Abstract
Background:
Osteoarthritis (OA) is a chronic disease that attacks joints and bones which can be caused by trauma or other joint diseases. Stem cell and Conditioned Medium (CM) of stem cells are developed for OA therapy, which is minimally invasive. It can decrease inflammation and be a replacement for knee surgery. This study aimed to utilize human Wharton’s Jelly-Mesenchymal Stem Cells (hWJMSCs) as an alternative OA therapy.
Methods:
CM from hWJMSCs induced by IGF1 was collected. The OA cells model (IL1β-CHON002) culture was treated as follows: 1) with hWJMSCs-CM 15% (v/v); 2) with hWJMSCs-CM 30% (v/v); 3) with IGF1-hWJMSCs (IGF1-hWJMSCs-CM) 15% (v/v); 4) with IGF1-hWJMSCs-CM 30% (v/v). Parameters including inflammatory cytokines (IL10 and TNFα), extracellular matrix degradation (MMP3 expression), and chondrogenic marker (COL2 expression) were determined.
Results:
The most significant increase in COL2 chondrogenic markers was found in IL1β-CHON002 treatment using 15% CM of hWJMSCs induced with IGF1. CM of hWJMSCs can reduce inflammatory cytokines (TNFα and IL10) and matrix degradation mediator MMP3. Better result was gained from IGF1-induced hWJMSCs-CM.
Conclusion:
CM of IGF1-hWJMSCs reduce inflammation while repairing injured joint in the human chondrocyte OA model.
Keywords: Chondrocyte, IGF1, Osteoarthritis, Proinflammatory, Wharton’s jelly
Introduction
The prevalence of Osteoarthritis (OA) and Rheumatoid Arthritis (RA) are increasing in linear to the growth of elderly population 1. Osteoarthritis is a chronic disease that attacks joints and bones which is caused by trauma as well as other joint diseases. Commonly, synovial inflammation can cause joint homeostasis disorders related to OA 2.
Interleukin-1β (IL1β) is a cytokine that can trigger OA through a variety of mechanisms, such as triggering an imbalance in cartilage repair process, triggering the formation of ROS including Nitric Oxide (NO), inflammatory mediators such as Prostaglandin E2 (PGE2) through increased expression of inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX2) 3. The formation of free radicals and the lack of an antioxidant defense system can trigger oxidative stress causing damage to joints in OA and RA 4. Clinical measures for OA therapy are usually based on the main symptoms and the main focus is to reduce pain from inflammation using NonSteroidal Anti-Inflammatory Drugs (NSAIDs) or total replacement of joints 5. OA therapy is not intended to regenerate articular cartilage. Continuous use of NSAIDs has side effects that can cause kidney, digestive and cardiovascular disorders 6,7.
Chondrocytes which are part of the cartilage are most widely used for OA therapy 8; however, treatment using autologous chondrocytes implantation has various disadvantages such as involving two-step surgeries which causes damage and degradation of the cartilage. Mesenchymal Stem Cells (MSCs) have the ability to differentiate into chondrocytes so they are suitable candidates for cartilage regeneration therapy. MSCs have a variety of abilities, one of which is modulating the microenvironment through anti-inflammatory and immunosuppressive functions. Diverse bioactive soluble factors excreted by MSCs can protect cartilage from damage and induce regeneration of remaining progenitor cells 9. The ability of homing possessed by MSCs causes MSCs to converge on the cartilage defect and they can proliferate to regenerate articular cartilage, reduce the concentration of synovial fluid from prostaglandins 10, and decrease the progressive nature of OA 11.
