Abstract
In the environment, bacteria show close association, such as interspecies interaction, with other bacteria as well as host organisms. The type VI secretion system (T6SS) in gram‐negative bacteria is involved in bacterial competition or virulence. The plant pathogen Burkholderia glumae BGR1, causing bacterial panicle blight in rice, has four T6SS gene clusters. The presence of at least one T6SS gene cluster in an organism indicates its distinct role, like in the bacterial and eukaryotic cell targeting system. In this study, deletion mutants targeting four tssD genes, which encode the main component of T6SS needle formation, were constructed to functionally dissect the four T6SSs in B. glumae BGR1. We found that both T6SS group_4 and group_5, belonging to the eukaryotic targeting system, act independently as bacterial virulence factors toward host plants. In contrast, T6SS group_1 is involved in bacterial competition by exerting antibacterial effects. The Δ tssD1 mutant lost the antibacterial effect of T6SS group_1. The ΔtssD1 mutant showed similar virulence as the wild‐type BGR1 in rice because the ΔtssD1 mutant, like the wild‐type BGR1, still has key virulence factors such as toxin production towards rice. However, metagenomic analysis showed different bacterial communities in rice infected with the ΔtssD1 mutant compared to wild‐type BGR1. In particular, the T6SS group_1 controls endophytic plant‐associated bacteria such as Luteibacter and Dyella in rice plants and may have an advantage in competing with endophytic plant‐associated bacteria for settlement inside rice plants in the environment. Thus, B. glumae BGR1 causes disease using T6SSs with functionally distinct roles.
Keywords: bacterial competition, bacterial virulence, Burkholderia glumae, interspecies interaction, metagenomic analysis, TssD, type VI secretion system
T6SS group_4 and T6SS group_5 affect the pathogenicity of Burkholderia glumae BGR1 as a completely independent eukaryotic targeting system, whereas T6SS group_1 is involved in bacterial competition by an antibacterial effect.

1. INTRODUCTION
Myriad bacteria demonstrate close associations with nearby bacteria, as well as with their host organisms (Freestone, 2013; Stubbendieck et al., 2016). Bacteria can persist and adapt in their ecological niche by continuously interacting with other organisms, and have developed many protective and infectious mechanisms against competitors and host organisms. Some of these, such as bacterial secretion systems, biofilms, and phytotoxins, provide survival benefits against bacterial and eukaryotic cells, and facilitate environmental adaptation (Backert and Meyer, 2006). In particular, bacterial secretion systems are considered the primary and effective means for communication and adaption to the surroundings (Russell et al., 2014).
Bacterial secretion systems are classified into nine groups (Tat system, types I–VII, type IX) according to their structures, functions, and specific effectors (Stanley et al., 2000; Whitney et al., 2013; Kneuper et al., 2014; Green and Mecsas, 2016). The type VI secretion system (T6SS) was identified as a bacterial secretion system in an opportunistic pathogen, Pseudomonas aeruginosa (Mougous et al., 2006). The T6SS is widespread in around 25% of gram‐negative bacteria and is easily found in pathogenic or symbiotic plant‐associated bacteria (Boyer et al., 2009; Bernal et al., 2018). The T6SS forms proteinaceous machinery that delivers substrates (effectors) to adjacent bacterial or eukaryotic cells in a contact‐dependent manner or releases effectors tothe extracellular environment. The effectors secreted by these systems are used to destroy and manipulate eukaryotic cells and/or to fight other bacteria to gain dominant status in the same ecological niche (Ma and Mekalanos, 2010). At least one T6SS gene cluster is present in some bacteria. The presence of two T6SS gene clusters in one organism increases the possibility that each system has a different function depending on the circumstances (Bingle et al., 2008). More recently, functional T6SSs were discovered in a broad spectrum of bacterial species and were found to contribute to virulence in host organisms, improve bacterial robustness, and enhance adaptation to polymicrobial environments through bacterial competition (Russell et al., 2014). For example, P. aeruginosa has H1‐T6SS, H2‐T6SS, and H3‐T6SS. H1‐T6SS contributes only to interactions between bacteria by the delivery of toxins and effector molecules (Hood et al., 2010). H2‐T6SS and H3‐T6SS also exhibit antibacterial activity by secreting trans‐kingdom effectors, but are involved in virulence against eukaryotes (Russell et al., 2013; Jiang et al., 2014). T6SS‐5 of Burkholderia thailandensis is also a major virulence factor in the murine model of acute melioidosis (Schwarz et al., 2010).
The T6SS is composed of a minimal set of 13 core components, TssA–TssM (type six secretion). Apart from these major components, several accessory proteins are encoded in the T6SS gene cluster, and work together with the T6SS effector to damage the inner membrane of prokaryotic target cells or participate in negative regulation of the T6SS (Miyata et al., 2013; Lin et al., 2018). These components are generally encoded by clustered genes; each cluster has a different gene organization despite the clusters being in a single organism. Among the core components, TssD, also known as Hcp (hemolysin co‐regulated protein), and VgrG (valine‐glycine repeat protein G) have been considered the hallmark apparatus of T6SS as well as secreted effectors in all bacteria with functional T6SSs (Pukatzki et al., 2007).
B. glumae is a gram‐negative bacterium known as a seedborne phytopathogenic bacterium that causes bacterial panicle blight as well as sheath and seedling rot in rice (Oryza sativa) (Goto, 1956; Goto et al., 1987). The major virulence factors of B. glumae are toxoflavin, lipase, type III effectors, and extracellular polysaccharides, along with cell motility (Iiyama et al., 1995; Suzuki et al., 2004; Kim et al., 2007; Kang et al., 2008; Lee et al., 2016; Jung et al., 2018). The T6SS was classified into six groups based on the distribution of 5 out of 13 T6SS components in 12 Burkholderia strains, including B. glumae (Seo et al., 2015). Of these six T6SS groups, four T6SS groups are found in B. glumae BGR1. However, their functions are still unexplored.
In this study, we report that T6SS group_1 has antibacterial effects but is dispensable for virulence, whereas T6SS group_4 and T6SS group_5 were found to directly affect the pathogenicity of B. glumae BGR1 targeting rice plants through phylogenetic analysis and a phenotypic assay. Furthermore, metagenomics analysis of the endophytic bacterial community inside plants identified different bacterial communities with the ΔtssD1 mutant compared to those with the wild‐type BGR1. This indicates that T6SS group_1 inhibits or limits the growth of endophytic plant‐associated bacteria, thereby helping pathogenic bacteria form a bacterial community easily. Our findings clearly describe the important role of T6SS in interspecies interactions, including pathogen–host interaction and interaction with other bacteria.
2. RESULTS
2.1. Evolutionary analysis of type VI secretion systems in Burkholderia species shows functional differentiation based on environmental adaptation
The evolutionary analysis of Burkholderia T6SS in B. mallei, B. pseudomallei, B. thailandensis, and B. cepacia, as well as in six rice‐pathogenic strains of B. plantarii, B. gladioli, and B. glumae, was performed to identify the distinct roles of the four T6SS in B. glumae BGR1, using at least 10 major core components of T6SS. The T6SS gene clusters of B. glumae BGR1, B. plantarii ATCC43733, and B. gladioli BSR3 were annotated in our previous study (Seo et al., 2015). All Burkholderia species have at least one T6SS gene cluster. However, we found that not all T6SS gene clusters of Burkholderia species were completely composed of 13 major elements. In the resulting phylogeny, six rice‐pathogenic Burkholderia species were highly conserved in at least one T6SS gene cluster, which includes T6SS group_1 of B. glumae, B. gladioli, and B. plantarii (Figure S1). These T6SS gene clusters are closely clustered with B. thailandensis E264 T6SS‐1, a well‐known bacterial cell targeting system (Schwarz et al., 2010). In addition, T6SS group_4 and T6SS group_5 of B. glumae BGR1 were clustered with the T6SS gene clusters of the eukaryotic cell targeting system, which are B. thailandensis E264 T6SS‐5 and B. pseudomallei K96243 T6SS‐5, in one or two subtrees (Figure S1) (Schwarz et al., 2010; Lennings et al., 2019). T6SS group_2 was clustered in one or two subtrees with T6SS‐2 of B. pseudomallei K96243 and B. thailandensis E264. However, T6SS group_2 was not clustered with the bacterial and eukaryotic cell targeting systems despite the presence of a complete T6SS gene cluster. Therefore, we assumed that the T6SS of B. glumae BGR1 is involved in the bacterial cell targeting system and the eukaryotic cell targeting system through T6SS group_1 and both T6SS group_4 and 5, respectively.
2.2. The tssD deletion mutants were constructed to evaluate the functions of four T6SS systems in B. glumae BGR1
B. glumae BGR1 has four T6SS gene clusters (T6SS group_1, T6SS group_2, T6SS group_4, and T6SS group_5) with different genetic architectures as well as Tss components (Figure 1). The T6SS group_2 and group_4 clusters possess all of the 13 T6SS core components. However, the T6SS group_1 cluster lacks TssI (VgrG) and the T6SS group_5 cluster lacks TssB, TssH, and TssJ. The tssD1 gene (bglu_1g03910) belongs to T6SS group_1 and is located in chromosome 1. The tssD2 gene (bglu_2g01440), tssD4 gene (bglu_2g11090), and tssD5 gene (bglu_2g07470) are located in chromosome 2 and belong to T6SS group_2, T6SS group_4, and T6SS group_5, respectively. To determine the functions of each T6SS, we constructed markerless deletion mutants targeting the four tssD genes via two homologous recombinations. After completion of the second recombination, tssD deletion mutant strains were confirmed by PCR (Figure S2 and Table S3). Single deletion mutants called ΔtssD1, ΔtssD2, ΔtssD4, and ΔtssD5, corresponding to the genes tssD1, tssD2, tssD4, and tssD5, respectively, were generated. Double deletion mutants, ΔtssD12 and ΔtssD45, were generated. In addition, a quadruple deletion mutant called ΔtssD1245 was also constructed. In order to complement the phenotype of tssD deletion mutants ΔtssD1, ΔtssD4, ΔtssD5, and ΔtssD45, the respective complementation strains, Δtss1D‐C, Δtss4D‐C, ΔtssD5‐C, and ΔtssD45‐C were generated.
