Abstract
Objective
The number of therapies for axial spondyloarthritis (axSpA) is increasing. Thus, it has become more challenging for patients and physicians to navigate the risk‐benefit profiles of the various treatment options. In this study, we used conjoint analysis—a form of trade‐off analysis that elucidates how people make complex decisions by balancing competing factors—to examine patient decision‐making surrounding medication options for axSpA.
Methods
We conducted an adaptive choice‐based conjoint analysis survey for patients with axSpA to assess the relative importance of medication attributes (eg, chance of symptom improvement, risk of side effects, route of administration, etc) in their decision‐making. We also performed logistic regression to explore whether patient demographics and disease characteristics predicted decision‐making.
Results
Overall, 397 patients with axSpA completed the conjoint analysis survey. Patients prioritized medication efficacy (importance score 26.8%), cost (26.3%), and route of administration (13.9%) as most important in their decision‐making. These were followed by risk of lymphoma (9.5%), dosing frequency (7.2%), risk of serious infection (6.0%), tolerability of side effects (5.3%), and clinic visit and laboratory test frequency (4.8%). In regression analyses, there were few significant associations between patients’ treatment preferences and sociodemographic and axSpA characteristics.
Conclusions
Treatment decision‐making in axSpA is highly individualized, and demographics and baseline disease characteristics are poor predictors of individual preferences. This calls for the development of online shared decision‐making tools for patients and providers, with the goal of selecting a treatment that is consistent with patients’ preferences.
INTRODUCTION
Axial spondyloarthritis (axSpA) is a chronic, progressive form of inflammatory arthritis affecting the axial skeleton and includes both nonradiographic axSpA and ankylosing spondylitis (AS) (radiographic form of axSpA) (1, 2). There are numerous treatments for axSpA, ranging from nonsteroidal anti‐inflammatory drugs (NSAIDs) to biologics (3, 4, 5, 6, 7), and it can be difficult for patients to navigate the array of options and choose a therapy that aligns with their preferences. Adding to this complexity is that even within medication classes, particularly for biologics, there are varying mechanisms of action, modes of administration, effectiveness, and side effects.
We thus sought to understand how patients with axSpA decide from among the different treatment options by using conjoint analysis—a technique that determines how people make complex decisions by balancing competing factors. Conjoint analysis is based on the idea that any product (eg, a service, test, or treatment) can be described by its attributes and is valued based on the levels of these attributes. It is administered via a computer‐based interactive exercise in which respondents evaluate competing profiles (eg, of axSpA treatments) and select their preferred profile (see example in Figure 1). In this study, we specifically aimed to quantify and rank‐order the relative importance of axSpA medication attributes (eg, efficacy, side effects, route of administration, cost, etc) in patients’ decision‐making, without making reference to any generic or branded products. Furthermore, we evaluated whether certain patient factors (ie, sociodemographic and axSpA characteristics) predicted preference patterns.
Figure 1.
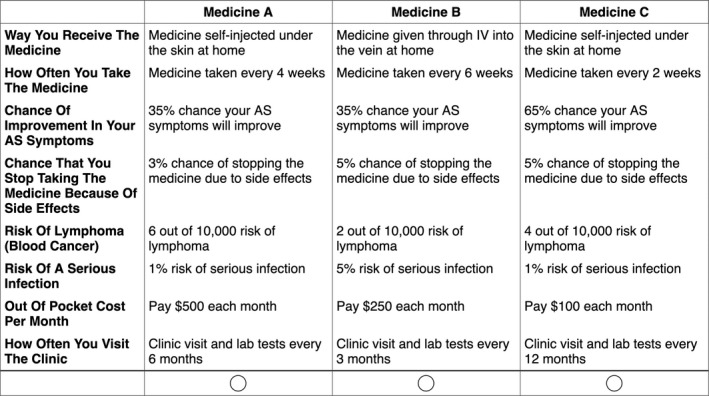
Sample choice tournament task in which participants consider three hypothetical medication profiles side by side and decide which medication they would prefer for treating their axial spondyloarthritis. Respondents were shown 20 different vignettes, each of which with varying attribute levels. Abbreviation: AS, ankylosing spondylitis; IV, intravenous.
Patients and Methods
Overview of conjoint analysis
Conjoint analysis is a method that quantifies how respondents make trade‐offs when considering competing factors (8). This approach assumes that decision‐making is based on the attributes of a product, each of which has multiple levels. A series of side‐by‐side profiles of unbranded hypothetical products are presented, with each profile having unique levels assigned to each attribute (Figure 1). Based on the respondent’s answer to the first comparison, an algorithm selects a new side‐by‐side comparison and asks the respondent to select the preferred profile. Conjoint analysis is used extensively in clinical research (8), and examples extend across diverse domains, including rheumatology (9, 10, 11), spinal surgery (12), diabetes management (13), and inflammatory bowel disease (IBD) (14).