MSCs are first isolated from cartilage [Bone Marrow Mesenchymal Stem Cells (BMMSCs)] and after that they can also be isolated from adipose tissue, placenta, umbilical cord, umbilical cord blood, dental pulp, amnion 12 and Wharton’s jelly 13. Various therapies for patients with OA are chondroprotective and they reduce inflammation and delay damage to the cartilage 14. When compared with BMMSCs, Adipose tissue-MSCs (ADMSCs) have lower chondrogenesis ability. Induction using Transforming Growth Factor-β2 (TGFβ2) and Insulin like Growth Factor-1 (IGF1) in ADMSCs can produce chondrocyte markers comparable to BMMSCs, which include collagen-1A (COL1-A), COL2A1, and SRY-related HMG-box (SOX9) 15. Plasmid-based overexpression from IGF1 in rabbit chondrocytes encapsulated using alginate and given in vivo shows the ability to repair cartilage and accelerate subchondral bone formation in osteochondral disorders 16.
OA treatment uses stem cells, and especially human Wharton’s Jelly Mesenchymal Stem Cells (hWJMSCs) have the potential to be applied in the treatment of OA because of their high regeneration power and are easily obtained because they come from the umbilical cord. The previous study by Sanchooli et al shows that conditioned medium from ADMSCs (ADMSCs-CM) has a high potential for bone healing. The effectiveness of MSCs-CM therapy is due to the presence of growth factors and cytokines which can inhibit apoptosis and increase cell proliferation and even stimulate mobilization and placement of stem cells to the site of injury 17.
Nevertheless, stem cells transplantation has some obstacles such as differentiation and low cell endurance. These problems can be overcome by using CM obtained from stem cell culture. Analysis shows that CM of hWJMSCs contains various important proteins such as cytokines, growth factors, and angiogenic factors 18,19. This study was conducted to evaluate the potential of CM of IGF1-induced hWJMSCs (IGF1-hWJMSCs-CM) for OA therapy.
Materials and Methods
Cultivation of hWJMSCs and CM collection
hWJMSCs were collected from the Stem Cell and Cancer Institute (Jakarta, Indonesia). The cells had been characterized by the cell multipotent differentiation and surface phenotype 13,20. Informed consent was obtained from the Institutional Ethics Committee at the Stem Cell and Cancer Institute, Jakarta, Indonesia. hWJMSCs at a density of 1×106/well were cultured in Minimum Essential Medium-α (MEM-α) (Gibco, 12561056) supplemented with Fetal Bovine Serum (20%) (FBS) (Gibco, 10270106) and 1% antibiotic and antimycotic (Gibco, 1772653). The cells were incubated in a humidified atmosphere with 5% CO2 at 37°C for 24 hr. The medium was discarded and washed with Phosphate Buffered Saline (PBS) (Gibco, 1740576). hWJMSCs at density of 3×105 cells/well were maintained in a complete medium. The cells were treated with IGF-1 (Biolegend, 590904) at concentrations of 0 and 150 ng/ml, and incubated at 5% CO2, 37°C for 7 days, to obtain IGF1-induced hWJMSCs cells (IGF1-hWJMSCs) for measuring COL2 gene expression. After inducing IGF1, hWJMSCs were harvested. The medium was collected and centrifuged at 3000 g for 4 min at room temperature, and the supernatant was filtered by a 0.22-mm filter (TPP, 99722) and used as CM of hWJMSCs (hWJMSCs-CM) and stored at −80°C 19–21.
OA model treated with CM of IGF1-hWJMSCs
Human chondrocyte CHON002 cell line (ATCC RL-2847) (5×105 cells) were obtained from Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung, Indonesia. The cells were seeded into T-25 flasks and incubated for 48 hr. The medium (hWJMSCs-CM) was replaced and the cells treated with recombinant IL1β (Biolegend, 579404) with concentrations of 0 (no treatment) and 10 ng/ml for 5 days in preparation for the OA model 20–22.
The experiment were conducted with 6 different groups as follow: 1) CHON002 without IL1β induction and without additions of CM (control); 2) IL1β-CHON002 without additions of hWJMSCs-CM; 3) IL1β-CHON002 treated with hWJMSCs-CM 15% (v/v); 4) IL1β-CHON002 treated with hWJMSCs-CM 30% (v/v); 5) IL1β-CHON002 treated with CM of IGF1-hWJMSCs (IGF1-hWJMSCs-CM) 15% (v/v); 6) IL1β-CHON002 treated with IGF1-hWJMSCs-CM 30% (v/v). The medium were replaced every 2 days. The experiment were carried for 1 and 2 weeks 21,23.
