Abstract
Objective
To compare the characteristics of patients with axial spondyloarthritis (axSpA) who had enthesitis versus those without enthesitis.
Methods
This study included adult patients with axSpA enrolled in the Corrona Psoriatic Arthritis/Spondyloarthritis Registry (March 2013 to August 2018). Enthesitis was assessed at enrollment via the Spondyloarthritis Research Consortium of Canada Enthesitis Index. Characteristics were compared between patients with and without enthesitis using t tests or Wilcoxon rank‐sum tests for continuous variables and χ2 or Fisher exact tests for categorical variables.
Results
Of 477 patients with axSpA, 121 (25.4%) had enthesitis (mean, 3.9 sites) at enrollment. Higher proportions of patients with enthesitis were female and had nonradiographic axSpA than those without enthesitis (both P < 0.05). Additionally, higher proportions of patients with enthesitis had prior biologic (38.8% vs 27.2%) and conventional synthetic disease‐modifying antirheumatic drug (csDMARD; 24.8% vs 13.3%) use and were currently receiving a combination of biologics and csDMARDs (28.6% vs 18.1%) than those without enthesitis. Patients with enthesitis had worse disease activity (tender and swollen joint counts, physician global assessment, Ankylosing Spondylitis Disease Activity Score, Bath Ankylosing Spondylitis Disease Activity Index, and Bath Ankylosing Spondylitis Functional Index), spinal mobility, and quality of life (pain, fatigue, Health Assessment Questionnaire, and EuroQol visual analog scale scores); greater work impairment; and had a history of depression and fibromyalgia than those without enthesitis (all P < 0.05).
Conclusion
In this US‐based real‐world study, enthesitis in patients with axSpA was associated with worse disease activity and quality of life than those with no enthesitis.
INTRODUCTION
Spondyloarthritis (SpA) comprises a group of rheumatic diseases that include ankylosing spondylosis (AS), reactive arthritis, psoriatic arthritis (PsA), juvenile SpA, and arthritis and spondylitis associated with inflammatory bowel disease, such as ulcerative colitis and Crohn disease (1). SpA with predominantly axial involvement is termed axial SpA (axSpA) and is classified into two subgroups based on presence (AS) or absence (nonradiographic axSpA [nr‐axSpA]) of radiographic sacroiliitis (1). Enthesitis, defined as inflammation of tendon, ligament, or joint capsule insertion sites into bone, or entheses, commonly occurs in patients with SpA (2). Enthesitis may be among the first symptoms of SpA (3) and was identified as a unique feature of SpA that could differentiate this group of rheumatic diseases from rheumatoid arthritis (RA) (4). Enthesitis predominantly occurs in extra‐articular structures prone to inflammation in SpA diseases, whereas RA is characterized by intra‐articular inflammation of the synovial membrane; both enthesitis and synovitis can occur simultaneously or independently of one another (2).
Enthesitis is a key clinical feature of SpA, with or without axial involvement (5). Among patients with AS and nr‐axSpA, the prevalence of enthesitis is reportedly 34% to 74% (6, 7, 8) and 35.4% (7), respectively. However, enthesitis is often underdiagnosed, and quantification of peripheral enthesitis in routine clinical practice lacks specificity and sensitivity (9, 10). Enthesitis is commonly observed in the lower extremities of patients with SpA, particularly the heel (11). In addition to the Achilles and plantar fascia insertions, other frequently observed sites of enthesitis include muscle attachments to the greater and lesser trochanters, insertion of the quadriceps tendon at the upper patellar pole, and insertions of the flexor and extensor tendons at the phalanges (9, 11).
Clinical evaluation of enthesitis is challenging. Correlation between clinical examination of tenderness at entheseal insertion sites and imaging techniques for evidence of entheseal inflammation is low (12). In addition, enthesis sites are not readily accessible to biopsy for verification of inflammatory change, unlike the synovium, skin, or gut; hence, physical examination of entheseal insertion sites may not reflect true inflammatory change but could reveal variable pain thresholds and the phenomenon of central sensitization (13). Despite its frequent occurrence among patients with axSpA and its correlation with disease severity, enthesitis is primarily evaluated as a secondary endpoint in randomized clinical trials or not evaluated at all (14, 15, 16).
There are limited real‐world studies on the characteristics of patients with axSpA and enthesitis. Additionally, relatively little is known about the impact of enthesitis on these patients. In this US‐based study, we sought to compare the characteristics of patients with axSpA who had physical evidence of enthesitis with those of patients without enthesitis. Because imaging methods were not used to verify the presence of enthesitis, the results reported in our study describe the characteristics and impact of clinical enthesitis.
METHODS
Data source
The Corrona PsA/SpA Registry is a large, independent, prospective, observational cohort of patients diagnosed with PsA or SpA by a rheumatologist. Patients were recruited from 44 private and academic practice sites across 27 states in the United States, with 46 participating rheumatologists. As of June 2019, data on approximately 3939 patients with PsA/SpA had been collected. The Corrona PsA/SpA Registry includes information on 15 806 patient visits and approximately 8423 patient‐years of follow‐up, with a mean patient follow‐up of 2.9 years (median, 2.4 years).
All participating investigators were required to obtain full board approval for conducting noninterventional research with a limited data set involving human participants. The Corrona PsA/SpA Registry and its investigators have been reviewed and approved by a central institutional review board (IRB; New England Independent Review Board No. 120160070). For academic investigative sites that did not receive a waiver to use the central IRB, approval was obtained from the respective governing IRBs. All research was conducted in compliance with the current (2013) Declaration of Helsinki. All registry participants were required to provide written informed consent and authorization prior to participating.
