Abstract
The association between increased serum urate and hypertension has been a subject of intense controversy. Extracellular uric acid drives uric acid deposition in gout, kidney stones, and possibly vascular calcification. Mendelian randomization studies, however, indicate that serum urate is likely not the causal factor in hypertension although it does increase the risk for sudden cardiac death and diabetic vascular disease. Nevertheless, experimental evidence strongly suggests that an increase in intracellular urate is a key factor in the pathogenesis of primary hypertension. Pilot clinical trials show beneficial effect of lowering serum urate in hyperuricemic individuals who are young, hypertensive, and have preserved kidney function. Some evidence suggest that activation of the renin–angiotensin system (RAS) occurs in hyperuricemia and blocking the RAS may mimic the effects of xanthine oxidase inhibitors. A reduction in intracellular urate may be achieved by lowering serum urate concentration or by suppressing intracellular urate production with dietary measures that include reducing sugar, fructose, and salt intake. We suggest that these elements in the western diet may play a major role in the pathogenesis of primary hypertension. Studies are necessary to better define the interrelation between uric acid concentrations inside and outside the cell. In addition, large-scale clinical trials are needed to determine if extracellular and intracellular urate reduction can provide benefit hypertension and cardiometabolic disease.
Keywords: blood pressure, fructose, hypertension, renin–angiotensin system, uric acid, xanthine oxidase
In the 1870s, a young medical student by the name of Frederick Akbar Mahomed took on a research project at Guy’s Hospital with the goal of measuring blood pressure. The sphygmograph had been recently invented by the French physiologist, Etienne-Jules Marey, but it was wieldy and not quantitative, so Mahomed constructed a lighter weight model that provided actual measurements. Using the machine, he confirmed the association of kidney disease (as noted by proteinuria) with high blood pressure, but he also noted that subjects could have high blood pressure in the absence of kidney disease, which turned out to be the first description of primary hypertension. He then noted that this latter condition was frequently associated with elevated serum urate, and he postulated that uric acid might have a causal role in the condition.1
Since Mahomed’s initial discovery, there have been thousands of articles that have evaluated the relationship of uric acid and hypertension with no clear consensus of the role of uric acid in this condition.2,3 In this review, we will summarize the current evidence for a causal role for the association of uric acid with hypertension, and discuss the major controversies in the literature.
URIC ACID BIOLOGY
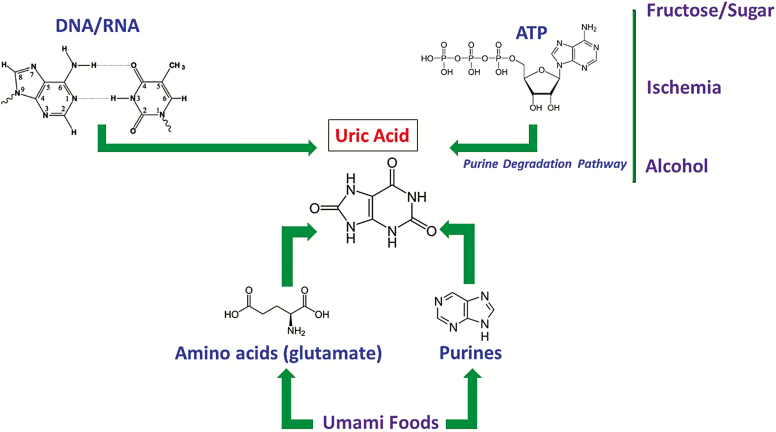
Uric acid is an end product of purine metabolism (Figure 1). Uric acid can be generated from amino acid precursors or from purines provided in the diet. Indeed, one source of foods that stimulates uric acid production are those that solicit the umami taste,4 which include glutamate-rich foods (i.e., glutamate is metabolized to uric acid in the liver) and purine-rich foods, especially those that contain inosine monophosphate (IMP), adenosine monophosphate (AMP), or uric acid itself. Uric acid can also be generated from the breakdown of DNA and RNA (such as occurs with tumor lysis syndrome) or from the breakdown of ATP (such as occurs with fructose metabolism or alcohol). We have recently shown that high salt diets and high glycemic diets can induce the expression of aldose reductase that leads to increased fructose generation and metabolism in the liver, resulting in an increase in intracellular uric acid production.5,6 Ischemia can also activate the transcription factor HIF1 alpha and NFAT5 that can induce aldose reductase and xanthine oxidase with the production of both endogenous fructose and uric acid generation.7 Similarly, heat stress and dehydration act through similar pathways to generate uric acid.8
Figure 1.
Generation of uric acid, a purine endproduct. Uric acid can be generated from precursors, primarily umami-based foods, as well as from ATP degradation, such as occurs with fructose-based sugars, ischemia, and alcohol, or from cell turnover and injury with release of RNA and DNA. The endogenous production of fructose can occur from high glycemic and high salt diets that activate aldose reductase.
Uric acid is both metabolized and excreted. In the remote past, our human ancestors metabolized most of the uric acid we produced with the enzyme uricase (also known as urate oxidase), generating 5-hydroxyisourate and eventually allantoin. However, uricase was fully mutated, resulting in it being nonfunctional in humans today.9 Nevertheless, uric acid can still be metabolized as it can react with oxidants, lipid radicals, with peroxynitrite to generate triuret, and with nitric oxide to make 6-aminouracil.10,11 These products are normally minimally generated but are overproduced in those people who smoke, have preeclampsia, or who have chronic kidney disease (CKD) or diabetes.10,12,13
Uric acid is excreted by the kidneys (two-thirds) and the gut (one-third). For the kidney, the normal fractional excretion is about 10% but it can increase as eGFR falls and this may increase the risk for crystallization in the setting of low urine pH. The primary drivers of urate reabsorption are the transporters URAT1 and Glut9, while ABCG2 pumps uric acid into the urine. However, in the gut, SLC2A9 and ABCG2 are important in secreting uric acid into the gut lumen where it is degraded by bacteria.
Genetics also play a role in uric acid metabolism, and recent GWAS studies have identified over 180 genes that influence serum uric acid levels, accounting for nearly 8% of the variance in serum urate levels.14 Genetic polymorphisms in SLC2A9 account for the majority of this variance.14 Most evidence supports diet and/or obesity as having the primary role in the rise in serum urate over the last century,15 but genetics may be playing a dominant role in the mild differences observed in those subjects on a stable western diet.16
URIC ACID AND HYPERTENSION: EXPERIMENTAL STUDIES
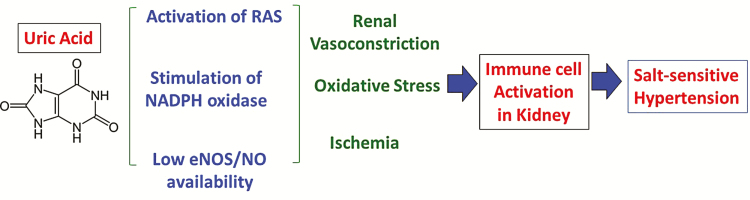
Since rats and mice, unlike humans, produce urate oxidase, the development of a hyperuricemic model has generally involved inhibiting or knocking down mouse or rat uricase. The mouse knockout model develops exuberant hyperuricemia and kidney disease, but studies have shown that they also develop modest metabolic abnormalities in insulin secretion (in male mice) with the development of hypertension and lipid abnormalities (in female mice).17 The more common model of hyperuricemia is performed by inhibiting rat uricase with oxonic acid as these rats develop modest hypertension that is prevented by a variety of agents that lower serum urate, as well as by inhibitors of the renin–angiotensin system (RAS), agents that block oxidative stress, or compounds that increase endothelial nitric oxide production.18–21 While initially the hypertension is fully reversible by uric acid lowering agents, as kidney disease develops (as noted by the development of an arteriolopathy and interstitial inflammation), the hypertension becomes salt-sensitive and uric acid-independent.22 These observations are consistent with recent studies that suggest hypertension may initially result from the generation of kidney vasoconstriction, ischemia, and oxidative stress which are followed by the activation of immune mechanisms cause a persistent inflammation in target organs that drive the increase in blood pressure (BP).23,24 A pathway showing the proposed mechanism of uric acid-induced hypertension is shown in Figure 2.
Figure 2.
Potential mechanism of uric acid induced hypertension. Uric acid induces oxidative stress, a decrease in endothelial nitric oxide availability, and activation of both plasma renin activity and intrakidney angiotensin activity, leading to kidney vasoconstriction, ischemia, and oxidative stress in the kidney. This triggers activation of the immune system that causes persistent kidney vasoconstriction and salt-sensitive hypertension.