Figure 1.
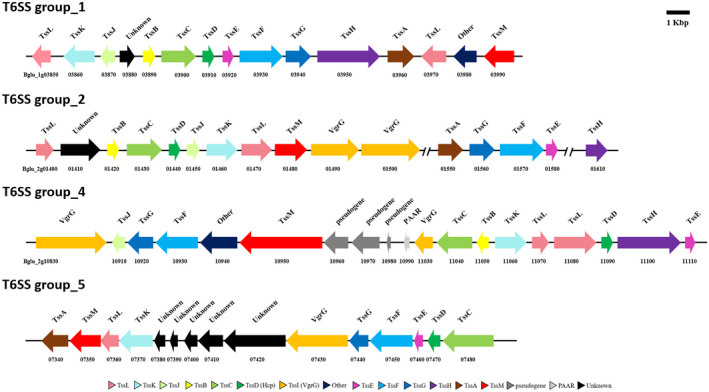
Genetic organization of the four type VI secretion system (T6SS) clusters in Burkholderia glumae BGR1. B. glumae BGR1 has four T6SS gene clusters in its genome. The genes are indicated by the locus ID (e.g., bglu_1g03850) and are to scale. The COGs of 13 conserved T6SSs from the SecreT6 database (http://db‐mml.sjtu.edu.cn/SecReT6/) are marked with specific colours in the four T6SS clusters of B. glumae BGR1 (Li et al., 2015)
2.3. Phenotypic characteristics such as motility and toxoflavin synthesis between the B. glumae BGR1 and the tssD deletion mutants are not different
There were no differences in the growth rate between wild‐type B. glumae BGR1 and the single or quadruple tssD deletion mutants (Figure S3). Several virulence‐related phenotypic characters were tested. However, no apparent difference in swarming motility was observed in the mutant strains compared to that in wild‐type BGR1, that is, comparable swarming motility was observed in all strains, as assessed by the formation of dendritic patterns (Figure S4a). Furthermore, none of the strains had any defects in the biosynthesis of toxoflavin (Lee et al., 2016) (Figure S4b). Thus, there were no changes in the major phenotypic characteristics, including the major virulence factors of B. glumae BGR1, in tssD deletion mutants despite the loss of T6SS functions.
2.4. T6SS group_4 and T6SS group_5 contribute to virulence toward rice plants
To determine whether the T6SS is involved in the eukaryotic cell‐targeting system of B. glumae BGR1, rice plants were infected by wild‐type B. glumae BGR1 and tssD mutants. Primary virulence assays were conducted towards rice plants to investigate the relationship between the T6SS and virulence in the vegetative stages of rice cultivated under greenhouse conditions. At 8 days post‐inoculation (dpi) of the tested tssD mutants (ΔtssD1, ΔtssD2, ΔtssD4, ΔtssD5, ΔtssD12, ΔtssD45, and ΔtssD1245), only ΔtssD4, ΔtssD5, ΔtssD45, and ΔtssD1245 showed reduced virulence (Figure 2a). These results suggest that the T6SSs group_4 and group_5 were involved in bacterial virulence towards rice plants as the eukaryotic cell‐targeting system.
Figure 2.
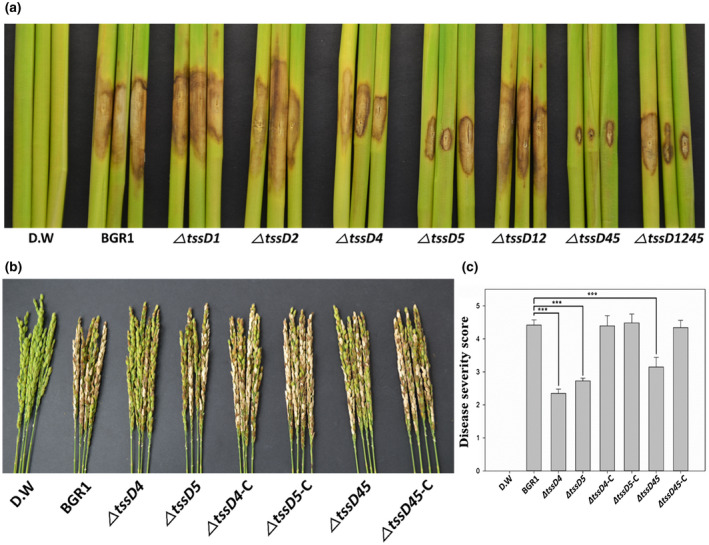
In vivo pathogenicity assay of the vegetative and reproductive stage. (a) T6SS deletion mutants were inoculated with 108 cfu/ml to assess the virulence of the T6SS cluster in the xylem of rice stem (Oryza sativa) at the vegetative stage. The experiments were conducted by three replicates (n = 3). (b) Bacterial suspensions were inoculated into rice panicles at the reproductive stage to assess the virulence of ΔtssD4, ΔtssD5, ΔtssD45, ΔtssD4‐C, ΔtssD5‐C, and ΔtssD45‐C. Representative of four replicates. (c) Disease severity on the rice panicles was calculated on a scale of 0 to 5 after inoculating the bacterial suspension. The data are presented as the mean ± SD of four replicates (n = 4). Mean values followed by the same letters are not significantly different according to Tukey's HSD test (*p < .05, **p < .01, ***p < .001). Disease symptoms at 8 days post‐inoculation. Distilled water (D.W.) was used as the negative control
Next, the bacterial suspensions of ΔtssD4, ΔtssD5, and ΔtssD45 were inoculated in the reproductive stage of rice plants to determine whether the T6SSs are the virulence factors in bacterial panicle blight caused by BGR1. By 8 dpi, most of the BGR1‐inoculated panicles had developed blight symptoms. The results of the virulence test for ΔtssD4, ΔtssD5, and ΔtssD45 in rice panicles were consistent with those obtained in the virulence assay at the vegetative stage of rice. Specifically, disease severity (0–5) was 4.42 ± 0.13 in wild‐type BGR1, and decreased to 2.35 ± 0.11 in ΔtssD4, to 2.73 ± 0.07 in ΔtssD5, and to 3.15 ± 0.24 in ΔtssD45. Furthermore, the complemented strains, ΔtssD4‐C, ΔtssD5‐C, and ΔtssD45‐C, almost completely recovered their virulence, and the disease severity of these strains was 4.40 ± 0.25, 4.48 ± 0.22, and 4.34 ± 0.18, respectively (Figure 2b,c). BGR1 pBBR1MCS2, the negative control strain of the complementation strain, showed virulence towards rice plants similar to the wild‐type BGR1 (Figure S5a–c). Thus, the genes tssD4 and tssD5 contribute to virulence towards rice plants regardless of the growth stage of the plant. However, the result of no attenuated virulence by ΔtssD45 showed no synergistic effect via tssD4 and tssD5.
2.5. T6SS group_1 is involved in antibacterial competition ability
The antibacterial competition ability was tested using apramycin‐resistant Escherichia coli cells as prey and cocultured B. glumae BGR1 as predators. The single E. coli cell cultures proliferated to approximately 9.33 × 107 cfu/ml, whereas E. coli cocultured with an equal number of wild‐type BGR1 cells at a 1:1 ratio for 6 hr showed a decrease to 2.67 × 103 cfu/ml. As a result, the surviving number of E. coli cells grown in pure culture was more than 104 times higher compared to those cocultured with wild‐type BGR1. To investigate whether the T6SS of B. glumae BGR1 is involved in this antibacterial effect, the numbers of E. coli cells surviving coculture with ΔtssD1245 were compared with those of E. coli cells cultured alone. The number of E. coli surviving coculture with ΔtssD1245, which proliferated to approximately 3.25 × 107 cfu/ml, was similar to that of E. coli cultured alone. Next, antibacterial competition assays with tssD1, tssD2, tssD4, and tssD5 single mutants as predators were conducted to determine which T6SS clusters were associated with the antibacterial effects (Figure 3a). Only the effect of ΔtssD1 was found to be comparable with that of ΔtssD1245. The numbers of E. coli cells surviving coculture with ΔtssD1 proliferated to approximately 2.67 × 107 cfu/ml. The complemented strain ΔtssD1‐C, with recovered function of tssD1, showed antibacterial activity almost completely restored to wild‐type levels, as reflected by a sharp decrease in the surviving number of E. coli prey cells (Figure 3b). The BGR1 pBBR1MCS2 strain, as the negative control of the complementation strain, showed an antibacterial effect similar to wild‐type BGR1 (Figure S5d). The antibacterial effect of ΔtssD1 and ΔtssD1245 was also observed using green fluorescent protein (GFP)‐labelled E. coli as prey (Figure 3c). Therefore, only the tssD1 gene contributed to the antibacterial effect in in vitro interbacterial competition assays.
Figure 3.