Study design and population
This cross‐sectional observational study included all patients aged 18 years or older enrolled in the Corrona PsA/SpA Registry between March 2013 and August 2018 who had rheumatologist‐diagnosed axSpA (AS or nr‐axSpA) according to the Assessment of Spondyloarthritis International Society classification criteria (17, 18). Patients who had a concomitant PsA diagnosis were excluded.
Patients were stratified by the presence or absence of clinical enthesitis at registry enrollment. Patients were classified as having enthesitis (yes/no) at any site using the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index, a measure of enthesitis based on presence of tenderness at 16 entheseal sites (19). The SPARCC Enthesitis Index assesses nine bilateral sites: medial epicondyle, lateral epicondyle, supraspinatus insertion into greater tuberosity of humerus, greater trochanter, quadriceps insertion into superior border of patella, patellar ligament insertion into inferior pole of patella or tibial tubercle, Achilles tendon insertion into calcaneum, and plantar fascia insertion into calcaneum. For scoring purposes, the inferior patella and tibial tuberosities were considered one site due to their anatomical proximity. Tenderness was recorded as present (ie, one) or absent (ie, zero) at each site. The sites were summed with the overall score ranging from 0 to 16; a score of 0 is indicative of absence of enthesitis, whereas patients with a score greater than 0 were classified as having enthesitis.
Outcomes and assessments
Data were collected at the registry enrollment visit and included patient demographics (age, sex, race/ethnicity, marital status, and patient‐reported work status), clinical characteristics (physician‐reported history of comorbidities and disease and symptom duration), treatment profiles (prior and current biologic and conventional synthetic disease‐modifying antirheumatic drug [csDMARD] use), disease activity measures (Ankylosing Spondylitis Disease Activity Score [ASDAS], Bath Ankylosing Spondylitis Disease Activity Index [BASDAI], Bath Ankylosing Spondylitis Functional Index [BASFI], C‐reactive protein [CRP] level, erythrocyte sedimentation rate [ESR], tender joint count [TJC], swollen joint count [SJC], SPARCC Enthesitis Index, dactylitis count, and physician global assessment), quality of life measures (patient global assessment visual analog scale [VAS], patient‐reported pain and fatigue, Health Assessment Questionnaire Disability Index [HAQ‐DI], HAQ for the Spondyloarthropathies [HAQ‐S] (20), EuroQol Quality of Life VAS [EQ‐VAS], and EuroQol 5‐dimension scale [EQ‐5D]), and work productivity measures (Work Productivity and Activity Impairment questionnaire).
Moderate positive correlations between obesity or higher body mass index (BMI) and enthesitis counts in patients with axSpA and PsA have been observed in previous studies (21, 22, 23, 24). To explore a potential relationship between obesity and enthesitis at specific sites in our study population, we evaluated the prevalence of enthesitis at each SPARCC enthesitis site among patients, with enthesitis stratified by BMI category (normal/underweight, BMI < 25 kg/m2; overweight, BMI 25 to < 30 kg/m2; obese, BMI ≥ 30 kg/m2).
Data analysis
Means and frequencies of characteristics in all patients with positive enthesitis scores were compared with those in patients without positive enthesitis scores (reference group) using χ2 or Fisher exact tests for categorical variables and t tests or Wilcoxon rank‐sum tests for continuous variables.
RESULTS
Baseline patient demographics, clinical characteristics, and treatment profiles
A total of 477 patients with axSpA met the inclusion criteria. The majority of patients were white, overweight (mean BMI, 29 kg/m2), worked full time, and had private insurance (Table 1). Overall, 121 patients (25.4%) had enthesitis and 356 (74.6%) did not. The prevalence of enthesitis at each site (mean, 3.9 sites) among patients with enthesitis is shown in Figure 1). Enthesitis was most prevalent in the greater trochanter (43.8%) followed by the lateral epicondyle (43.0%) and supraspinatus insertion into the greater tuberosity of the humerus (34.7%). There was no significant relationship between BMI categories and presence of enthesitis (Supplementary Table 1).
Table 1.
Baseline demographics of patients with axSpA at enrollment, stratified by enthesitis status
| Characteristic | Patients with axSpAa | P valueb | |
|---|---|---|---|
| With enthesitis (n = 121) | No enthesitis (n = 356) | ||
| Nonradiographic axSpA, n (%) | 34 (28.1) | 56 (15.7) | <0.01 |
| Age, mean (SD), yearsc | 47.3 (13.1) | 47.3 (14.1) | 0.99 |
| Female, n (%) | 61 (50.4) | 112 (31.9) | <0.01 |
| White, n (%) | 105 (88.2) | 319 (92.7) | 0.16 |
| Attended college/university, n (%) | 92 (76.7) | 236 (67.0) | 0.09 |
| Private insurance, n (%) | 96 (82.1) | 276 (81.9) | 0.12 |
| Work status, n (%) | n = 121 | n = 351 | 0.30 |
| Full time | 69 (57.0) | 214 (61.0) | |
| Part time | 7 (5.8) | 22 (6.3) | |
| Disabled | 24 (19.8) | 42 (12.0) | |
| Retired | 12 (9.9) | 44 (12.5) | |
| Other | 9 (7.4) | 29 (8.3) | |
| BMI, mean (SD), kg/m2 | 29.9 (6.9) | 29.8 (6.8) | 0.83 |
| BMI (in kg/m2) categories, n (%) | n = 120 | n = 345 | 0.46 |
| Normal/underweight (<25) | 33 (27.5) | 86 (24.9) | |
| Overweight (25 to <30) | 33 (27.5) | 116 (33.6) | |
| Obese (≥30) | 54 (45.0) | 143 (41.4) | |
Abbreviation: axSpA, axial spondyloarthritis; BMI, body mass index.