Analysis of COL2 gene expression
RNA was extracted using Aurum RNA kit (Bio-Rad, 7326820) based on the manufacturer’s instructions. The concentration and purity of RNA of each sample was determined at 260/280 nm (Table 1). Primer sequences can be seen in table 2. The synthesis of cDNA from the RNA was carried out using iScript cDNA synthesis kit (Bio-Rad, 1708890) at 25°C for 5 min, 42°C for 30 min, and 85°C for 5 min for the final step. The end-product was stored at −20°C. Quantitative gene expression was conducted using Thermo Scientific PikoReal Real-time PCR System (Thermo Fisher). PCR included pre-incubation cycle at 95°C for 5 min, 40 cycles of denaturation at 95°C for 1 min, annealing at 53°C for 40 s, and extension at 72°C for 1 min. The reaction mix that was used to perform qPCR was from Evagreen master mix (Bio-Rad, 1725200). Table 2 shows the primers used in this research 21,23.
Table 1.
Concentration and purity of RNA
| Treatment | RNA concentration (ng/ml) | RNA purity (λ260/λ280 nm) | ||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 1 | Week 2 | |
| Normal cell (CHON002) | 38.32 | 147.00 | 2.18 | 2.29 |
| IL1β-CHON002 | 39.36 | 162.38 | 2.13 | 2.26 |
| hWJMSCs-CM 15%+IL1β-CHON002 | 36.76 | 134.22 | 2.10 | 2.25 |
| hWJMSCs-CM 30%+IL1β-CHON002, | 61.64 | 230.02 | 2.11 | 2.26 |
| IGF1-hWJMSCs-CM 15%+IL1β-CHON002 | 61.04 | 233.00 | 2.13 | 2.29 |
| IGF1-hWJMSCs-CM 30%+IL1β-CHON002 | 49.48 | 194.24 | 2.19 | 2.27 |
Table 2.
Primer sequence of COL2 and GAPDH gene
| Gene symbols | Primer sequence (5′ to 3′) upper strand: sense lower strand: antisense | Product size (bp) | Annealing (°C) | Cycle | References |
|---|---|---|---|---|---|
| COL2 | 5′-TTTCCCAGGTCAAGATGGTC-3′ 5′-CTGCAGCACCTGTCTCACCA-3′ |
377 | 53 | 40 | NM_001844.4 |
| GAPDH | 5′-GGGCTGCTTTTAACTCTGGT-3′ 5′-TGGCAGGTTTTTCTAGACGG-3′ |
702 | 51 | 40 | NM_001289745.1 |
Quantification of IL10, TNFα, and MMP3 level in OA model treated with hWJMSCs-CM
Secretion of IL10, TNFα, MMP3 was assessed using ELISA Kit IL10 (Elabsci, E-EL-H0103), TNFα (Elabsci, E-EL-H0109), and MMP3 (Elabsci, E-ELH1446). The procedure was in accordance with manufacturer’s protocol. Sample absorbances were read at 450 nm using microplate reader (Multiskan GO, ThermoScientific). IL10, TNFα, MMP3 concentration were calculated based on a protein standard curve 19,21,24.
Results
COL2 gene expression level
COL2 is a monomer protein that forms the main formation of the cartilage matrix and is the main target of tissue that is attacked by OA. The long assembly process of COL2 in the cytoplasm will eventually be transported out of the cell to form a cartilage matrix and its expression culminates in the ripening of chondrocytes 25. COL2 expression can be seen in figure 1 and has increased from the first week to the second week. The highest expression was found in the IGF1-hWJMSCs-CM 15% treated group.
Figure 1.