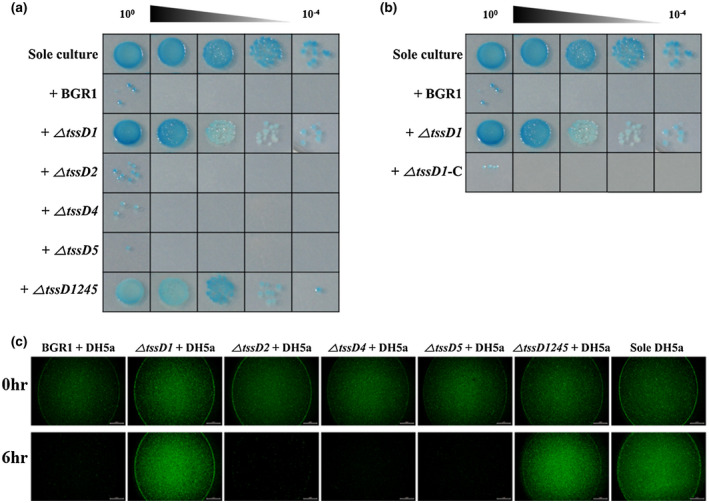
Interbacterial interaction between Escherichia coli DH5α and four tssD mutants of Burkholderia glumae BGR1. (a) and (b) Antibacterial effects of the four T6SS clusters in B. glumae BGR1. Survival of prey cell was strongly decreased by coculturing with wild‐type BGR1, ΔtssD2, ΔtssD4, and ΔtssD5. The survival of prey cells by coculture with ΔtssD1 and ΔtssD1245 was similar to that of pure culture of prey without predators (sole). The ΔtssD1 complementation strains restored the wild‐type antibacterial activity. The experiments were conducted by three replicates (n = 3). (c) Observation of living prey cells expressing green fluorescent protein (GFP) by spotting the prey and predator mixture. Except in the coculture of ΔtssD1 and ΔtssD1245, the intensity of GFP expressed in living prey cells was found to disappear after 6 hr. The experiments were conducted using three replicates (n = 3). This is representative of the results from independent experiments with three replicates showing the same pattern
2.6. The presence of prey increases the gene expression levels of tssD1 but not tssD2, tssD4, and tssD5
Quantitative reverse transcription PCR (RT‐qPCR) was used to assess the expression levels of the four tssD genes in the presence of prey. In the presence of E. coli as prey, the relative gene expression level of only tssD1 was increased whereas the relative gene expression levels of the tssD2, tssD4, and tssD5 genes showed little difference regardless of the presence of prey (Figure 4).
Figure 4.
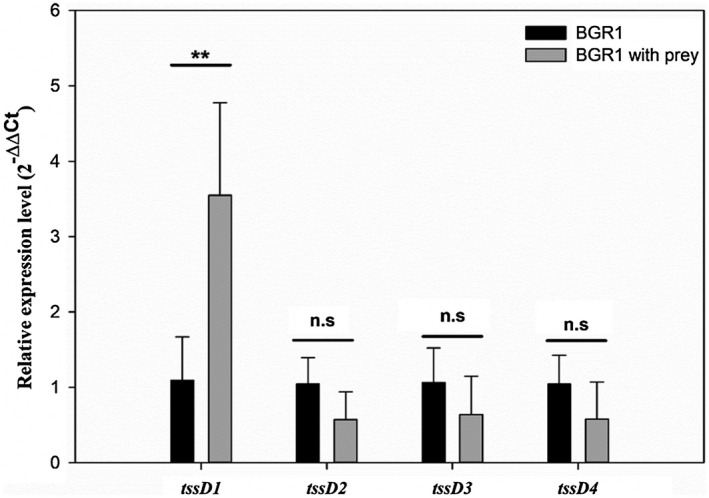
Analysis of relative gene expression level of tssD1, tssD2, tssD4, and tssD5 with or without prey. The relative gene expression levels of the four tssD genes of Burkholderia glumae BGR1 in the absence or presence of Escherichia coli as prey are presented. Only tssD1 gene expression was increased in the presence of E. coli as prey. The experiments were conducted by four replicates (n = 4). Mean values followed by the same letters are not significantly different according to Tukey's HSD test (ns, no statistical significance, **p < .01)
2.7. The endophytic bacterial community composition in noninfected rice, rice infected with B. glumae BGR1, and rice infected with ΔtssD1 differ because of the antibacterial activity of T6SS group_1
Metagenomic analysis of endophytic bacterial communities was performed among three groups: noninfected rice, rice infected with BGR1, and rice infected with ΔtssD1. Alpha diversity was used to measure the diversity of each sample by calculating a value for each sample. Alpha diversity was quantified based on Chao1, the observed operational taxonomic units (OTUs), the phylogenetic diversity (PD) whole tree, and the Simpson index (Figure 5a). Alpha diversity between rice infected with BGR1 and rice infected with ΔtssD1 showed that the observed OTUs (P observed OTUs = 0.003) and PD whole tree (P PD whole tree = 0.008) were significantly decreased in rice infected with BGR1, even though statistical testing using Chao1 (P Chao1 = 0.052) and the Simpson index (P Simpson = 0.408) showed no difference (Figure 5a). All alpha diversity indices of noninfected rice were higher than the other groups. The relative abundance of the top 20 genera showed that the genus Pantoea dominated the noninfected rice sample, followed by the genus Burkholderia. In contrast, the most abundant genera in rice infected with BGR1 and ΔtssD1 were Burkholderia followed by Achromobacter (Figure S6). Bacteria in the genera Luteibacter, Dyella, and Xanthomonas were relatively more abundant in the samples of noninfected rice and rice infected with ΔtssD1 than in rice infected with BGR1 (Figure 5b).
Figure 5.
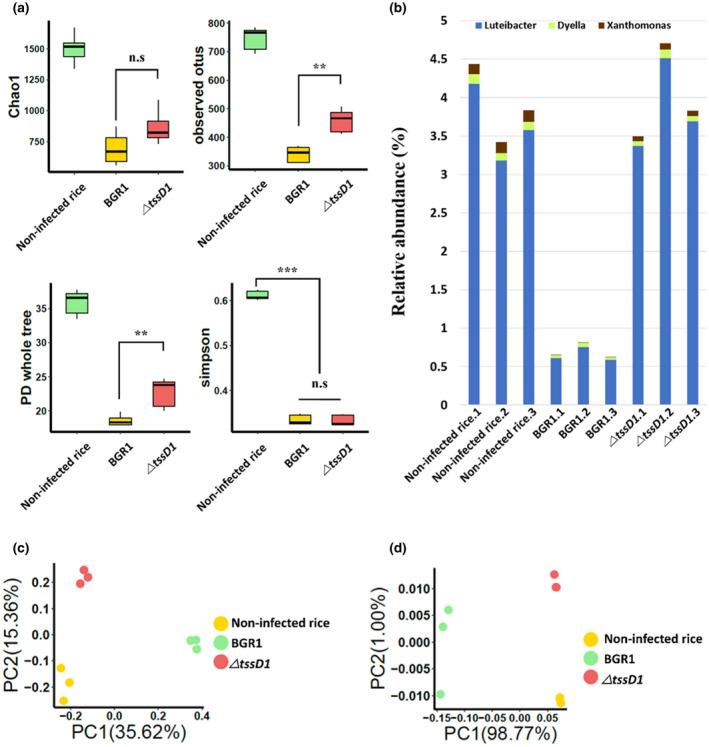
Alpha diversity and beta diversity in noninfected rice, rice infected by Burkholderia glumae BGR1 and the ΔtssD1 mutant. (a) Species richness (Chao1), observed operational taxonimoc units (OTUs), phylogenetic diversity (PD), and Simpson evenness was estimated. Alpha diversity was higher in the noninfected rice samples than in the other two groups. Apart from the Simpson index, the three indices showed that rice infected by ΔtssD1 samples showed greater diversity than that in rice infected by BGR1. The box‐and‐whiskers plot represents the range of the upper quartile and lower quartile, and the median value. (b) Luteibacter, Dyella, and Xanthomonas were the most dominant in noninfected rice and in rice infected rice with ΔtssD1 in the relative abundance of the top 20 bacteria at genus level. Principal coordinate analysis plots of (c) unweighted UniFrac and (d) weighted UniFrac distance of bacterial community composition. There was a significant difference in bacterial composition by the group (p = .001, p = .006, respectively, ANOSIM) in both weighted and unweighted UniFrac distances
Principal coordinate analysis (PCoA) plots showed the difference in the communities of the noninfected rice, rice infected with BGR1, and rice infected with ΔtssD1 based on the distance metrics obtained from UniFrac analysis (Figure 5c,d). Endophytic bacterial communities in the noninfected rice, rice infected by BGR1, and rice infected by ΔtssD1 were separated clearly by a principal coordinate plot of distance based on the analysis of a weighted and unweighted UniFrac (p = .001, .006, respectively) (Figure 5c,d). Among these three groups, linear discriminant analysis (LDA) effect size (LEfSe) was used to find the strongest effects for group differentiation using imbalanced OTUs. Heatmap abundance showed differential abundance and frequency of the total bacterial groups (p < .05, LDA score > 2.5) among the three groups (Figure 6). PCoA plots and heatmap abundance (p < .05, LDA score > 2.5) showed that the three groups constituted their own communities and presence or absence of antibacterial effects led to differences in the community of rice infected with BGR1 and ΔtssD1 at 8 dpi (Figures 5 and 6). At the order level, Enterobacteriales, Flavobacteriales, Bacillales, and Rickettsiales were enriched in noninfected rice, whereas Rhizobiales and Sphingobacteriales were enriched in rice infected by ΔtssD1. In rice infected by BGR1, only Burkholderiales was observed at the order level. At the family level, six bacterial groups, Enterobacteriaceae, Sphingomonadaceae, Flavobacteriaceae, Rickettsiaceae, Rhizobiaceae, and Staphylococcaceae, were enriched in noninfected rice; five bacterial groups, Sphingobacteriaceae, Brucellaceae, Oxalobacteraceae, Burkholderiaceae, and Comamonadaceae, were enriched in rice infected by ΔtssD1; and only one bacterial group, Alcaligenaceae, was enriched in rice infected rice by BGR1. In particular, the order Flavobacteriales in noninfected rice and the order Sphingobacteriales in rice infected by ΔtssD1 were abundant in the phylum Bacteroidetes, whereas at the phylum level, Proteobacteria were enriched in rice infected by BGR1.