All values were calculated based on available data and had less than 20% missing data.
P value for comparison between patients with versus those without enthesitis. The Wilcoxon rank‐sum test was used for continuous variables, whereas the χ2 test was used for categorical variables. For variables where more than 20% of the cells had expected counts less than five, the Fisher exact test was used.
t test for age comparison between pairs of groups.
Figure 1.
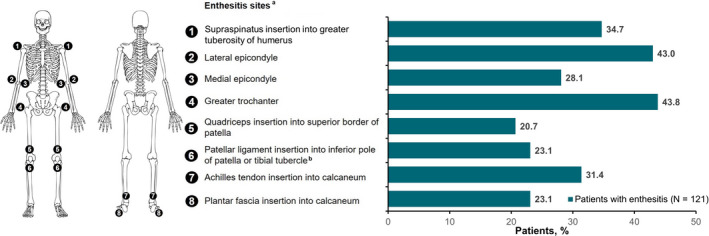
Distribution of enthesitis sites among patients with axial spondyloarthritis. aPercentages listed are a total of the left and right affected sites. bFor scoring purposes, the inferior patella and tibial tuberosities are considered one site due to their anatomical proximity.
Higher proportions of patients with enthesitis were female and had nr‐axSpA than those without enthesitis (both P < 0.05; Table 1). Patients with enthesitis were more likely to have a history of depression, serious infections, and fibromyalgia than those without enthesitis (all P < 0.05; Table 2). With regard to treatment, higher proportions of patients with enthesitis had a history of prior biologic (38.8% vs 27.2%) and csDMARD (24.8% vs 13.2%) use than those without enthesitis (both P < 0.05; Table 2). The proportion of patients currently receiving biologic therapy was comparable between groups. However, among patients currently receiving a tumor necrosis factor inhibitor (TNFi), a higher proportion of patients with enthesitis were receiving a TNFi in combination with csDMARDs than those without enthesitis (28.6% vs 18.1%).
Table 2.
Baseline clinical characteristics and treatment history of patients with axSpA at enrollment, stratified by enthesitis status
| Characteristic | Patients with axSpAa | P valueb | |
|---|---|---|---|
| With enthesitis (n = 121) | No enthesitis (n = 356) | ||
| Symptom duration, mean (SD), y | 17.3 (13.1) | 16.8 (11.8) | 0.67 |
| Disease duration, mean (SD), y | 8.2 (10.3) | 10.2 (10.7) | 0.09 |
| History of comorbidities, n (%) | |||
| Cardiovascular disease | 56 (46.3) | 140 (39.3) | 0.18 |
| Hypertension | 37 (30.6) | 109 (30.6) | 0.99 |
| Depression | 29 (24.0) | 53 (14.9) | 0.02 |
| Hyperlipidemia | 21 (17.4) | 52 (14.6) | 0.47 |
| Uveitis | 13 (10.7) | 40 (11.2) | 0.88 |
| Metabolic syndrome | 9 (7.4) | 27 (7.6) | 0.96 |
| Psoriasis | 7 (5.8) | 22 (6.2) | 0.88 |
| Diabetes mellitus | 11 (9.1) | 20 (5.6) | 0.18 |
| Ulcerative colitis | 3 (2.5) | 18 (5.1) | 0.31 |
| Serious infections | 12 (9.9) | 16 (4.5) | 0.03 |
| Any cancer (excluding NMSC) | 5 (4.1) | 16 (4.5) | 0.87 |
| Crohn disease | 6 (5.0) | 14 (3.9) | 0.63 |
| Fibromyalgia | 11 (9.1) | 11 (3.1) | 0.01 |
| Anxiety | 6 (5.0) | 7 (2.0) | 0.08 |
| History of prior biologic use, n (%) | 47 (38.8) | 97 (27.2) | 0.02 |
| No. of prior biologics used, n (%) | 0.01 | ||
| 0 | 74 (61.2) | 259 (72.8) | |
| 1 | 26 (21.5) | 68 (19.1) | |
| ≥2 | 21 (17.4) | 29 (8.1) | |
| History of prior csDMARD use, n (%) | 30 (24.8) | 47 (13.2) | <0.01 |
| No. of prior csDMARDs used, n (%) | 0.01 | ||
| 0 | 91 (75.2) | 309 (86.8) | |
| 1 | 22 (18.2) | 37 (10.4) | |
| ≥2 | 8 (6.6) | 10 (2.8) | |
| Current biologic use, n (%) | 84 (69.4) | 237 (66.6) | 0.56 |
| TNFi use | 0.05 | ||
| Monotherapy | 55 (71.4) | 185 (81.9) | |
| Combination therapy | 22 (28.6) | 41 (18.1) | |
| Non‐TNFi use | 0.47 | ||
| Monotherapy | 6 (100) | 6 (75) | |
| Combination therapy | 0 | 2 (25) | |
| Current overall csDMARD use, n (%)c | 34 (28.1) | 72 (20.2) | 0.07 |
| Current csDMARD use only (without biologic or tsDMARD use), n (%) | 12 (9.9) | 30 (8.4) | 0.62 |
Abbreviation: axSpA, axial spondyloarthritis; csDMARD, conventional synthetic disease‐modifying antirheumatic drug; NMSC, nonmelanoma skin cancer; TNFi, tumor necrosis factor inhibitor; tsDMARD, targeted synthetic disease‐modifying antirheumatic drug.