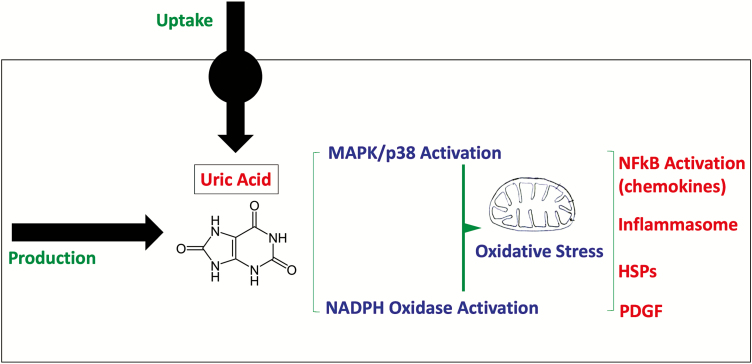
The cellular mechanisms driving the hypertensive response involve either intracellular production of uric acid (via xanthine oxidase) or uptake of urate into the target cell, followed by the induction of mitogen activated protein kinases (such as p38) and a burst of oxidative stress mediated by NADPH oxidase. This is associated with oxidative stress in the mitochondria, resulting in the inhibition of aconitase and enoyl CoA hydratase, as well as activation of stress pathways involved in inflammation and immune activation (NFKB, chemokines, inflammasomes, heat shock proteins), cell proliferation (PDGF), and vasoconstriction pro(renin) and intracellular angiotensin system, endothelin, thromboxanes.25–34 While the response varies, inflammatory effects of soluble uric acid have been demonstrated in many cell types, including monocytes, vascular endothelial and smooth muscle cells, kidney tubular cells, hepatocytes, adipocytes, and pancreatic islet cells. Cellular pathways of inflammation are shown in Figure 3.
Figure 3.
Intracellular mechanisms of uric acid induced inflammation. Uric acid can be produced inside the cell by activation of xanthine oxidoreductase or by uptake via specific urate transporters. Once uric acid levels increase, there is activation of both mitogen activated protein kinases (MAPK) as well as stimulation of NADPH oxidase that triggers mitochondrial and cytoplasmic oxidative stress. This is associated with activation of inflammatory pathways (including NFκB activation, inflammasome activation, release of growth factors and heat shock proteins) as well as a down regulation of energy production by the mitochondria with a shift toward glycolysis.
URIC ACID AND HYPERTENSION: EPIDEMIOLOGICAL STUDIES
Hyperuricemia is common in people who present with primary hypertension, and tends to be especially common in those with accelerated (or malignant) hypertension.35 Some of the hyperuricemia might represent coexistent CKD or the use of thiazide diuretics that increase serum uric acid levels. However, hyperuricemia can be present in individuals in the absence of these findings. In children, the cutoff for an elevated serum urate is in the 5.2 to 5.5 mg/dl range, and children and adolescents presenting with new onset hypertension often have serum urate levels above this cutoff.36–38 Similarly, in pregnancy, serum urate usually falls to less than 4 mg/dl, but many patients presenting with hypertension or preeclampsia tend to have serum urate levels >4 mg/dl.39 In hypertensive individuals who have hyperuricemia, there is also an increased frequency to be hypertensive during sleep (nondipping type)40,41 and serum urate tends to correlated better with central BP than pulsatile pressure.42 People with hyperuricemia and hypertension also tend to have elevated plasma renin activity.43,44 Another aspect of uric acid and BP is that the relationship tends to be linear between the 3 and 10 mg/dl range.45,46 Sugary beverages containing fructose also increase serum urate and are associated with hypertension.47 Indeed intake of fructose-containing sweetened beverages is dose-dependently associated with incident hypertension, although this association was not found when other food sources containing fructose were analyzed.48 The discrepancy may relate to the fact that fructose contained in fluids is absorbed more rapidly in the gut, resulting in greater concentrations in the portal circulation and liver, in comparison with solid foods in which fructose is absorbed more slowly due to presence of fiber and other foods.49
Hyperuricemia is also a potent independent predictor of hypertension, with an approximately two-fold increased risk within 5–10 years.50–52 The risk is less evident in the elderly or who have pre-existent kidney disease.
Hyperuricemia also is a potent independent predictor of incident CKD, of metabolic syndrome and its various components, and of nonalcoholic fatty liver disease.53–55 Thus, these studies support the hypothesis that hyperuricemia might predict the development of hypertension.
URIC ACID AND HYPERTENSION: MENDELIAN RANDOMIZATION STUDIES
Genome wide association studies (GWAS) have been used to identify genetic polymorphisms that control serum urate levels, allowing the development of a genetic scoring system to evaluate the risk for hypertension in large population studies. In an approach called Mendelian randomization, the genetic risk scores for hyperuricemia have been found to predict the risk for gout, but failed to predict the development of hypertension, CKD, and cardiometabolic diseases.56–58 Results in studies that have linked genetic scores for hyperuricemia with hypertension and metabolic diseases are thought to be due to pleiotropy, in which the genetic polymorphisms influence both serum urate and cardiometabolic disease through common pathways.14,59,60 However, this interpretation is linked with similar assumptions as multivariable analysis that assume variables are independent and not causally linked via bidirectional or multidirectional pathways.61 Only two Mendelian Randomization studies found the genetic score to be associated with cardiovascular disease (sudden cardiac death)62 and diabetic vascular disease.63 However, smaller studies have linked polymorphisms with increased risk for obesity, hypertension, and CKD.64–67
Mendelian randomization studies provide strong evidence that serum urate is not causal in cardiometabolic disease, but one must remember that it is the intracellular uric acid that has the biological effects while extracellular uric acid is thought to be involved primarily as a risk factor for gout. Indeed, most of the genetic score is based on polymorphisms that affect urate transport as opposed to uric acid generation, but it is not known how these transporters regulate intracellular urate. It is known that the polymorphisms in the major transporter (SLC2A9) affecting the genetic score have widely different effects depending on where it is knocked out, with it causing hyperuricemia without hypertension in the liver specific knockout, hyperuricemia with hypertension in the intestinal knockout, and hypouricemia if it is the systemic knockout.68–70 Likewise, our group has found that high salt diets induce intracellular fructose metabolism and intracellular hepatic urate generation with a rise in BP and development of metabolic syndrome but without the development of hyperuricemia,6 thus showing how even diet can have opposing effects on intracellular vs. serum urate. It is therefore of interest that polymorphisms in xanthine oxidase that drives urate production have been reported to predict the development of hypertension.71–74
CLINICAL TRIALS OF LOWERING URIC ACID IN HYPERTENSION
To date there have been only a few studies with small numbers of subjects that have investigated whether uric acid lowering therapy can reduce BP in hypertension associated with hyperuricemia (Table 1).
Table 1.
Brief summary of clinical trials of lowering BP in hypertension
| Subjects | Agent | Effects | Reference |
|---|---|---|---|
| Children and adolescent with hypertension | AP | A reduction in BP, plasma renin activity and systemic vascular resistance | 43 |
| Prehypertensive obese adolescents | AP | A reduction in BP and body weight | 75 |
| Adolescent with primary hypertension | AP (with ACEi) | A reduction in BP (further reduction with ACEi) | 76 |
| Hypertensive adults | Fx | Fail to lower BP except in those with normal kidney function (40% subject were on RAS inhibitor) | 77 |
| Older subjects with recent ischemic attack or stroke | AP | A reduction in BP Less progression of carotid intimal thickness | 78 |
| Type 2 diabetic with normal renal function | AP | Improvement in blood pressure, kidney function, insulin resistance, and inflammation | 79 |
| Prehypertensive with mild hyperuricemia | AP | Dipping pattern of BP were observed | 80 |
| Gouty patients | Pegloticase | A reduction in BP | 81 |
| Normotensive subjects | Minor reduction in systolic BP in subject with asymptomatic hyperuricemia | 82,83,84 | |
| Normotensive subjects with anti-hypertensive agents | XO inhibitor | No effect on BP A reduction in plasma renin activity and plasma aldosterone in hyperuricemic subjects | 85,86 |
| Hypertensive and CKD patients | Febuxostat | A reduction in BP | 87 |
| CKD subjects | Removing AP | BP and renal function got worse in subjects who were not on RAS inhibitors. | 88 |
| Volunteers on low fructose diet | AP | A reduction in BP and serum UA | 89 |
| Overweight | Low fructose diet (with AP) | A reduction in BP (BP was further reduced with AP) | 80 |
Hypertension in children and adolescents
The strongest data have been observed in hyperuricemic children with primary hypertension. In an 8-week, double blind, placebo-controlled crossover study, allopurinol was found to reduce both clinic and ambulatory BP in adolescents with newly diagnosed hypertension compared with controls, and this was associated with a significant reduction in systemic vascular resistance with a fall in plasma renin activity.43 In those subjects who achieved serum urate levels at the goal of <5.0 mg/dl, allopurinol normalized the BP of 19 of 22 subjects while placebo treatment resulted in normal BP in only one of 30 subjects. In a second parallel study in obese prehypertensive subjects, both allopurinol and probenecid also showed a significant fall in systemic BP in prohypertensive obese adolescents. An added benefit was that the lowering of uric acid was associated with a decrease in weight (1 kg) in the allopurinol group while the placebo group gained 2 kg over the 8-week study period.75 A third study showed that the benefit of allopurinol in adolescents with primary hypertension was additive to the effects of an ACE inhibitor.76 These studies suggest that xanthine oxidase inhibitors might be a useful therapy in childhood-onset hypertension.