Figure 6.
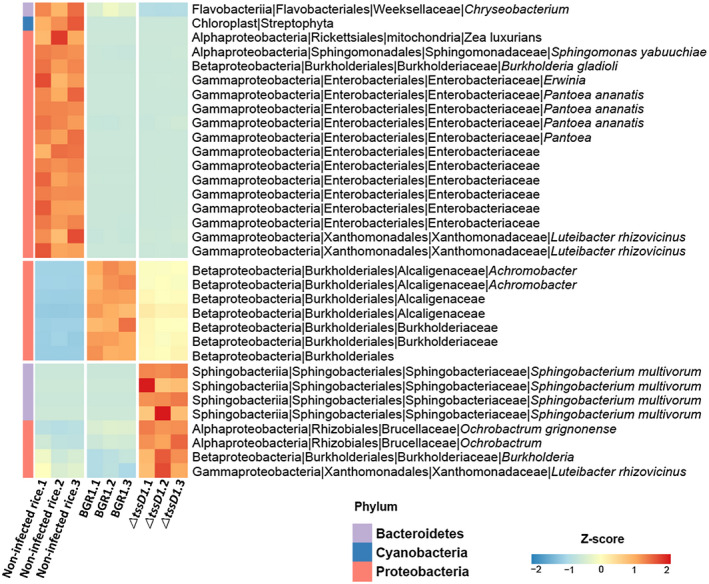
Identifying taxa with the most differences abundance from LEfSe analysis. LefSe analysis was performed with p < .05, LDA score > 2.5 with the relative abundance of bacterial OTUs. Blue represents lower abundance and red represents higher abundance. Relative abundances were normalized to z‐score to indicate differences between samples. The side bar on the left of the heatmap indicates the bacterial phyla
3. DISCUSSION
Type VI secretion systems (T6SSs) have been discovered in a variety of gram‐negative bacteria. The T6SS is known to have two distinct features: antibacterial effects as bacterial interaction and bacterial virulence as eukaryotic interaction. In many Burkholderia species having at least one T6SS, the T6SS has evolved into the most complex secretion pathway and has an essential role in their interaction with the environment (Nguyen et al., 2018). Based on the evolutionary analysis of T6SSs in Burkholderia species, T6SS group_1 of B. glumae BGR1 was clustered in bacterial interaction with antibacterial effect, whereas T6SS group_4 and group_5 were clustered in eukaryotic interaction with bacterial virulence; the function of T6SS group_2 could not be predicted through evolutionary analysis. To identify the T6SSs of B. glumae BGR1, the approach of evolutionary relationships is useful for inferring the function and role of a specific relationship. However, additional information is needed to determine whether the evolutionary relationship of a T6SS suggests general predictable factors for the function of the T6SS in eukaryotic and bacterial cell interactions.
B. glumae BGR1 has four T6SS gene clusters, namely two complete T6SS gene clusters (T6SS group_2, 4) and two incomplete T6SS gene clusters (T6SS group_1, 5), in its genome. The T6SS group_1 gene cluster is incomplete due to lack of the vgrG gene. However, some vgrG genes are located outside the T6SS gene cluster and are used as major components of the T6SS (Zong et al., 2019). The T6SS group_5 gene cluster is also incomplete due to three major components being missing. Although Yersinia pestis CO92 also has five incomplete T6SS gene clusters among six T6SS gene clusters, the five incomplete T6SS clusters seem to be involved in host cell cytotoxicity based on the results of infection attenuation with the incomplete T6SS deletion mutants (Andersson et al., 2017; Yang et al., 2018). Thus, an incomplete T6SS cluster, such as T6SS group_1 and 5, could be functional.
Based on our evolutionary analysis, we hypothesized that the four T6SS gene clusters of B. glumae BGR1 are evolutionarily differentiated and play distinct roles in the environment. The construction of deletion mutants to knock out the function of the T6SS needle were attempted by targeting tssD genes, which are present in each of the four T6SS clusters of B. glumae BGR1. The TssD protein, which forms an inner tube of stacked hexamers with helical symmetry on the assembly baseplate to transport effectors, is essential to form the T6SS needle to puncture adjacent cells (Pell et al., 2009; Cascales and Cambillau, 2012). The T6SS needle, with the VgrG‐PAAR spike complex at its end, comprises an inner tube and a TssB‐TssC ring surrounding the inner tube. Interestingly, Hcp (TssD) and VgrG proteins are mutually dependent when the surface is assembled (Pukatzki et al., 2007; Zheng and Leung, 2007; Hachani et al., 2011). Consequently, tssD deletion leads to loss of function of the T6SS in B. glumae BGR1 because the TssD protein is required to build an intact T6SS.
The T6SS is involved in bacterial virulence as a eukaryotic cell targeting system for plants or animals. The T6SSs as a eukaryotic cell targeting system of animals have been reported a to be essential elements for the virulence of bacterial pathogens (Keynan and Rubinstein, 2007; Suarez et al., 2010; Russell et al., 2013). Furthermore, the T6SSs, as a eukaryotic cell targeting system of plants, have been identified important for bacterial virulence based on an observable decrease of bacterial virulence in plants in the absence of the T6SS (Zhang et al., 2014; Shyntum et al., 2015). An in vivo pathogenicity assay using tssD deletion mutants was performed in rice stems at the vegetative stage to determine whether the T6SSs of B. glumae BGR1 are required for bacterial virulence. The attenuated virulence of ΔtssD4 and ΔtssD5 in the in vivo pathogenicity assay of stems suggests that T6SS group_4, including the tssD4 gene, and T6SS group_5, including the tssD5 gene, are related to bacterial virulence and pathogenicity in rice plants. The results of rice panicle blight disease by an in vivo pathogenicity assay at the reproductive stage were consistent with those from the stems at the vegetative stage, confirming that tssD4 and tssD5 are required for the full virulence of B. glumae BGR1 in rice plants. However, the results of no attenuated virulence by ΔtssD45, compared to ΔtssD4 and ΔtssD5, indicate the absence of a synergistic effect in causing bacterial panicle blight by the genes tssD4 and tssD5 (Figure 2b,c). Thus, T6SS group_4 and T6SS group_5 contribute to bacterial virulence as a completely independent eukaryotic targeting system.
The T6SS, involved in the eukaryotic targeting system, is activated when certain conditions are met on entering the host. For example, T6SS‐1 of B. pseudomallei, which is involved in virulence in mice and hamsters, is not expressed during culture in rich media but is expressed following uptake by the host cell (Shalom et al., 2007; Burtnick et al., 2011; Chen et al., 2011). Only T6SS group_5 of B. glumae was expressed and activated during infection of rice in Table S1 (Kim et al., 2014). However, the exact mechanism for the involvement of T6SS group_4 and T6SS group_5 in the infection process and for the activation of T6SSs remains unclear. For a comprehensive understanding of the T6SS activation mechanisms, bioinformatics and comparative secretome approaches need to be employed in future studies.
In addition to engaging in bacterial virulence toward host organisms, the T6SS is involved in bacterial competition via antibacterial effects by T6SS‐dependent antibacterial effectors like peptidoglycan hydrolases, phospholipases, nucleases, and NAD(P)+‐glycohydrolases (Koskiniemi et al., 2013; Russell et al., 2013; Whitney et al., 2013; Tang et al., 2018). Bacterial competition between species appears to be defined as interactions for occupying a predominant position in the bacterial community. In general, bacterial competition occurs within the framework of growth suppression or limitation. Our in vitro interbacterial competition assay showed that only T6SS group_1 plays an important role in bacterial competition with E. coli through suppression or limitation of bacterial growth. The similar survival rate of prey cells between coculture with ΔtssD1 and ΔtssD1245 indicated that the other T6SS groups have no synergistic effects with T6SS group_1 (Figure 2a). Although we conducted a bacterial competition assay under limited conditions using E. coli or GFP‐labelled E. coli as prey, it may be a suitable approach to investigate interbacterial interactions of T6SS group_1 in B. glumae BGR1. T6SS‐related recognition of Proteus mirabilis requires cell‐to‐cell contact for the usage of T6SSs, which discriminate the strains and orchestrate the neighbouring cells (Saak and Gibbs, 2016; Gallique et al., 2017), Thus, we performed a RT‐qPCR analysis to explore the expression pattern of four tssD genes of B. glumae BGR1 in the presence of E. coli as prey. When B. glumae BGR1 was present with E. coli as prey, the expression level of only the tssD1 gene was increased. Consequently, the high expression level of tssD1 suggests that B. glumae BGR1 recognizes external stimuli such as the presence of E. coli as prey, and activates the T6SS group_1, which is involved in the antibacterial effect.
