Abstract
Nipah virus (NiV; family Paramyxoviridae, genus Henipavirus) infection can cause severe respiratory and neurological disease in humans. The pathophysiology of disease is not fully understood, and it may vary by presentation and clinical course. In this study, we investigate changes in blood chemistry in NiV-infected Syrian hamsters that survived or succumbed to disease. Increased sodium and magnesium and decreased albumin and lactate levels were detected in animals euthanized with severe clinical disease compared with mock-infected controls. When subjects were grouped by clinical syndrome, additional trends were discernable, highlighting changes associated with either respiratory or neurological disease.
Keywords: blood, neurological, Nipah virus, respiratory, Syrian hamster
Nipah virus (NiV; family Paramyxoviridae, genus Henipavirus) infection can result in severe disease in humans with case fatality rates upwards of 70%. NiV was first identified in a large outbreak in Malaysia and Singapore in 1998 and later in Bangladesh and India in 2001 during a retrospective investigation of an outbreak of encephalitis [1]. After an incubation period of 5–14 days, patients usually develop non-specific clinical signs and symptoms (fever, headache, myalgia, vomiting, and sore throat), but they may rapidly progress to present with severe respiratory disease or altered consciousness (drowsiness, disorientation, obtundation) and other neurological signs indicative of encephalitis. In Malaysia, patients presented primarily with encephalitis. In Bangladesh and subsequent outbreaks, it has been recognized that patients may present with respiratory illness during the early part of infection and that approximately half of the patients with severe neurological signs also show pulmonary signs.
Alterations in blood chemistry in acute and convalescent patients have not been investigated in detail. These data are also limited in animal models of disease, including hamsters, ferrets, and non-human primates. In this study, using a large dataset (~215 samples) from NiV-Malaysia-infected Syrian hamsters (Mesocricetus auratus), which recapitulate both respiratory and encephalitic disease [2], we investigate blood chemistry in animals with terminal disease and in survivors, and we describe associations between analyte disturbances and clinical presentations of NiV infection.
METHODS
All animal procedures were approved by the Centers for Disease Control and Prevention (CDC) Institutional Animal Care and Use Committee and conducted in accordance with the Guide for the Care and Use of Laboratory Animals. The CDC is fully accredited by AAALAC International. Procedures conducted with NiV or NiV-infected animals were performed in the CDC biosafety level-4 laboratory. All recombinant virus work was approved by the CDC Institutional Biosafety Committee.
Female Syrian hamsters (5- to 7 weeks old; HsdHan: AURA, no. 8902F [Envigo]) were intranasally (100 μL total; divided between nares) or intraperitoneally (500 μL or 2 mL) inoculated with 103–107 50% tissue culture infective dose (TCID50) of wild-type (passaged on Vero-E6 + 1, Vero +1) or recombinant NiV-Malaysia (based on wild-type [GenBank accession no. AF212302]; rescued on BSR-T7/5 with Vero, passaged on Vero +1). All viruses were sequence-confirmed and mycoplasma-negative. Data represent untreated and/or unvaccinated NiV-infected experimental groups and associated mock-infected control animals from 5 independent studies (2 unpublished by S.R.W. et al., 2019, and [3–5]). Average age of subjects at time of euthanasia was 59.5 days (range, 42–85 days). Full details on age, dose, route, virus, clinical score, and outcome for each animal are found in Supplementary Table 1.
Clinical signs were quantified by the following scoring system: 2 points each for quiet/dull/responsive disposition, hunched back/ruffled coat, hypoactivity, or mild neurological signs (abnormal gait or mild head tilt); 3 points for dehydration/decreased skin turgor; 5 points each for dyspnea, hypothermia (<34°C), or moderate neurological signs (moderate head tilt, tremors, ataxia, and/or circling); and 10 points each for inability to bear weight, frank hemorrhage, severe neurological signs (severe head tilt, seizures), or weight loss of >25% baseline (at −1 day postinfection [dpi]). Infected animals with a score of ≥10, or at completion of study (27–30 dpi for mock-infected, or 28 dpi for all NiV-infected), were humanely euthanized under deep isoflurane anesthesia via intracardiac administration of sodium pentobarbital solution or by isoflurane vapor overdose alone.