Hypertension in adults in the absence of kidney disease
A randomized trial of 120 subjects randomized to febuxostat or placebo in adults with hyperuricemia and hypertension reported that the lowering of serum urate in the hypertensive general population did not lower ambulatory BP in the overall analysis. However, there was a significant reduction in BP in the prespecified group with normal kidney function.77 In this trial, approximately 40% of subjects were on blockers of the RAS.77 In another study of older (mean age 68 years) subjects with a recent transient ischemic attack or stroke, a randomized trial comparing allopurinol to placebo showed a greater fall in BP in the allopurinol group along with less progression of carotid intimal thickness at 12 months.78 In a 3-year study of type 2 diabetic subjects with hyperuricemia and normal kidney function, the use of allopurinol was associated with an improvement in BP as well as kidney function, insulin resistance (HOMA-IR), and inflammation (hs-CRP levels).79 Another placebo-controlled study reported that allopurinol could lower ambulatory systolic BP in prehypertensive subjects with modest elevations in serum urate (mean serum urate 6–6.2 mg/dl) over 4 weeks compared with placebo, and this was associated with an increase in a dipping pattern of BP and greater weight loss.80 A post hoc analysis of a study in subjects with gout also reported an improvement in BP in subjects receiving pegloticase every 2 weeks who were responders (meaning their serum urates fell significantly in response to treatment) compared with the controls. Importantly, in these hyperuricemic subjects serum urate levels tended to fall to less than 3 mg/dl.81
Uric acid lowering therapy has also been given to individuals whose BP was in the normal range, either because they were normotensive or because they were receiving anti-hypertensive agents. The lowering of serum urate does not appear to significantly lower BP in normouricemic subjects with normal BP, nor is there an effect on plasma renin activity,83 while minor reductions in systolic BP were observed in subjects with asymptomatic hyperuricemia and normal BP.82,84 Likewise, hypertensive subjects with normal blood pressures on anti-hypertensive treatment also do not show further improvement in BP with xanthine oxidase inhibition,85,86 although in the study in which mean serum urate values were in the hyperuricemic range, some reduction in plasma renin activity and plasma aldosterone was observed.86 These studies suggest that xanthine oxidase inhibitors are unlikely to lower BP in normotensive subjects, especially if the individuals are not hyperuricemic.
Hypertension in the setting of kidney disease
Several studies that have evaluated the effect of lowering serum urate on BP in subjects with CKD have not noted any significant BP lowering effect compared with controls.91–93 One study of subjects with stage 2 and stage 3 CKD did note a greater change in systolic BP in the febuxostat group (−13 mm Hg) compared with the control group (−4 mm Hg) although differences in final absolute BP did not reach significance.87 On the basis of the experimental studies, the reason may relate to immune mechanisms becoming dominant in driving BP in subjects with kidney disease, and also because inhibitors of the RAS are commonly used in subjects with kidney disease and may mimic some of the effects of xanthine oxidase inhibitors. Indeed, Talaat and el-Sheikh88 performed an interesting study in which subjects with CKD who were on allopurinol were taken off allopurinol for 12 months to determine if it had any effect on BP. The striking finding was a marked increase in systolic and diastolic BP with a worsening of kidney function in the subjects with CKD who were not on RAS blockade, whereas those who were on ACE inhibitors or angiotensin receptor blockers (ARBs) remained stable. Furthermore, Shi et al.90 performed a randomized clinical trial in subjects with IgA nephropathy in which use of RAS inhibitors was not allowed, and in this study a significant decrease in BP occurred in the subjects given allopurinol who were not on antihypertensive agents along with a reduction in anti-hyperpertensive agents in those who were on medication, whereas no effect on BP was observed in the control group.
The effect of modulating uric acid levels by diet or direct infusion of uric acid
Soluble uric acid has also been administered intravenously to humans, but despite raising serum urate by more than 2 mg/dl, acute changes in BP were not found.94 Similarly, clinical trials in which inosine has been given orally to subjects with multiple sclerosis or Parkinson’s disease have not observed an effect of raising serum uric acid on BP.95,96 In contrast, when intracellular urate is increased, such as by fructose,97 a hypertensive response can be observed.98 Perez-Pozo et al. performed a trial in which 200 g of fructose was given daily for 2 weeks to with or without allopurinol. The group receiving fructose alone had a marked rise in serum urate as well as a rise in clinic BP that were both prevented in the group receiving allopurinol.89 Madero et al. conducted a pilot study with overweight subjects in which a low fructose diet or isocaloric control diet was given for 4 weeks. The low fructose group showed a reduction in BP that was further decreased when the subjects were additionally treated with allopurinol for 4 weeks.80 These studies suggest that intracellular urate may be more important in the BP response that simply raising serum urate.
Special settings
Lead intoxication is known to cause saturnine gout and to be associated with hyperuricemia, hypertension, and kidney disease.99 It is not known if the hypertension associated with lead poisoning in humans is amenable to urate lowering therapy, although in experimental animals the hypertension is uric acid-dependent.100 In addition, some of the cardiovascular benefits of SGLT2 inhibitors101 and of losartan (an ARB)102 may relate to their ability to also lower serum urate.
ADDITIONAL BENEFITS
Some studies suggest additional benefits of lowering serum urate, including possible beneficial effects on insulin resistance and HbA1c values, on weight gain, and on kidney function.75,79,103,104 Some studies also suggest the benefit on cardiovascular and kidney outcomes is best observed if the treatment using a xanthine oxidase inhibitor is maintained for 3 years or more.79,103,105 Currently, there is a large placebo-controlled study evaluating whether allopurinol use provides additional cardiovascular benefit in subjects with preexisting ischemic heart disease (the ALL-HEART study).106
SAFETY CONCERNS
Currently, all uric acid-lowering therapies have potential toxicities. For example, allopurinol can result in a hypersensitivity syndrome similar to a Stevens–Johnson reaction, and this is observed almost exclusively in individuals who carry the HLA-B*58 genotype, especially Chinese Han people (3–7%), followed by African Americans (3%) and Caucasians (0.5%). We recommend genotyping Asian individuals before initiating allopurinol in this group. Febuxostat does not appear to carry this risk. However, a secondary endpoint analysis in the Cardiovascular Safety of Febuxostat and Allopurinol in Patients With Gout and Cardiovascular Morbidities (CARES) Study reported that in subjects at cardiovascular risk, febuxostat carried greater risk for cardiovascular mortality than allopurinol.107 In particular, febuxostat was associated with a higher risk for sudden cardiac death than allopurinol (2.7% vs. 1.8%). This has led to an FDA black box warning on the use of febuxostat in subjects at cardiovascular risk, which characterizes many subjects with hyperuricemia. However, this study was associated with an excessively high dropout (57%) rate, and an analysis of the cardiovascular mortality documented that many of the events occurred when febuxostat or allopurinol were stopped, with an 18-fold increased rate in the first 30 days following withdrawal.108 This suggests that the withdrawal of xanthine oxidase inhibitors might be associated with a rebound effect. One possibility is that chronic inhibition of xanthine oxidase might lead to increased xanthine oxidase expression that leads to an exuberant response when the inhibitors are withdrawn. Based on the study by Talaat and el-Sheikh,88 this might relate to enhanced stimulation of the RAS, and argues for placing an individual on an ACE inhibitor or ARB if a xanthine oxidase inhibitor is stopped in a subject at cardiovascular risk.
