Abstract
In the absence of approved vaccines and therapeutics for use in humans, Nipah virus (NiV) continues to cause fatal outbreaks of encephalitis and respiratory disease in Bangladesh and India on a near-annual basis. We determined that a single dose of a lipid nanoparticle nucleoside-modified messenger RNA vaccine encoding the soluble Hendra virus glycoprotein protected up to 70% of Syrian hamsters from lethal NiV challenge, despite animals having suboptimally primed immune responses before challenge. These data provide a foundation from which to optimize future messenger RNA vaccination studies against NiV and other highly pathogenic viruses.
Keywords: Nipah virus, mRNA vaccine, soluble Hendra virus glycoprotein, virus, hemorrhagic fever, Syrian hamster, disease, lung, brain, CNS, neurological, respiratory
Nipah virus (NiV) is a highly pathogenic zoonotic henipavirus harbored by pteropid bats, from which spillover into the human population has caused near-annual outbreaks of severe encephalitis and respiratory disease in Bangladesh and India since 2001 [1]. Despite the demonstrated in vivo efficacy of viral vector–based and protein subunit–based vaccine platforms encoding henipavirus glycoproteins against NiV infection in non-human primates (NHPs) [2, 3], there is currently no approved vaccine for human use against NiV infection.
Recent technological advances have facilitated development of messenger RNA (mRNA)-based vaccines as a promising platform alongside DNA and protein-based platforms. mRNA vaccines offer a number of advantages with regard to safety, efficacy, and production, which include reducing the immunogenicity of the RNA through nucleoside modification to maximize antigen expression, as well as protecting the mRNA from nucleases by complexing it into lipid nanoparticles (LNPs) (reviewed in [4]). A 2017 study evaluating a single-dose mRNA LNP vaccine against Zika virus demonstrated complete protection from virus challenge in both mice and NHPs [5].
Because the soluble Hendra virus glycoprotein (sHeVG) subunit vaccine has demonstrated efficacy against NiV challenge in multiple animal models [2, 6], we evaluated the single-dose efficacy of an LNP-encapsulated mRNA vaccine encoding the sHeVG (sHeVG mRNA LNP) against NiV challenge in the Syrian hamster model. All animal work was approved by the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committee and was performed by certified staff in AAALAC International-approved biosafety level 4 facilities at CDC.
METHODS
To generate the sHeVG mRNA LNP vaccine, we first purified in vitro-transcribed, nucleoside-modified, codon-optimized mRNA transcripts encoding the sHeVG antigen [7], using fast-performance liquid chromatography [8], and then complexed the mRNA into LNPs as described elsewhere [9]. For vaccination, 6-week-old female Syrian hamsters (Mesocricetus auratus; Envigo) were anesthetized with isoflurane and inoculated intramuscularly in the right quadriceps muscle with a 30-µL volume of either phosphate-buffered saline vehicle control (“NO VAX” group), a 10-µg dose of sHeVG mRNA LNP (“VAX LO” group), or a 30-µg dose of sHeVG mRNA LNP (“VAX HI” group), with 10 animals in each group. At 25 days after vaccination, approximately 0.3–0.4 mL of blood was collected from each animal to measure levels of NiV cross-reactive and neutralizing antibodies.
On day 30 after vaccination (challenge day 0), all hamsters were inoculated via the intraperitoneal route with a uniformly lethal challenge dose (approximately 105 times the median tissue culture infective dose per hamster [>1000 times the median lethal dose]) of NiV (genotype M; NiV M) [10]. They were followed up until 28 days after infection. Weight, temperature, water intake (measured by cage and averaged by the number of animals), and clinical scores were assessed daily (Supplementary Figure 1B–1D). The following scores were allotted for each listed clinical sign: 2 indicated quiet, dull, responsive disposition, hunched back, ruffled coat, abnormal huddling or hypoactivity; 3, dehydration/decreased skin turgor; 5, dyspnea, anemia, or moderate neurological signs (moderate head tilt, tremors, ataxia, and/or circling); 10, inability to bear weight, frank hemorrhage, severe neurological signs (severe head tilt, seizures, paralysis), or weight loss >25% baseline (1 day before infection). Hypothermia (<34°C), when present with other clinical signs, was also considered as an indicator for euthanasia. Animals with a score ≥10, or at completion of the study (28 days after infection), were humanely euthanized while under deep isoflurane anesthesia by intracardiac administration of sodium pentobarbital solution, or by isoflurane vapor overdose alone.
