Abstract
Introduction
Coronavirus disease 2019 (COVID-19) represents a serious threat to patients on maintenance dialysis. The clinical setting, mortality rate, and prognostic factors in these patients have not been well established.
Methods
We included all dialyzed patients with COVID-19 referred to our dialysis center between March 11 and April 11, 2020. Data were obtained through the review of the medical records and were censored at the time of data cutoff, on May 11, 2020.
Results
Forty-four patients on maintenance dialysis with COVID-19 were referred to our dialysis unit during the COVID-19 epidemic. Median age was 61 years (interquartile range [IQR]: 51.5–72.5); 65.9% were men. Comorbidities included hypertension (97.7%), diabetes mellitus (50%), and chronic cardiac (38.6%) and respiratory (27.3%) diseases. Initial symptoms were fever (79.5%), shortness of breath (29.5%), cough (43.2%), and diarrhea (13.6%). Three profiles of severity were distinguished based on the World Health Organization (WHO) progression scale. Forty-one (93.2%) were hospitalized and only 3 were maintained on outpatient hemodialysis. Thirty-three (75%) patients required oxygen therapy, including 15 (45.5%) who were referred to the intensive care unit. Overall, 27.3% of patients died, and 58.5% were discharged from hospital, including only 2 (13.3%) of those admitted to the intensive care unit. By multivariate analysis, cough, thrombopenia <120 g/l, lactate dehydrogenase (LDH) level greater than 2 times the upper limit of normal, and blood C-reactive protein (CRP) >175 mg/l were significantly associated with death.
Conclusion
A major outbreak of COVID-19 occurred in the Paris region, and spread among dialyzed patients. Our study underscores the severity of COVID-19 in these patients and identified prognostic markers.
Keywords: coronavirus, COVID-19, hemodialysis, kidney, peritoneal dialysis
See Commentary on Page 1381
In December 2019, a novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan, Hubei Province, China, and rapidly spread worldwide.1,2 COVID-19 is transmitted via droplets during the incubation period and throughout the course of illness.3 Up to 20% of infected patients develop moderate-to-severe forms and require hospitalization, and 5% to 10% are admitted to intensive care unit (ICU) for ventilation support.4, 5, 6, 7, 8 Elderly individuals, patients with comorbidities, and pregnant women are particularly at risk for severe forms. COVID-19 thus represents a special threat to patients on maintenance dialysis, who frequently display multiple comorbidities, and are particularly vulnerable to infection.9 Unlike other individuals who can observe a strict lockdown, they still need to come to the dialysis center thrice-weekly.10 Strategies have been established to mitigate the risk of infection among patients and staff.10 Unfortunately, a major outbreak of COVID-19 recently occurred in the Paris region, and rapidly spread among patients on dialysis. So far, the clinical setting and disease course of COVID-19 in dialyzed patients has not been well established. Our study aimed at describing the clinical presentation, management, and outcome of patients referred to our dialysis center between March 11 and April 11, 2020.
Patients and Methods
Study Population
We included all consecutive patients with COVID-19 on maintenance dialysis (hemodialysis or peritoneal dialysis) who were referred to the in-patient dialysis center of Bicêtre University Hospital (Assistance Publique-Hôpitaux de Paris, Le Kremlin-Bicêtre, Paris region, France) between March 11 and April 11, 2020. At this time, the national and local policies were to screen only symptomatic patients for SARS-CoV-2 infection. A case of COVID-19 was defined by a positive result on a reverse-transcriptase polymerase chain reaction (RT-PCR) assay based on the WHO standard and targeting the SARS-CoV-2 E gene and RdRp gene of a specimen collected on a nasopharyngeal swab. In case of negative RT-PCR, a second RT-PCR could be performed. Alternatively, the diagnosis also could be established on the results of a chest computed tomography scan, showing ground-glass opacities with or without consolidative abnormalities highly suggestive of COVID-19 infection. The study was approved by the local institutional review board.
Data Collection
Patients’ data were obtained through the retrospective review of the medical electronic records. Demographic data and comorbidities included age, sex, hypertension, diabetes mellitus, body mass index, cause of chronic kidney disease, duration on dialysis before admission, cardiovascular diseases, and other notable past medical history. The use of angiotensin receptor blockers or angiotensin-converting enzyme inhibitors was also recorded. Date and nature of presenting signs, and oxygen saturation and oxygen level to reach an oxygen saturation ≥ 94% were collected. The WHO progression scale (Supplementary Table S1) was used to establish 3 groups of severity on admission and during follow-up, including (i) ambulatory or hospitalized patients who did not require oxygen therapy (WHO score = 2–4), (ii) hospitalized patients who needed oxygen therapy up to 6 l/min by mask or nasal prongs (WHO score = 5), and (iii) hospitalized patients who required higher amounts of oxygen therapy by noninvasive ventilation or intubation and mechanical ventilation (WHO score ≥6). The maximum level of oxygen before ICU transfer, use of noninvasive and mechanical ventilation, severe events during hospitalization, including acute respiratory distress syndrome, thrombosis, bleeding, cardiac arrhythmia, and death were also recorded. Dates of admission, transfer to the ICU, mechanical ventilation, and discharge from ICU and from hospital were collected. All laboratory tests and radiologic assessments were performed at the discretion of the physician. Laboratory data included the result of the SARS-CoV-2 RT-PCR, and the values of biological parameters on admission and during hospitalization (extreme values): serum levels of electrolytes, albumin, LDH, ferritin, CRP, procalcitonin, D-Dimers, fibrinogen, and hemoglobin, lymphocyte, neutrophil, and platelet counts. The administration of antibiotics and other notable drugs, such as anti–interleukin-6 antibodies or hydroxychloroquine, was also recorded. Patient data were censored at the time of data cutoff, which occurred on May 11, 2020.