Other uric acid lowering therapies may also carry some risk. For example, uricosuric agents (especially if given alone) can result in uric acid kidney stones or rarely acute kidney injury, possibly related to the effects of high urinary urate levels with or without crystal formation.109 In contrast, recombinant uricase (such as rasburicase or pegylated uricase) can be associated with allergic reactions resulting from an immune response to the uricase peptides that can rarely result in anaphylaxis and may also impair efficacy.110
EVOLUTIONARY CONSIDERATIONS
The mutation of uricase in the Homo lineage occurred during a period when our ancestors were starving and close to extinction in Europe, and may have occurred as a survival mechanism to maintain BP and enhance fat stores.111 Recent studies suggest that there is a primary survival pathway used by many species that is mediated by either dietary or endogenously produced fructose. This pathway is initiated by a drop in cellular energy associated with the stepwise degradation of ATP and the generation of uric acid that leads to a reduction in mitochondrial metabolism with an increased stimulation of glycolysis.112 Studies suggest that the uricase mutation, which approximately doubled serum uric acid from 1–2 to 3–4 mg/dl, acted to amplify this pathway as a survival mechanism at a time when food (and especially fructose) was scarce.9
A trade-off of the uricase mutation was the reduced ability to control serum and intracellular urate levels, and in the setting of high fructose, and umami-rich high purine foods led to hyperuricemia with its risk for extracellular urate deposition (gout and kidney stones) and high intracellular urate levels (increasing the risk for obesity, diabetes, and hypertension). Indeed, while intracellular urate may drive some of kidney hemodynamic effects (glomerular hypertension and systemic vascular resistance),27,113 the necessity for kidney excretion increases the risk for urate crystalluria and acute kidney injury, especially in the setting of heat stress, dehydration, diabetes, or increased purine load.114–116 This may be why certain polymorphisms that lower serum urate by increasing urate excretion can be associated with increased risk for CKD.117
It has also been suggested that the uricase mutation increased extracellular anti-oxidant activity due to the ability of uric acid to scavenge peroxynitrite (to form triuret) and to react with singlet oxygen (1O2), lipid-derived radicals and ferric iron (urate-Fe3+-urate).118,119 In the latter reaction, uric acid serves to diminish the oxidizing potential of Fe3+ and thus may reduce overall oxidant burden. While the reaction with 1O2 and lipid radicals does appear protective, uric acid generates oxygen-free radicals when it binds to peroxynitrite120 or participates in myeloperoxidase (MPO)-based reactions.121 The antioxidant activity of uric acid has been proposed to provide neural protection, and that in conditions, such as multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease, the presence of a low serum urate may increase risk for the disease and/or its progression. However, recent studies suggest that uric acid levels are high in the sera and cerebral spinal fluid of subjects with multiple sclerosis and reflect mitochondrial dysfunction122,123 and is consistent with the lack of beneficial effect observed with inosine therapy to raise uric acid in this disorder.124 Likewise, while serum urate levels were originally reported to be low in Alzheimer’s disease, this may reflect the effect of chronic disability to impair adequate food intake, and meta-analyses suggest no difference in serum uric acid in this condition.125 Indeed, in Alzheimer’s disease there may be evidence for intracerebral activation of fructose metabolism resulting in cerebral insulin resistance and mitochondrial dysfunction, with potential toxic effects of intracerebral urate.126–129 Only in Parkinson’s disease is there any evidence that low serum urate may confer risk and that orally administered inosine may provide benefit by raising serum urate, at least in women.130
Likewise, countervailing associations between uric acid and stroke have been realized where hyperuricemia is reported to be predictive of an ischemic event as well as poorer outcomes131,132 whereas intravenous administration of uric acid post stroke (with and/or without TPA) appears to afford benefit.131,133 A potential explanation for protective effects of uric acid in stroke may be its antioxidant capacity as described above; however, the fact that UA, itself, is an xanthine oxidase (XO) inhibitor may also play a role. For example, XO inhibition by UA has been shown in human plasma (IC50 = 300 µM)134,135 well-within the physiologic range and thus, this product-based inhibition may produce salutary actions in an ischemic and subsequently reperfused setting where XO has been reported to be a major source of oxidant generation. In summary, the role assumed by uric acid in these disease processes may be determined not only by its concentration, but importantly by its abundance in the circulation vs. its presence in the intracellular compartment.
SUMMARY
The role of uric acid in the pathogenesis of human disease depends on whether the increase in urate concentration is inside the cell, where it may have a role in hypertension and metabolic disease, or outside the cell, where it causes extracellular urate depositions diseases, such as gout and nephrolithiasis. The observation that genetic scores for serum urate predict sudden cardiac death62 and diabetic vascular disease63 may relate to the ability of extracellular urate to deposit in plaque and to form crystalline deposits in coronary and other major blood vessels where it may also act as a nidus for calcification.136 Indeed, the observation for increased sudden cardiovascular death following withdrawal of xanthine oxidase inhibitors108 may relate to the rapid rebound of serum urate with its potential for crystallization at sites of plaque similar to a gout attack or possibly a surge in plasma renin activity. High serum uric acid may also predispose to increased urinary excretion that can be exacerbated with purine loads or fructose intake114 which could lead to kidney injury especially in the setting of dehydration or heat stress. Intracellular urate will also be more likely elevated when serum urate is elevated, due to the ability of urate to enter into cells via specific transporters.137 Thus, extracellular uric acid may also carry cardiometabolic risk, despite intracellular urate having the dominant role. Dissociation of intracellular and extracellular levels is possible, and increased intracellular urate production may be driven by either polymorphisms in urate transporters or by diet (fructose, glutamate and purine rich foods, high glycemic carbohydrates and high salt diets). The complex relationship between extracellular and intracellular urate concentration is central in the interpretation of the controversies raised by apparently contrasting studies on the pathogenic role of uric acid levels in primary hypertension.
FINAL RECOMMENDATIONS
We believe that young Frederick Mahomed was correct when he suggested in the 1870s that uric acid might have a contributory and causal role in primary hypertension.1 We suspect that the rise in hypertension over the last century largely reflects the rise in sugar and salt intake and other components of the western diet that can influence intracellular urate levels. Reducing sugar, fructose, and salt intake,80,138–140 and increasing water intake to suppress vasopressin141 have emerged as potential strategies that deserve further investigation. The mechanisms and strategies for decreasing intracellular urate require further study. At this time, specific recommendations cannot be given, but it is reasonable to initiate urate lowering therapy in subjects with hyperuricemia and hypertension provided the patient is aware of the associated safety risks of using the various therapies. In the event that the xanthine oxidase inhibitor are to be discontinued, awareness of the rebound of serum urate levels is needed and we suggest slowly weaning the dose while maintaining the individuals on RAS blockers.
ACKNOWLEDGMENTS
We would like to thank the many collaborators and colleagues who have been involved in uric acid research over the years. This article was supported in part by National Institutes of Health National Institute of Diabetes Digestive and Kidney Disease (1RO1DK108408 to R.J.J.) and (DK108859 to M.A.L.) and American Heart Association (19TPA34850089 to E.E.K.). P.B. is funded by National Institute of Diabetes Digestive and Kidney Diseases (DK116720 and U2CDK114886), in addition to research support from Juvenile Diabetes Research Foundation (2-SRA-2018-627-M-B, 2-SRA-2019-845-S-B), Thrasher Research Fund, NIH/NIDDK Diabetes Complications Consortium, International Society of Pediatric and Adolescent Diabetes, Diabetes Guild, Center for Women’s Health Research at University of Colorado, Children’s Hospital Colorado Research Institute, and Colorado Clinical and Translational Sciences Institute. B.R.I. is the recipient of the “Catedra Salvador Zubiran” from the Faculty of Medicine, Universidad Nacional Autónoma de México and the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
DISCLOSURE
R.J.J. has equity with XORTX Therapeutics which is a startup company generating novel xanthine oxidase inhibitors. R.J.J., M.A.L., and L.G.S. are also members of Colorado Research Partners LLC that is developing novel inhibitors of fructose metabolism. P.B. has acted as a consultant for Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Sanofi, Novo Nordisk, and Horizon Pharma. P.B. serves on the advisory board of XORTX and Boehringer Ingelheim. All support was outside the submitted work. All other authors have indicated they have no relationships relevant to this article to disclose.