In order to investigate the antibacterial effect of T6SS group_1 involved in bacterial competition in host plant by using infection of ΔtssD1, metagenomic analysis was performed by focusing on the different bacterial communities in rice plants infected with the ΔtssD1 mutant compared to the wild‐type BGR1. Metagenomic analysis of the endophytic bacterial community showed upon infection by the plant‐pathogenic B. glumae BGR1 in rice plants, the existing bacterial population in the plant collapsed, and B. glumae BGR1 formed the dominant population causing plant disease. The bacterial population in noninfected rice plants had a much higher diversity compared to the bacterial population in rice infected by B. glumae BGR1 and by ΔtssD1. There was no significant difference in the Simpson index, reflecting the relative abundance and the number of species between the groups of rice infected by B. glumae BGR1 and by ΔtssD1 due to the high relative abundance of above 80% of genus Burkholderia. However, the differences in the unique OTUs and PD whole tree between the rice infected by B. glumae BGR1 and ΔtssD1 is a result of deletion of the tssD1 gene from T6SS group_1. Relative abundance at the genus level also showed that the genera Luteibacter and Dyella, belonging to Gammaproteobacteria, were more abundant in rice infected by ΔtssD1 than by BGR1. In particular, the genus Luteibacter contains L. rhizovicinus, a bacterial species that promotes plant growth and development, and contributes to protection against pathogenic bacteria (Innerebner et al., 2011; Guglielmetti et al., 2013). Consequentially, the T6SS group_1 was shown to play a role in the antibacterial effect by targeting endophytic plant‐associated bacteria within rice plants and created an easily accessible environment for other pathogenic bacteria by controlling plant growth‐promoting bacteria. However, these results have limitations in explaining the differences in the overall bacterial population between the groups of rice infected with B. glumae BGR1 and ΔtssD1.
Although the disease severity in rice infected with B. glumae BGR1 and ΔtssD1 was similar, LEfSe analysis showed differences in the bacterial community among the three groups, noninfected rice, rice infected by B. glumae BGR1, and rice infected by ΔtssD1. In particular, endophytic plant‐associated bacteria in the orders Enterobacteriales, Flavobacteriales, Sphingomonadales, Bacillales, Rickettsiales, Sphingobacteriales, and Rhizobiales were dominant in noninfected rice and in rice infected by ΔtssD1 (Ivanova et al., 2000; Delmotte et al., 2009). In contrast, in rice infected by B. glumae BGR1, the order Burkholderiales, which contains several pathogenic bacterial species, prevailed (Hauser et al., 2011; Voronina et al., 2015). Thus, T6SS group_1 of B. glumae BGR1 is not involved in bacterial virulence, but has the function of exerting antibacterial effects by recognizing certain surrounding endophytic plant‐associated bacteria like Luteibacter and Dyella as prey; the growth inhibition of endophytic plant‐associated bacteria by antibacterial effects is important for bacterial competition in rice plants. In particular, bacterial competition is more important to form dominant populations of plant pathogens like B. glumae BGR1 during their initial colonization in plants to cause plant disease.
These results allow us to infer the distinct role of T6SSs in the infection process of B. glumae BGR1. Moreover, investigation of tssD gene expression profiles provided insights into the role of these genes in vitro and in planta. B. glumae BGR1 must reach a sufficient cell population to cause disease. B. glumae BGR1 recognizes the presence of surrounding bacteria and uses T6SS group_1 to gain an advantage in the ecological niche by competing with adjacent bacteria inside rice plants. In the next step, B. glumae BGR1, predominant in rice, demonstrates interaction between bacteria and plants through bacterial virulence by targeting the eukaryotic cells of the rice plant either directly or indirectly through T6SS group_4 and T6SS group_5.
In the current study, we provide the first functional description of different T6SSs in the phytopathogenic B. glumae and provide evidence for their roles in interbacterial interaction and virulence towards rice plants. The T6SS group_1 confers bacterial competition ability in rice plants, whereas T6SS group_4 and T6SS group_5 are involved in the virulence toward host plants. Our results indicate that the T6SSs of B. glumae BGR1 are involved in the plant–bacteria interaction and in the interbacteria interaction, and play a functionally distinct role as efficient and indiscriminate weapons in causing plant disease.
4. EXPERIMENTAL PROCEDURES
4.1. Analysis of type VI secretion systems in the genome of Burkholderia species using the maximum‐likelihood method
The available T6SS clusters, including Clusters of Orthologous Groups of proteins (COG) of T6SS major components, which are COG3515; TssA, COG3516; TssB, COG3517; TssC, COG3157; TssD (Hcp), COG3518; TssE, COG3519; TssF, COG3520; TssG, COG0542; TssH, COG3501; TssI (VgrG), COG3521; TssJ, COG3522; TssK, COG3455; TssL; and COG3523; TssM in some bacterial genomes, were acquired from the SecreT6 database (http://db‐mml.sjtu.edu.cn/SecReT6/) (Li et al., 2015) and their nucleotide sequences were extracted from the National Center for Biotechnology Information (NCBI). Next, these T6SS component genes were detected in the genome of 10 Burkholderia species, which includes B. glumae BGR1, using the T6SS‐BLASTP program of SecreT6. All T6SS component genes in Burkholderia species were confirmed in detail. In particular, the COGs of 13 conserved T6SSs were found in four different clusters on the genome of B. glumae BGR1 among 10 Burkholderia species and were assigned to each gene encoding similar proteins in the four clusters (Figure 1). Several types of T6SS cluster, including at least 10 major components, were used to elucidate the relationship among all detected T6SS clusters of Burkholderia species. The protein sequences of all T6SS clusters were aligned using the ClustalW algorithm in MEGA6. The relationships of Burkholderia T6SS were constructed based on the maximum‐likelihood method and the Jones‐Taylor‐Thornton matrix‐based model with 1,000 bootstrap replicates (Jones et al., 1992). The relationship and evolutionary rate differences among sites (five categories [+G, parameter = 4.9]) were designated with a discrete gamma distribution.
4.2. In vivo pathogenicity assay at vegetative stage and reproductive stage
To examine the eukaryotic‐targeting system of B. glumae BGR1 in rice plants at the vegetative stage and reproductive stage, cultured bacterial cells at the mid‐logarithmic phase were harvested by centrifugation, washed, and resuspended with distilled water. The optical density of bacterial suspensions was adjusted to OD600 nm = 0.8. The rice plants used in this experiment were grown under greenhouse conditions (average 30°C in the day and 25°C at night). Next, the stems of rice plants in the vegetative stage were inoculated with bacterial suspensions using a syringe and grown for 8 days. The disease severity was observed at the inoculated area. Suspensions for each bacterial stain were prepared and adjusted to OD600 nm = 0.5 to confirm the bacterial panicle blight disease at the reproductive stage of rice. At the flowering stage, the rice panicles were inoculated by dipping them into 50 ml of bacterial suspensions for 1 min. At 8 dpi, the disease severity in rice panicles was evaluated using the following scale: 0, healthy panicle; 1, 0%–20% diseased panicle; 2, 21%–40% diseased panicle; 3, 41%–60% diseased panicle; 4, 61%–80% diseased panicle; 5, 81%–100% diseased panicle. Disease severity was calculated using the following formula: disease severity = Σ(number of samples per rating × rating value)/total number of panicles. BGR1 pBBR1MCS2, which contains an empty vector, was used as a negative control of the complementation strain in the in vivo pathogenicity assay.
4.3. In vitro interbacterial competition assay
B. glumae strains (Table S1) were cocultured with E. coli DH5α, used as prey cells. The E. coli DH5α cells harboured the pCRISPomyces‐2 plasmid, allowing survival in apramycin medium and alpha complementation for β‐galactosidase. The B. glumae strains were co‐incubated with E. coli at a 1:1 ratio in the form of patches on Luria Bertani (LB) plates for 6 hr at 37°C. Following incubation, the patches were recovered and resuspended in LB broth. To assess the survival of the E. coli prey, the resuspended mixtures were serially diluted from 0 to 10−7 and spotted on LB plates containing apramycin (100 μg/ml) and 5‐bromo‐4‐chloro‐indolyl‐β‐d‐galactopyranoside (X‐Gal, 40 μg/ml). The number of blue colonies on the plate indicated the number of surviving E. coli. BGR1 pBBR1MCS2, which contains an empty vector, was used as a negative control of the complementation strain in the in vitro interbacterial competition assay. In addition, an interbacterial competition assay with GFP was performed with the same method using GFP‐labelled E. coli DH5α. The gfp gene was amplified from the commercial plasmid pUBN‐GFP‐DEST (Addgene) using the primer pair GFP_HindIII_F and GFP_BamHI_R (Table S3). The amplified gfp fragment and pBBR1P2 plasmid were ligated. Recombinant plasmids were transformed into E. coli DH5α. The density of GFP‐labelled E. coli was observed at 0 and 6 hr of bacterial competition under an Optinity KB‐600F fluorescent microscope with a CoolLED pE300‐WHITE illumination (CoolLED). Images were acquired with the KCS3‐23S CMOS sensor camera (KoreaLabTech).