At the time of euthanasia, approximately 2–3 mL of heparinized blood was collected from each hamster by cardiac puncture for serological and viral RNA analyses. Necropsies were performed to collect liver, lung, spleen, kidney, heart, ovarian, eye, and brain tissues, which were inactivated in MagMAX RNA lysis buffer (Thermo Fisher Scientific) for subsequent RNA extraction and real-time reverse-transcription polymerase chain reaction analysis, as described elsewhere [10].
RESULTS
All animals were clinically normal throughout the vaccination period, having relatively uniform weight change, body temperature, and water consumption across the experimental groups (Supplementary Figure 1A).
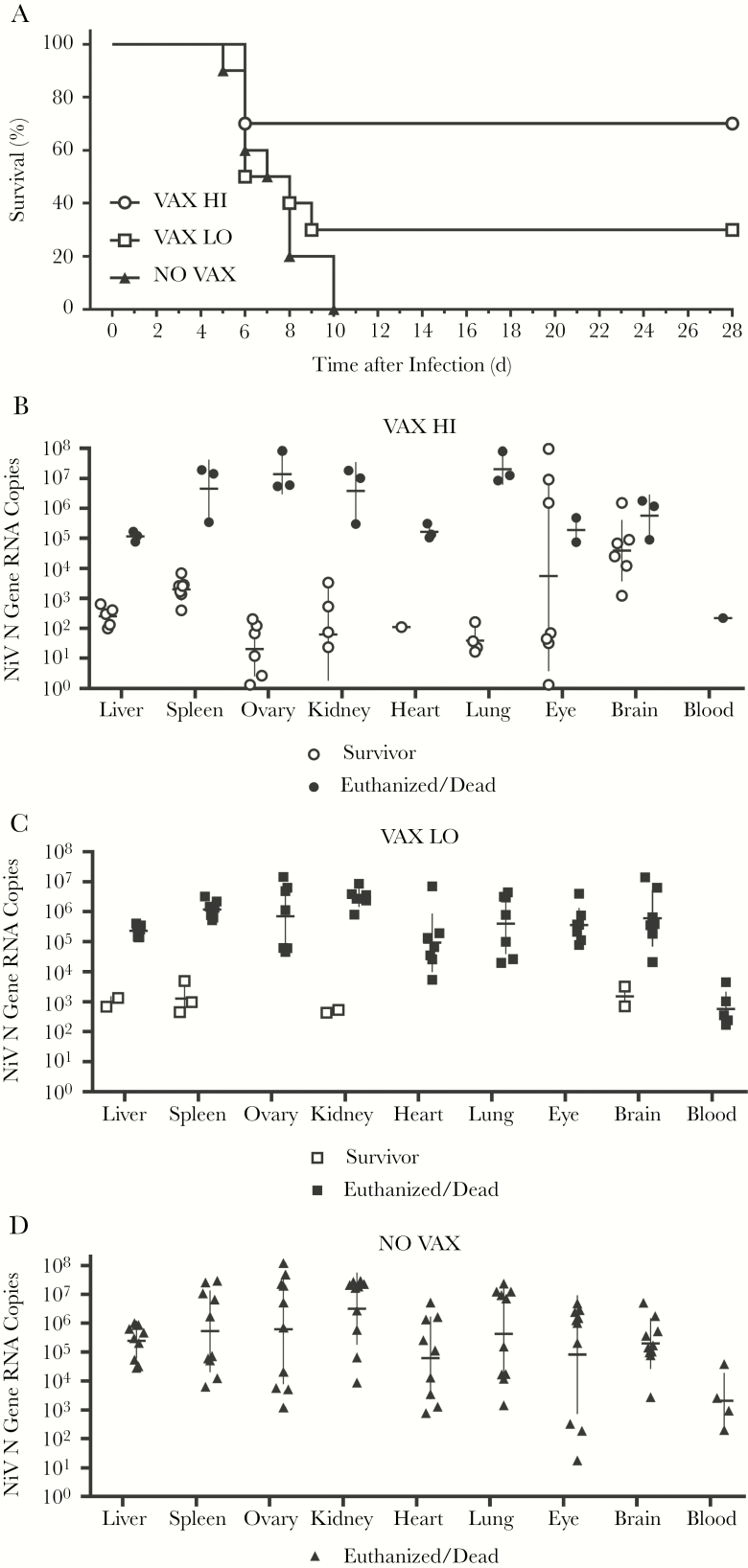
Animals in the NO VAX group met euthanasia criteria based on severe clinical signs beginning at 5 days after infection, and by 10 days after infection all animals in the group either died or were euthanized (Figure 1A). In the VAX HI group, 7 of 10 animals survived NiV challenge (Figure 1A). Although the majority of VAX HI survivors (6 of 7) showed some clinical signs during the course of the study, 6 of the 7 survivors had no observable clinical signs by the end of the study (Supplementary Figure 1B). The 3 non-surviving animals in the VAX HI group succumbed 6 days after infection, within the same time frame as observed for non-survivors in the NO VAX and VAX LO group. In the VAX LO group, 7 of 10 animals died or were euthanized 6–9 days after infection. Of the 3 survivors in the VAX LO group, 1 showed no clinical signs throughout the study (Figure 1A and Supplementary Figure 1C).
Figure 1.