All values were calculated based on available data and had less than 20% missing data.
P value for comparison between patients with versus those without enthesitis. The Wilcoxon rank‐sum test was used for continuous variables, whereas the χ2 test was used for categorical variables. For variables where more than 20% of the cells had expected counts less than 5, the Fisher exact test was used.
A patient may be on multiple csDMARDs.
Baseline disease activity measures
Patients with enthesitis had worse disease activity than those without enthesitis, including higher mean TJC (8.2 vs 1.3) and SJC (1.3 vs 0.5); higher ASDAS (2.9 vs 2.5), BASDAI (5.3 vs 4.1), BASFI (4.5 vs 3.3), and physician global assessment (36.6 vs 23.5) scores; and more restricted spinal mobility as measured by mean lateral lumbar flexion (28.1 vs 21.5 cm) (all P < 0.05; Table 3). Additionally, patients with enthesitis had lower CRP levels (6.9 vs 10.4 mg/L) and ESR (14.2 vs 15.5 mm/h) than those without enthesitis, but these differences were not statistically significant (Table 3).
Table 3.
Baseline disease activity measures in patients with axSpA at enrollment, stratified by enthesitis status
| Characteristic | Patients with axSpAa | P valueb | |
|---|---|---|---|
| With enthesitis (n = 121) | No enthesitis (n = 356) | ||
| ASDAS | 2.9 (0.9) | 2.5 (1.2) | <0.01 |
| BASDAI (0‐10) | 5.3 (2.2) | 4.1 (2.4) | <0.01 |
| BASFI (0‐10) | 4.5 (2.8) | 3.3 (2.7) | <0.01 |
| Tender joint count (0‐68) | 8.2 (10.8) | 1.3 (4.2) | <0.01 |
| Swollen joint count (0‐66) | 1.3 (3.3) | 0.5 (2.0) | <0.01 |
| Dactylitis, n (%) | 4 (3.3) | 7 (2.0) | 0.40 |
| Dactylitis (1‐20) | 2.0 (2.0) | 3.6 (3.9) | 0.35 |
| SPARCC Enthesitis Index (1‐16) | 3.9 (2.9) | 0 | … |
| Inflammatory back pain, n (%) | 78 (64.5) | 205 (57.6) | 0.18 |
| Lateral lumbar flexion (average of left and right), cm | 28.1 (21.1) | 21.5 (18.8) | 0.01 |
| CRP, mg/L | 6.9 (7.0) | 10.4 (24.1) | 0.32 |
| ESR, mm/h | 14.2 (16.4) | 15.5 (18.9) | 0.84 |
| Physician global assessment | 36.6 (21.9) | 23.5 (21.9) | <0.01 |
Abbreviation: ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; SPARCC, Spondyloarthritis Research Consortium of Canada.
All values were calculated based on available data and are presented as mean (SD) unless otherwise stated and had less than 20% missing data except for ESR and CRP.
P value for comparison between patients with versus those without enthesitis. The Wilcoxon rank‐sum test was used for continuous variables, whereas the χ2 test was used for categorical variables. For variables where more than 20% of the cells had expected counts less than 5, the Fisher exact test was used.
Baseline patient‐reported outcome measures and work productivity
Patients with enthesitis had worse quality of life than those without enthesitis, including higher mean pain (57.5 vs 43.5), fatigue (56.5 vs 45.0), HAQ‐DI (0.9 vs 0.6), and HAQ‐S (0.9 vs 0.6) scores; additionally, they had a lower EQ‐VAS score at enrollment (59.7 vs 66.4), indicating a worse health state (all P < 0.01; Table 4). With regard to work productivity and activity impairment, patients with enthesitis had a significantly greater percentage of impairment while working (38.3% vs 25.3%), percentage of overall work impairment (41.4% vs 27.6%), and percentage of activity impairment (49.8% vs 35.6%) than those without enthesitis (all P < 0.01).
Table 4.
Baseline patient‐reported outcome measures in patients with axSpA at enrollment, stratified by enthesitis status
| Characteristic | Patients with axSpAa | P valueb | |
|---|---|---|---|
| With enthesitis (n = 121) | No enthesitis (n = 356) | ||
| Patient pain (VAS 0‐100) | 57.5 (26.7) | 43.5 (29.5) | <0.01 |
| Patient fatigue (VAS 0‐100) | 56.5 (27.5) | 45.0 (28.6) | <0.01 |
| Patient global assessment (VAS 0‐100) | 49.8 (29.7) | 54.1 (32.9) | 0.56 |
| HAQ‐DI (0‐3) | 0.9 (0.7) | 0.6 (0.6) | <0.01 |
| HAQ‐S (0‐3) | 0.9 (0.7) | 0.6 (0.6) | <0.01 |
| EQ‐5D (0‐1) | 0.7 (0.2) | 0.7 (0.2) | <0.01 |
| EQ (VAS 0‐100): patient health today | 59.7 (22.7) | 66.4 (22.0) | <0.01 |
| WPAI‐GH domains | |||
| % Work time missed | 9.0 (18.8) | 5.8 (16.8) | 0.06 |
| % Impairment while working | 38.3 (27.4) | 25.3 (24.4) | <0.01 |
| % Overall work impairment | 41.4 (29) | 27.6 (26.6) | <0.01 |
| % Activity impairment | 49.8 (29.5) | 35.6 (29.3) | <0.01 |
| Current employment, n (%) | 75 (63.0) | 241 (68.7) | 0.26 |
| Morning stiffness, n (%) | n = 117 | n = 318 | 0.72 |
| <30 min | 28 (23.9) | 71 (22.3) | |
| ≥30 min | 89 (76.1) | 247 (77.7) | |
Abbreviation: axSpA, axial spondyloarthritis; EQ‐5D, EuroQol 5‐dimension questionnaire; HAQ‐DI, Health Assessment Questionnaire Disability Index; HAQ‐S, Health Assessment Questionnaire for the Spondyloarthropathies; VAS, visual analog scale; WPAI‐GH, Work Productivity and Activity Impairment Questionnaire‐General Health.