Adaptive choice‐based conjoint analysis for therapy decision‐making in axSpA
We employed adaptive choice‐based conjoint (ACBC) analysis software (Sawtooth Software) to determine how patients with axSpA make decisions when selecting from among the various treatment options. Table 1 displays the eight attributes and their associated levels that were tested in the ACBC analysis survey; these were based on characteristics of axSpA therapies, including NSAIDs and biologics. The eight attributes were further organized in four categories: 1) medication characteristics (route of administration, dosing frequency, frequency of clinic appointments, and laboratory testing), 2) efficacy (ie, chance of improvement in axSpA symptoms), 3) side effect profile (tolerability of unwanted side effects or reactions, risk of lymphoma, and risk of serious infection), and 4) out‐of‐pocket costs per month. These attributes were selected based on input from the literature (10, 15, 16, 17, 18); our axSpA social media netnography research, which examined patient concerns and perceptions regarding biologics (19); axSpA content experts on the research team; and on US Food and Drug Administration labels (including “black box warnings”) for currently marketed products to ensure accurate representation of factors important in the decision‐making process.
Table 1.
Medication attributes and levels included in the conjoint analysis survey
| Attribute Category | Specific Attribute | Attribute Levels |
|---|---|---|
| Medication characteristics | Route of administration |
|
| Dosing frequency |
|
|
| Frequency of clinic appointments and laboratory tests |
|
|
| Efficacy | Chance of improvement in AS symptoms |
|
| Side effect profile | Tolerability of unwanted side effects (ie, chance of stopping the medication because of side effects) |
|
| Risk of lymphoma a |
|
|
| Risk of serious infection |
|
|
| Cost | Out‐of‐pocket costs per month |
|
Abbreviation: AS, ankylosing spondylitis.
Respondents were informed that the baseline risk of lymphoma without biologics is 2 of 10 000.
The ACBC analysis software uses the inputted attributes and levels to create a series of side‐by‐side profiles of hypothetical axSpA therapies as part of a “choice tournament” with 20 distinct decisions (Figure 1). After participants complete the survey, the software uses hierarchical Bayes regression to estimate individual‐level utility coefficients (20, 21). These coefficients—called part‐worth utilities—are generated for each attribute level, and levels with greater importance in the decision‐making process have higher part‐worth utilities. The ACBC analysis software also generates importance scores, which are derived by calculating the Δ between the part‐worth utilities for the most important and least important levels of each attribute (21). A larger Δ in part‐worth utilities correlates with a larger importance of the attribute in the decision‐making process. More information about ACBC analysis, part‐worth utilities, and importance scores (14) can be found at the following link: http://links.lww.com/AJG/A215.
Survey design
Prior to the full launch of the survey, the entire instrument was pilot tested with five patients with axSpA to ensure understandability and usability. Once respondents accessed the survey, we first assessed their eligibility (see Participants section for details). Eligible participants then proceeded through the choice tournament exercise as described earlier. Before seeing the first set of side‐by‐side profiles, participants were shown descriptions of the medication attributes used in the survey to facilitate their understanding of each characteristic (Supplementary Figure 1). This information was also available during the choice tournament exercise when participants hovered their cursor over the attribute labels.
After the conjoint vignettes, the survey presented questions regarding sociodemographic information (eg, age, sex, insurance, etc) and axSpA characteristics, including time since axSpA symptoms started, symptom severity within the past week as measured by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (22), and current and prior axSpA treatments. These data were collected to explore potential correlations between patient factors and therapy preferences. This study was approved at all sites using the Streamlined, Multisite, Accelerated Resources for Trials Institutional Review Board (SMART IRB) Reliance platform, with the Cedars‐Sinai Institutional Review Board serving as the main site (Pro50046).
Participants
Participants aged 18 years and older with axSpA were recruited to complete the online survey between March 14, 2018, and June 21, 2019. We recruited patients with axSpA receiving care at Cedars‐Sinai Medical Center, University of Massachusetts (UMass) Memorial Medical Center, and University of California, San Francisco (UCSF) Medical Center. We also partnered with the Spondylitis Association of America (SAA), who included the survey invitation in their June 2018 eSUN newsletter to more than 25 000 recipients as well as posted the survey link on their website’s “Participate in Spondylitis Research” page. No honorarium was awarded to respondents from the above panels for completing the survey. Participants also needed to confirm that they had axSpA by answering “yes” to the question “Have you been diagnosed with AS by a physician?” Although we asked about AS diagnosis in the survey, we use the axSpA umbrella term throughout this article because we did not have access to radiographs from those recruited through the SAA or Cint (see details below) for distinguishing between AS and nonradiographic axSpA.
We also recruited patients in partnership with Cint, a US survey research firm. Because users who complete studies through Cint were provided an honorarium, the Cint version of the survey included a blinded screening question. Respondents were asked if they had been diagnosed with any of the following conditions (presented in random order) by a physician: AS, juvenile idiopathic arthritis, lupus, osteoarthritis, polymyalgia rheumatica, rheumatoid arthritis, or none of the above. Only those who stated that they were diagnosed with AS were allowed to proceed. By using a screener with six rheumatologic conditions, we hoped to maximize the likelihood that respondents had been diagnosed with axSpA and were not simply seeking compensation by participating in a survey. Of note, we performed sensitivity analyses with and without the SAA and Cint cohorts to confirm lack of systematic differences between self‐reported (ie, those from SAA and Cint) and medically confirmed axSpA (ie, patients from Cedars‐Sinai, UMass, and UCSF medical centers).