A single dose of soluble Hendra virus glycoprotein (sHeVG) messenger RNA (mRNA) lipid nanoparticle (LNP) mRNA vaccine provides partial protection against lethal Nipah virus (NiV) challenge in a dose-dependent manner. (A), Survival of female Syrian hamsters vaccinated with phosphate-buffered saline vehicle control (NO VAX) or either 10 µg (VAX LO), or 30 µg (VAX HI) of sHeVG mRNA LNP and challenged intraperitoneally with 6.84 × 104 times the median tissue culture infective dose of NiV genotype M (NiV M) (n = 10 in each group). (B–D), Copy numbers of NiV nucleoprotein (NiV N) gene RNA were measured from blood and tissues of VAX HI (B), VAX LO (C), and NO VAX (D) experimental groups. Horizontal bars represent geometric means for survivors (open symbols) and non-survivors (closed symbols); vertical lines, standard deviations. RNA levels were detected from 5 µL of RNA.
Although single-dose vaccination with sHeVG mRNA LNP did not provide complete protection from lethal NiV challenge for both VAX LO and VAX HI groups, geometric mean copy numbers of NiV N gene RNA in surviving animals were expectedly lower than in their non-surviving group counterparts (Figure 1B–1D). Whereas RNA copy numbers in tissues from nonsurvivors across all groups showed notable variation (typically ranging from approximately 1 × 103 to >108 copies), copy numbers from most tissues of survivors were typically <104 copies (Figure 1B–1D and Supplementary Table 1). We conducted statistical analyses to compare NiV RNA copies in tissues among non-survivors from both VAX HI and VAX LO groups with those from NO VAX group non-survivors, and we failed to find significant decreases in the vaccine groups. Among several VAX HI survivors, however, we detected levels of NiV RNA in eye and brain tissues that were either nearly equivalent to or greater than those in their non-survivors counterparts (Figure 1B and Supplementary Table 1).