All values were calculated based on available data and are presented as mean (SD) unless otherwise stated and had less than 20% missing data except for patient global assessment, HAQ‐DI, HAQ‐S, and WPAI‐GH1 to WPAI‐GH3.
P value for comparison between patients with versus those without enthesitis. The Wilcoxon rank‐sum test was used for disease activity measurements, whereas the χ2 test was used for categorical variables.
DISCUSSION
In this US‐based real‐world study of patients with axSpA, the presence of clinical enthesitis was associated with higher disease activity and worse quality of life than the absence of enthesitis. We also found that patients with enthesitis were more likely to be receiving combination therapy, further suggesting that this population had more severe or burdensome disease. These results are consistent with previous studies showing that presence of enthesitis in patients with axSpA is correlated with increased disease severity and poorer quality of life (25, 26, 27). Additionally, consistent with previous reports (27, 28, 29), we observed more frequent enthesitis among female patients (50%) versus male patients (32%). Presence of enthesitis in patients with PsA has been linked to axial and peripheral joint damage, likelihood of developing joint ankyloses, overall higher disease activity, more pain, worse quality of life and functional status, sleep disturbance, and patient‐reported pain and fatigue (30, 31, 32). Additionally, in PsA, enthesitis has been regarded as a predictor of poor disease outcomes and may represent a lower probability of achieving remission and low disease activity (22, 30).
The higher prevalence of pain or tenderness at entheseal sites among female patients with axSpA may contribute to the greater disease burden in female than in male patients, despite slower radiographic progression in female patients (27, 28, 29). However, in painful chronic disorders, pain can result not only from inflammation and mechanical irritation but from a combination of mechanisms, including central sensitization, which is defined as amplification of neural signaling that leads to pain hypersensitivity (33). Central sensitization contributes to the pain hypersensitivity phenotype of several disorders, including fibromyalgia, which represents a state of persistent central sensitization (13, 33). The American College of Rheumatology introduced fibromyalgia classification criteria in 1990 based on the scoring of 11 out of 18 tender points (34); these sites overlap with those often assessed for enthesitis (35). Subsequent iterations of the criteria have removed the tender points assessment, but evaluation of tender points is still commonly performed in clinical practice (36). Because central pain sensitization likely occurs in some patients with axSpA and is associated with higher disease activity (37), patient‐reported outcome measures may be amplified by presence of fibromyalgia, which can occur along with axSpA. As in our study, the proportion of women with fibromyalgia in previous studies of patients with axSpA was higher than that of men (13). Because enthesitis was defined in our study as presence of tenderness at 18 entheseal points based on physical examination without ultrasound or magnetic resonance imaging (MRI) corroboration of inflammation, the difference in frequency between men and women may partially reflect a higher prevalence of central sensitization in women, which correlated with symptoms such as higher TJC, depression, and fatigue. Therefore, distinguishing fibromyalgia from axSpA is important and may be addressed using currently available patient‐reported outcome tools for fibromyalgia, such as the Widespread Pain Index and Symptom Severity Score or the Fibromyalgia Rapid Screening Tool (36). However, it may be challenging to differentiate entheseal site tenderness due to inflammatory enthesitis from tenderness due to central sensitization using only clinical examination; thus, more specific tools for diagnosis of fibromyalgia or central sensitization are needed, such as validated questionnaires as mentioned above and/or advanced imaging techniques, including ultrasound or MRI of the entheses, neuroimaging techniques, or quantitative sensory testing (13).
Because enthesitis may be one of the pivotal pathological processes in SpA associated with poor disease outcomes (30, 38), detection of early enthesitis lesions may be critical for SpA diagnosis. MRI has shown that extracapsular inflammation of joints represents enthesitis with varying extents of soft tissue and bone marrow edema (39, 40). Recently, Poggenborg and colleagues demonstrated the utility of whole‐body MRI in the detection of subclinical axial and peripheral enthesitis (41). Detection of enthesitis using ultrasound has also been useful, particularly for early diagnosis of SpA (42).
Several indices are available for clinical measurement of inflammatory activity and treatment response of enthesitis in axSpA and peripheral SpA. The Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), University of California, San Francisco Index, and Berlin Index have been used in axSpA studies (43, 44, 45). MASES was initially developed for identification of entheses specific for axial disease in AS (46) and has performed well in many AS and axSpA trials (45, 47, 48). The SPARCC Enthesitis Index was initially developed for use in both axial and peripheral SpA, including PsA (19). Although it has principally been used in PsA and has not been used in axSpA clinical trials, we have successfully used the SPARCC Enthesitis Index for enthesitis evaluation in previous studies of patients with axSpA (30, 49, 50, 51).