Statistical Analysis
Descriptive statistics were used to summarize the data. Results are reported as medians with IQRs for continuous variables and counts and percentages for categorical variables. No imputation was made for missing data. Analysis was performed with STATA 11.2 software (StataCorp, College Station, TX). Univariate analyses were performed using the Kruskal-Wallis test, and the χ2 or Fisher exact test, as appropriate. A P value < 0.05 was considered as significant. Kaplan-Meier curves were used to illustrate the survival rate according to relevant covariates. Survival analysis was performed using Cox regression method. Covariates with a P value < 0.10 in the univariate Cox analysis were included in the multivariate Cox proportional hazard ratio model. A backward method was applied to identify covariates independently associated with survival, with or without adjusting for age, known to be a major risk factor for COVID-related death. Statistical significance was assumed at P < 0.05.
Results
Diagnosis of COVID-19 in Patients on Dialysis
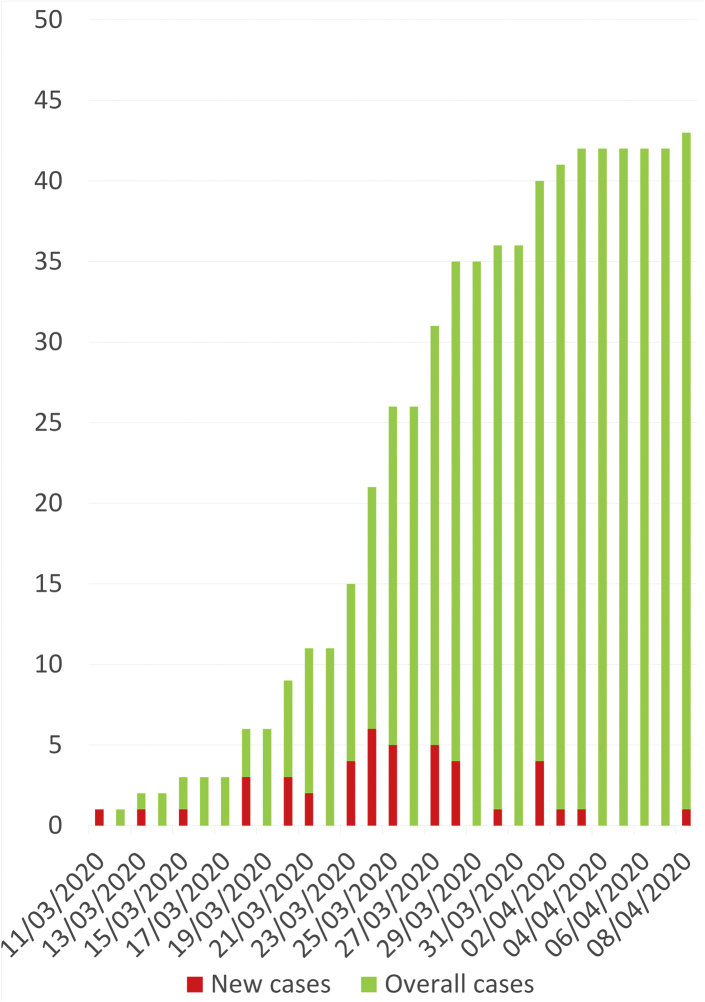
During the study period, 44 patients with COVID-19 infection, including 29 men and 15 women, were admitted to our in-patient dialysis center (Figure 1). Only symptomatic patients were screened for SARS-CoV-2 infection before being referred to our unit irrespectively of the initial requirement of oxygen therapy. RT-PCR assay of a nasopharyngeal swab was positive in all but 1 patient, who had highly suspicious chest computed tomography scan findings. Patients were referred from 11 other dialysis facilities (Supplementary Figure S1). No official policy was established for referring dialyzed patients with COVID-19 to hospital. Patients were most often hospitalized because of clinical severity, coexisting comorbidities, and other medical issues. Overall, only 3 patients with mild symptoms, no oxygen requirement, and no inflammatory syndrome were not admitted to hospital but maintained in our dialysis unit as outpatients.
Figure 1.
Cumulative incidence of COVID-19 dialyzed patients referred to the in-hospital dialysis unit of Bicêtre University Hospital (APHP).
Clinical Characteristics and Radiologic Findings
Median age at diagnosis was 61 years (IQR: 51.5–72.5) with 65.9% men. The demographic and clinical characteristics of the patients, and the underlying causes of chronic kidney disease are listed in Table 1. Median duration of maintenance dialysis before COVID-19 was 36.5 months (IQR: 12–75). Main coexisting conditions were hypertension (97.7%), dyslipidemia (59.1%), diabetes mellitus (50%), smoking (34.1%), and obesity (34.1%). Almost 40% and 30% of patients had a history of chronic cardiac or respiratory disease, respectively. Thirty-eight patients (86.4%) had more than 1 coexisting medical condition. The most common symptoms on admission are detailed in Table 2 and included fever and chills (79.5%), cough (43.2%), shortness of breath (29.5%), and diarrhea (13.6%). Most patients (59.1%) presented with anorexia and weigh loss.