REFERENCES
- 1. Mahomed FA. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; I:398–404. [Google Scholar]
- 2. Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 2018; 71: 851– 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart DJ, Langlois V, Noone D. Hyperuricemia and hypertension: links and risks. Integr Blood Press Control 2019; 12:43–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson RJ, Nakagawa T, Sánchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, Ishimoto T, Thomas J, Inaba S, Kitagawa W, Rivard CJ. Umami: the taste that drives purine intake. J Rheumatol 2013; 40:1794–1796. [DOI] [PubMed] [Google Scholar]
- 5. Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Ruzicky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sanchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Johnson RJ. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013; 4:2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lanaspa MA, Kuwabara M, Andres-Hernando A, Li N, Cicerchi C, Jensen T, Orlicky DJ, Roncal-Jimenez CA, Ishimoto T, Nakagawa T, Rodriguez-Iturbe B, MacLean PS, Johnson RJ. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc Natl Acad Sci USA 2018; 115:3138–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, Le MT, Wempe MF, Milagres T, Ishimoto T, Fini M, Nakagawa T, Johnson RJ, Lanaspa MA. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun 2017; 8:14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, González MA, Aragón A, Wesseling C, Sánchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 2014; 86:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, Ortlund EA, Johnson RJ, Gaucher EA. Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci USA 2014; 111:3763–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gersch C, Palii SP, Imaram W, Kim KM, Karumanchi SA, Angerhofer A, Johnson RJ, Henderson GN. Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids 2009; 28:118–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids 2008; 27:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim KM, Henderson GN, Frye RF, Galloway CD, Brown NJ, Segal MS, Imaram W, Angerhofer A, Johnson RJ. Simultaneous determination of uric acid metabolites allantoin, 6-aminouracil, and triuret in human urine using liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kand’ár R, Záková P, Muzáková V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin Chim Acta 2006; 365:249–256. [DOI] [PubMed] [Google Scholar]
- 14. Tin A, Marten J, Halperin Kuhns VL, Li Y, Wuttke M, Kirsten H, Sieber KB, Qiu C, Gorski M, Yu Z, Giri A, Sveinbjornsson G, Li M, Chu AY, Hoppmann A, O’Connor LJ, Prins B, Nutile T, Noce D, Akiyama M, Cocca M, Ghasemi S, van der Most PJ, Horn K, Xu Y, Fuchsberger C, Sedaghat S, Afaq S, Amin N, Ärnlöv J, Bakker SJL, Bansal N, Baptista D, Bergmann S, Biggs ML, Biino G, Boerwinkle E, Bottinger EP, Boutin TS, Brumat M, Burkhardt R, Campana E, Campbell A, Campbell H, Carroll RJ, Catamo E, Chambers JC, Ciullo M, Concas MP, Coresh J, Corre T, Cusi D, Felicita SC, de Borst MH, De Grandi A, de Mutsert R, de Vries APJ, Delgado G, Demirkan A, Devuyst O, Dittrich K, Eckardt KU, Ehret G, Endlich K, Evans MK, Gansevoort RT, Gasparini P, Giedraitis V, Gieger C, Girotto G, Gögele M, Gordon SD, Gudbjartsson DF, Gudnason V, Haller T, Hamet P, Harris TB, Hayward C, Hicks AA, Hofer E, Holm H, Huang W, Hutri-Kähönen N, Hwang SJ, Ikram MA, Lewis RM, Ingelsson E, Jakobsdottir J, Jonsdottir I, Jonsson H, Joshi PK, Josyula NS, Jung B, Kähönen M, Kamatani Y, Kanai M, Kerr SM, Kiess W, Kleber ME, Koenig W, Kooner JS, Körner A, Kovacs P, Krämer BK, Kronenberg F, Kubo M, Kühnel B, La Bianca M, Lange LA, Lehne B, Lehtimäki T, Liu J, Loeffler M, Loos RJF, Lyytikäinen LP, Magi R, Mahajan A, Martin NG, März W, Mascalzoni D, Matsuda K, Meisinger C, Meitinger T, Metspalu A, Milaneschi Y, O’Donnell CJ, Wilson OD, Gaziano JM, Mishra PP, Mohlke KL, Mononen N, Montgomery GW, Mook-Kanamori DO, Müller-Nurasyid M, Nadkarni GN, Nalls MA, Nauck M, Nikus K, Ning B, Nolte IM, Noordam R, O’Connell JR, Olafsson I, Padmanabhan S, Penninx BWJH, Perls T, Peters A, Pirastu M, Pirastu N, Pistis G, Polasek O, Ponte B, Porteous DJ, Poulain T, Preuss MH, Rabelink TJ, Raffield LM, Raitakari OT, Rettig R, Rheinberger M, Rice KM, Rizzi F, Robino A, Rudan I, Krajcoviechova A, Cifkova R, Rueedi R, Ruggiero D, Ryan KA, Saba Y, Salvi E, Schmidt H, Schmidt R, Shaffer CM, Smith AV, Smith BH, Spracklen CN, Strauch K, Stumvoll M, Sulem P, Tajuddin SM, Teren A, Thiery J, Thio CHL, Thorsteinsdottir U, Toniolo D, Tönjes A, Tremblay J, Uitterlinden AG, Vaccargiu S, van der Harst P, van Duijn CM, Verweij N, Völker U, Vollenweider P, Waeber G, Waldenberger M, Whitfield JB, Wild SH, Wilson JF, Yang Q, Zhang W, Zonderman AB, Bochud M, Wilson JG, Pendergrass SA, Ho K, Parsa A, Pramstaller PP, Psaty BM, Böger CA, Snieder H, Butterworth AS, Okada Y, Edwards TL, Stefansson K, Susztak K, Scholz M, Heid IM, Hung AM, Teumer A, Pattaro C, Woodward OM, Vitart V, Köttgen A; German Chronic Kidney Disease Study ; Lifelines Cohort Study; V. A. Million Veteran Program. Target genes, variants, tissues and transcriptional pathways influencing human serum urate levels. Nat Genet 2019; 51:1459–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ. Uric acid, evolution and primitive cultures. Semin Nephrol 2005; 25:3–8. [DOI] [PubMed] [Google Scholar]
- 16. Major TJ, Topless RK, Dalbeth N, Merriman TR. Evaluation of the diet wide contribution to serum urate levels: meta-analysis of population based cohorts. BMJ 2018; 363:k3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X, Ma L, Cheng X, Xin Y, Wang C, Zhang K, Wang X, Ren W, Sun R, Jia Z, Tian Z, Mi QS, Li C. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int 2018; 93:69–80. [DOI] [PubMed] [Google Scholar]
- 18. Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 2002; 282:F991–F997. [DOI] [PubMed] [Google Scholar]
- 19. García-Arroyo FE, Gonzaga G, Muñoz-Hernández I, Blas-Marron M, Silverio O, Tapia E, Soto V, Ranganathan N, Ranganathan P, Vyas U, Irvin A, Ir D, Robertson CE, Frank DN, Johnson RJ, Sánchez-Lozada LG. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS One 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol 2008; 295:F1134–F1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez-Lozada LG, Tapia E, Lopez-Molina R, Nepomuceno T, Soto V, Avila-Casado C, Nakagawa T, Johnson RJ, Herrera-Acosta J, Franco M. Effects of acute and chronicl-arginine treatment in experimental hyperuricemia. Am J Physiol Renal Physiol 2007; 292:F1238–1244. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, Mazzali M, Johnson RJ. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 2002; 40:355–360. [DOI] [PubMed] [Google Scholar]
- 23. Pons H, Ferrebuz A, Quiroz Y, Romero-Vasquez F, Parra G, Johnson RJ, Rodriguez-Iturbe B. Immune reactivity to heat shock protein 70 expressed in the kidney is cause of salt-sensitive hypertension. Am J Physiol Renal Physiol 2013; 304:F289–F299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev 2017; 97:1127–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin–angiotensin system. J Hypertens 2008; 26:269–275. [DOI] [PubMed] [Google Scholar]
- 26. Eräranta A, Kurra V, Tahvanainen AM, Vehmas TI, Kööbi P, Lakkisto P, Tikkanen I, Niemelä OJ, Mustonen JT, Pörsti IH. Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. J Hypertens 2008; 26:1661–1668. [DOI] [PubMed] [Google Scholar]
- 27. Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH. Oxidative stress with an activation of the renin–angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens 2010; 28:1234–1242. [PubMed] [Google Scholar]
- 28. Verzola D, Ratto E, Villaggio B, Parodi EL, Pontremoli R, Garibotto G, Viazzi F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One 2014; 9:e115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhou Y, Fang L, Jiang L, Wen P, Cao H, He W, Dai C, Yang J. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One 2012; 7:e39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiao J, Zhang XL, Fu C, Han R, Chen W, Lu Y, Ye Z. Soluble uric acid increases NALP3 inflammasome and interleukin-1β expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med 2015; 35:1347–1354. [DOI] [PubMed] [Google Scholar]