The SPARCC Enthesitis Index has been used in several PsA clinical trials with success (52, 53, 54). An additional index, the Leeds Enthesitis Index (LEI), was developed for enthesitis assessment in PsA (55). In the ABILITY‐2 trial, which assessed the efficacy of adalimumab in patients with peripheral SpA, the LEI, SPARCC, and MASES indices were compared for their discriminatory capacity in assessing enthesitis and treatment response; the LEI and SPARCC Enthesitis Index outperformed the MASES, most likely because the MASES primarily evaluates axial sites, whereas the LEI and SPARCC Enthesitis Index evaluate more peripheral sites (46).
This study has some limitations that should be considered. Patients enrolled in this study are routinely seen and treated by rheumatologists voluntarily participating in the Corrona PsA/SpA Registry and may not be representative of all US patients with axSpA. Because presence of enthesitis in our study was based on physical examination without imaging corroboration, we cannot truly distinguish between central sensitization, as characterized by tender entheseal sites, and enthesitis. Accordingly, our findings describe features and impact of clinical enthesitis. Additionally, the presence of depression and fibromyalgia was based on investigator judgement and was not objectively measured. Going forward, the Corrona Registry will be incorporating the Widespread Pain Index and Symptom Severity Scale, a validated quantitative tool to measure central sensitization (36, 56), to provide a more objective assessment of the presence and impact of central sensitization in future analyses. All comparisons were descriptive; no adjustments were made to account for differences in patient characteristics, such as age and sex, which may have influenced the other differences observed between patients with and those without enthesitis.
In conclusion, enthesitis was associated with greater overall burden of axSpA, including worse disease activity and quality of life. Enthesitis may represent the primary underlying process for SpA diseases, such as axSpA. Therefore, identification, evaluation, and management of enthesitis is critical to ameliorate the overall burden of disease. Additionally, more research is needed to guide treatment options specific to enthesitis and to better discriminate between enthesitis and central sensitization.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, approved the final version to be published, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mease, Rebello, McLean, Yi, Park, Ogdie.
Acquisition of data
Mease, Liu, Rebello, Hua, McLean, Ogdie.
Analysis and interpretation of data
Mease, Liu, Rebello, Hua, McLean, Yi, Park, Ogdie.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank all the investigators, their clinical staff, and patients who participate in the Corrona PsA/SpA Registry. Support for third‐party writing assistance for this manuscript, furnished by Kheng Bekdache, PhD, of Health Interactions, Inc, was provided by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey.
This study was sponsored by Corrona, LLC, and financially supported by Novartis. The design and conduct of the study were a collaborative effort between Corrona, LLC, and Novartis. Novartis participated in the interpretation of data and review and approval of the abstract. Corrona, LLC, has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Crescendo, Eli Lilly and Company, Genentech, Gilead, GlaxoSmithKline, Janssen, Merck, Momenta Pharmaceuticals, Novartis, Ortho Dermatologics, Pfizer Inc, Regeneron, Roche, Sun, and UCB. Dr. Mease has received research grants from Celgene, Novartis, AbbVie, Amgen, Bristol‐Myers Squibb, Lilly, Pfizer, and UCB. Dr. Ogdie has received grant support from the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Psoriasis Foundation, Rheumatology Research Foundation, Pfizer, and Novartis.
Philip J. Mease, MD, MACR: Swedish Medical Center/Providence St. Joseph Health and University of Washington, Seattle, Washington; 2Mei Liu, PhD, Sabrina Rebello, MPH, Winnie Hua, MA, MS, Robert R. McLean, DSc, MPH: Corrona, LLC, Waltham, Massachusetts; 3Esther Yi, PharmD, Yujin Park, PharmD: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey; 4Alexis Ogdie, MD, MSCE: University of Pennsylvania, Philadelphia, Pennsylvania.
Dr. Mease has received consulting fees from Celgene, Corrona, Novartis, AbbVie, Amgen, Bristol‐Myers Squibb, Galapagos, Gilead, Janssen, Lilly, Merck, Pfizer, Sun, and UCB; and speakers bureau fees from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, Genentech, Janssen, Pfizer, and UCB. Dr. Ogdie has received consulting fees from Amgen, AbbVie, Bristol‐Myers Squibb, Celgene, Lilly, Novartis, and Pfizer. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos‐Vargas R, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68 Suppl 2:ii1–44. [DOI] [PubMed] [Google Scholar]
- 2. Schett G, Lories RJ, D'Agostino MA, Elewaut D, Kirkham B, Soriano ER, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 2017;13:731–41. [DOI] [PubMed] [Google Scholar]
- 3. Godfrin B, Zabraniecki L, Lamboley V, Bertrand‐Latour F, Sans N, Fournié B. Spondyloarthropathy with entheseal pain. A prospective study in 33 patients. Joint Bone Spine 2004;71:557–62. [DOI] [PubMed] [Google Scholar]
- 4. Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis 1971;30:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 6. Ciurea A, Scherer A, Exer P, Bernhard J, Dudler J, Beyeler B, et al. Tumor necrosis factor α inhibition in radiographic and nonradiographic axial spondyloarthritis: results from a large observational cohort. Arthritis Rheum 2013;65:3096–106. [DOI] [PubMed] [Google Scholar]