Table 1.
Baseline characteristics of dialyzed patients with COVID-19
| Characteristics | All patients (n = 44) |
WHO score (2–4) |
WHO score = 5 |
WHO score ≥ 6 |
P |
|---|---|---|---|---|---|
| No oxygen (n = 11, 25%) |
O2 ≤ 6 l/min (n = 14, 31.8%) |
O2 ≥ 9 l/min (n = 19, 43.2%) |
|||
| Demographic data | |||||
| Men | 29 (65.9) | 4 (36.4) | 11 (78.6) | 14 (73.7) | 0.06 |
| Age, yr | 61 (51.5–72.5) | 52 (39–69) | 65.5 (58–76) | 64 (52–73) | ns |
| >65 yr | 19 (43.2) | 3 (27.3) | 7 (50) | 9 (47.4) | ns |
| BMI, kg/m2 | 26.3 (23.45–31.1) | 24.1 (19.8–31.4) | 28.45 (24–31.5) | 26.5 (23.5–30.9) | ns |
| Duration of dialysis, mo | 36.5 (12–75) | 43 (9–58) | 50 (10–75) | 36 (14–84) | ns |
| Underlying CKD | |||||
| Diabetic nephropathy | 9 (20.5) | 2 (18.2) | 2 (14.3) | 5 (26.3) | ns |
| Vascular nephropathy | 9 (20.5) | 2 (18.2) | 4 (28.6) | 3 (15.8) | ns |
| Mixt nephropathya | 7 (15.9) | 1 (9.1) | 2 (14.3) | 4 (21.1) | ns |
| Glomerular disease | 6 (13.6) | 0 (0.0) | 2 (14.3) | 4 (21.1) | ns |
| Polycystic kidney disease | 4 (9.1) | 2 (18.2) | 2 (14.3) | 0 (0.0) | ns |
| Undetermined | 5 (11.4) | 3 (27.3) | 1 (7.1) | 1 (5.3) | ns |
| Other | 4 (9.1) | 1 (9.1) | 1 (7.1) | 2 (10.5) | ns |
| Coexisting comorbid conditions | |||||
| Obesity, BMI >30 kg/m2 | 15 (34.1) | 3 (27.3) | 6 (42.9) | 6 (31.6) | ns |
| Hypertension | 43 (97.7) | 11 (100.0) | 14 (100.0) | 18 (94.7) | ns |
| ACEI | 13 (29.5) | 3 (27.3) | 6 (42.9) | 4 (21.1) | ns |
| ARB | 8 (18.2) | 1 (9.1) | 3 (21.4) | 4 (21.1) | ns |
| Diabetes mellitus | 22 (50) | 5 (45.5) | 4 (28.6) | 13 (68.4) | 0.07 |
| Dyslipidemia | 26 (59.1) | 5 (45.5) | 9 (64.3) | 12 (63.2) | ns |
| Smoking | 15 (34.1) | 10 (90.9) | 9 (64.3) | 10 (52.6) | ns |
| Chronic respiratory disease | 12 (27.3) | 3 (27.3) | 4 (28.6) | 5 (26.3) | ns |
| Chronic cardiac disease | 17 (38.6) | 3 (27.3) | 7 (50.0) | 7 (36.8) | ns |
| Cancer | 8 (18.2) | 1 (9.1) | 4 (28.6) | 3 (15.8) | ns |
| ≥2 comorbidities | 38 (86.4) | 8 (72.7) | 12 (85.7) | 18 (94.7) | ns |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; ns, nonsignificant P value; WHO, World Health Organization.
Data are n (%) or median (interquartile range).
Mixt refers to diabetic and vascular nephropathy.
Table 2.
Common features at presentation in dialyzed patients with COVID-19
| Features | All patients (n = 44) |
WHO score (2–4) |
WHO score = 5 |
WHO score ≥ 6 |
P |
|---|---|---|---|---|---|
| No oxygen (n = 11, 25%) |
O2 ≤ 6 l/min (n = 14, 31.8%) |
O2 ≥ 9 l/min (n = 19, 43.2%) |
|||
| Clinical symptoms | |||||
| Fever / chills | 35 (79.5) | 8 (72.7) | 10 (71.4) | 17 (89.5) | Ns |
| Oxygen requirement | 20 (45.5) | 0 (0.0) | 6 (42.9) | 14 (73.7) | |
| Cough | 19 (43.2) | 4 (36.4) | 5 (35.7) | 10 (52.63) | Ns |
| Shortness of breath | 13 (29.5) | 0 (0.0) | 4 (28.6) | 9 (47.4) | Ns |
| Diarrhea | 6 (13.6) | 0 (0.0) | 3 (21.4) | 3 (15.8) | Ns |
| Anorexia and weigh loss | 26 (59.1) | 8 (72.7) | 10 (71.4) | 8 (42.1) | Ns |
| SARS-CoV-2 RT-PCR | |||||
| Positive | 43 (97.7) | 10 (90.9) | 14 (100) | 19 (100) | Ns |