Statistical analyses
Based on conjoint analysis sample size precedents and recommendations from the software provider (23), we aimed to recruit at least 300 patients with axSpA to complete the conjoint analysis survey. Statistical analyses were performed using Stata 13.1 (StataCorp LP). A two‐tailed P value of less than 0.05 was considered significant. Descriptive analyses were used for patient sociodemographic and disease characteristics, importance scores, and patient‐level preferences report ratings.
We used multivariable logistic regression models to adjust for potentially confounding factors and to calculate odds ratios and 95% confidence intervals (CIs). The outcomes in the models were whether individuals reported the following attribute categories as the most important factor in their decision‐making: 1) medication characteristics (route of administration, dosing frequency, frequency of clinic appointments), 2) efficacy, 3) side effect profile (tolerability of unwanted side effects or reactions, risk of lymphoma, risk of serious infection), and 4) cost. The regressions included patient‐level sociodemographic variables and axSpA clinical variables as covariates.
Results
Study population
Supplementary Figure 2 displays the flow diagram of enrolled patients, stratified by recruitment source. Demographic and disease characteristics of the 397 patients with axSpA included in the final analysis are presented in Table 2.
Table 2.
Study population demographics
| Variable | All Respondents (N = 397) | Patients With Medically Confirmed axSpA a (n = 122) |
|---|---|---|
| Age, mean (SD), y | 44.4 (15.7) | 51.1 (14.3) |
| Male sex, % | 49.4 | 68.0 |
| Race/ethnicity, % | ||
| Non‐Hispanic white | 78.8 | 70.5 |
| Non‐Hispanic black, Latino, non‐Hispanic Asian, or other | 21.2 | 29.5 |
| Education, % | ||
| High school or less | 10.8 | 5.7 |
| Some college | 19.9 | 12.3 |
| College degree | 34.3 | 35.3 |
| Graduate degree | 35.0 | 46.7 |
| Married or long‐term relationship, % | 74.3 | 68.9 |
| Employed or full‐time student, % | 68.3 | 69.7 |
| Total household income, % | ||
| ≤$50 000 | 22.2 | 12.3 |
| $50 001‐$100 000 | 24.9 | 17.2 |
| $100 001‐$200 000 | 31.0 | 27.1 |
| ≥$200 001 | 14.1 | 29.5 |
| Prefer not to say | 7.8 | 13.9 |
| Has insurance, % | 97.5 | 100.0 |
| Physical activity vs. others, % | ||
| Much less active | 14.1 | 3.3 |
| Less active | 19.9 | 14.8 |
| Similar | 27.0 | 30.3 |
| More active | 23.9 | 32.0 |
| Much more active | 15.1 | 19.7 |
| Duration of axSpA symptoms, mean (SD), y | 16.4 (14.6) | 26.6 (14.5) |
| BASDAI score, b mean (SD) | 4.7 (2.3) | 3.4 (2.3) |
| Nonbiologic medication use, % | ||
| Nonselective NSAID | 47.6 | 36.9 |
| COX‐2 inhibitor (celecoxib) | 19.1 | 9.0 |
| Methotrexate | 18.6 | 5.7 |
| Leflunomide | 12.1 | 0.8 |
| Sulfasalazine | 14.6 | 4.9 |
| Glucocorticoids | 18.6 | 5.7 |
| Other | 12.9 | 11.5 |
| Biologic medication exposure, % | ||
| Biologic naïve | 30.0 | 21.3 |
| Prior use of biologics | 11.1 | 10.7 |
| Currently using biologics | 58.9 | 68.0 |
| Recruitment source, % | ||
| Cedars‐Sinai Medical Center | 15.1 | 49.2 |
| UMass Memorial Medical Center | 3.0 | 9.8 |
| UCSF Medical Center | 12.6 | 41.0 |
| Spondylitis Association of America | 27.5 | … |
| Cint (survey research firm) | 41.8 | … |
Abbreviation: axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; NSAID, nonsteroidal anti‐inflammatory drug; UCSF, University of California, San Francisco; UMass, University of Massachusetts.
Patients receiving care at Cedars‐Sinai Medical Center, UMass Memorial Medical Center, or UCSF Medical Center.
Higher BASDAI score corresponds to more severe symptoms.
Overall rank‐ordering of medication attribute importance
The average importance scores calculated and ranked by the ACBC analysis algorithm are shown in Figure 2. On average, patients prioritized medication efficacy, cost, and route of administration as the three most important factors when selecting from among the various options. These were followed by risk of lymphoma, dosing frequency, risk of serious infection, tolerability of side effects, and clinic visit and laboratory test frequency.
Figure 2.

Average attribute importance scores for patients with axial spondyloarthritis (N = 397). The mean importance of each medication attribute is based on part‐worth utilities. Chance of symptom improvement, cost, and route of administration were the most important factors and accounted for 26.8%, 26.3%, and 13.9% of decision‐making, respectively.
When grouping the eight attributes into four overarching categories, 177 (44.6%) respondents reported efficacy to be the most important factor when choosing from among the options. One hundred fifty‐two (38.3%) and 56 (14.1%) participants reported cost and medication characteristics (route of administration, dosing frequency, clinic visit and laboratory test frequency), respectively, as the predominant factor. Conversely, only 12 (3.0%) prioritized side effect profile (tolerability of unwanted side effects or reactions, risk of lymphoma, risk of serious infection) in their decision‐making.