- 7. De Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra‐articular disease in ankylosing spondylitis versus non‐radiographic axial spondyloarthritis: a meta‐analysis. Arthritis Res Ther 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vander Cruyssen B, Ribbens C, Boonen A, Mielants H, de Vlam K, Lenaerts J, et al. The epidemiology of ankylosing spondylitis and the commencement of anti‐TNF therapy in daily rheumatology practice. Ann Rheum Dis 2007;66:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Agostino MA, Aegerter P, Bechara K, Salliot C, Judet O, Chimenti MS, et al. How to diagnose spondyloarthritis early? Accuracy of peripheral enthesitis detection by power Doppler ultrasonography. Ann Rheum Dis 2011;70:1433–40. [DOI] [PubMed] [Google Scholar]
- 10. Weill C, Norregaard J, Szkudlarek M, Hasselquist M, Moller JM, Terslev L, et al. Ultrasonography of finger joints, tendons and entheses in patients with spondyloarthropathy: a comparison with clinical examination and MRI. Ann Rheum Dis 2005;64:327. [Google Scholar]
- 11. D'Agostino MA, Olivieri I. Enthesitis. Best Pract Res Clin Rheumatol 2006;20:473–86. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Liang J, Qiu J, Wang F, Sun L. Ultrasonographic evaluation of enthesitis in patients with ankylosing spondylitis. J Biomed Res 2017;31:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mease PJ. Fibromyalgia, a missed comorbidity in spondyloarthritis: prevalence and impact on assessment and treatment. Curr Opin Rheumatol 2017;29:304–10. [DOI] [PubMed] [Google Scholar]
- 14. Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti‐interleukin‐17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;382:1705–13. [DOI] [PubMed] [Google Scholar]
- 15. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin‐17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 16. Marzo‐Ortega H, Sieper J, Kivitz A, Blanco R, Cohen M, Martin R, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active ankylosing spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken) 2017;69:1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rudwaleit M, Landewé R, van der Heijde D, Listing J, Brandt J, Braun J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal [published erratum appears in Ann Rheum Dis 2011;70:1519]. Ann Rheum Dis 2009;68:770–6. [DOI] [PubMed] [Google Scholar]
- 18. Rudwaleit M, van der Heijde D, Landewé R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection [correction published in Ann Rheum Dis 2019;78:e59]. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 19. Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) Enthesitis Index. Ann Rheum Dis 2009;68:948–53. [DOI] [PubMed] [Google Scholar]
- 20. Daltroy LH, Larson MG, Roberts NW, Liang MH. A modification of the Health Assessment Questionnaire for the spondyloarthropathies [published erratum appears in J Rheumatol 1991;18:305]. J Rheumatol 1990;17:946–50. [PubMed] [Google Scholar]
- 21. Klingberg E, Bilberg A, Björkman S, Hedberg M, Jacobsson L, Forsblad‐d'Elia H, et al. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: an interventional study. Arthritis Res Ther 2019;21:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaeley GS, Eder L, Aydin SZ, Gutierrez M, Bakewell C. Enthesitis: a hallmark of psoriatic arthritis. Semin Arthritis Rheum 2018;48:35–43. [DOI] [PubMed] [Google Scholar]
- 23. Bakirci S, Dabague J, Eder L, McGonagle D, Aydin SZ. The role of obesity on inflammation and damage in spondyloarthritis: a systematic literature review on body mass index and imaging. Clin Exp Rheumatol 2020;38:144–8. [PubMed] [Google Scholar]
- 24. Micheroli R, Hebeisen M, Wildi LM, Exer P, Tamborrini G, Bernhard J, et al. Impact of obesity on the response to tumor necrosis factor inhibitors in axial spondyloarthritis. Arthritis Res Ther 2017;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Winter JJ, Paramarta JE, de Jong HM, van de Sande MG, Baeten DL. Peripheral disease contributes significantly to the level of disease activity in axial spondyloarthritis. RMD Open 2019;5:e000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palominos PE, de Campos AP, Ribeiro SL, Xavier RM, Xavier JW, de Oliveira FB, et al. Correlation of enthesitis indices with disease activity and function in axial and peripheral spondyloarthritis: a cross‐sectional study comparing MASES, SPARCC and LEI. Adv Rheumatol 2019;59:23. [DOI] [PubMed] [Google Scholar]
- 27. Tournadre A, Pereira B, Lhoste A, Dubost JJ, Ristori JM, Claudepierre P, et al. Differences between women and men with recent‐onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res (Hoboken) 2013;65:1482–9. [DOI] [PubMed] [Google Scholar]
- 28. Lubrano E, Perrotta FM, Manara M, D'Angelo S, Addimanda O, Ramonda R, et al. The sex influence on response to tumor necrosis factor‐α inhibitors and remission in axial spondyloarthritis. J Rheumatol 2018;45:195–201. [DOI] [PubMed] [Google Scholar]
- 29. Shahlaee A, Mahmoudi M, Nicknam MH, Farhadi E, Fallahi S, Jamshidi AR. Gender differences in Iranian patients with ankylosing spondylitis. Clin Rheumatol 2015;34:285–93. [DOI] [PubMed] [Google Scholar]
- 30. Mease PJ, Karki C, Palmer JB, Etzel CJ, Kavanaugh A, Ritchlin CT, et al. Clinical characteristics, disease activity, and patient‐reported outcomes in psoriatic arthritis patients with dactylitis or enthesitis: results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. Arthritis Care Res (Hoboken) 2017;69:1692–9. [DOI] [PubMed] [Google Scholar]