| Delay from initial signs, d | 1 (0–2) | 0 (0–1) | 1 (0–3) | 1 (1–5) | 0.04 |
| Chest CT-scan | |||||
| Delay from initial signs, d | 2 (0–5) | 0 (0–6) | 2 (0–3) | 2.5 (1–6) | Ns |
| Normal | 8 (19.5) | 3 (33.3) | 2 (14.3) | 3 (16.7) | Ns |
| Mild | 9 (22.0) | 2 (22.2) | 5 (35.7) | 2 (11.1) | Ns |
| Moderate | 11 (26.8) | 2 (22.2) | 5 (35.7) | 4 (22.2) | Ns |
| Severe | 13 (31.7) | 2 (22.2) | 2 (14.3) | 9 (50.0) | 0.08 |
| Biochemical parameters | |||||
| Sodium level, mmol/l | 138 (136.5–140) | 137 (135–140) | 139 (137–141) | 138 (135–139) | Ns |
| Bicarbonate level, mmol/l | 24 (21–26) | 25 (21–25) | 24.5 (23–26) | 24 (20–26) | Ns |
| Albumin level, g/l | 32.5 (24–38) | 35 (28–39) | 33 (23–40) | 32 (21–35) | Ns |
| LDH level, n < 250 UI/l | 313 (206–377) | 198 (141–320) | 313 (254–438) | 315.5 (239–328) | Ns |
| CRP level, mg/l | 65 (6–123) | 5 (5–44) | 71.5 (18–120) | 91.5 (16–138) | 0.01 |
| Fibrinogen level, g/l | 5.4 (4.25–6.1) | 4.35 (3.4–5.4) | 5.6 (4.4–6.5) | 5.4 (4.3–6.2) | Ns |
| D-dimers level, UI/l | 715 (515–970) | 715 (515–970) | 1690 (540–2830) | 1640 (880–3575) | Ns |
| Hematological parameters | |||||
| Hemoglobin level, g/dl | 10.4 (9.45–11.8) | 11.8 (9.3–12.5) | 9.95 (9.5–11.0) | 10.4 (8.9–11.7) | Ns |
| Platelet count, g/l | 196.5 (136–249) | 250 (164–364) | 165 (123–237) | 195 (133–218) | 0.07 |
| Lymphocyte count, g/l | 0.6 (0.46–1.04) | 0.68 (0.53–1.2) | 0.60 (0.48–1.28) | 0.57 (0.41–0.97) | Ns |
| Neutrophil count, g/l | 3.91 (2.39–6.00) | 2.85 (2.14–4.92) | 4.23 (2.42–6.2) | 4.38 (3.26–7.56) | Ns |
CRP, C-reactive protein; COVID-19, coronavirus disease 2019; CT, computed tomography; LDH, lactate dehydrogenase; ns, a nonsignificant P value; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data are n (%) or median (interquartile range).
Chest computed tomography scan was performed in 41 patients (93.2%) within the first 2 days (IQR 0–5) after initial symptoms and showed bilateral ground-glass opacities with or without consolidations in 80.5% of cases. Radiological findings were classified as mild, moderate, or severe in 22%, 26.8%, and 31.7% of cases, respectively. Eight computed tomography scans were normal and were performed within the 2 days following initial symptoms.
Laboratory Findings
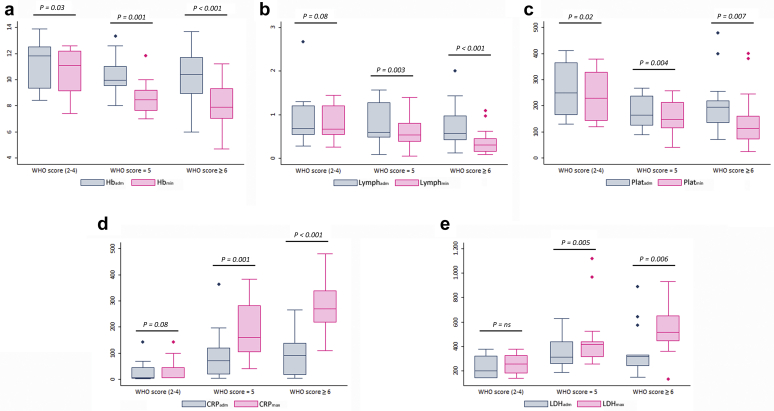
Laboratory findings on admission and during hospitalization are detailed in Tables 2 and 3. Oxygen-free patients (WHO score = 2–4) had only mild biological abnormalities compared with those requiring oxygen (WHO score = 5 and ≥ 6). Hematological parameters were also more severe in these patients compared with those who did not require oxygen therapy. Most patients presented with lymphopenia with a median of 0.6 g/l (IQR: 0.46–1.04), whereas only patients requiring oxygen therapy had a trend for low platelet counts. Interestingly, worsening of hematological and inflammatory markers during disease course was most notable in patients requiring oxygen therapy (Figure 2).
Table 3.