4.4. Isolation of bacteria from rice plants and preparation of metagenomic DNA
Stems of rice plant at the vegetative stage were inoculated with each bacterial suspension as explained above (adjusted to OD600 nm = 0.8). At 8 dpi, bacteria were isolated from the noninfected and infected stems of rice. The noninfected or infected rice stem segments at 5 cm from the upper and lower parts of the inoculation site were gathered. The surface of each 10 cm rice stem segment was sterilized to eliminate the bacterial cells attached to the surface of the plant tissue by wiping it down with 70% ethanol. Next, about 6 g of six rice stem segments were squeezed tightly in a 15‐ml conical tube, and centrifuged at 4°C and 1,800 × g for 7 min directly to harvest a pellet of the bacteria inside the stem segments. The bacterial pellet acquired after centrifugation was immediately used to extract DNA following the classic cetyltrimethyl ammonium bromide‐based protocol with modifications (Murray and Thompson, 1980). The extracted DNA was amplified to obtain paired‐end sequences of the 16S rRNA variable regions using Herculase II fusion DNA polymerase Nextera XT Index Kit v. 2 on the Illumina MiSeq platform at Macrogen (Seoul, South Korea). The following primer pairs were used for amplification: (F), 5′‐TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGN‐GGCWGCAG‐3′; (R), 5′‐GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC‐3′.
4.5. Bacterial community analysis
The paired‐end sequences with a p value of 0.3, obtained from Illumina MiSeq, were merged using meren/illumina‐utils (https://github.com/meren/illumina‐utils) (Eren et al., 2013). The merged sequences were analysed using the QIIME (Quantitative Insights into Microbial Ecology) pipeline v. 1.9.1 (Caporaso et al., 2010b). The total merged sequences were clustered into de novo OTUs with a sequence identity of 97% using UCLUST (Edgar, 2010). The representative sequences were designated using the most abundant sequences within each OTU. The representative sequences were aligned using PyNAST aligner (Caporaso et al., 2010a), and FastTree (Price et al., 2009) was used to construct a phylogenetic tree. The representative sequences of OTUs were assigned to a Greengenes database (DeSantis et al., 2006) using an RDP (Ribosomal Database Project Ⅱ (http://rdp.cme.msu.edu)) Classifier (Wang et al., 2007) with a threshold confidence of 50%. OTUs assigned as chloroplasts and mitochondria were excluded from further analysis. As B. glumae was not included in the Greengene database, BLASTn was performed using the representative sequences against the NCBI ref‐seq database to identify sequences with more than 97% sequence identity as B. glumae. Beta diversity was estimated using the unweighted and weighted UniFrac distance matrices with a phylogenetic tree. A PCoA was conducted to identify the bacterial community differences among samples using UniFrac distance matrices. Alpha diversity was estimated with Chao1, PD whole tree, observed OTUs, and Simpson indices using rarefied OTU tables (96,526 sequences per sample).
4.6. Statistical analysis
All experiments except bacterial community analysis were conducted twice with at least three replicates. Bacterial community analysis was conducted three times with six replicates. Analysis of variance was conducted using the generalized linear model procedure and means were compared using the least significant difference test at p < .05 according to Tukey's HSD test. To identify the significant differences in bacterial features among the three groups, LEfSe was performed. A threshold of LDA scores of 2.0, and α = .05 for the Kruskal–Wallis test were applied to estimate the effect size of each of the differently abundant OTU features (Segata et al., 2011).
Supporting information
FIGURE S1 Relationship of Burkholderia type VI secretion systems. The relationship of Burkholderia type VI secretion systems was generated using 13 T6SS core components in the T6SS clusters of Burkholderia species. The relationship was inferred using the maximum likelihood method and Jones‐Taylor‐Thornton matrix‐based model. The percentages of replicate trees in which the associated taxa were clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. A discrete gamma distribution was used to model the evolutionary rate differences among sites (five categories [+G, parameter = 4.9003]). Evolutionary analyses were conducted in MEGA X. The eukaryotic targeting system is marked in red and the bacterial targeting system is marked in blue
FIGURE S2 Confirmation of tssD mutants using PCR. (a) Deletion mutants were generated and PCR was conducted using primers targeting the 5′‐upstream and 3′‐downstream regions of each tssD region to verify the mutant strains. (b) Agarose gel electrophoresis of the PCR products was performed to distinguish the wild‐type and mutant strains based on different fragment lengths. The PCR product size for each mutant strain was smaller than that obtained from wild‐type BGR1
FIGURE S3 Growth curve of Burkholderia glumae BGR1 and tssD mutants. Wild‐type BGR1, tssD single to quadruple deletion mutants, and tssD complementation strains were grown overnight at 37 °C. Overnight bacterial cultures were diluted into fresh Luria broth and then incubated at 37 °C. Bacterial growth was monitored using samples withdrawn every 2 hr and measuring the optical density (600 nm). Compared to the growth pattern of the wild type, no difference was observed in the growth pattern of the mutants. Points indicate the mean values and error bars indicate standard deviations (n = 4)
FIGURE S4 Phenotype assay of wild‐type Burkholderia glumae BGR1 and the tssD mutants. (a) Swarming motility by wild‐type BGR1 and the mutants ΔtssD1, ΔtssD2, ΔtssD4, ΔtssD5, and ΔtssD1245. The swarming motility assay was performed on 0.5% agar plates. The image is representative of three independent replicates (n = 3). (b) Biosynthesis of toxoflavin by wild‐type BGR1 and mutants ΔtssD1, ΔtssD2, ΔtssD4, ΔtssD5, and ΔtssD1245. Thin‐layer chromatography analysis was used to detect toxoflavin from the bacterial culture supernatant. The produced toxoflavin was examined visually in daylight and under UV at 365 nm. This is representative of the results from independent experiments with three replicates showing the same pattern
FIGURE S5 Comparison of in vivo pathogenicity assay and antibacterial effect between wild‐type BGR1 and BGR1 pBBR1MCS2. (a) Wild‐type BGR1 and BGR1 containing empty vector were inoculated with 108 cfu/ml to assess the virulence in rice stem at the vegetative stage. (b) Bacterial suspension of wild‐type BGR1 and BGR1 pBBR1MCS2, which contains empty vector of pBBR1MCS2, were inoculated into rice panicles (Oryza sativa) at the reproductive stage to comparison of the virulence. (c) Disease severity on the rice panicles was calculated on a scale of 0 to 5 after inoculating the bacterial suspension. The data are presented as the mean ± SD of three replicates (n = 3). Mean values followed by the same letters are not significantly different according to Tukey’s HSD test (ns, no statistical significance, *p < .05, **p < .01, ***p < .001). At the reproductive and vegetative stage, the disease severity of rice infected with wild‐type BGR1 was not different from that of rice infected with BGR1 pBBR1MCS2. Disease symptoms at 8 days post inoculation. Distilled water was used as the negative control. (d) Antibacterial effects in wild‐type BGR1 and BGR1 pBBR1MCS2. Survival of prey cell was decreased by coculturing with wild‐type BGR1 and BGR1 pBBR1MCS2. Prey cell survival by coculturing with BGR1 pBBR1MCS2 was similar to prey cell survival by coculturing with wild‐type BGR1. This is representative of the results from independent experiments with three replicates showing the same pattern. BGR1 pBBR1MCS2 was used as the negative control of complementation strains. The data were conducted with three replicates
FIGURE S6 Relative abundance of the top 20 genera at the genus level. Relative abundance (RA) of the top 20 bacteria at the genus level and the remaining bacteria labelled as “others”. In the noninfected rice samples, Pantoea was the most dominant whereas in the rice infected by BGR1 and ΔtssD1, Burkholderia was the most dominant
TABLE S1 The representative up‐regulated genes clustered in T6SS group_5 in Burkholderia glumae BGR1 within rice plants
TABLE S2 Bacterial strains and plasmids used in this study
TABLE S3 Oligonucleotide primers used in this study
TEXT S1 Bacterial strains, plasmids, and growth conditions; bacterial DNA extraction and PCRs; generation of markerless deletion mutants and complementation strains in Burkholderia glumae BGR1 and its several phenotypic characteristics; and quantitative reverse transcription PCR
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Programme through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF‐2019R1A2C2006779) and by grants from the Strategic Initiative for Microbiomes in Agriculture and Food, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (no. 918019‐04).
Kim N, Kim JJ, Kim I, et al. Type VI secretion systems of plant‐pathogenic Burkholderia glumae BGR1 play a functionally distinct role in interspecies interactions and virulence. Molecular Plant Pathology. 2020;21:1055–1069. 10.1111/mpp.12966
DATA AVAILABILITY STATEMENT
The sequencing data that support the findings of this study are openly available in the NCBI Bioproject database at https://www.ncbi.nlm.nih.gov/bioproject/, accession number PRJNA622913. Sample information is in the NCBI BioSample database at https://www.ncbi.nlm.nih.gov/biosample/, accession numbers SRR11475244, SRR11475245, SRR11475246, SRR11475247, SRR11475248, SRR11475249, SRR11475250, SRR11475251 and SRR11475252.