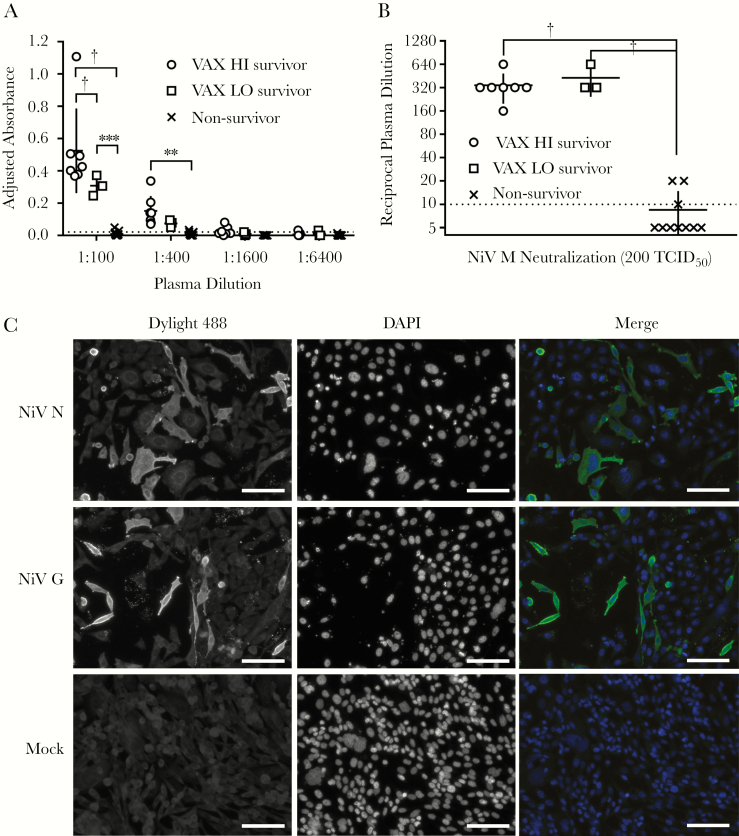
To better understand the level of protection provided by sHeVG mRNA LNP vaccination, we analyzed plasma samples collected both before and after virus challenge for total levels of anti-NiV immunoglobulin G (IgG) antibodies and for virus-neutralizing antibodies. We did not detect any anti-NiV IgG or virus-neutralizing activity from any of the plasma collected from the VAX HI or VAX LO groups before NiV challenge (data not shown). In contrast, plasma samples collected from all post-challenge survivors tested positive for anti-NiV IgG antibodies by enzyme-linked immunosorbent assay, with the mean magnitude of the responses (as measured by adjusted absorbance) from VAX HI survivors being significantly higher than that from VAX LO survivors at the initial 1:100 dilution (Figure 2A and Supplementary Table 1). Levels of virus-neutralizing activity, however, were similar across VAX HI and VAX LO survivors, with reciprocal neutralization titers ranging between 160 and 640, whereas all euthanized animals had minimal to undetectable neutralizing activity (Figure 2B and Supplementary Table 1).
Figure 2.