Laboratory findings during COVID-19 course
| Characteristics | All patients (n = 44) |
WHO score (2–4) |
WHO score = 5 |
WHO score ≥ 6 |
P |
|---|---|---|---|---|---|
| No oxygen (n = 11, 25%) |
O2 ≤ 6 l/min (n = 14, 31.8%) |
O2 ≥ 9 l/min (n = 19, 43.2%) |
|||
| Biochemical parameters | |||||
| Hyponatremia < 135 mmol/l | 18 (41.9) | 3 (27.3) | 3 (21.4) | 12 (66.7) | 0.02 |
| Metabolic acidosis <21 mmol/l | 230 (53.5) | 2 (18.2) | 6 (42.9) | 15 (83.3) | 0.002 |
| Low albumin level ≤ 20 g/l | 6 (15.0) | 1 (12.5) | 1 (7.1) | 4 (22.2) | ns |
| LDH > 2N | 14 (31.8) | 1 (9.1) | 3 (21.4) | 10 (52.6) | 0.03 |
| Ferritin level > 1000 | 31 (70.5) | 8 (72.7) | 9 (64.3) | 14 (73.7) | ns |
| CRP level > 175 mg/l | 23 (52.3) | 1 (9.1) | 6 (42.9) | 16 (84.2) | <0.001 |
| Fibrinogen level > 5 g/l | 34 (77.3) | 5 (45.5) | 10 (71.4) | 19 (100) | 0.002 |
| D-dimer level > 2000 UI/l | 25 (56.8) | 4 (36.4) | 8 (57.1) | 13 (68.4) | ns |
| Hematological parameters | |||||
| Anemia < 10 g/dl | 34 (77.3) | 4 (36.4) | 13 (92.9) | 17 (89.5) | 0.001 |
| Thrombopenia < 120 g/l | 16 (34.9) | 1 (9.1) | 6 (42.9) | 9 (47.4) | 0.09 |
| Lymphopenia < 0.5 g/l | 24 (54.5) | 2 (18.2) | 7 (50.0) | 15 (78.9) | 0.005 |
| Neutrophilia > 12 g/l | 11 (25.0) | 0 (0) | 3 (21.4) | 8 (42.1) | 0.04 |
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; LDH, lactate dehydrogenase; min, minimal; ns, nonsignificant P value; WHO, World Health Organization.
Data are n (%) or median (interquartile range).
Figure 2.
Comparison of main laboratory data between admission and worst value by World Health Organization (WHO) score group. (a) Hemoglobin level (g/dl) on admission (Hbadm) and minimum (Hbmin) value during hospitalization. (b) Lymphocyte count (g/l) on admission (Lymphadm) and minimum (Lymphmin) value during hospitalization. (c) Platelet count (g/l) on admission (Platadm) and minimum (Platmin) value during hospitalization. (d) Blood C-reactive protein level (mg/l) on admission (CRPadm) and maximum (CRPmax) value during hospitalization. (e) Lactate dehydrogenases level (UI/l) on admission (LDHadm) and maximum (LDHmax) value during hospitalization.
Initial Management
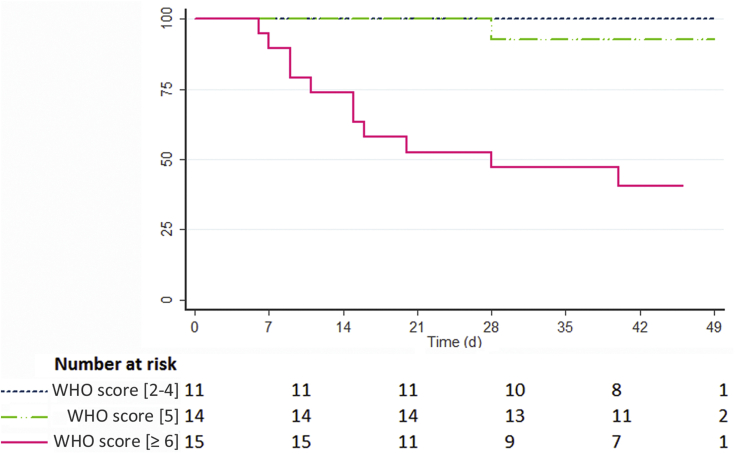
On admission, 20 patients (45.5%) required continuous intermittent nasal catheter oxygen inhalation to reach a saturation of ≥ 94% with a median level of 2 l/min ranging from 1 to 15 l/min. By contrast, 24 patients (54.5%) did not require oxygen therapy and had a median oxygen saturation level in ambient air of 98% (IQR: 96–100). Overall, 41 patients were hospitalized, including 33 who finally required oxygen therapy during disease course, with a delay ranging from 0 to 9 days after initial symptoms. Three patients were maintained on outpatient hemodialysis from initial referral to last follow-up because of only mild symptoms with an oxygen saturation of 99% to 100% in ambient air and no inflammatory biological syndrome. Three patients initially on peritoneal dialysis were switched to hemodialysis at the time of ICU referral. Most were administered empiric antibiotherapy during hospitalization, based on penicillin, 3G cephalosporin combined with macrolides (Table 4). Only a minority of patients received hydroxychloroquine (6.8%) or anti–interleukin-6 antibodies (9.1%), and none had antiviral therapy. All patients were prescribed prophylactic heparin and patients who developed pulmonary embolism or cardiac arrhythmia received therapeutic dosing of heparin. During hospitalization, 3 profiles of severity could be distinguished on the basis of WHO progression scale, which were correlated with outcome (Figure 3). Eleven patients (25%) remained oxygen-free during the whole disease course (WHO score between 2 and 4). Fourteen (31.8%) with a WHO score equal to 5 required up to 6 l/min of oxygen therapy, whereas 19 (43.2%) with a WHO score ≥ 6 required higher amounts (≥ 9/l). Of these, 15 (34.1%) were transferred to the ICU for noninvasive (20%) or mechanical (80%) ventilation, with a median delay of 4 days (IQR 2–9) after initial symptoms. Four patients with high oxygen requirement were not transferred to the ICU because of a do-not-resuscitate order on admission.
Table 4.
