite outgrowth resulting from endoplasmic reticulum stress. J Neurosci Res 92: 1122–1133. 10.1002/jnr.23389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GM, Gatie MI (2017) Mechanisms regulating stemness and differentiation in embryonal carcinoma cells. Stem Cells Int 2017: 3684178 10.1155/2017/3684178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D, Samali A, Jager R (2015) Methods for studying ER stress and UPR markers in human cells. Methods Mol Biol 1292: 3–18. 10.1007/978-1-4939-2522-3_1 [DOI] [PubMed] [Google Scholar]
- Kuge O, Hara-Kuge S, Orci L, Ravazzola M, Amherdt M, Tanigawa G, Wieland FT, Rothman JE (1993) zeta-COP, a subunit of coatomer, is required for COP-coated vesicle assembly. J Cell Biol 123: 1727–1734. 10.1083/jcb.123.6.1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, Valen E (2019) CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res 47: W171–W174. 10.1093/nar/gkz365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Pritchard JK (2016) Coregulation of tandem duplicate genes slows evolution of subfunctionalization in mammals. Science 352: 1009–1013. 10.1126/science.aad8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Xing Y, Nguyen D, Xie J, Lee CJ, Black DL (2007) Depolarization and CaM kinase IV modulate NMDA receptor splicing through two essential RNA elements. PLoS Biol 5: e40 10.1371/journal.pbio.0050040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Custer SK, Gilson T, Hao le T, Beattie CE, Androphy EJ (2015) alpha-COP binding to the survival motor neuron protein SMN is required for neuronal process outgrowth. Hum Mol Genet 24: 7295–7307. 10.1093/hmg/ddv428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe M, Kreis TE (1995) In vitro assembly and disassembly of coatomer. J Biol Chem 270: 31364–31371. 10.1074/jbc.270.52.31364 [DOI] [PubMed] [Google Scholar]
- Lowe M, Kreis TE (1996) In vivo assembly of coatomer, the COP-I coat precursor. J Biol Chem 271: 30725–30730. 10.1074/jbc.271.48.30725 [DOI] [PubMed] [Google Scholar]
- Luo PM, Boyce M (2019) Directing traffic: Regulation of COPI transport by post-translational modifications. Front Cell Dev Biol 7: 190 10.3389/fcell.2019.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J (2007) The transport signal on Sec22 for packaging into COPII-coated vesicles is a conformational epitope. Mol Cell 26: 403–414. 10.1016/j.molcel.2007.03.017 [DOI] [PubMed] [Google Scholar]
- Mancias JD, Goldberg J (2008) Structural basis of cargo membrane protein discrimination by the human COPII coat machinery. EMBO J 27: 2918–2928. 10.1038/emboj.2008.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelleken J, Malsam J, Betts MJ, Movafeghi A, Reckmann I, Meissner I, Hellwig A, Russell RB, Sollner T, Brugger B, et al. (2007) Differential localization of coatomer complex isoforms within the Golgi apparatus. Proc Natl Acad Sci U S A 104: 4425–4430. 10.1073/pnas.0611360104 [DOI] [PubMed] [Google Scholar]
- Moll T, Shaw PJ, Cooper-Knock J (2019) Disrupted glycosylation of lipids and proteins is a cause of neurodegeneration. Brain 143: 1332–1340. 10.1093/brain/awz358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morassutti DJ, Staines WA, Magnuson DS, Marshall KC, McBurney MW (1994) Murine embryonal carcinoma-derived neurons survive and mature following transplantation into adult rat striatum. Neuroscience 58: 753–763. 10.1016/0306-4522(94)90452-9 [DOI] [PubMed] [Google Scholar]
- Nakagomi S, Barsoum MJ, Bossy-Wetzel E, Sutterlin C, Malhotra V, Lipton SA (2008) A Golgi fragmentation pathway in neurodegeneration. Neurobiol Dis 29: 221–231. 10.1016/j.nbd.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski CM, Urano F (2011) Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490: 71–92. 10.1016/b978-0-12-385114-7.00004-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389. 10.1038/sj.cdd.4401373 [DOI] [PubMed] [Google Scholar]
- Pachernik J, Bryja V, Esner M, Kubala L, Dvorak P, Hampl A (2005) Neural differentiation of pluripotent mouse embryonal carcinoma cells by retinoic acid: Inhibitory effect of serum. Physiol Res 54: 115–122. http://www.biomed.cas.cz/physiolres/pdf/54/54_115.pdf [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Helms JB, Beckers CJ, Orci L, Rothman JE (1993) Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem 268: 12083–12089. https://www.jbc.org/content/268/16/12083.long [PubMed] [Google Scholar]