Blood samples collected at the time of euthanasia were analyzed on Abaxis Piccolo Xpress chemistry analyzers using the Metlac 12 Panel (n = 102; lithium heparinized whole blood, within 1 hour of collection) and/or the General Chemistry 13 Panel (n = 113; lithium heparinized plasma, 1 freeze/thaw cycle). The Metlac 12 panel provides data for assessing electrolyte values and preliminary acid-base status, whereas the General Chemistry 13 panel provides more complete insight into hepatic and renal function. To assess similarity of values for shared analytes in the 2 panels (blood glucose, blood urea nitrogen [BUN], creatinine [CRE], total calcium, and albumin) from paired samples (n = 99), the Wilcoxon matched-pairs test was used. Although both panels used in this study are from the same manufacturer, marginal discrepancies in values were anticipated based on different sample type (whole blood vs. plasma) and inherent variation between parallel or sequential sample runs [6]. As expected, small but consistent differences were noted among the shared analytes when comparing matched samples, but these differences were not found to significantly affect overall findings on data analyses. For shared analytes, results from the General Chemistry 13 are reported and presented in figures.
Statistical analyses were conducted using GraphPad Prism, version 8.2.1 (La Jolla, CA). The Mann-Whitney test was used to compare mock-infected control animals to those euthanized due to disease, whereas the Kruskal-Wallis test was used to compare data categorized by clinical syndrome at euthanasia. Findings were deemed statistically significant at P < .01.
For viral ribonucleic acid (RNA) detection (data also reported in [3–5]), RNA was extracted from lithium heparinized whole blood and homogenized tissue samples using the MagMAX-96 Total RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA) on a 96-well ABI MagMAX extraction platform with a DNaseI treatment step. The RNA was quantified using a one-step, real-time, reverse-transcription polymerase chain reaction (RT-PCR) targeting the N gene sequence and normalized to 18S with a SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher Scientific) according to manufacturer’s instructions (primer and probe sequences available on request). Relative copy number was determined based on a dilution series of an in vitro RNA transcript of known copy number run in parallel (Supplementary Table 1).
RESULTS
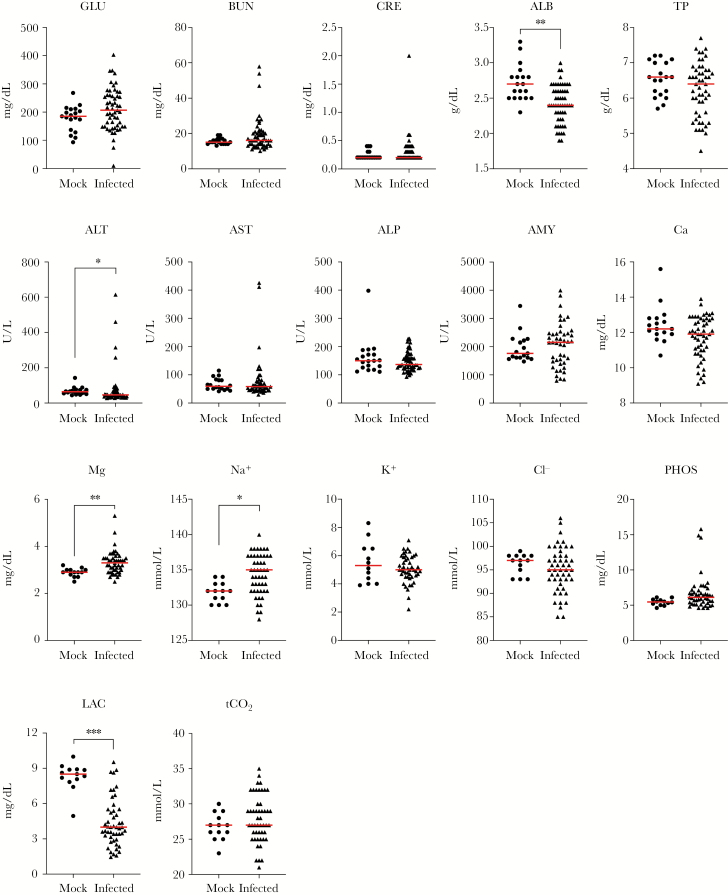
Compared to age-matched mock-infected controls that were housed and handled in parallel, NiV-infected hamsters that succumbed to disease had significantly increased magnesium and sodium and decreased albumin and lactate levels (Figure 1). Despite a small subset of individuals (4 of 55; 7%) with elevated alanine aminotransferase (ALT), a significant decrease in median ALT was observed. However, because the median value was <2- to 3-fold higher than normal range, this finding was not considered clinically significant. The other analytes examined (blood glucose, blood urea nitrogen [BUN], creatinine [CRE], total protein [TP], aspartate aminotransferase [AST], alkaline phosphatase, amylase, calcium, potassium, chloride, phosphorus, and total CO2 [tCO2]) were not statistically different between mock-infected and NiV-infected animals that succumbed to disease.
Figure 1.