- 31. Kim SM, Lee SH, Kim YG, Kim SY, Seo JW, Choi YW, Kim DJ, Jeong KH, Lee TW, Ihm CG, Won KY, Moon JY. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am J Physiol Renal Physiol 2015; 308:F993–F1003. [DOI] [PubMed] [Google Scholar]
- 32. Xu C, Lu A, Lu X, Zhang L, Fang H, Zhou L, Yang T. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension 2017; 69:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang X, Gu J, Lv H, Li H, Cheng Y, Liu Y, Jiang Y. Uric acid induced inflammatory responses in endothelial cells via up-regulating(pro)renin receptor. Biomed Pharmacother 2019; 109:1163–1170. [DOI] [PubMed] [Google Scholar]
- 34. Joosten LAB, Crişan TO, Bjornstad P, Johnson RJ. Asymptomatic hyperuricaemia: a silent activator of the innate immune system. Nat Rev Rheumatol 2020; 16:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyperuricemia in primary and renal hypertension. N Engl J Med 1966; 275:457–464. [DOI] [PubMed] [Google Scholar]
- 36. Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 2003; 42:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prebis JW, Gruskin AB, Polinsky MS, Baluarte HJ. Uric acid in childhood essential hypertension. J Pediatr 1981; 98:702–707. [DOI] [PubMed] [Google Scholar]
- 38. Loeffler LF, Navas-Acien A, Brady TM, Miller ER III, Fadrowski JJ. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999–2006. Hypertension 2012; 59:811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schuster E, Weppelmann B. Plasma urate measurements and fetal outcome in preeclampsia. Gynecol Obstet Invest 1981; 12:162–167. [DOI] [PubMed] [Google Scholar]
- 40. Turak O, Ozcan F, Tok D, Işleyen A, Sökmen E, Taşoğlu I, Aydoğdu S, Sen N, McFann K, Johnson RJ, Kanbay M. Serum uric acid, inflammation, and nondipping circadian pattern in essential hypertension. J Clin Hypertens (Greenwich) 2013; 15:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giallauria F, Predotti P, Casciello A, Grieco A, Russo A, Viggiano A, Citro R, Ravera A, Ciardo M, Guglielmi M, Maggio M, Vigorito C. Serum uric acid is associated with non-dipping circadian pattern in young patients (30–40 years old) with newly diagnosed essential hypertension. Clin Exp Hypertens 2016; 38:233–237. [DOI] [PubMed] [Google Scholar]
- 42. Lepeytre F, Lavoie PL, Troyanov S, Madore F, Agharazii M, Goupil R. Uric acid association with pulsatile and steady components of central and peripheral blood pressures. J Hypertens 2018; 36:495–501. [DOI] [PubMed] [Google Scholar]
- 43. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008; 300:924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saito I, Saruta T, Kondo K, Nakamura R, Oguro T, Yamagami K, Ozawa Y, Kato E. Serum uric acid and the renin–angiotensin system in hypertension. J Am Geriatr Soc 1978; 26:241–247. [DOI] [PubMed] [Google Scholar]
- 45. Brand FN, McGee DL, Kannel WB, Stokes J III, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: the Framingham Study. Am J Epidemiol 1985; 121:11–18. [DOI] [PubMed] [Google Scholar]
- 46. Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med 1973; 132:401–410. [DOI] [PubMed] [Google Scholar]
- 47. Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 2009; 154:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Q, Ayoub-Charette S, Khan TA, Au-Yeung F, Blanco Mejia S, de Souza RJ, Wolever TMS, Leiter LA, Kendall CWC, Sievenpiper JL. Important food sources of fructose-containing sugars and incident hypertension: a systematic review and dose–response meta-analysis of prospective cohort studies. J Am Heart Assoc 2019; 8:e010977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sundborn G, Thornley S, Merriman TR, Lang B, King C, Lanaspa MA, Johnson RJ. Are liquid sugars different from solid sugar in their ability to cause metabolic syndrome? Obesity (Silver Spring) 2019; 27:879–887. [DOI] [PubMed] [Google Scholar]
- 50. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011; 63:102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feig DI, Madero M, Jalal DI, Sanchez-Lozada LG, Johnson RJ. Uric acid and the origins of hypertension. J Pediatr 2013; 162:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kuwabara M, Niwa K, Hisatome I, Nakagawa T, Roncal-Jimenez CA, Andres-Hernando A, Bjornstad P, Jensen T, Sato Y, Milagres T, Garcia G, Ohno M, Lanaspa MA, Johnson RJ. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: five-year Japanese cohort study. Hypertension 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 2014; 15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J, Gao P, Tian XJ, Liu JS, Zhu ZH, Huang K, Zhang C. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One 2013; 8:e56864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 2018; 68:1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jordan DM, Choi HK, Verbanck M, Topless R, Won HH, Nadkarni G, Merriman TR, Do R. No causal effects of serum urate levels on the risk of chronic kidney disease: a Mendelian randomization study. PLoS Med 2019; 16:e1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pfister R, Barnes D, Luben R, Forouhi NG, Bochud M, Khaw KT, Wareham NJ, Langenberg C. No evidence for a causal link between uric acid and type 2 diabetes: a Mendelian randomisation approach. Diabetologia 2011; 54:2561–2569. [DOI] [PubMed] [Google Scholar]
- 58. Yang Q, Köttgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Nalls M, Hernandez D, Arking DE, Boerwinkle E, Grove ML, Li M, Linda Kao WH, Chonchol M, Haritunians T, Li G, Lumley T, Psaty BM, Shlipak M, Hwang SJ, Larson MG, O’Donnell CJ, Upadhyay A, van Duijn CM, Hofman A, Rivadeneira F, Stricker B, Uitterlinden AG, Paré G, Parker AN, Ridker PM, Siscovick DS, Gudnason V, Witteman JC, Fox CS, Coresh J. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet 2010; 3:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism 2005; 54:1435–1441. [DOI] [PubMed] [Google Scholar]
- 60. Li X, Meng X, He Y, Spiliopoulou A, Timofeeva M, Wei WQ, Gifford A, Yang T, Varley T, Tzoulaki I, Joshi P, Denny JC, Mckeigue P, Campbell H, Theodoratou E. Genetically determined serum urate levels and cardiovascular and other diseases in UK Biobank cohort: a phenome-wide Mendelian randomization study. PLoS Med 2019; 16:e1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Johnson RJ. Finding the truth: multivariable analysis and the assassination of Abraham Lincoln. J R Coll Physicians Edinb 2018; 48:153–154. [DOI] [PubMed] [Google Scholar]
- 62. Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Krämer BK, Ritz E, März W. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol 2015; 26:2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan D, Wang J, Jiang F, Zhang R, Wang T, Wang S, Peng D, He Z, Chen H, Bao Y, Hu C, Jia W. A causal relationship between uric acid and diabetic macrovascular disease in Chinese type 2 diabetes patients: a Mendelian randomization analysis. Int J Cardiol 2016; 214:194–199. [DOI] [PubMed] [Google Scholar]
- 64. Shafiu M, Johnson RJ, Turner ST, Langaee T, Gong Y, Chapman AB, Gums JG, Johnson JA. Urate transporter gene SLC22A12 polymorphisms associated with obesity and metabolic syndrome in Caucasians with hypertension. Kidney Blood Press Res 2012; 35:477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, Sanguedolce MC, Parlongo RM, Tripepi G, Zoccali C. A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens 2014. [DOI] [PubMed] [Google Scholar]
- 66. Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, Sanguedolce MC, Parlongo RM, Tripepi G, Zoccali C. A genetic marker of uric acid level, carotid atherosclerosis, and arterial stiffness: a family-based study. Am J Kidney Dis 2015; 65:294–302. [DOI] [PubMed] [Google Scholar]