- Pavel J, Harter C, Wieland FT (1998) Reversible dissociation of coatomer: Functional characterization of a beta/delta-coat protein subcomplex. Proc Natl Acad Sci U S A 95: 2140–2145. 10.1073/pnas.95.5.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser I, Sarov M, Hutchins JR, Heriche JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, et al. (2008) BAC TransgeneOmics: A high-throughput method for exploration of protein function in mammals. Nat Methods 5: 409–415. 10.1038/nmeth.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praschberger R, Lowe SA, Malintan NT, Giachello CNG, Patel N, Houlden H, Kullmann DM, Baines RA, Usowicz MM, Krishnakumar SS, et al. (2017) Mutations in membrin/GOSR2 reveal stringent secretory pathway demands of dendritic growth and synaptic integrity. Cell Rep 21: 97–109. 10.1016/j.celrep.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308. 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M, Chan EY, Tooze SA (2009) Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol 185: 305–321. 10.1083/jcb.200810098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlmuller MC, Strating JR, Beck R, Eckert P, Popoff V, Haag M, Hellwig A, Berger I, Brugger B, Wieland FT (2011) Recombinant heptameric coatomer complexes: Novel tools to study isoform-specific functions. Traffic 12: 682–692. 10.1111/j.1600-0854.2011.01177.x [DOI] [PubMed] [Google Scholar]
- Shtutman M, Baig M, Levina E, Hurteau G, Lim CU, Broude E, Nikiforov M, Harkins TT, Carmack CS, Ding Y, et al. (2011) Tumor-specific silencing of COPZ2 gene encoding coatomer protein complex subunit zeta 2 renders tumor cells dependent on its paralogous gene COPZ1. Proc Natl Acad Sci U S A 108: 12449–12454. 10.1073/pnas.1103842108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Roninson IB (2011) A subunit of coatomer protein complex offers a novel tumor-specific target through a surprising mechanism. Autophagy 7: 1551–1552. 10.4161/auto.7.12.17659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines WA, Morassutti DJ, Reuhl KR, Ally AI, McBurney MW (1994) Neurons derived from P19 embryonal carcinoma cells have varied morphologies and neurotransmitters. Neuroscience 58: 735–751. 10.1016/0306-4522(94)90451-0 [DOI] [PubMed] [Google Scholar]
- Styers ML, O’Connor AK, Grabski R, Cormet-Boyaka E, Sztul E (2008) Depletion of beta-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. Am J Physiol Cell Physiol 294: C1485–C1498. 10.1152/ajpcell.00010.2008 [DOI] [PubMed] [Google Scholar]
- Tippmann SC, Ivanek R, Gaidatzis D, Scholer A, Hoerner L, van Nimwegen E, Stadler PF, Stadler MB, Schubeler D (2012) Chromatin measurements reveal contributions of synthesis and decay to steady-state mRNA levels. Mol Syst Biol 8: 593 10.1038/msb.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Cotrufo T, Ricolo D, Soriano E, Araujo SJ (2018) SNARE complex in axonal guidance and neuroregeneration. Neural Regen Res 13: 386–392. 10.4103/1673-5374.228710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Lin C, Lu D, Ning Z, Cox T, Melvin D, Wang X, Bradley A, Liu P (2008) Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci U S A 105: 9290–9295. 10.1073/pnas.0801017105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkin LB, Jessen B, Wiszniewski W, Vece TJ, Jan M, Sha Y, Thamsen M, Santos-Cortez RL, Lee K, Gambin T, et al. (2015) COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet 47: 654–660. 10.1038/ng.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann D, Hess P, Baier C, Wieland FT, Reinhard C (2004) Novel isotypic gamma/zeta subunits reveal three coatomer complexes in mammals. Mol Cell Biol 24: 1070–1080. 10.1128/mcb.24.3.1070-1080.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kedlaya R, Higuchi H, Ikeda S, Justice MJ, Setaluri V, Ikeda A (2010) Mutation in archain 1, a subunit of COPI coatomer complex, causes diluted coat color and Purkinje cell degeneration. PLoS Genet 6: e1000956 10.1371/journal.pgen.1000956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN (2007) Growing dendrites and axons differ in their reliance on the secretory pathway. Cell 130: 717–729. 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche B, Heidenreich M, Mohanraju P, Fedorova I, Kneppers J, DeGennaro EM, Winblad N, Choudhury SR, Abudayyeh OO, Gootenberg JS, et al. (2017) Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35: 31–34. 10.1038/nbt.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Source Data for Figure 1LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Source Data for Figure 2LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Source Data for Figure 4LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Source Data for Figure S3LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Source Data for Figure S4LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Source Data for Figure 6LSA-2020-00714_SdataF1_F2_F4_F6_FS3_FS4.tif (9MB, tif)
Table S1 Materials used in this study. (9.2KB, xlsx)
Table S2 List of sgRNAs/crRNAs. (9KB, xlsx)
Table S3 Plasmids used in this study. (9.4KB, xlsx)
Table S4 Antibodies used in this study. (9.5KB, xlsx)
Table S5 List of primers used in qPCR experiments. (9.3KB, xlsx)