Alterations in blood chemistry in terminal Nipah virus (NiV)-infected animals. Age-matched female Syrian hamsters were mock-infected (Dulbecco’s modified Eagle’s medium only, circles; n = 13 for Metlac 12 analytes or n = 19 for General Chemistry 13 analytes) or infected with NiV-Malaysia (n = 49–55, triangles). Samples from mock-infected controls and NiV-infected animals that were euthanized due to severe disease, regardless of route (intranasal or intraperitoneal), dose (103–107 50% tissue culture infective dose [TCID50]), or virus stock (wild-type or recombinant), were analyzed using the Piccolo General Chemistry 13 (plasma) or Metlac 12 (whole blood) reagent discs. Individual values and median (red bar) are depicted.*, P < .01; **, P < .001; ***, P < .0001. ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, total calcium; Cl−, chloride; CRE, creatinine; GLU, blood glucose; K+, potassium; LAC, lactate; Mg, magnesium; Na+, sodium; PHOS, phosphorus; tCO2, total carbon dioxide; TP, total protein.
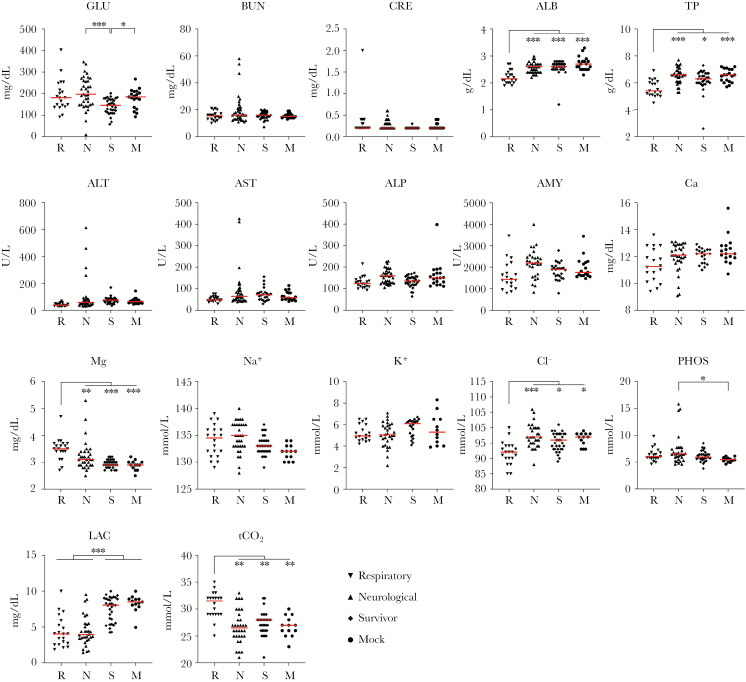
To further dissect whether clinical signs are associated with particular changes in analytes, data were grouped and analyzed by predominant clinical syndrome or by severity of disease (clinical score) at euthanasia. Animals grouped by clinical syndrome were classified as either respiratory (dyspnea, tachypnea, with or without serosanguinous froth in the lungs), neurological (head tilt, circling locomotion, loss of righting reflex, seizures, limb paresis or paralysis, or obtunded or moribund mentation), or survivors (non-specific signs or absence of clinical signs at study completion). Subjects with both respiratory and neurological signs were excluded from analyses. Survivors were found to have decreased blood glucose but no other significant findings compared with mock-infected animals (Figure 2). Animals with respiratory disease had lower albumin, TP, and chloride ions and higher magnesium and tCO2 compared with all other groups (Figure 2).
Figure 2.
Alterations in blood chemistry in NiV-infected hamsters euthanized with respiratory or neurological disease and in survivors. Age-matched female Syrian hamsters were mock-infected (Dulbecco’s days post infection modified Eagle’s medium only, circles; n = 13 for Metlac 12 analytes or n = 19 for General Chemistry 13 analytes) or infected with NiV-Malaysia and followed for up to 28 days post infection. Samples collected at euthanasia were analyzed using the Piccolo General Chemistry 13 (plasma) or Metlac 12 (whole blood) reagent discs. NiV-infected hamsters were grouped based on clinical presentation at time of euthanasia (respiratory [n = 21, inverted triangles] or neurological [n = 36, triangles]) or as survivors (n = 34, diamonds). Subjects with both respiratory and neurological signs were excluded from analyses (n = 5). Individual values and median (red bar) are depicted. *, P < .01; **, P < .001; ***, P < .0001. ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Ca, total calcium; Cl−, chloride; CRE, creatinine; GLU, blood glucose; K+, potassium; LAC, lactate; Mg, magnesium; Na+, sodium; PHOS, phosphorus; tCO2, total carbon dioxide; TP, total protein.