REFERENCES
- Andersson, J.A. , Sha, J. , Erova, T.E. , Fitts, E.C. , Ponnusamy, D. , Kozlova, E.V. et al (2017) Identification of new virulence factors and vaccine candidates for Yersinia pestis . Frontiers in Cellular and Infection Microbiology, 7, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert, S. and Meyer, T.F. (2006) Type IV secretion systems and their effectors in bacterial pathogenesis. Current Opinion in Microbiology, 9, 207–217. [DOI] [PubMed] [Google Scholar]
- Bernal, P. , Llamas, M.A. and Filloux, A. (2018) Type VI secretion systems in plant‐associated bacteria. Environmental Microbiology, 20, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle, L.E.H. , Bailey, C.M. and Pallen, M.J. (2008) Type VI secretion: a beginner’s guide. Current Opinion in Microbiology, 11, 3–8. [DOI] [PubMed] [Google Scholar]
- Boyer, F. , Fichant, G. , Berthod, J. , Vandenbrouck, Y. and Attree, I. (2009) Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics, 10, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick, M.N. , Brett, P.J. , Harding, S.V. , Ngugi, S.A. , Ribot, W.J. , Chantratita, N. et al (2011) The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei . Infection and Immunity, 79, 1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Bittinger, K. , Bushman, F.D. , DeSantis, T.Z. , Andersen, G.l. and Knight, R. (2010a) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics, 26, 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Kuczynski, J. , Stombaugh, J. , Bittinger, K. , Bushman, F.D. , Costello, E.K. et al (2010b) QIIME allows analysis of high‐throughput community sequencing data. Nature Methods, 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales, E. and Cambillau, C. (2012) Structural biology of type VI secretion systems. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Wong, J. , Sun, G.W. , Liu, Y. , Tan, G.‐Y.G. and Gan, Y.‐H. (2011) Regulation of type VI secretion system during Burkholderia pseudomallei infection. Infection and Immunity, 79, 3064–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmotte, N. , Knief, C. , Chaffron, S. , Innerebner, G. , Roschitzki, B. , Schlapbach, R. et al (2009) Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proceedings of the National Academy of Sciences of the United States of America, 106, 16428–16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis, T.Z. , Hugenholtz, P. , Larsen, N. , Rojas, M. , Brodie, E.l. , Keller, K. et al (2006) Greengenes, a chimera‐checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology, 72, 5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, R.C. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- Eren, A.M. , Vineis, J.H. , Morrison, H.G. and Sogin, M.L. (2013) A filtering method to generate high quality short reads using Illumina paired‐end technology. PLoS ONE, 8, e66643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freestone, P. (2013) Communication between bacteria and their Hosts. Scientifica, 2013, 361073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallique, M. , Bouteiller, M. and Merieau, A. (2017) The type VI secretion system: a dynamic system for bacterial communication? Frontiers in Microbiology, 8, e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto, K. (1956) New bacterial diseases of rice‐bacterial brown stripe and bacterial grain rot. Annals of the Phytopathological Society of Japan, 21, 46–47. [Google Scholar]
- Goto, T. , Nishiyama, K. and Ohata, K. (1987) Bacteria causing grain rot of rice. Japanese Journal of Phytopathology, 53, 141–149. [Google Scholar]
- Green, E.R. and Mecsas, J. (2016) Bacterial secretion systems: an overview. Microbiology Spectrum, 4, VMBF‐0012‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti, S. , Basilico, R. , Taverniti, V. , Arioli, S. , Piagnani, C. and Bernacchi, A. (2013) Luteibacter rhizovicinus MIMR1 promotes root development in barley (Hordeum vulgare L.) under laboratory conditions. World Journal of Microbiology and Biotechnology, 29, 2025–2032. [DOI] [PubMed] [Google Scholar]
- Hachani, A. , Lossi, N.S. , Hamilton, A. , Jones, C. , Bleves, S. , Albesa‐Jové, D. et al (2011) Type VI secretion system in Pseudomonas aeruginosa: secretion and multimerization of VgrG proteins. Journal of Biological Chemistry, 286, 12317–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, A.R. , Jain, M. , Bar‐Meir, M. and McColley, S.A. (2011) Clinical significance of microbial infection and adaptation in cystic fibrosis. Clinical Microbiology Reviews, 24, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, R.D. , Singh, P. , Hsu, F. , Güvener, T. , Carl, M.A. , Trinidad, R.R.S. (2010) A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host and Microbe, 7, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiyama, K. , Furuya, N. , Takanami, Y. and Matsuyama, N. (1995) A role of phytotoxin in virulence of Pseudomonas glumae Kurita et Tabei. Japanese Journal of Phytopathology, 61, 470–476. [Google Scholar]
- Innerebner, G. , Knief, C. and Vorholt, J.A. (2011) Protection of Arabidopsis thaliana against leaf‐pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Applied and Environmental Microbiology, 77, 3202–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova, E.G. , Doronina, N.V. , Shepelyakovskaya, A.O. , Laman, A.G. , Brovko, F.A. and Trotsenko, Y.A. (2000) Facultative and obligate aerobic methylobacteria synthesize cytokinins. Microbiology, 69, 646–651. [PubMed] [Google Scholar]
- Jiang, F. , Waterfield, N.R. , Yang, J. , Yang, G. and Jin, Q. (2014) A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host and Microbe, 15, 600–610. [DOI] [PubMed] [Google Scholar]
- Jones, D.T. , Taylor, W.R. and Thornton, J.M. (1992) The rapid generation of mutation data matrices from protein sequences. Bioinformatics, 9, 275–282. [DOI] [PubMed] [Google Scholar]
- Jung, B. , Park, J. , Kim, N. , Li, T. , Kim, S. , Bartley, L.E. et al (2018) Cooperative interactions between seed‐borne bacterial and air‐borne fungal pathogens on rice. Nature Communications, 9, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Y. , Kim, J. , Kim, S. , Kim, H. , Lim, J.Y. , Kim, M. et al (2008) Proteomic analysis of the proteins regulated by HrpB from the plant pathogenic bacterium Burkholderia glumae . Proteomics, 8, 106–121. [DOI] [PubMed] [Google Scholar]
- Keynan, Y. and Rubinstein, E. (2007) The changing face of Klebsiella pneumoniae infections in the community. International Journal of Antimicrobial Agents, 30, 385–389. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Kang, Y. , Choi, O. , Jeong, Y. , Jeong, J.‐E. , Lim, J.Y. et al (2007) Regulation of polar flagellum genes is mediated by quorum sensing and FlhDC in Burkholderia glumae . Molecular Microbiology, 64, 165–179. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Park, J. , Lee, J. , Shin, D. , Park, D.‐S. , Lim, J.‐S. et al (2014) Understanding pathogenic Burkholderia glumae metabolic and signaling pathways within rice tissues through in vivo transcriptome analyses. Gene, 547, 77–85. [DOI] [PubMed] [Google Scholar]
- Kneuper, H. , Cao, Z.P. , Twomey, K.B. , Zoltner, M. , Jäger, F. , Cargill, J.S. et al (2014) Heterogeneity in ess transcriptional organization and variable contribution of the Ess/Type VII protein secretion system to virulence across closely related Staphylocccus aureus strains. Molecular Microbiology, 93, 928–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskiniemi, S. , Lamoureux, J.G. , Nikolakakis, K.C. , t'Kint de Roodenbeke, C. , Kaplan, M.D. , Low, D.A. et al (2013) Rhs proteins from diverse bacteria mediate intercellular competition. Proceedings of the National Academy of Sciences of the United States of America, 110, 7032–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. , Park, J. , Kim, S. , Park, I. and Seo, Y.S. (2016) Differential regulation of toxoflavin production and its role in the enhanced virulence of Burkholderia gladioli . Molecular Plant Pathology, 17, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennings, J. , West, T.E. and Schwarz, S. (2019) The Burkholderia type VI secretion system 5: composition, regulation and role in virulence. Frontiers in Microbiology, 10, e3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Yao, Y. , Xu, H.H. , Hao, L. , Deng, Z. , Rajakumar, K. et al (2015) SecReT6: a web‐based resource for type VI secretion systems found in bacteria. Environmental Microbiology, 17, 2196–2202. [DOI] [PubMed] [Google Scholar]
- Lin, J.‐S. , Pissaridou, P. , Wu, H.‐H. , Tsai, M.‐D. , Filloux, A. and Lai, E.‐M. (2018) TagF‐mediated repression of bacterial type VI secretion systems involves a direct interaction with the cytoplasmic protein Fha. Journal of Biological Chemistry, 293, 8829–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, A.T. and Mekalanos, J.J. (2010) In vivo actin cross‐linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proceedings of the National Academy of Sciences of the United States of America, 107, 4365–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata, S.T. , Unterweger, D. , Rudko, S.P. and Pukatzki, S. (2013) Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae . PLoS Pathogens, 9, e1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougous, J.D. , Cuff, M.E. , Raunser, S. , Shen, A. , Zhou, M. , Gifford, C.A. et al (2006) A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science, 312, 1526–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, M.G. and Thompson, W.F. (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 8, 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T.T. , Lee, H.H. , Park, I. and Seo, Y.S. (2018) Genome‐wide analysis of type VI system clusters and effectors in Burkholderia species. Plant Pathology Journal, 34, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell, L.G. , Kanelis, V. , Donaldson, L.W. , Howell, P.L. and Davidson, A.R. (2009) The phage λ major tail protein structure reveals a common evolution for long‐tailed phages and the type VI bacterial secretion system. Proceedings of the National Academy of Sciences of the United States of America, 106, 4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, M.N. , Dehal, P.S. and Arkin, A.P. (2009) Fasttree: computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution, 26, 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukatzki, S. , Ma, A.T. , Revel, A.T. , Sturtevant, D. and Mekalanos, J.J. (2007) Type VI secretion system translocates a phage tail spike‐like protein into target cells where it cross‐links actin. Proceedings of the National Academy of Sciences of the United States of America, 104, 15508–15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, A.B. , LeRoux, M. , Hathazi, K. , Agnello, D.M. , Ishikawa, T. , Wiggins, P.A. et al (2013) Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature, 496, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, A.B. , Peterson, S.B. and Mougous, J.D. (2014) Type VI secretion system effectors: poisons with a purpose. Nature Reviews Microbiology, 12, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saak, C.C. and Gibbs, K.A. (2016) The self‐identity protein IdsD is communicated between cells in swarming Proteus mirabilis colonies. Journal of Bacteriology, 198, 3278–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, S. , West, T.E. , Boyer, F. , Chiang, W.