Management and outcome of dialyzed patients with COVID-19
| Events | All patients (n = 44) |
WHO score (2–4) |
WHO score = 5 |
WHO score ≥ 6 |
P |
|---|---|---|---|---|---|
| No oxygen (n = 11, 25%) |
O2 ≤ 6 l/min (n = 14, 31.8%) |
O2 ≥ 9 l/min (n = 19, 43.2%) |
|||
| Hospitalization | 41 (93.2) | 8 (72.7) | 14 (100) | 19 (100) | 0.008 |
| Delay from initial symptoms | 0 (0–3) | 0 (0–0) | 2 (0–3) | 1 (0–3) | ns |
| Pharmacological treatment | |||||
| Antibiotherapy | 29 (65.9) | 2 (18.2) | 8 (57.1) | 19 (100) | <0.001 |
| HCQ or chloroquine | 3 (6.8) | 0 (0.0) | 1 (7.1) | 2 (10.5) | ns |
| Anti-IL6 antibody | 4 (9.1) | 0 (0.0) | 0 (0.0) | 4 (21.1) | 0.06 |
| Oxygen therapy | |||||
| Max O2 level, l/min | 4.5 (0.5–15) | NA | 3 (2–4) | 15 (15–15) | <0.001 |
| Delay to max O2 level | 5 (2–7) | NA | 5 (2–13) | 5 (2–7) | ns |
| ICU transfer | 15 (34.1) | 0 (0.0) | 0 (0.0) | 15 (78.9) | <0.001 |
| Delay from initial symptoms | 6 (2–8) | NA | NA | 6 (2–8) | |
| Mechanical ventilation | 12 (27.3) | 0 (0.0) | 0 (0.0) | 12 (63.2) | <0.001 |
| Duration in the ICU | 10 (7–21) | NA | NA | 10 (7–21) | |
| Severe adverse events | |||||
| ARDS | 12 (27.3) | 0 (0.0) | 0 (0.0) | 12 (63.2) | <0.001 |
| Hemodynamic instability | 10 (22.7) | 0 (0.0) | 0 (0.0) | 10 (52.6) | <0.001 |
| Cardiac arrythmia | 5 (11.4) | 0 (0.0) | 2 (14.3) | 3 (15.8) | ns |
| Severe bleeding | 4 (9.1) | 0 (0.0) | 0 (0.0) | 4 (21.1) | 0.06 |
| Pulmonary embolism | 2 (4.5) | 1 (9.1) | 0 (0.0) | 1 (5.3) | ns |
| Final outcome | |||||
| Follow-up | 40 (28–46) | 42 (38–49) | 44 (40–47) | 28 (11–44) | ns |
| Death | 12 (27.3) | 0 (0.0) | 1 (7.1) | 11 (57.9) | 0.001 |
| Delay from initial signs | 15 (9–24) | NA | 28 | 15 (9–20) | ns |
| Hospital discharge | 24 (58.5) | 8 (100) | 12 (85.7) | 4 (21.1) | <0.001 |
| Duration of hospital staya | 12 (7–18) | 9 (2–12) | 12 (9–14) | 29 (21–36)b | ns |
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; HCQ, hydroxychloroquine; ICU, intensive care unit; IL6, interleukin 6; max, maximal; NA, not applicable; ns, nonsignificant P value; WHO, World Health Organization.
Data are n (%) or median (interquartile range).
All delays and durations are provided in days.
At last follow-up (May 11, 2020), 6 patients are still hospitalized, including 3 in the ICU, thus underestimating the median duration of hospital stay, particularly in the group of patients with a WHO score ≥ 6.
Figure 3.
Overall severity by World Health Organization (WHO) progression scale and impact on mortality.
Outcome
Patients were maintained on hospitalization until complete resolution of clinical symptoms and withdrawal of oxygen therapy for at least 24 hours. Patients were then referred home with instructions to minimize the risk of infection transmission at home, including isolation in a separate room at home and use of face masks for 10 days after complete resolution of clinical signs. Discharged patients from hospital were maintained dialyzed in our unit for at least 14 days after initial symptoms and 10 days after complete resolution of clinical signs, before being referred back to their initial outpatient center. A regular follow-up was undertaken in a tight collaboration with the referral nephrologist. The median duration of hospital stay was 12 days (IQR 7–18), and the median length of ICU stay was 10 days (IQR 7–21). Overall, 58.5% of hospitalized patients, but only 4 (21.1%) of those patients with a WHO score ≥ 6, were discharged home. At the time of data cutoff (May 11, 2020), only 2 patients of those admitted to the ICU were discharged from hospital after 18 and 33 days, respectively. Two other patients are still in the ICU, and 2 have been discharged from the ICU but are still hospitalized. Overall, 12 patients (27.3%) died, including 1 who had a hepatocellular carcinoma progression with only mild COVID-19 infection. Importantly, the overall case fatality rate was 36.4% in patients who required oxygen therapy, and 60.0% in those admitted to the ICU. For comparison, 1377 nondialyzed patients with COVID-19 were admitted to our hospital during the recent outbreak with an overall mortality rate of 12.9% compared with 27.3% for dialyzed patients (P = 0.006). Overall, 294 (22.7%) nondialyzed patients were transferred to the ICU compared with 15 (34.1%) dialyzed patients (P = 0.04), and in-ICU mortality was 20.7% in the nondialyzed population compared with 60% in dialyzed patients (P = 0.002).