Alterations in animals with neurological disease included notably increased blood glucose values; these elevations were not statistically different from mock-infected or those in animals with respiratory disease, but they were significantly higher compared with survivors, which had the lowest values of all groups. Phosphorus was higher in animals with neurological disease than in mock-infected, but not compared with other groups. In addition, a subset of animals with neurological disease (4 of 36; 11%) had clinically significant elevations in both AST and ALT compared with mock-infected animals, consistent with hepatitis (>3-fold higher). Of note, although these animals had detectable viral RNA in the liver (102–104 copies), overall, copy number was not correlated to elevated liver enzymes. Changes observed in both respiratory and neurological disease included decreased lactate values, which were remarkably lower than in mock-infected or survivors (Figure 2).
Two approaches were used when grouping animals by severity of disease at euthanasia, both of which were then compared with mock-infected animals: (1) score of ≤2 or ≥8, or (2) score of <5 or ≥5. The more polarized scoring method, separating those that were asymptomatic or had mild signs from those were euthanized due to severe disease, did not yield more granular detail than the <5 or ≥5 approach. Analyte changes were only observed in animals scoring ≥5 or ≥8 (Supplementary Figures 1 and 2) and reflected the changes described earlier when comparing mock-infected to NiV-infected animals that succumbed to disease (Figure 1).
DISCUSSION
In Syrian hamsters, NiV disease manifests as a respiratory syndrome, a neurological syndrome, or a combination of both. A subset of infected animals (~20%–30% depending on dose and route) present with less severe, more generalized clinical signs (ruffled fur, hunched posture, decreased activity level) despite presence of viral RNA in a variety of tissues, including brain, lung, liver, spleen, gonads, eye, kidney, and heart, at time of euthanasia [3]. Disease in humans is characterized as a febrile illness with encephalitis and/or respiratory involvement that may progress to acute respiratory distress syndrome [7]. To date, clinical data, including histopathology [8], are based on a relatively small number of case reports [9, 10]. Hematologic changes that may occur during infection include thrombocytopenia, leukopenia, and increased hepatic (ALT and AST) and renal (BUN and CRE) markers. Blood chemistry data are similarly limited in animal models: hypoalbuminemia, hyperglycemia, and increased BUN are reported in ferrets [11]; hypoalbuminemia and increased BUN, CRE, AST, and gamma-glutamyl transferase are reported in African green monkeys (Chlorocebus aethiops) [12].
Similar to NiV infection in humans and African green monkeys [12], hypoalbuminemia (and correspondingly hypoproteinemia) is a key finding in infected hamsters, in particular those with respiratory disease (Figure 2). Potential mechanisms for this finding include poor nutritional state due to anorexia or critical illness, hepatic insufficiency, body cavity transudates, and hemodilution as a result of fluid loss into the respiratory spaces. In a cohort of human patients with recrudescent neurological disease, hyponatremia was noted, but otherwise no bloodwork changes were found [13]. In this study, animals exhibiting neurological disease had slightly higher sodium levels compared with mock-infected animals, although these values were not consistent with hypernatremia (defined as >145 mmol/L). Potential explanations for this finding include decreased body water stores (e.g., from reduced ability to drink due to neurological signs) and third-space loss into tissues from other NiV-related processes. Although we found many similarities to previous reports, we did not detect hyponatremia or consistent significant changes in BUN or CRE in these hamsters. These differences may reflect anatomical and physiological characteristics in hamsters compared with humans (higher metabolic rate, fermenting nonglandular forestomach, and hindgut, inability to vomit, and desert-acclimated behaviors and physiology), or other species, or may be due to low availability of clinical chemistry data in both human cases and animal studies.
The availability of a large, controlled, hamster data set supports potential identification of previously unrecognized NiV-mediated alterations in blood chemistry. One such finding includes the elevated tCO2 in hamsters with compromised respiratory function. Virus is detected in the lungs of animals that succumb to respiratory disease [3], and longitudinal studies report pulmonary anti-NiV immunohistochemistry staining predominantly in the interstitium and pulmonary vessels, with rare staining in bronchi. In addition, radiological findings include moderate interstitial infiltrates and areas of consolidation, followed by rapidly progressive increases of pulmonary infiltrates throughout the lung fields and formation of air bronchograms [14]. Our proposed mechanism for the elevated tCO2 is inefficient gas exchange resulting in hypoxia and hypercapnia secondary to viral pneumonitis. However, other physiologic processes may also explain the findings. A lack of other acid-base disorder signifiers, including partial pressure of carbon dioxide and blood pH, precludes full clinical interpretation at this time. Another unexpected finding was the significantly decreased lactate values for euthanized animals compared with survivors and mock-infected animals. Elevated lactate values are often observed in acutely ill patients, most frequently due to poor peripheral perfusion of tissues; decreased lactate is not a common finding in disease. The clinical relevance and physiological processes contributing to this relative decrease in animals with lethal NiV disease are unclear; lack of blood pressure data and other cardiovascular parameters prevent further assessment of this finding at this time.