Route of administration: oral versus subcutaneous versus intravenous
In the part‐worth utilities assessment, we found that 198 (49.9%) participants preferred an oral axSpA medication. The remaining respondents desired a parenterally delivered medication. One hundred twenty‐eight (32.2%) participants preferred subcutaneous administration, whereas 71 (17.9%) participants selected an intravenous‐infused medication.
Uniqueness of individual preferences report
The conjoint software rank‐ordered the relative importance scores of all eight medication attributes for each respondent, which, taken together, represents an individual preferences report. For example, a participant may have the following medication attribute rank order: 1) cost, 2) efficacy, 3) route of administration…8) risk of serious infection. When comparing the reports among participants, we found a high level of uniqueness. Figure 3 shows the proportion of unique decision‐making profiles stratified by the number of included attributes. When evaluating the rank order of all eight attributes, nearly three‐quarters of respondents had a ranking that did not match that of anyone else. Even when limiting the analysis to only four attributes, approximately one‐third of participants still had a unique report.
Figure 3.
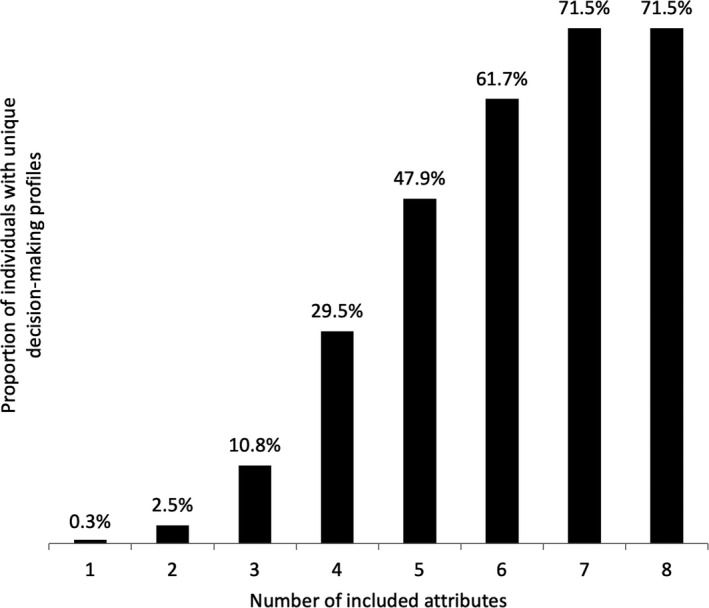
Proportion of unique decision‐making profiles stratified by number of included attributes (N = 397). For each respondent, the conjoint software rank‐ordered the importance of the eight medication attributes as he or she selected among the various options. When considering individuals’ top three attributes, only 10.8% of respondents had a unique decision‐making profile (ie, rank‐ordering of top three attributes did not match anyone else’s). However, when including all eight medication attributes, 71.5% had unique profiles.
Predictors of therapy decision‐making
Results from the multivariable regressions assessing independent predictors of patient preferences are listed in Table 3. Overall, sociodemographic and clinical factors largely did not predict treatment preferences, save for a few exceptions. Individuals from high‐income households (ie, greater than or equal to $200 001 per year) had significantly higher odds of noting medication efficacy as the most important factor when selecting from among the options and were less likely to prioritize cost or medication characteristics. Increasing duration of axSpA symptoms was associated with increased odds for valuing medication efficacy. We also found that increasing levels of physical activity was inversely associated with the odds for prioritizing medication cost. Moreover, those who reported being more or similarly active as compared with others had higher odds for prioritizing medication efficacy when compared with those who were much less active. The remaining variables largely were not predictive of decision‐making.
Table 3.