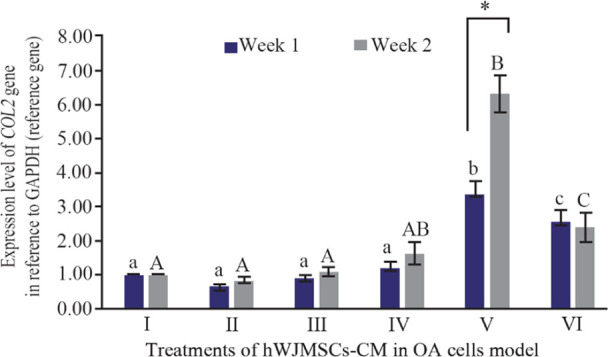
Effect of hWJMSCs-CM, IGF1-hWJMSCs-CM toward COL2 gene expression on OA cells model. (I) Normal cell (CHON002), (II) IL1β-CHON002, (III) hWJMSCs-CM 15% + IL1β-CHON002 (IV), hWJMSCs-CM 30% + IL1β-CHON002, (V) IGF1-hWJMSCs-CM 15% + IL1β-CHON002, (VI) IGF1-hWJMSCs-CM 30%+IL1β-CHON002. The histograms are presented as mean± standard deviation, the treatment was done triplicate. The data were analyzed with ANOVA and Tukey post hoc test. Different letters (a, b, c) indicate significant differences in 1 week incubation (Blue color) and different letters (A, AB, B, C) indicate significant differences in 2 week incubation (Gray color). The symbol (*) presents significant differences between week 1 and week 2 based on paired t-test (p<0.05).
Level of TNFα, IL10, and MMP3
TNFα, together with IL1β, is considered an inflammatory cytokine which is a key in the pathophysiological process that occurs during OA. TNFα is secreted by the same cells as cells that synthesize IL1β 26,27. Figure 2 shows TNFα levels using the ELISA method. It can be seen that IL1β-CHON002 has the highest level of TNFα among others, while addition of CM of IGF1-hWJMSCs 15% shows a significant decrease in TNFα (p<0.05). TNFα also experienced elevated levels in the treatment for 2 weeks.
Figure 2.
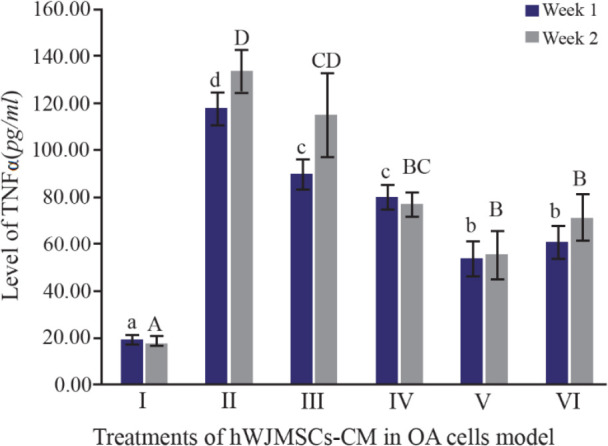
Effect of hWJMSCs-CM, IGF1-hWJMSCs-CM toward TNFα on OA cells model. (I) Normal cell (CHON002), (II) IL1β-CHON002, (III) hWJMSCs-CM 15%+IL1β-CHON002, (IV) hWJMSCs-CM 30%+IL1β-CHON002, (V) IGF1-hWJMSCs-CM 15%+ IL1β-CHON002, (VI) IGF1-hWJMSCs-CM 30%+IL1β-CHON002. The histograms are presented as mean±standard deviation, the treatment was done triplicate. The data were analyzed with ANOVA and Tukey post hoc test. Different letters (a, b, c, d) indicate significant differences in 1 week incubation (Blue color) and different letters (A, B, BC, CD, D) indicate significant differences in 2 week incubation (Gray color).
IL10 is a cytokine that acts as an anti-inflammatory agent and it is one of the cytokines that shows a chondroprotective effect on OA 28. The IL10 cytokines and IL10R receptors are expressed by chondrocytes 29. IL10 works by stimulating antagonist proteins against IL1β, namely IL1Ra, metalloproteinase inhibitors (TIMP1), and also as growth factors. IL10 levels can be seen in figure 3. IL10 as an anti-inflammatory mediator was found with the highest levels in IL1β-CHON002. However, addition of hWJMSCs-CM with or without IGF1 induction shows a decrease in IL10 levels compared to OA cells model.