Survivors among recipients of soluble Hendra virus glycoprotein mRNA LNP vaccine (10 µg [VAX LO] or 30 µg [VAX HI]) in NiV challenge show robust anti–NiV-specific antibody responses. (A), Adjusted absorbance readings from 4-fold dilutions of hamster plasma in a NiV-specific immunoglobulin G (enzyme-linked immunosorbent assay. Horizontal bars represent means for survivor groups and non-survivors (from all groups); vertical lines, standard deviations; dotted line, cutoff value for diagnostic determination of a positive sample. *P < .01; †P < .001 (2-way analysis of variance [ANOVA] with Tukey multiple comparisons test). (B), NiV neutralization by plasma samples from individual hamsters. Reciprocal plasma dilutions in which hamster plasma completely neutralized 200 times the median tissue culture infective dose (TCID50) of NiV genotype M (NiV M) across 4 replicates were plotted according to group-specific indications. Horizontal bars represent geometric means for survivor groups and non-survivors (from all groups); vertical lines, standard deviation; dotted line at 10, limit of detection for virus neutralization assay. Plasma samples with undetectable neutralizing activity were plotted as having a titer of 5. Owing to insufficient samples, only 3 plasma samples were tested from recipients of phosphate-buffered saline vehicle control. *P < .01; †P < .001 (1-way ANOVA with multiple comparisons Kruskal-Wallis test). (C), Immunofluorescence assay depicted with representative micrographs from a VAX HI survivor. Plasma samples collected from representative NiV challenge survivors, diluted 1:40 in phosphate-buffered saline with 5% skim milk, were incubated with either NiV nucleoprotein (NiV N), NiV attachment glycoprotein (NiV G), or mock-transfected CHO-K1 cells. Goat anti-hamster Dylight 488–conjugated antibodies were used to detect primary hamster antibodies, and nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI). Scale bar represent 100 µm.
Finally, we used representative post-vaccination, pre-challenge, and post-challenge plasma samples from each group to perform immunofluorescence assay against Chinese hamster ovary cells (CHO-K1; CCL-61 from ATCC) that were either mock-transfected or transfected with a plasmid expressing the NiV nucleoprotein (NiV N) or the attachment glycoprotein (NiV G). Representative pre-challenge plasma samples collected across all groups and post-challenge plasma samples from euthanized animals had no detectable reactivity against either NiV N– or NiV G–transfected cells (data not shown), whereas post-challenge plasma from both VAX HI and VAX LO survivors had robust reactivity against both NiV N and NiV G (Figure 2C).
DISCUSSION
Our study documents the partial efficacy of a single-dose sHeVG mRNA LNP vaccine against lethal NiV challenge. We chose the sHeVG antigen for this initial study not only for its demonstrated efficacy against NiV challenge but also for its versatility to protect against both Hendra virus and NiV infections. In contrast to robust protective immune responses elicited by single-dose LNP mRNA vaccines encoding Zika virus envelope or influenza A virus hemagglutinin proteins [5, 11], the lack of any detectable NiV-specific antibodies in the plasma of all sHeVG mRNA LNP–vaccinated animals before virus challenge likely indicated a suboptimally primed immune response. This lesser response may be attributed to several factors, including the use of a different animal model (to our knowledge the current study is the first to evaluate the mRNA LNP platform in hamsters) and, by corollary, a potentially suboptimal route of vaccination. We chose the intramuscular route of inoculation over the untested method of performing multiple-site intradermal inoculation into the backs of hamsters (which have excessive amounts of skin compared with mice and NHPs), not only owing to familiarity [10], but also for the sake of safety in high containment biosafety level 4 conditions.
Another factor that may have adversely affected protection in this study is the inherently suboptimal immunogenicity of the sHeVG antigen itself. It is noteworthy that every in vivo study that demonstrated the protective efficacy of the sHeVG protein subunit vaccine against NiV challenge not only used the powerful Toll-like receptor 9 agonist CpG as an adjuvant but also used prime-boost regimens before NiV challenge [2, 6]. Our results suggest that whereas a single dose of sHeVG mRNA LNP partially protects animals from NiV infection (likely by means of cell-mediated immunity [12]), a second booster dose would probably provide complete protection, as has been observed with Ebola virus infection in the guinea pig model [13]. Even so, given the level of protection we observed with sHeVG mRNA LNP, despite suboptimal immune responses before challenge, achieving single-dose efficacy against NiV is still feasible.