ORs for reporting medication efficacy, cost, or characteristics as the most important factor in the decision‐making process (N = 397) a
| Variable | Most Important Medication Attribute | |||||
|---|---|---|---|---|---|---|
| Efficacy | Cost | Medication Characteristics b | ||||
| n (%) c | OR (95% CI) d | n (%) c | OR (95% CI) d | n (%) c | OR (95% CI) d | |
| Age, y | … | 0.99 (0.97‐1.01) | … | 1.02 (0.99‐1.04) | … | 0.99 (0.96‐1.02) |
| Sex | ||||||
| Male | 90 (45.9) | Reference | 71 (36.2) | Reference | 32 (16.3) | Reference |
| Female | 87 (43.3) | 1.11 (0.70‐1.75) | 81 (40.3) | 0.99 (0.62‐1.56) | 24 (11.9) | 0.66 (0.35‐1.25) |
| Race/ethnicity | ||||||
| Non‐Hispanic white | 136 (43.5) | Reference | 130 (41.5) | Reference | 37 (11.8) | Reference |
| Non‐Hispanic black, Latino, non‐Hispanic Asian, or other | 41 (48.8) | 1.12 (0.63‐1.97) | 22 (26.2) | 0.58 (0.32‐1.06) | 19 (22.6) | 1.96 (0.96‐4.00) |
| Education | ||||||
| High school or less | 16 (37.2) | Reference | 17 (39.5) | Reference | 9 (20.9) | Reference |
| Some college | 34 (43.0) | 1.13 (0.49‐2.56) | 32 (40.5) | 1.05 (0.46‐2.39) | 11 (13.9) | 0.75 (0.26‐2.16) |
| College degree | 53 (39.0) | 0.78 (0.35‐1.73) | 63 (46.3) | 1.66 (0.75‐3.66) | 16 (11.8) | 0.59 (0.21‐1.61) |
| Graduate degree | 74 (53.2) | 1.11 (0.49‐2.52) | 40 (28.8) | 0.90 (0.39‐2.08) | 20 (14.4) | 0.84 (0.29‐2.37) |
| Relationship status | ||||||
| Married or long‐term relationship | 130 (44.1) | Reference | 113 (38.3) | Reference | 44 (14.9) | Reference |
| Not married | 47 (46.1) | 1.26 (0.73‐2.20) | 39 (38.2) | 0.85 (0.48‐1.51) | 12 (11.8) | 0.81 (0.37‐1.80) |
| Employment status | ||||||
| Unemployed | 56 (44.4) | Reference | 48 (38.1) | Reference | 18 (14.3) | Reference |
| Employed or student | 121 (44.7) | 0.83 (0.47‐1.47) | 104 (38.4) | 1.56 (0.86‐2.82) | 38 (14.0) | 0.59 (0.26‐1.32) |
| Total household income | ||||||
| ≤$50 000 | 33 (37.5) | Reference | 41 (46.6) | reference | 13 (14.8) | Reference |
| $50 001‐$100 000 | 37 (37.4) | 0.95 (0.48‐1.88) | 45 (45.5) | 1.03 (0.52‐2.02) | 13 (13.1) | 0.73 (0.28‐1.89) |
| $100 001‐$200 000 | 43 (35.0) | 0.97 (0.47‐1.99) | 51 (41.5) | 0.75 (0.36‐1.55) | 25 (20.3) | 1.28 (0.49‐3.33) |
| ≥$200 001 | 44 (78.6) | 6.38 (2.51‐16.22) | 10 (17.9) | 0.27 (0.10‐0.71) | 2 (3.6) | 0.14 (0.03‐0.76) |
| Prefer not to say | 20 (64.5) | 2.72 (1.08‐6.87) | 5 (16.1) | 0.26 (0.09‐0.80) | 3 (9.7) | 0.45 (0.11‐1.94) |
| Physical activity vs. others | ||||||
| Much less active | 15 (26.8) | Reference | 34 (60.7) | Reference | 4 (7.1) | Reference |
| Less active | 33 (41.8) | 2.15 (0.95‐4.88) | 30 (38.0) | 0.39 (0.18‐0.84) | 11 (13.9) | 1.81 (0.51‐6.49) |
| Similar | 53 (49.5) | 2.70 (1.22‐5.96) | 38 (35.5) | 0.38 (0.18‐0.80) | 15 (14.0) | 2.11 (0.61‐7.25) |
| More active | 50 (52.6) | 2.39 (1.04‐5.52) | 30 (31.6) | 0.40 (0.18‐0.89) | 14 (14.7) | 2.45 (0.68‐8.78) |
| Much more active | 26 (43.3) | 1.47 (0.59‐3.62) | 20 (33.3) | 0.51 (0.22‐1.21) | 12 (20.0) | 3.37 (0.89‐12.79) |
| Duration of axSpA symptoms, y | … | 1.030 (1.009‐1.052) | … | 0.978 (0.957‐0.999) | … | 0.988 (0.958‐1.018) |
| BASDAI score | … | 0.99 (0.88‐1.10) | … | 1.07 (0.95‐1.20) | … | 0.94 (0.81‐1.10) |
| Biologic medication use | ||||||
| Biologic naïve | 46 (38.7) | Reference | 51 (42.9) | Reference | 17 (14.3) | Reference |
| Prior use of biologics | 17 (38.7) | 1.04 (0.48‐2.27) | 22 (50.0) | 1.34 (0.62‐2.89) | 5 (11.4) | 0.73 (0.23‐2.32) |
| Currently using biologics | 114 (48.7) | 1.49 (0.87‐2.56) | 79 (33.8) | 0.71 (0.41‐1.23) | 34 (14.5) | 0.94 (0.44‐1.97) |
Abbreviation: axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CI, confidence interval; OR, odds ratio.
We did not conduct a regression on reporting of the side effect profile as the most important factor in the decision‐making process because only 12 individuals prioritized it in the study.
Includes route of administration, dosing frequency, and clinic visit and laboratory test frequency.
Represents the number of persons prioritizing the respective factor as the most important in their decision‐making process.
The multivariable logistic regression model included all covariates in the table. Insurance status was not included in the model because 97.5% of respondents had health insurance.
Subgroup analyses
We performed a subgroup analysis among the 122 patients with axSpA receiving care at Cedars‐Sinai, UMass, or UCSF (Table 2). When compared against the primary analyses, the overall rank‐ordering of medication attribute importance was similar: symptom improvement, 32.3%; cost, 23.8%; route of administration, 14.9%; risk of lymphoma, 9.8%; risk of serious infection, 5.5%; dosing frequency, 5.3%; tolerability of unwanted side effects, 4.5%; clinic visit and laboratory test frequency, 3.9%. We also continued to see a high level of uniqueness with respect to respondents’ preferences report; when considering their rankings of the eight attributes, we found that 90 (73.8%) participants had a report that did not match anyone else’s.