Figure 3.

Effect of hWJMSCs-CM, CM of IGF1-hWJMSCs toward IL10 level on OA cells model. (I) Normal cell (CHON002), (II) IL-1β-CHON002, (III) hWJMSCs-CM 15%+IL1β- CHON002 (IV), hWJMSCs-CM 30%+IL1β-CHON002, (V) IGF1-hWJMSCs-CM 15%+IL1β-CHON002, (VI) IGF1-hWJMSCs-CM 30%+IL1β-CHON002. The histograms are presented as mean±standard deviation, the treatment was done triplicate. The data were analyzed with ANOVA and Tukey post hoc test. Different letters (a, b, c) indicate significant differences in 1 week incubation (Blue color) and different letters (A, B, C, D) indicate significant differences in 2 week incubation (Gray color). The symbol (*) presents significant differences between week 1 and week 2 based on paired t-test (p<0.05).
The MMPs are expressed in joint tissues of patients with OA and RA. The MMP3 is secreted from chondrocyte and synovial cells and MMP3 can reduce various extracellular matrix. Figure 4 shows MMP3 levels using ELISA method. The lower MMP3 level was shown by treatment with the addition of 15%, 30% of IGF1-hWJMSC-CM during 1 and 2 weeks of incubation, while the lowest MMP3 levels was during 2 weeks of incubation of IGF1-hWJMSCs-CM 15%. This result shows that IGF1-hWJMSCs-CM can reduce levels of MMP3 which plays a role in matrix degradation.
Figure 4.
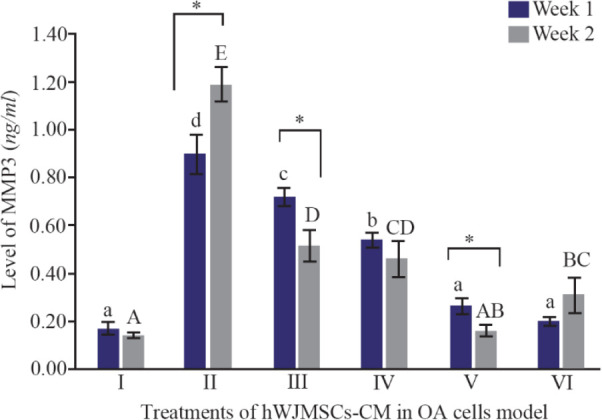
Levels of MMP3 in cells of OA models cultured in hWJMSCs-CM. (I) Normal cell (CHON002), (II) IL1β-CHON002, (III) hWJMSCs-CM 15%+IL1β-CHON002 (IV), hWJMSCs-CM 30%+IL1β-CHON002, (V) IGF1-hWJMSCs-CM 15%+IL1β-CHON002, (VI) IGF1-hWJMSCs-CM 30%+IL1β-CHON002. The histograms are presented as mean ± standard deviation, the treatment was done triplicate. The data were analyzed with ANOVA and Tukey post hoc test. Different letters (a, b, c, d) indicate significant differences in 1 week incubation (Blue color) and different letters (A, AB, BC, C, CD, D, E) indicate significant differences in 2 week incubation (Gray color). The symbol (*) presents significant differences between week 1 and week 2 based on paired t-test (p<0.05).