Identification of Prognostic Factors Associated With Death
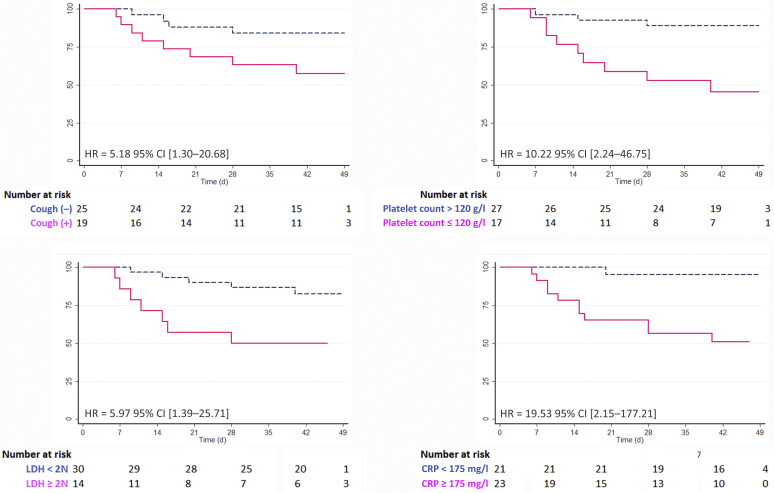
By univariate Cox survival analysis, acute respiratory syndrome, neutrophil count ≥ 10 g/l, thrombopenia ≤ 120 g/l, metabolic acidosis < 21 mmol/l, LDH level ≥ 2 times the upper normal limit, blood CRP level ≥ 175 mg/l, and D-dimer level > 4000 UI/l were associated with a higher risk of death (Table 5). Other variables, including dyspnea, cough, and lymphocyte count ≤ 0.5 g/l also tended to significance (P < 0.10). By contrast, age was not associated with mortality. By multivariate analysis (Table 5 and Figure 4), cough (5.18, 95% confidence interval: 1.30–20.68), thrombopenia ≤ 120 g/l (10.22, 95% confidence interval: 2.24–46.75), LDH ≥ 2N (5.97, 95% confidence interval: 1.39–25.71), and blood CRP ≥ 175 mg/l (19.53, 95% confidence interval: 2.15–177.21) remained significantly and independently associated with death, with or without adjustment of the multivariate model for age.
Table 5.
Covariates associated with death by Cox survival analysis
| Covariates | Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|---|
| P | HR | 95% CI | P | HR | 95% CI | |
| Age | 0.339 | 1.01 | 0.97–1.06 | |||
| Dyspnea | 0.076 | 2.86 | 0.925–8.88 | |||
| Cough | 0.06 | 3.04 | 0.92–10.12 | 0.02 | 5.18 | 1.30–20.68 |
| ARDS | 0.01 | 4.44 | 1.40–14.03 | |||
| Neutrophil count ≥ 10 g/l | 0.01 | 4.49 | 1.34–14.93 | |||
| Platelet count ≤ 120 g/l | 0.003 | 6.06 | 1.64–22.49 | 0.003 | 10.22 | 2.24–46.75 |
| Lymphocyte count ≤ 0.5 g/l | 0.09 | 2.84 | 0.77–10.51 | |||
| Metabolic acidosis ≤ 21 mmol/l | 0.02 | 11.18 | 1.43–87.51 | |||
| LDH ≥ 2Nb | 0.010 | 3.99 | 1.26–12.63 | 0.016 | 5.97 | 1.39–25.71 |
| D-dimer ≥ 4000 UI/l | 0.03 | 4.44 | 1.11–11.03 | |||
| CRP ≥ 175 mg/l | <0.001 | 13.06 | 1.68–101.41 | 0.008 | 19.53 | 2.15–177.21 |
ARDS, acute respiratory distress syndrome; CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; LDH, lactate dehydrogenase.
Results of multivariate analysis are provided after adjustment for age.
2N refers to 2 times the upper normal limit.
Figure 4.
Kaplan-Meier survival curves of significant covariates associated with death by multivariate analysis. Hazard ratios (HR) and 95% confidence intervals (CI) are detailed after adjustment for age. CRP, C-reactive protein; LDH, lactate dehydrogenase.
Discussion
The present series describes the demographics, clinical course of COVID-19 in patients on maintenance dialysis during the recent epidemic in the Paris region. We established 3 profiles of severity based on the WHO progression scale that were tightly correlated with disease outcome and identified prognostic factors significantly associated with death.