Future studies should not only involve optimizing the route of vaccination; they should also include characterization and assessment of efficacy and immunogenicity by LNP RNA based on sHeVG, compared with those expressing either the soluble NiV attachment glycoprotein and/or the NiV fusion glycoprotein [14]. Furthermore, the documented ability of mRNA LNP vaccines to induce robust T-cell and B-cell responses [11] warrants more detailed characterization of antihenipavirus immune responses in animal models for which immunology reagents are more readily available. Another interesting observation from our study was the apparent persistence of viral RNA in the eye and brain tissues from the majority of vaccinated survivors from the VAX HI group. Because residual neurological deficits have been documented in human survivors of NiV infection, long-term viral persistence studies should be conducted not only to determine the molecular markers potentially associated with persistence but also to elucidate the consequences of maintaining significant viral loads in the brain of hamsters surviving acute NiV infection.
The mRNA LNP vaccine platform has already shown promise against viral diseases of human importance, including Zika, influenza A, and respiratory syncytial virus, among others (reviewed in [4]). Earlier this year, the Coalition for Epidemic Preparedness Innovations granted up to $34 million toward developing a mRNA LNP vaccine platform to be rapidly deployed in response to new and previously unknown pathogens (referred to by the World Health Organization as “disease X”) [15]. Our findings from the current study support further development of this platform and provide a foundation for optimizing mRNA vaccines against NiV and other highly pathogenic viruses.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. We thank members of the Centers for Disease Control and Prevention’s (CDC’s) Comparative Medicine Branch for providing care for the animals.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by an appointment to the CDC’s Research Participation Program, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC (S. R. W.), and by CDC Emerging Infectious Disease Research Core funds.
Potential conflicts of interest. D. W. is listed as an inventor on the patent “Suppression of RNA Recognition by Toll-Like Receptors; The Impact of Nucleoside Modification and the Evolutionary Origin of RNA,” with royalties paid to CellScript, Moderna, and BioNTech. D. W. also reports financial relationship with CellScript outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Spiropoulou CF. Nipah virus outbreaks: still small but extremely lethal. J Infect Dis 2019; 219:1855–7. [DOI] [PubMed] [Google Scholar]
- 2. Bossart KN, Rockx B, Feldmann F, et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci Transl Med 2012; 4:146ra07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prescott J, DeBuysscher BL, Feldmann F, et al. Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine 2015; 33:2823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 2018; 17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pardi N, Hogan MJ, Pelc RS, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017; 543:248–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pallister JA, Klein R, Arkinstall R, et al. Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months. Virol J 2013; 10:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bossart KN, Crameri G, Dimitrov AS, et al. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J Virol 2005; 79:6690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weissman D, Pardi N, Muramatsu H, Karikó K. HPLC purification of in vitro transcribed long RNA. Methods Mol Biol 2013; 969:43–54. [DOI] [PubMed] [Google Scholar]
- 9. Pardi N, Tuyishime S, Muramatsu H, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release 2015; 217:345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lo MK, Bird BH, Chattopadhyay A, et al. Single-dose replication-defective VSV-based Nipah virus vaccines provide protection from lethal challenge in Syrian hamsters. Antiviral Res 2014; 101:26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med 2018; 215:1571–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stachowiak B, Weingartl HM. Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PLoS One 2012; 7:e30855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer M, Huang E, Yuzhakov O, Ramanathan P, Ciaramella G, Bukreyev A. Modified mRNA-based vaccines elicit robust immune responses and protect guinea pigs from Ebola virus disease. J Infect Dis 2018; 217:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dang HV, Chan YP, Park YJ, et al. An antibody against the F glycoprotein inhibits Nipah and Hendra virus infections. Nat Struct Mol Biol 2019; 26:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christodoulou M. CEPI awards US $34million contract to CureVac to advance The RNA Printer™—a mRNA vaccine platform that can rapidly combat multiple diseases https://cepi.net/news_cepi/cepi-awards-contract-to-curevac-to-advance-the-rna-printer-a-mrna-vaccine-platform-that-can-rapidly-combat-multiple-diseases/. Accessed 27 February 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.