When grouping the eight attributes into four overarching categories, we found that participants prioritized the following as the most important factor in their decision‐making: efficacy (76 [62.3%]), cost (28 [23.0%]), medication characteristics (16 [13.1%]), and side effect profile (2 [1.6%]). Similar to the primary regression analyses, patient sociodemographic and axSpA characteristics largely were not predictive of decision‐making (Supplementary Table 1). The one exception was income; those from households making greater than or equal to $200 001 per year had 8.34 (95% CI 1.24‐56.11) times the odds of prioritizing medication efficacy compared with those making less than or equal to $50 000 per year.
Discussion
Using conjoint analysis, we found that on average, patients with axSpA prioritize medication efficacy, cost, and route of administration when selecting from among the various options. However, we also found that treatment decision‐making is highly individualized; patient sociodemographic and axSpA clinical characteristics poorly predict medication preferences.
Our analysis is one of a small number of studies that have analyzed how patients with axSpA make decisions regarding potential therapies. Nolla et al (10) conducted a conjoint analysis assessing attribute preferences of biologics used in the treatment of rheumatologic diseases in Spain. Among patients with axSpA, the authors found that patients prioritize pain relief, followed by risk of adverse events (high vs. low risk), administration method, and, lastly, duration of effects. Conversely, our study—which also included costs and specific side effects and modeled both biologic and nonbiologic options—found that patients value symptom improvement the most, followed by cost, route of administration, and risk of lymphoma.
To our knowledge, our study is the first to assess the impact of medication out‐of‐pocket costs on decision‐making in axSpA, which ranks second highest in importance among patients. Not surprisingly, household income influences how cost is factored into decision‐making. Individuals reporting a household income of less than or equal to $50 000 are more likely to prioritize cost compared with those from households making more than $200 000. Of note, prior studies in rheumatoid arthritis have assessed the role of medication costs in decision‐making (24, 25). Augustovski et al (24) found that cost is the most important consideration in choosing a rheumatoid arthritis medication among Argentinians, whereas Louder et al (25) noted that cost is the fourth most important of seven attributes in commercially insured Americans. Additional research examining how medication costs impact treatment decision‐making, adherence, and outcomes in axSpA is warranted.
Our finding of the highly individualized nature of selecting an axSpA treatment is consistent with our prior study examining biologic decision‐making among patients with IBD (14). However, when responses between the cohorts are considered, some differences are noted. Although the proportions of individuals with axSpA (45%) and IBD (41%) who reported efficacy as the most important factor in the decision‐making process were similar, a higher percentage of patients with IBD (38%) prioritized avoidance of side effects compared with those with axSpA (3%) (14). This suggests that patients with axSpA may approach treatment decision‐making differently from those with IBD. Of note, the conjoint analysis survey administered to those with IBD did not include cost, which was highly valued among individuals with axSpA. It is possible that the proportion of patients with IBD who prioritized avoidance of side effects would have decreased had cost been included as an attribute in the survey. Regardless, this calls for the need for further research to better understand how those with axSpA consider medication side effects when choosing a therapy.
In our analyses, when attempting to identify predictors of individual choices, we found that sociodemographic and axSpA clinical factors were rarely helpful in predicting medication preferences, except in a few cases. For example, we found that those with total household incomes greater than $200 000 and patients with a longer duration of axSpA symptoms were more likely to prioritize efficacy when choosing from among the different treatment options. We also discovered that individuals who reported higher levels of physical activity as compared with others were less likely to note out‐of‐pocket cost as the most important factor. These patients may be willing to spend more money on axSpA therapies to maintain their active lifestyles. Interestingly, axSpA symptom severity, as measured by the BASDAI, is not associated with decision‐making; worse symptoms do not predict prioritization of a medication’s efficacy when choosing an axSpA therapy. Moreover, when considering each respondent’s preferences report, we found that nearly three‐quarters had a completely unique decision‐making profile. These results emphasize that providers cannot rely on demographic or clinical variables to accurately predict which therapy will align with a patient’s personal values.
Because of the highly individualized nature of decision‐making in axSpA, along with health care’s increased emphasis on shared decision‐making between patients and providers (11, 26, 27), it is important for clinicians to identify what matters most to patients when choosing from among therapeutic options. Yet, in the context of a brief clinical visit, it can be challenging for rheumatologists to determine a patient’s unique preferences profile while also engaging in detailed discussions around each treatment’s risks, benefits, and trade‐offs. Thus, there is a need for simple and efficient decision tools that elicit individual preferences and support the patient‐provider interaction. In IBD, we developed an online decision aid called IBD&me (www.ibdandme.org) that uses conjoint analysis to assess a patient’s priorities when selecting a biologic medicine and then generates a personalized report that displays the rank order of attributes that matter most to an individual (14). The patient can share the report with his or her physician, who can review the information to quickly understand the patient’s preferences.