‐C. , Carl, M.A. , Hood, R.D. et al (2010) Burkholderia type vi secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathogens, 6, 77–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata, N. , Izard, J. , Waldron, L. , Gevers D., Miropolsky, L. , Garrett, W.S. and Huttenhower C. (2011) Metagenomic biomarker discovery and explanation. Genome Biology, 12, R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, Y.‐S. , Lim, J.Y. , Park, J. , Kim, S. , Lee, H.‐H. , Cheong, H. et al (2015) Comparative genome analysis of rice‐pathogenic Burkholderia provides insight into capacity to adapt to different environments and hosts. BMC Genomics, 16, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom, G. , Shaw, J.G. and Thomas, M.S. (2007) In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology, 153, 2689–2699. [DOI] [PubMed] [Google Scholar]
- Shyntum, D.Y. , Theron, J. , Venter, S.N. , Moleleki, L.N. , Toth, I.K. and Coutinho, T.A. (2015) Pantoea ananatis utilizes a type VI secretion system for pathogenesis and bacterial competition. Molecular Plant‐Microbe Interactions, 28, 420–431. [DOI] [PubMed] [Google Scholar]
- Stanley, N.R. , Palmer, T. and Berks, B.C. (2000) The twin arginine consensus motif of Tat signal peptides is involved in Sec‐independent protein targeting in Escherichia coli . Journal of Biological Chemistry, 275, 11591–11596. [DOI] [PubMed] [Google Scholar]
- Stubbendieck, R.M. , Vargas‐Bautista, C. and Straight, P.D. (2016) Bacterial communities: interactions to scale. Frontiers in Microbiology, 7, e1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez, G. , Sierra, J.C. , Kirtley, M.L. and Chopra, A.K. (2010) Role of Hcp, a type 6 secretion system effector, of Aeromonas hydrophila in modulating activation of host immune cells. Microbiology, 156, 3678–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, F. , Sawada, H. , Azegami, K. and Tsuchiya, K. (2004) Molecular characterization of the tox operon involved in toxoflavin biosynthesis of Burkholderia glumae . Journal of General Plant Pathology, 70, 97–107. [Google Scholar]
- Tang, J.Y. , Bullen, N.P. , Ahmad, S. and Whitney, J.C. (2018) Diverse NADase effector families mediate interbacterial antagonism via the type VI secretion system. Journal of Biological Chemistry, 293, 1504–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina, O.L. , Kunda, M.S. , Ryzhova, N.N. , Aksenova, E.I. , Semenov, A.N. , Lasareva, A.V. et al (2015) The variability of the order Burkholderiales representatives in the healthcare units. BioMed Research International, 2015, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Garrity, G.M. , Tiedje, J.M. and Cole, J.R. (2007) Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73, 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney, J.C. , Chou, S. , Russell, A.B. , Biboy, J. , Gardiner, T.E. , Ferrin, M.A. et al (2013) Identification, structure, and function of a novel type VI secretion peptidoglycan glycoside hydrolase effector‐immunity pair. Journal of Biological Chemistry, 288, 26616–26624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Pan, J. , Wang, Y. and Shen, X. (2018) Type VI secretion systems present new insights on pathogenic Yersinia . Frontiers in Cellular and Infection Microbiology, 31, e260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Xu, J. , Xu, J. , Zhang, H. , He, L. and Feng, J. (2014) TssB is essential for virulence and required for type VI secretion system in Ralstonia solanacearum . Microbial Pathogenesis, 74, 1–7. [DOI] [PubMed] [Google Scholar]
- Zheng, J. and Leung, K.Y. (2007) Dissection of a type VI secretion system in Edwardsiella tarda . Molecular Microbiology, 66, 1192–1206. [DOI] [PubMed] [Google Scholar]
- Zong, B. , Zhang, Y. , Wang, X. , Liu, M. , Zhang, T. , Zhu, Y. et al (2019) Characterization of multiple type‐VI secretion system (T6SS) VgrG proteins in the pathogenicity and antibacterial activity of porcine extra‐intestinal pathogenic Escherichia coli . Virulence, 10, 118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Relationship of Burkholderia type VI secretion systems. The relationship of Burkholderia type VI secretion systems was generated using 13 T6SS core components in the T6SS clusters of Burkholderia species. The relationship was inferred using the maximum likelihood method and Jones‐Taylor‐Thornton matrix‐based model. The percentages of replicate trees in which the associated taxa were clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. A discrete gamma distribution was used to model the evolutionary rate differences among sites (five categories [+G, parameter = 4.9003]). Evolutionary analyses were conducted in MEGA X. The eukaryotic targeting system is marked in red and the bacterial targeting system is marked in blue
FIGURE S2 Confirmation of tssD mutants using PCR. (a) Deletion mutants were generated and PCR was conducted using primers targeting the 5′‐upstream and 3′‐downstream regions of each tssD region to verify the mutant strains. (b) Agarose gel electrophoresis of the PCR products was performed to distinguish the wild‐type and mutant strains based on different fragment lengths. The PCR product size for each mutant strain was smaller than that obtained from wild‐type BGR1
FIGURE S3 Growth curve of Burkholderia glumae BGR1 and tssD mutants. Wild‐type BGR1, tssD single to quadruple deletion mutants, and tssD complementation strains were grown overnight at 37 °C. Overnight bacterial cultures were diluted into fresh Luria broth and then incubated at 37 °C. Bacterial growth was monitored using samples withdrawn every 2 hr and measuring the optical density (600 nm). Compared to the growth pattern of the wild type, no difference was observed in the growth pattern of the mutants. Points indicate the mean values and error bars indicate standard deviations (n = 4)
FIGURE S4 Phenotype assay of wild‐type Burkholderia glumae BGR1 and the tssD mutants. (a) Swarming motility by wild‐type BGR1 and the mutants ΔtssD1, ΔtssD2, ΔtssD4, ΔtssD5, and ΔtssD1245. The swarming motility assay was performed on 0.5% agar plates. The image is representative of three independent replicates (n = 3). (b) Biosynthesis of toxoflavin by wild‐type BGR1 and mutants ΔtssD1, ΔtssD2, ΔtssD4, ΔtssD5, and ΔtssD1245. Thin‐layer chromatography analysis was used to detect toxoflavin from the bacterial culture supernatant. The produced toxoflavin was examined visually in daylight and under UV at 365 nm. This is representative of the results from independent experiments with three replicates showing the same pattern
FIGURE S5 Comparison of in vivo pathogenicity assay and antibacterial effect between wild‐type BGR1 and BGR1 pBBR1MCS2. (a) Wild‐type BGR1 and BGR1 containing empty vector were inoculated with 108 cfu/ml to assess the virulence in rice stem at the vegetative stage. (b) Bacterial suspension of wild‐type BGR1 and BGR1 pBBR1MCS2, which contains empty vector of pBBR1MCS2, were inoculated into rice panicles (Oryza sativa) at the reproductive stage to comparison of the virulence. (c) Disease severity on the rice panicles was calculated on a scale of 0 to 5 after inoculating the bacterial suspension. The data are presented as the mean ± SD of three replicates (n = 3). Mean values followed by the same letters are not significantly different according to Tukey’s HSD test (ns, no statistical significance, *p < .05, **p < .01, ***p < .001). At the reproductive and vegetative stage, the disease severity of rice infected with wild‐type BGR1 was not different from that of rice infected with BGR1 pBBR1MCS2. Disease symptoms at 8 days post inoculation. Distilled water was used as the negative control. (d) Antibacterial effects in wild‐type BGR1 and BGR1 pBBR1MCS2. Survival of prey cell was decreased by coculturing with wild‐type BGR1 and BGR1 pBBR1MCS2. Prey cell survival by coculturing with BGR1 pBBR1MCS2 was similar to prey cell survival by coculturing with wild‐type BGR1. This is representative of the results from independent experiments with three replicates showing the same pattern. BGR1 pBBR1MCS2 was used as the negative control of complementation strains. The data were conducted with three replicates
FIGURE S6 Relative abundance of the top 20 genera at the genus level. Relative abundance (RA) of the top 20 bacteria at the genus level and the remaining bacteria labelled as “others”. In the noninfected rice samples, Pantoea was the most dominant whereas in the rice infected by BGR1 and ΔtssD1, Burkholderia was the most dominant
TABLE S1 The representative up‐regulated genes clustered in T6SS group_5 in Burkholderia glumae BGR1 within rice plants
TABLE S2 Bacterial strains and plasmids used in this study
TABLE S3 Oligonucleotide primers used in this study
TEXT S1 Bacterial strains, plasmids, and growth conditions; bacterial DNA extraction and PCRs; generation of markerless deletion mutants and complementation strains in Burkholderia glumae BGR1 and its several phenotypic characteristics; and quantitative reverse transcription PCR
Data Availability Statement
The sequencing data that support the findings of this study are openly available in the NCBI Bioproject database at https://www.ncbi.nlm.nih.gov/bioproject/, accession number PRJNA622913. Sample information is in the NCBI BioSample database at https://www.ncbi.nlm.nih.gov/biosample/, accession numbers SRR11475244, SRR11475245, SRR11475246, SRR11475247, SRR11475248, SRR11475249, SRR11475250, SRR11475251 and SRR11475252.