Discussion
MSCs have been used as one of the candidates for tissue engineering which have the ability in repair, replacement, and regeneration of cartilage tissue, because of their high proliferation and differentiation 29. One type of them originates from Wharton’s jelly 13,19. Previous studies reported that hWJMSCs can differentiate into chondrocytes 21,24, skeletal muscle cells, heart muscle cells, osteoblasts, adipocytes, β cells on the islets of Langerhans, and endothelial cells in vitro 30,31. Therefore, these cells can be used as alternatives in the treatment of chronic degenerative disorders and prevent cartilage degradation in OA patients. In this study, the induction of hWJMSCs using IGF1 increased COL2 expression. The COL2 gene which is the cartilage matrix gene has been indicated to be regulated by SOX9 32,33, and is involved in the structure and function of articular cartilage. This is in accordance with previous studies, where the inducing of IGF1 in hWJ-MSCs increased the expression of the SOX9 gene which means that the expression of the COL2 gene will also increase 21. SOX9 is present in the presumed cartilage during embryonic development. COL2 downregulation in OA is likely to contribute to cartilage pathology. IGF1 can stabilize the chondrocyte phenotype in pathological conditions, and also has mitogenic properties in the articular cartilage of adults and strongly stimulate the production of chondrocyte extracellular matrix components 21. COL2 is a chondrogenic gene marker in the joint cartilage, associated with Extracellular Matrix Secretion (ECM). In OA, early changes in articular cartilage are characterized by proteoglycan loss and a decrease in the expression of the COL2 gene without altering the regularity of the articular tissue structure 34. After treatment, as shown in figure 1, there were significant improvements in COL2 gene expression in OA model, indicating that there might be repairing process in the ECM. The highest expression of COL2 is by IGF1-hWJMSCs-CM 15%+IL1β-CHON-002 which is significantly different from other treatments. It is known that the expression of COL2 is closely related to SOX9.
The effect of TNFα in many cases coincides with the action of IL1β, and a relationship can be detected that occurs during OA between two cytokines 28. This effect is the result of activating the same group of intracellular signaling pathways, which in turn triggers effects that increase inflammation in joint tissues 35. In contrast, IL10 is a cytokine that acts as an anti-inflammatory agent and it is one of the cytokines that shows a chondroprotective effect on OA and works by stimulating antagonist proteins against IL1β as pro-inflammatory cytokine 28.
In the present study, the highest levels of TNFα were found in OA cells without treatment using CM of hWJMSCs and IGF1-hWJMSCs. This is possible because the production of proinflammatory cytokines is directly proportional to the production of anti-inflammatory cytokines, when high levels of proinflammatory cytokines occur and the body adjusts to provide a regulatory response to levels of anti-inflammatory cytokines. Furthermore, hWJMSCs-CM and IGF1-hWJMSCs-CM in the OA cells model decrease TNFα level which is responsible for OA inflammation; however, administration of them to IL10 did not show an increase in IL10 levels responsible for inhibiting pro-inflammatory cytokines. This is in line with the results of Al-Banna et al who state that the induction of inflammatory cytokines is followed by an increase in the level of anti-inflammatory cytokines 36.
Cell therapies can directly aid repair by forming new functional tissues, or support tissue repair through paracrine mechanisms, for instance by secreting growth factors, immunomodulatory molecules, and Extracellular Vesicles (EVs). EVs can mediate cell-cell communication and are involved in many processes, including immune signaling, angiogenesis, stress response, senescence, proliferation, and cell differentiation 36. In vitro passaging of MSCs results in cell enlargement, differentiation, and decrease in proliferation within 10 passages, and causes a strong response to micro-environment stiffness, affecting cell morphology, and function 37,38.
Metabolic imbalances between degradation and synthesis of articular cartilage are the main reason for degeneration in OA sufferers. MMP is a protein that is responsible for the degradation of the extracellular matrix and basement membrane components 39. MMP is a endopeptidase that is connected with zinc ions and is localized in various connective tissues; it can degrade various components of the ECM 40. In the present study, OA model (IL1β-CHON002) has shown high MMP3 levels, and after treatment using hWJMSCs-CM and IGF1-hWJMSCs-CM, showed a decrease in MMP3 levels (Figure 4) and the lowest MMP3 level was shown by treatment with the addition of IGF1-hWJMSCs-CM 30%. This indicates that the administration of hWJMSCs-CM with the addition of IGF1 can reduce MMP3 levels which are the cause of the degradation of the extracellular matrix.