Our study has limitations. First, we only recruited subjects from the United States; our findings may not be generalizable to patients with axSpA in other parts of the world. As many international rheumatology societies stress the importance of shared decision‐making between patients and providers when choosing a treatment (3, 7, 28), additional studies in other countries examining axSpA therapy decision‐making are warranted. Second, our study included patients with self‐reported axSpA. However, in sensitivity analyses that only included data from those confirmed to have the condition at academic medical centers, the findings were largely similar to the primary analyses. Third, we limited the study to eight attributes. Patients may have other considerations when selecting an axSpA therapy (eg, length of time since drug approval, mechanism of action, risk of hepatitis B reactivation, etc) that were not included in the survey. However, this was by design because ACBC analysis surveys can become unwieldy with too many attributes, and we decided to focus on eight core attributes that were deliberately chosen based on prior literature (15, 16, 17, 18, 19, 29, 30) and input from content experts to capture the most important considerations patients weigh when selecting a therapy. Fourth, our conjoint analysis included medication profiles modeled after oral (ie, NSAIDs) as well as parenteral (ie, biologics) therapies. It is possible that patients’ decision‐making would have differed had the survey only included biologic options; this is worthy of further study, particularly among patients with axSpA who remain symptomatic despite NSAIDs and are considering biologics. Fifth, despite the interactive design of the survey and limitation to 20 conjoint vignettes, patients may have found the serial decision‐making challenging. Similarly, patients with lower numeracy skills may have experienced difficulty interpreting the risks provided. It can be argued, however, that this exercise is at least comparable to clinic‐based decision‐making for determining patient preferences because patients may complete the survey at their own pace while carefully considering trade‐offs.
In conclusion, we found that patients with axSpA highly value medication efficacy, cost, and route of administration when selecting from among the various therapeutic options. We also discovered that the decision‐making process is highly individualized because sociodemographic and clinical characteristics poorly predict preferences. With the increasing number of therapeutic options becoming available for patients, these results underscore the need for development of treatment decision tools to enhance communication and shared decision‐making between patients and providers. This will optimize selection of therapies that match patients’ unique preferences and may ultimately improve medication adherence and outcomes.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Almario had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Joo, Almario, Ishimori, Park, Noah, Weisman, Spiegel.
Acquisition of data
Joo, Almario, Ishimori, Jusufagic, Noah, Gensler, Venuturupalli, Kay, Spiegel.
Analysis and interpretation of data
Joo, Almario, Ishimori, Park, Jusufagic, Gensler, Venuturupalli, Kay, Weisman, Spiegel.
Role of the Study Sponsor
Novartis Pharmaceuticals Corporation had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Novartis Pharmaceuticals Corporation.
Supporting information
Fig S1
Fig S2
Table S1
Acknowledgments
We would like to thank and acknowledge Elin Aslanyan at the SAA for her support in our recruitment efforts.
Supported by Novartis Pharmaceuticals Corporation. The Cedars‐Sinai Center for Outcomes Research and Education is supported by The Marc and Sheri Rapaport Fund for Digital Health Sciences and Precision Health. Dr. Almario’s work was supported by a career development award from the American College of Gastroenterology. Drs. Almario, Ishimori, and Spiegel’s work was supported by a Clinical and Translational Science Institute grant from the National Center for Advancing Translational Sciences at the NIH (grant UL1‐TR‐00188101).
Drs. Joo and Almario contributed equally to this work.
Dr. Gensler has received research support to her institution from AbbVie, Amgen, Novartis, and UCB and consulting fees from Janssen (less than $10,000), Lilly (less than $10,000), Novartis (more than $10,000), and UCB (more than $10,000). Dr. Kay has received research support paid to his institution from Pfizer and UCB and consulting fees from AbbVie (less than $10,000), Alvotech Suisse AG (more than $10,000), Boehringer Ingelheim GmbH (less than $10,000), Celltrion Healthcare Co. Ltd. (less than $10,000), Merck & Co., Inc. (more than $10,000), Mylan Inc. (less than $10,000), Novartis AG (more than $10,000), Samsung Bioepis (less than $10,000), Sandoz (more than $10,000), and UCB (more than $10,000). Dr. Weisman has received consulting fees from ACEA Biosciences (less than $10,000), GlaxoSmithKline (less than $10,000), Novartis (more than $10,000), Setpoint (less than $10,000), Takeda Pharmaceuticals (more than $10,000), and UCB (less than $10,000).