- 67. Voruganti VS, Laston S, Haack K, Mehta NR, Cole SA, Butte NF, Comuzzie AG. Serum uric acid concentrations and SLC2A9 genetic variation in Hispanic children: the Viva La Familia Study. Am J Clin Nutr 2015; 101:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DeBosch BJ, Kluth O, Fujiwara H, Schürmann A, Moley K. Early-onset metabolic syndrome in mice lacking the intestinal uric acid transporter SLC2A9. Nat Commun 2014; 5:4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dinour D, Gray NK, Campbell S, Shu X, Sawyer L, Richardson W, Rechavi G, Amariglio N, Ganon L, Sela BA, Bahat H, Goldman M, Weissgarten J, Millar MR, Wright AF, Holtzman EJ. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol 2010; 21:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Preitner F, Pimentel A, Metref S, Berthonneche C, Sarre A, Moret C, Rotman S, Centeno G, Firsov D, Thorens B. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int 2015; 87:940–947. [DOI] [PubMed] [Google Scholar]
- 71. Chaves FJ, Corella D, Blesa S, Mansego ML, Marín P, Portoles O, Sorlí JV, González-Albert V, Tormos MC, García-García AB, Sáez G, Redon J. Xanthine oxidoreductase polymorphisms: influence in blood pressure and oxidative stress levels. Pharmacogenet Genomics 2007; 17:589–596. [DOI] [PubMed] [Google Scholar]
- 72. Yang J, Kamide K, Kokubo Y, Takiuchi S, Horio T, Matayoshi T, Yasuda H, Miwa Y, Yoshii M, Yoshihara F, Nakamura S, Nakahama H, Tomoike H, Miyata T, Kawano Y. Associations of hypertension and its complications with variations in the xanthine dehydrogenase gene. Hypertens Res 2008; 31:931–940. [DOI] [PubMed] [Google Scholar]
- 73. Wu B, Hao Y, Shi J, Geng N, Li T, Chen Y, Sun Z, Zheng L, Li H, Li N, Zhang X, Sun Y. Association between xanthine dehydrogenase tag single nucleotide polymorphisms and essential hypertension. Mol Med Rep 2015; 12:5685–5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheepers LE, Wei FF, Stolarz-Skrzypek K, Malyutina S, Tikhonoff V, Thijs L, Salvi E, Barlassina C, Filipovský J, Casiglia E, Nikitin Y, Kawecka-Jaszcz K, Manunta P, Cusi D, Boonen A, Staessen JA, Arts IC. Xanthine oxidase gene variants and their association with blood pressure and incident hypertension: a population study. J Hypertens 2016; 34:2147–2154. [DOI] [PubMed] [Google Scholar]
- 75. Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012; 60:1148–1156. [DOI] [PubMed] [Google Scholar]
- 76. Assadi F. Allopurinol enhances the blood pressure lowering effect of enalapril in children with hyperuricemic essential hypertension. J Nephrol 2014; 27:51–56. [DOI] [PubMed] [Google Scholar]
- 77. Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, Feig DI. Effect of febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 randomized placebo-controlled study. J Am Heart Assoc 2017; 6:pili: e006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, Dawson J. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart 2014; 100:1085–1092. [DOI] [PubMed] [Google Scholar]
- 79. Liu P, Chen Y, Wang B, Zhang F, Wang D, Wang Y. Allopurinol treatment improves renal function in patients with type 2 diabetes and asymptomatic hyperuricemia: 3-year randomized parallel-controlled study. Clin Endocrinol (Oxf) 2015; 83:475–482. [DOI] [PubMed] [Google Scholar]
- 80. Madero M, Rodríguez Castellanos FE, Jalal D, Villalobos-Martín M, Salazar J, Vazquez-Rangel A, Johnson RJ, Sanchez-Lozada LG. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: a randomized placebo controlled trial. J Am Soc Hypertens 2015; 9:837–844. [DOI] [PubMed] [Google Scholar]
- 81. Johnson RJ, Choi HK, Yeo AE, Lipsky PE. Pegloticase treatment significantly decreases blood pressure in patients with chronic gout. Hypertension 2019. [DOI] [PubMed] [Google Scholar]
- 82. Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol 2007; 39:1227–1233. [DOI] [PubMed] [Google Scholar]
- 83. McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory BP: a randomized controlled trial. Clin J Am Soc Nephrol 2017; 12:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, Duranay M, Covic A, Johnson RJ. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol 2011; 6:1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Segal MS, Srinivas TR, Mohandas R, Shuster JJ, Wen X, Whidden E, Tantravahi J, Johnson RJ. The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J Am Soc Hypertens 2015; 9:610–619.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tani S, Nagao K, Hirayama A. Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: a prospective, open-label, pilot study. Clin Drug Investig 2015; 35:823–831. [DOI] [PubMed] [Google Scholar]
- 87. Sircar D, Chatterjee S, Waikhom R, Golay V, Raychaudhury A, Chatterjee S, Pandey R. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 2015; 66:945–950. [DOI] [PubMed] [Google Scholar]
- 88. Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol 2007; 27:435–440. [DOI] [PubMed] [Google Scholar]
- 89. Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes (Lond) 2010; 34:454–461. [DOI] [PubMed] [Google Scholar]
- 90. Shi Y, Chen W, Jalal D, Li Z, Chen W, Mao H, Yang Q, Johnson RJ, Yu X. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res 2012; 35:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, Arroyo D, Luño J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010; 5:1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006; 47:51–59. [DOI] [PubMed] [Google Scholar]
- 93. Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, Ito S, Yamamoto T, Tomino Y, Ohno I, Shibagaki Y, Iimuro S, Imai N, Kuwabara M, Hayakawa H, Ohtsu H, Ohashi Y; FEATHER Study Investigators . Febuxostat Therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018; 72:798–810. [DOI] [PubMed] [Google Scholar]
- 94. Waring WS, Adwani SH, Breukels O, Webb DJ, Maxwell SR. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart 2004; 90:155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Parkinson Study Group S-PDI, Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014; 71:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Spitsin S, Markowitz CE, Zimmerman V, Koprowski H, Hooper DC. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens 2010; 24:359–362. [DOI] [PubMed] [Google Scholar]
- 97. Kim KM, Henderson GN, Ouyang X, Frye RF, Sautin YY, Feig DI, Johnson RJ. A sensitive and specific liquid chromatography-tandem mass spectrometry method for the determination of intracellular and extracellular uric acid. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877:2032–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 2008; 294:R730–R737. [DOI] [PubMed] [Google Scholar]
- 99. Emmerson BT. The clinical differentiation of lead gout from primary gout. Arthritis Rheum 1968; 11:623–634. [DOI] [PubMed] [Google Scholar]
- 100. Roncal C, Mu W, Reungjui S, Kim KM, Henderson GN, Ouyang X, Nakagawa T, Johnson RJ. Lead, at low levels, accelerates arteriolopathy and tubulointerstitial injury in chronic kidney disease. Am J Physiol Renal Physiol 2007; 293:F1391–F1396. [DOI] [PubMed] [Google Scholar]
- 101. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab 2019; 21:1291–1298. [DOI] [PubMed] [Google Scholar]
- 102. Miao Y, Ottenbros SA, Laverman GD, Brenner BM, Cooper ME, Parving HH, Grobbee DE, Shahinfar S, de Zeeuw D, Lambers Heerspink HJ. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension 2011; 58:2–7. [DOI] [PubMed] [Google Scholar]
- 103. Kojima S, Matsui K, Hiramitsu S, Hisatome I, Waki M, Uchiyama K, Yokota N, Tokutake E, Wakasa Y, Jinnouchi H, Kakuda H, Hayashi T, Kawai N, Mori H, Sugawara M, Ohya Y, Kimura K, Saito Y, Ogawa H. Febuxostat for Cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur Heart J 2019; 40:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sato Y, Feig DI, Stack AG, Kang DH, Lanaspa M, Ejaz AA, Sanchez Lozada LG, Kuwabara M, Borghi C, Johnson RJ. The case for uric acid-lowering treatment in patients with hyperuricaemia and CKD. Nature Rev Nephrol 2019. [DOI] [PubMed] [Google Scholar]