Conclusion
In conclusion, CM of IGF1-induced hWJMSCs increases COL2 gene expression compared with CM of IGF1-uniduced hWJMSCs. CM of IGF1-hWJMSCs actively reduce the levels of pro-inflammatory cytokines of TNFα, IL10, MMP3 compared with CM of IGF1-uniduced hWJMSCs. CM of IGF1-hWJMSCs also increases chondrogenesis and can subsequently be an alternative for the treatment of OA. Further studies on animal models must be carried out for validation of IGF1-hWJMSCs effect.
Acknowledgement
This study was supported by the Grants-in-Aid Insinas Riset Pratama Individu 2019, from Ministry of Research, Technology and Higher Education of the Republic of Indonesia. The authors like to thank to Rr. Anisa Siwianti Handayani, Alya Mardhotillah Azizah, and Jenifer Kiem Aviani from Biomolecular and Biomedical Research Center, Aretha Medika Utama, Ban-dung, West Java, Indonesia for their technical assistants.
Footnotes
Conflict of Interest
There are no conflict of interest.
References
- 1.Ahmed U, Anwar A, Savage RS, Costa ML, Mackay N, Filer A, et al. Biomarkers of early stage osteoarthritis, rheumatoid arthritis and musculoskeletal health. Sci Rep 2015;5:9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smoleńska Ż, Kaznowska Z, Zarown D, Simmonds HA, Smoleński RT. Effect of methotrexate on blood purine and pyrimidine levels in patients with rheumatoid arthritis. Rheumatology 1999;38(10):997–1002. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1β-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Rad Biol Med 2002;33(8): 1097–1105. [DOI] [PubMed] [Google Scholar]
- 4.Ozkan Y, Yardým-Akaydýn S, Sepici A, Keskin E, Sepici V, Simsek B. Oxidative status in rheumatoid arthritis. Clin Rheumatol 2007;26(1):64–68. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 2001;27(2):269–281. [DOI] [PubMed] [Google Scholar]
- 6.Kremers HM, Nicola P, Crowson CS, O’fallon WM, Gabriel GS. Therapeutic strategies in rheumatoid arthritis over a 40-year period. J Rheumatol 2004;31:2366–2373. [PubMed] [Google Scholar]
- 7.Leong DJ, Choudhury M, Hanstein R, Hirsh DM, Kim SJ, Majeska RJ, et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthris Res Ther 2014;16(6):508. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 8.Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 2011;39(12):2566–2574. [DOI] [PubMed] [Google Scholar]
- 9.Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 2012;3(4):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthr Res Ther 2012;14(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells treatment of osteoarthritis. J Orthop Res 2009;27(12):1675–1680. [DOI] [PubMed] [Google Scholar]
- 12.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widowati W, Wijaya L, Bachtiar I, Gunanegar RF, Sugeng SU, Irawan YA, et al. Effect of oxygen tension on proliferation and characteristics of Wharton’s jelly-derived mesenchymal stem cells. BGM 2014;6(1):43–48. [Google Scholar]
- 14.Adcocks C, Collin P, Buttle DJ. Catechins from green tea (Camellia sinensis) inhibit bovine and human cartilage proteoglycan and type ii collagen degradation in vitro. J Nutr 2002;132(3):341–346. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Im GI. Chondrogenic differentiation of adipose tissue-derived mesenchymal stem cells: Greater doses of growth factor are necessary. J Orthop Res 2009;27(5): 612–619. [DOI] [PubMed] [Google Scholar]
- 16.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 2005;12(15):1171–1179. [DOI] [PubMed] [Google Scholar]
- 17.Sanchooli T, Norouzian M, Ardeshirylajimi A, Ghoreishi S, Amin Abdollahifar M, Nazarian H, et al. Adipose derived stem cells conditioned media in combination with Bioceramic-collagen scaffolds improved calvarial bone healing in hypothyroid rats. Iranian Red Crescent Med J 2017;19(5):e45516. [Google Scholar]