References
- 1. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 2016;374:2563–74. [DOI] [PubMed] [Google Scholar]
- 2. Sieper J, Braun J, Rudwaleit M, Boonen A, Zink A. Ankylosing spondylitis: an overview. Ann Rheum Dis 2002;61 Suppl 3:iii8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2016;68:282–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen J, Lin S, Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev 2014:CD004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Veras MM, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev 2013:CD004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Denderen JC, van der Paardt M, Nurmohamed MT, de Ryck YM, Dijkmans BA, van der Horst‐Bruinsma IE. Double blind, randomised, placebo controlled study of leflunomide in the treatment of active ankylosing spondylitis. Ann Rheum Dis 2005;64:1761–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van der Heijde D, Ramiro S, Landewe R, Baraliakos X, van den Bosch F, Sepriano A, et al. 2016 update of the ASAS‐EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 8. Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health: a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403–13. [DOI] [PubMed] [Google Scholar]
- 9. Kievit W, van Hulst L, van Riel P, Fraenkel L. Factors that influence rheumatologists' decisions to escalate care in rheumatoid arthritis: results from a choice‐based conjoint analysis. Arthritis Care Res (Hoboken) 2010;62:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nolla JM, Rodriguez M, Martin‐Mola E, Raya E, Ibero I, Nocea G, et al. Patients' and rheumatologists' preferences for the attributes of biological agents used in the treatment of rheumatic diseases in Spain. Patient Prefer Adherence 2016;10:1101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neogi T, Aletaha D, Silman AJ, Naden RL, Felson DT, Aggarwal R, et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: phase 2 methodological report. Arthritis Rheum 2010;62:2582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bederman SS, Mahomed NN, Kreder HJ, McIsaac WJ, Coyte PC, Wright JG. In the eye of the beholder: preferences of patients, family physicians, and surgeons for lumbar spinal surgery. Spine (Phila Pa 1976) 2010;35:108–115. [DOI] [PubMed] [Google Scholar]
- 13. Porzsolt F, Clouth J, Deutschmann M, Hippler HJ. Preferences of diabetes patients and physicians: a feasibility study to identify the key indicators for appraisal of health care values. Health Qual Life Outcomes 2010;8:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almario CV, Keller MS, Chen M, Lasch K, Ursos L, Shklovskaya J, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol 2018;113:58–71. [DOI] [PubMed] [Google Scholar]
- 15. Bolge SC, Eldridge HM, Lofland JH, Ravin C, Hart PJ, Ingham MP. Patient experience with intravenous biologic therapies for ankylosing spondylitis, Crohn's disease, psoriatic arthritis, psoriasis, rheumatoid arthritis, and ulcerative colitis. Patient Prefer Adherence 2017;11:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cinar FI, Cinar M, Yilmaz S, Simsek I, Erdem H, Pay S. Thoughts and perceptions of ankylosing spondylitis patients with regard to TNF inhibitors. Rheumatol Int 2014;34:979–86. [DOI] [PubMed] [Google Scholar]
- 17. Fajri DW, Brand CA, Dharmage SC, Martin BJ, Buchanan RR, Schachna L. What factors determine patients' preference for tumour necrosis factor inhibitors in ankylosing spondylitis? [Brief Report]. Clin Rheumatol 2009;28:599–602. [DOI] [PubMed] [Google Scholar]
- 18. Scalone L, Sarzi‐Puttini P, Sinigaglia L, Montecucco C, Giacomelli R, Lapadula G, et al. Patients', physicians', nurses', and pharmacists' preferences on the characteristics of biologic agents used in the treatment of rheumatic diseases. Patient Prefer Adherence 2018;12:2153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dzubur E, Khalil C, Almario CV, Noah B, Minhas D, Ishimori M, et al. Patients' concerns and perceptions regarding biologic therapies in ankylosing spondylitis: insights from a large‐scale survey of social media platforms. Arthritis Care Res (Hoboken) 2019;71:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cunningham CE, Deal K, Chen Y. Adaptive choice‐based conjoint analysis: a new patient‐centered approach to the assessment of health service preferences. Patient 2010;3:257–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lichtenstein GR, Waters HC, Kelly J, McDonald SS, Zanutto EL, Hendricks D, et al. Assessing drug treatment preferences of patients with Crohn’s disease: a conjoint analysis. Patient 2010;3:113–23. [Google Scholar]
- 22. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 23. Orme BK. Getting started with conjoint analysis: strategies for product design and pricing research. 2nd ed Madison (WI): Research Publishers; 2010. [Google Scholar]
- 24. Augustovski F, Beratarrechea A, Irazola V, Rubinstein F, Tesolin P, Gonzalez J, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete‐choice experiment. Value Health 2013;16:385–93. [DOI] [PubMed] [Google Scholar]
- 25. Louder AM, Singh A, Saverno K, Cappelleri JC, Aten AJ, Koenig AS, et al. Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Health Drug Benefits 2016;9:84–93. [PMC free article] [PubMed] [Google Scholar]
- 26. Oshima Lee E, Emanuel EJ. Shared decision making to improve care and reduce costs. N Engl J Med 2013;368:6–8. [DOI] [PubMed] [Google Scholar]
- 27. Siegel CA. Shared decision making in inflammatory bowel disease: helping patients understand the tradeoffs between treatment options. Gut 2012;61:459–65. [DOI] [PubMed] [Google Scholar]
- 28. Wei JC, Liu CH, Tseng JC, Hsieh LF, Chen CH, Chen HH, et al. Taiwan Rheumatology Association consensus recommendations for the management of axial spondyloarthritis. Int J Rheum Dis 2020;23:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stockdale J, Goodacre L. 'It's magic stuff': the experiences of patients with ankylosing spondylitis taking anti‐TNF‐α medication. Musculoskeletal Care 2009;7:162–77. [DOI] [PubMed] [Google Scholar]
- 30. Madsen M, Jensen KV, Esbensen BA. Men's experiences of living with ankylosing spondylitis: a qualitative study. Musculoskeletal Care 2015;13:31–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1