Of note, potassium and phosphorus values, even in mock-infected animals, were approximately half of the reference intervals for comparably aged adult (7- to 8-week-old) female hamsters reported by the vendor (Envigo, https://www.envigo.com/resources/data-sheets/hamster-female-cbc.pdf); however, they were more similar to published reference intervals for hamsters [15]. These changes may be due to differences in sampling technique, testing platform, or husbandry practices and emphasize the importance of negative control animals in experiments such as these.
CONCLUSIONS
In summary, our data are consistent with alterations noted in existing literature in other species for a subset of analytes (increased blood glucose and liver enzymes and decreased albumin), whereas we did not observe other changes previously reported (we found normal to slightly elevated sodium, and no significant changes in median BUN or CRE). To our knowledge, we are the first to report elevated tCO2 and decreased lactate in NiV disease. Altogether, these data provide additional support for hematological abnormalities consistent with NiV disease for both humans and animals, and they highlight additional analytes to investigate in clinical cases that may aid in our understanding of viral pathophysiology and in case management.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge Tatyana Klimova for assistance editing the manuscript and members of the Centers for Disease Control and Prevention’s Comparative Medicine Branch for assistance with animal husbandry.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was partially funded by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention (CDC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and CDC (to S. R. W.), the DARPA INTERfering and Co-Evolving Prevention and Therapy (INTERCEPT) program (DARPA-BAA-16-35), and by CDC Emerging Infectious Disease Research Core Funds.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Nikolay B, Salje H, Hossain MJ, et al. Transmission of Nipah virus - 14 years of investigations in Bangladesh. N Engl J Med 2019; 380:1804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rockx B. Recent developments in experimental animal models of henipavirus infection. Pathog Dis 2014; 71:197–204. [DOI] [PubMed] [Google Scholar]
- 3. Welch SR, Scholte FE, Harmon JR, et al. In situ imaging of fluorescent Nipah virus respiratory and neurological tissue tropism in the Syrian hamster model. J Infect Dis 2020;221(S4):S448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lo M, Spengler J, Welch SR, et al. Evaluation of a single-dose nucleoside-modified mRNA vaccine platform against lethal Nipah virus infection in Syrian hamsters. J Infect Dis 2020;221(S4):S493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lo M, Spengler J, Krumpe L, et al. Griffithsin inhibits Nipah virus entry and fusion, and can protect Syrian hamsters from lethal Nipah virus challenge. J Infect Dis 2020; 221(S4):S480–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spengler JR, Welch SR, Genzer SC, et al. Suboptimal handling of Piccolo samples or reagent discs for consideration in Ebola response. Emerg Infect Dis 2019; 25:2017–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee S, Gupta N, Kodan P, et al. Nipah virus disease: a rare and intractable disease. Intractable Rare Dis Res 2019; 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong KT, Shieh WJ, Kumar S, et al. ; Nipah Virus Pathology Working Group Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am J Pathol 2002; 161:2153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim CC, Lee WL, Leo YS, et al. Late clinical and magnetic resonance imaging follow up of Nipah virus infection. J Neurol Neurosurg Psychiatry 2003; 74:131–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goh KJ, Tan CT, Chew NK, et al. Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med 2002; 342:1229–35. [DOI] [PubMed] [Google Scholar]
- 11. Satterfield BA, Cross RW, Fenton KA, et al. et al. Nipah virus C and W proteins contribute to respiratory disease in ferrets. J Virol 2016; 90:6326–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geisbert TW, Mire CE, Geisbert JB, et al. Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody. Sci Transl Med 2014; 6:242ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong SC, Ooi MH, Wong MN, Tio PH, Solomon T, Cardosa MJ. Late presentation of Nipah virus encephalitis and kinetics of the humoral immune response. J Neurol Neurosurg Psychiatry 2001; 71:552–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rockx B, Brining D, Kramer J, et al. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J Virol 2011; 85:7658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loeb W, Quimby F, eds. The Clinical Chemistry of Laboratory Animals. 2nd ed Philadelphia, PA: Taylor and Francis; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.