- 18.Amable PR, Teixeira MVT, Carias RBV, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther 2014;5(3):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widowati W, Widyastuti H, Murti H, Laksmitawati DR, Kusuma HSW, Rizal R, et al. Interleukins and VEGF secretome of human wharton’s Jelly mesenchymal stem cells-conditioned medium (hWJMSCs-CM) in different passages and oxygen tensions. Biosci Res 2017;14:776–787. [Google Scholar]
- 20.Widowati W, Wijaya L, Murti H, Widyastuti H, Agustina D, Laksmitawati DR, et al. Conditioned medium from normoxia (WJMSCs-norCM) and hypoxia-treated WJMSCs (WJMSCs-hypoCM) in inhibiting cancer cell proliferation. BGM 2015;7:8–17. [Google Scholar]
- 21.Widowati W, Afifah E, Mozef T, Sandra F, Rizal R, Amalia A, et al. Effects of insulin-like growth factor-induced Wharton jelly mesenchymal stem cells toward chondrogenesis in an osteoarthritis model. Iran J Basic Med Sci 2018;21(7):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater Sci Eng: C 2004;24:391–396. [Google Scholar]
- 23.Afifah E, Mozef T, Sandra F, Arumwardana S, Rihibiha DD, Nufus H, et al. Induction of matrix metalloproteinases in chondrocytes by interleukin IL-1β as an osteoarthritis model. Journal of Mathematical and Fundamental Sciences 2019;51(2):103–111. [Google Scholar]
- 24.Noverina R, Widowati W, Ayuningtyas W, Kurniawan D, Afifah E, Laksmitawati DR, et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin Nutr Exp 2019;24:34–44. [Google Scholar]
- 25.Gadjanski I, Spiller K, Vunjak-Novakovic G. Time-dependent processes in stem cell-based tissue engineering of articular cartilage. Stem Cell Rev Rep 2012;8(3): 863–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massicotte F, Lajeunesse D, Benderdour M, Pelletier JP, Hilal G, Duval N, et al. Can altered production of interleukin-1β, interleukin-6, transforming growth factor-β and prostaglandin E2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients. Osteoarthr Cartilage 2002;10(6):491–500. [DOI] [PubMed] [Google Scholar]
- 27.Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthr Res Ther 2012;14(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iannone F, De Bari C, Dell Accio F, Covelli M, Cantatore FP, Patella V, et al. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol 2001;19(2):139–146. [PubMed] [Google Scholar]
- 30.Seo S, Na K. Mesenchymal stem cell-based tissue engineering for chondrogenesis. J Biomed Biotechnol 2011; 2011:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007;25(11):2886–2895. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P, Jimenez SA, Stokes DG. Regulation of human COL9A1 gene expression. J Biol Chem 2003;278(1): 117–123. [DOI] [PubMed] [Google Scholar]
- 33.Kou I, Ikegawa S. SOX9-dependent and -independent transcriptional regulation of human cartilage link protein. J Biol Chem 2004;279:50942–50948. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho). Arthritis Rheum 2003;48(9):2509–2518. [DOI] [PubMed] [Google Scholar]
- 35.Marcu KB, Otero M, Olivotto E, Maria Borzi R, Goldring MB. NF-κB signaling: multiple angles to target OA. Curr Drug Targets 2010;11(5):599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Banna N, Raghupathy R, Albert MJ. Correlation of proinflammatory and anti-inflammatory cytokine levels with histopathological changes in an adult mouse lung model of campylobacter jejuni infection. Clin Vaccine Immunol 2008;15(12):1780–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 2004;351(12): 1187–1196. [DOI] [PubMed] [Google Scholar]
- 38.Siddappa R, Licht R, van Blitterswijk C, de Boer J. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res 2007;25(8):1029–1041. [DOI] [PubMed] [Google Scholar]
- 39.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006;1 26(4):677–689. [DOI] [PubMed] [Google Scholar]
- 40.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthr Rheum 2001;44 (3):585–594. [DOI] [PubMed] [Google Scholar]