- 105. Singh JA, Yu S. Allopurinol reduces the risk of myocardial infarction (MI) in the elderly: a study of Medicare claims. Arthritis Res Ther 2016; 18:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mackenzie IS, Ford I, Walker A, Hawkey C, Begg A, Avery A, Taggar J, Wei L, Struthers AD, MacDonald TM; ALL-HEART study group . Multicentre, prospective, randomised, open-label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL-HEART study. BMJ Open 2016; 6:e013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, Hunt B, Castillo M, Gunawardhana L; CARES Investigators . Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378:1200–1210. [DOI] [PubMed] [Google Scholar]
- 108. Johnson TA, Kamatani N, Kuwabara M. Xanthine oxidase inhibitor withdrawal syndrome? comment on the article by Choi et al. Arthritis Rheumatol 2019; 71:1966–1967. [DOI] [PubMed] [Google Scholar]
- 109. Sanchez-Niño MD, Zheng-Lin B, Valiño-Rivas L, Sanz AB, Ramos AM, Luño J, Goicoechea M, Ortiz A. Lesinurad: what the nephrologist should know. Clin Kidney J 2017; 10:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Guttmann A, Krasnokutsky S, Pillinger MH, Berhanu A. Pegloticase in gout treatment—safety issues, latest evidence and clinical considerations. Ther Adv Drug Saf 2017; 8:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Johnson RJ, Andrews P. The fat gene: a genetic mutation in prehistoric apes may underlie today’s pandemic of obesity and diabetes. Sci Am 2015; 313:64–69. [Google Scholar]
- 112. Johnson RJ, Stenvinkel P, Andrews P, Sánchez-Lozada LG, Nakagawa T, Gaucher E, Andres-Hernando A, Rodriguez-Iturbe B, Jimenez CR, Garcia G, Kang DH, Tolan DR, Lanaspa MA. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med 2020; 287:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol 2012; 121:e71–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Clifford AJ, Riumallo JA, Youn VR, Scrimshaw NS. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J Nutr 1976; 106:428–450. [Google Scholar]
- 115. Bjornstad P, Maahs DM, Roncal CA, Snell-Bergeon JK, Shah VN, Milagres T, Ellis SL, Hatch M, Chung LT, Rewers MJ, Garg S, Cherney DZ, Pyle L, Nadeau KJ, Johnson RJ. Role of bicarbonate supplementation on urine uric acid crystals and diabetic tubulopathy in adults with type 1 diabetes. Diabetes Obes Metab 2018; 20:1776–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Roncal-Jimenez C, García-Trabanino R, Barregard L, Lanaspa MA, Wesseling C, Harra T, Aragón A, Grases F, Jarquin ER, González MA, Weiss I, Glaser J, Sánchez-Lozada LG, Johnson RJ. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on mesoamerican nephropathy. Am J Kidney Dis 2016; 67:20–30. [DOI] [PubMed] [Google Scholar]
- 117. Hughes K, Flynn T, de Zoysa J, Dalbeth N, Merriman TR. Mendelian randomization analysis associates increased serum urate, due to genetic variation in uric acid transporters, with improved renal function. Kidney Int 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981; 78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J 1986; 235:747–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Imaram W, Gersch C, Kim KM, Johnson RJ, Henderson GN, Angerhofer A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic Biol Med 2010; 49:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Meotti FC, Jameson GN, Turner R, Harwood DT, Stockwell S, Rees MD, Thomas SR, Kettle AJ. Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J Biol Chem 2011; 286:12901–12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Amorini AM, Petzold A, Tavazzi B, Eikelenboom J, Keir G, Belli A, Giovannoni G, Di Pietro V, Polman C, D’Urso S, Vagnozzi R, Uitdehaag B, Lazzarino G. Increase of uric acid and purine compounds in biological fluids of multiple sclerosis patients. Clin Biochem 2009; 42:1001–1006. [DOI] [PubMed] [Google Scholar]
- 123. Lazzarino G, Amorini AM, Petzold A, Gasperini C, Ruggieri S, Quartuccio ME, Lazzarino G, Di Stasio E, Tavazzi B. Serum compounds of energy metabolism impairment are related to disability, disease course and neuroimaging in multiple sclerosis. Mol Neurobiol 2017; 54:7520–7533. [DOI] [PubMed] [Google Scholar]
- 124. Muñoz García D, Midaglia L, Martinez Vilela J, Marín Sánchez M, López González FJ, Arias Gómez M, Dapena Bolaño D, Iglesias Castañón A, Alonso Alonso M, Romero López J. Associated inosine to interferon: results of a clinical trial in multiple sclerosis. Acta Neurol Scand 2015; 131:405–410. [DOI] [PubMed] [Google Scholar]
- 125. Chen X, Guo X, Huang R, Chen Y, Zheng Z, Shang H. Serum uric acid levels in patients with Alzheimer’s disease: a meta-analysis. PLoS One 2014; 9:e94084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Desideri G, Gentile R, Antonosante A, Benedetti E, Grassi D, Cristiano L, Manocchio A, Selli S, Ippoliti R, Ferri C, Borghi C, Giordano A, Cimini A. Uric acid amplifies Aβ amyloid effects involved in the cognitive dysfunction/dementia: evidences from an experimental model in vitro. J Cell Physiol 2017; 232:1069–1078. [DOI] [PubMed] [Google Scholar]
- 127. Xu J, Begley P, Church SJ, Patassini S, McHarg S, Kureishy N, Hollywood KA, Waldvogel HJ, Liu H, Zhang S, Lin W, Herholz K, Turner C, Synek BJ, Curtis MA, Rivers-Auty J, Lawrence CB, Kellett KA, Hooper NM, Vardy ER, Wu D, Unwin RD, Faull RL, Dowsey AW, Cooper GJ. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep 2016; 6:27524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sims B, Powers RE, Sabina RL, Theibert AB. Elevated adenosine monophosphate deaminase activity in Alzheimer’s disease brain. Neurobiol Aging 1998; 19:385–391. [DOI] [PubMed] [Google Scholar]
- 129. Pase MP, Himali JJ, Jacques PF, DeCarli C, Satizabal CL, Aparicio H, Vasan RS, Beiser AS, Seshadri S. Sugary beverage intake and preclinical Alzheimer’s disease in the community. Alzheimers Dement 2017; 13:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schwarzschild MA, Macklin EA, Bakshi R, Battacharyya S, Logan R, Espay AJ, Hung AY, Bwala G, Goetz CG, Russell DS, Goudreau JL, Parashos SA, Saint-Hilaire MH, Rudolph A, Hare JM, Curhan GC, Ascherio A; Parkinson Study Group SURE-PD Investigators . Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial. Neurology 2019; 93:e1328–e1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Karagiannis A, Mikhailidis DP, Tziomalos K, Sileli M, Savvatianos S, Kakafika A, Gossios T, Krikis N, Moschou I, Xochellis M, Athyros VG. Serum uric acid as an independent predictor of early death after acute stroke. Circ J 2007; 71:1120–1127. [DOI] [PubMed] [Google Scholar]
- 132. Hozawa A, Folsom AR, Ibrahim H, Nieto FJ, Rosamond WD, Shahar E. Serum uric acid and risk of ischemic stroke: the ARIC study. Atherosclerosis 2006; 187:401–407. [DOI] [PubMed] [Google Scholar]
- 133. Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab 2007; 27:14–20. [DOI] [PubMed] [Google Scholar]
- 134. Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta 1992; 1122:178–182. [DOI] [PubMed] [Google Scholar]
- 135. Tan S, Radi R, Gaudier F, Evans RA, Rivera A, Kirk KA, Parks DA. Physiologic levels of uric acid inhibit xanthine oxidase in human plasma. Pediatr Res 1993; 34:303–307. [DOI] [PubMed] [Google Scholar]
- 136. Klauser AS, Halpern EJ, Strobl S, Gruber J, Feuchtner G, Bellmann-Weiler R, Weiss G, Stofferin H, Jaschke W. Dual-energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol 2005; 25:425–433. [DOI] [PubMed] [Google Scholar]
- 138. Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Batch BC, Anderson CA, Appel LJ. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation 2010; 121:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Madero M, Arriaga JC, Jalal D, Rivard C, McFann K, Pérez-Méndez O, Vázquez A, Ruiz A, Lanaspa MA, Jimenez CR, Johnson RJ, Lozada LG. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metabolism 2011; 60:1551–1559. [DOI] [PubMed] [Google Scholar]
- 140. Brymora A, Flisiński M, Johnson RJ, Goszka G, Stefańska A, Manitius J. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol Dial Transplant 2012; 27:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Enhörning S, Brunkwall L, Tasevska I, Ericson U, Persson Tholin J, Persson M, Lemetais G, Vanhaecke T, Dolci A, Perrier ET, Melander O. Water supplementation reduces copeptin and plasma glucose in adults with high copeptin: the H2O metabolism pilot study. J Clin Endocrinol Metab 2019; 104:1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]