France has been on a lockdown since March 17, 2020. Unlike other individuals, dialyzed patients could not respect strict confinement, and were potentially exposed to circulating coronavirus by going to the dialysis center. A significant proportion was likely infected before the measures to mitigate infection spread were effective.11, 12, 13 Data from other parts of the world suggest that approximately 15% of dialyzed patients develop symptomatic forms of COVID-19.14, 15, 16
Although more than 50% of patients do not require oxygen therapy on admission, a significant proportion may abruptly worsen, particularly at the end of the first week.17 In our study, only 25% remained oxygen-free along disease course. Only a few patients received a specific therapy in our series because the local policy was to restrict the off-label use of such treatments and dialyzed patients were most often excluded from randomized trials. By contrast, more than 75% of patients in the Italian, Spanish, and New York cohorts received antivirals and hydroxychloroquine.16, 17, 18 Despite such heterogeneity in the therapeutic approach, the mortality is remarkably consistent across the different cohorts and approximates 30% to 40%.15, 16, 17 These data contrast with the initial report of only mild disease in China,19 and a mortality rate of 18.9% in Chinese hemodialyzed patients with COVID-19.14 Case fatality rate is also strikingly higher than the 3% to 4% reported in the general population.20 Nevertheless, because only symptomatic patients were screened for SARS-CoV-2 infection in most studies, this difference is likely overestimated. Interestingly, we were able to compare the rates of ICU transfer and case fatality between nondialyzed and dialyzed patients admitted to our hospital. In our series, 34.1% of dialyzed patients were transferred to the ICU with an ICU mortality of 60% compared with 21.4% and 20.7% in the general hospitalized population. The in-hospital mortality and ICU admission in the French general population are approximately 16% and 17%, respectively (data from Santé Publique France, accessed on May 12, 2020).21 Moreover, the ICU case fatality rate due to COVID-19 has been estimated at 24%, which is in line with our results and the ICU mortality rate of 26% reported in other cohorts.8,22,23 The policy for ICU transfer and mechanical ventilation and the rate of a “do-not-resuscitate” order as observed in the Spanish and New York cohorts are also to take into account when interpreting such data.17,18 Such increase in mortality risk in the dialyzed patients could be explained by a greater vulnerability to infections,9 and the coexistence of multiple comorbidities known to be associated with more severe forms of COVID-19.10
Our survival analysis showed that cough, thrombopenia ≤ 120 g/l, increased LDH level above 2 times the upper normal limit, and blood CRP level above 175 mg/l were independently associated with death. Dialysis vintage, low lymphocyte count, and high LDH level were identified predictors of mortality in the Spanish series, whereas fever and cough at disease onset, and CRP at baseline > 50 mg/l were significantly associated with death in the Italian series.16,17 It is quite surprising that symptoms as subjective as cough and shortness of breath were associated with poor outcomes, whereas imaging data did not in our series and other cohorts.16,17 Moreover, neither age nor common cardiovascular risk factors were associated with mortality, contrary to the general population.17 Altogether, our data and recent reports indicate that COVID-19–related mortality in dialyzed patients seems more driven by disease presentation and subsequent severity than a patient’s comorbid status. Our results are thus useful to recognize most at-risk dialyzed patients to die from COVID-19.15, 16, 17
Our study has some limitations mainly due to the retrospective design and the size of the cohort, which increases the risk of overfitting of our multivariate analysis. Nevertheless, our findings were quite similar to those reported by the Italian and Spanish groups, providing further consistency of our results. Moreover, at data cutoff, several patients were still hospitalized, resulting in a potential underestimation of overall morbidity and mortality.
In conclusion, COVID-19 is burdened by a high mortality rate in dialyzed patients compared with the general population and is also associated with dramatic complications and prolonged hospitalization. The use of clinical and biological parameters as early prognostic markers needs further validation but will be helpful to guide future management strategies.
Disclosure
All the authors declared no competing interests.
Ackowledgments
We thank Ms. Valérie Gérard and Ms. Nadia Corre, and all the colleagues and nurses of our department and institution who were involved in the management of patients during the recent epidemic. We also thank Marie Frank and Jérémy Laurent from the Département d’Information Médicale Paris Saclay for supplying the data regarding the ICU mortality rate in our institution
Footnotes
Table S1. WHO progression scale.
Figure S1. Geographic provenance of patients with COVID-19 referred to the in-hospital dialysis unit of Bicêtre University Hospital (APHP).
Supplementary Material
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauer S.A., Grantz K.H., Bi Q. The incubation period of Coronavirus Disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Livingston E., Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 9.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliger A.S., Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15:707–709. doi: 10.2215/CJN.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/dialysis.html Published February 11, 2020. Available at:
- 12.COVID-19. https://www.theisn.org/covid-19#recommendations-for-the-novel-coronavirus-2019-epidemic Available at:
- 13.COVID-19 News and Information. ERA-EDTA. https://www.era-edta.org/en/covid-19-news-and-information/ Available at:
- 14.Ma Y, Diao B, Lv X, et al. 2019 novel coronavirus disease in hemodialysis (HD) patients: Report from one HD center in Wuhan, China [e-pub ahead of print]. medRxiv.https://doi.org/10.1101/2020.02.24.20027201. Accessed April 26, 2020.
- 15.Scarpioni R., Manini A., Valsania T. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol. 2020;37 2020-vol2. [PubMed] [Google Scholar]
- 16.Alberici F., Delbarba E., Manenti C. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goicoechea M., Cámara L.A.S., Macías N. COVID-19: Clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int. 2020;98:27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher M, Yunes M, Mokrzycki MH, et al. Chronic hemodialysis patients hospitalized with COVID-19 - Short-term outcomes in Bronx, New York [e-pub ahead of print]. Kidney360.https://doi.org/10.34067/KID.0003672020. Accessed June 4, 2020. [DOI] [PMC free article] [PubMed]
- 19.Wang R., Liao C., He H. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis. 2020;76:141–143. doi: 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson N., Kvalsvig A., Barnard L.T., Baker M.G. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020;26:1339–1441. doi: 10.3201/eid2606.200320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infection au nouveau Coronavirus (SARS-CoV-2), COVID-19, France et Monde. Available at: https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/articles/infection-au-nouveau-coronavirus-sars-cov-2-covid-19-france-et-monde. Accessed May 12, 2020.
- 22.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 23.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.