- 31. Polachek A, Li S, Chandran V, Gladman DD. Clinical enthesitis in a prospective longitudinal psoriatic arthritis cohort: incidence, prevalence, characteristics, and outcome [published erratum appears in Arthritis Care Res (Hoboken) 2019;71:574]. Arthritis Care Res (Hoboken) 2017;69:1685–91. [DOI] [PubMed] [Google Scholar]
- 32. Polachek A, Cook R, Chandran V, Gladman DD, Eder L. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther 2017;19:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 35. Roussou E, Ciurtin C. Clinical overlap between fibromyalgia tender points and enthesitis sites in patients with spondyloarthritis who present with inflammatory back pain. Clin Exp Rheumatol 2012;30 Suppl 74:24–30. [PubMed] [Google Scholar]
- 36. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, et al. 2016 revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- 37. Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum 2013;65:1494–503. [DOI] [PubMed] [Google Scholar]
- 38. Mease PJ, Liu M, Rebello S, Hua W, McLean RR, Hur P. Disease characteristics, quality of life, and work productivity by enthesitis sites: real‐world data from the US Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol 2018;70 Suppl 10 URL: https://acrabstracts.org/abstract/disease‐characteristics‐quality‐of‐life‐and‐work‐productivity‐by‐enthesitis‐sites‐real‐world‐data‐from‐the‐us‐corrona‐psoriatic‐arthritis‐spondyloarthritis‐psa‐spa‐registry/ [DOI] [PubMed] [Google Scholar]
- 39. Marzo‐Ortega H, McGonagle D, O'Connor P, Emery P. Efficacy of etanercept in the treatment of the entheseal pathology in resistant spondylarthropathy: a clinical and magnetic resonance imaging study. Arthritis Rheum 2001;44:2112–7. [DOI] [PubMed] [Google Scholar]
- 40. McGonagle D, Marzo‐Ortega H, O'Connor P, Gibbon W, Pease C, Reece R, et al. The role of biomechanical factors and HLA‐B27 in magnetic resonance imaging‐determined bone changes in plantar fascia enthesopathy. Arthritis Rheum 2002;46:489–93. [DOI] [PubMed] [Google Scholar]
- 41. Poggenborg RP, Eshed I, Østergaard M, Sørensen IJ, Møller JM, Madsen OR, et al. Enthesitis in patients with psoriatic arthritis, axial spondyloarthritis and healthy subjects assessed by ‘head‐to‐toe’ whole‐body MRI and clinical examination. Ann Rheum Dis 2015;74:823–9. [DOI] [PubMed] [Google Scholar]
- 42. De Miguel E, Muñoz‐Fernández S, Castillo C, Cobo‐Ibáñez T, Martín‐Mola E. Diagnostic accuracy of enthesis ultrasound in the diagnosis of early spondyloarthritis. Ann Rheum Dis 2011;70:434–9. [DOI] [PubMed] [Google Scholar]
- 43. Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. [DOI] [PubMed] [Google Scholar]
- 44. Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor α. N Engl J Med 2002;346:1349–56. [DOI] [PubMed] [Google Scholar]
- 45. Heuft‐Dorenbosch L, Spoorenberg A, van Tubergen A, Landewe R, van der Tempel H, Mielants H, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mease PJ, Van den Bosch F, Sieper J, Xia Y, Pangan AL, Song IH. Performance of 3 enthesitis indices in patients with peripheral spondyloarthritis during treatment with adalimumab. J Rheumatol 2017;44:599–608. [DOI] [PubMed] [Google Scholar]
- 47. Van der Heijde D, Braun J, Deodhar A, Inman RD, Xu S, Mack ME, et al. Comparison of three enthesitis indices in a multicentre, randomized, placebo‐controlled trial of golimumab in ankylosing spondylitis (GO‐RAISE). Rheumatology (Oxford) 2013;52:321–5. [DOI] [PubMed] [Google Scholar]
- 48. Rudwaleit M, Claudepierre P, Kron M, Kary S, Wong R, Kupper H. Effectiveness of adalimumab in treating patients with ankylosing spondylitis associated with enthesitis and peripheral arthritis. Arthritis Res Ther 2010;12:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mease PJ, Van Der Heijde D, Karki C, Palmer JB, Liu M, Pandurengan R, et al. Characterization of patients with ankylosing spondylitis and nonradiographic axial spondyloarthritis in the US‐based Corrona registry. Arthritis Care Res (Hoboken) 2018;70:1661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mease PJ, Palmer JB, Liu M, Kavanaugh A, Pandurengan R, Ritchlin CT, et al. Influence of axial involvement on clinical characteristics of psoriatic arthritis: analysis from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol 2018;45:1389–96. [DOI] [PubMed] [Google Scholar]
- 51. Mease PJ, van der Heijde D, Karki C, Liu M, Park Y, Greenberg JD. Tumor necrosis factor inhibitor discontinuation in patients with ankylosing spondylitis: an observational study from the US‐based Corrona registry. Rheumatol Ther 2018;5:537–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐month and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kirkham B, McInnes IB, Mease P, Kremer J, Kandala S, Pricop L, et al. Secukinumab is effective in reducing dactylitis and enthesitis using multuple measures in patients with psoriatic arthritis: data from a phase 3 randomized, multicenter, double‐blind, placebo‐controlled study (FUTURE 2). Ann Rheum Dis 2015;74:351. [Google Scholar]
- 54. Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24‐week results of a phase 3 double‐blind randomised placebo‐controlled study (RAPID‐PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 56. Wolfe F, Butler SH, Fitzcharles M, Häuser W, Katz RL, Mease PJ, et al. Revised chronic widespread pain criteria: development from and integration with fibromyalgia criteria. Scand J Pain 2019;20:77–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


