Abstract
Background
Objectively defined early onset hypertension, based on repeated blood pressure measurements, is a strong risk factor for cardiovascular disease (CVD). We aimed to assess if also self-reported hypertension onset age is associated with hypertension-mediated organ damage (HMOD). Additionally, we evaluated the agreement between self-reported and objectively defined hypertension onset age.
Methods
We studied 2,649 participants (50 ± 4 years at the time of outcome assessment, 57% women) of the Coronary Artery Risk Development in Young Adults (CARDIA) study who underwent measurements for echocardiographic left ventricular hypertrophy (LVH), left ventricular diastolic dysfunction (LVDD), coronary calcification, and albuminuria. We divided the participants into groups according to self-reported hypertension onset age (<35 years, 35–44 years, ≥45 years, and no hypertension). We used multivariable-adjusted logistic regression models to assess the relation between self-reported hypertension onset age with the presence of HMOD, with those who did not report hypertension as the referent group.
Results
Compared with individuals without self-reported hypertension, self-reported hypertension onset at <35 years was associated with LVH (odds ratio (OR), 2.38; 95% confidence interval (CI), 1.51–3.76), LVDD (OR, 2.32; 95% CI, 1.28–4.18, coronary calcification (OR, 2.87; 95% CI, 1.50–5.47), and albuminuria (OR, 1.62; 95% CI, 0.81–3.26). Self-reported hypertension onset at ≥45 years was only associated with LVDD (OR, 1.81; 95% CI, 1.06–3.08). The agreement between self-reported and objectively defined hypertension onset age groups was 78–79%.
Conclusions
Our findings suggest that self-reported hypertension onset age, a pragmatically feasible assessment in clinical practice, is a reasonable method for assessing risk of HMOD and CVD.
Keywords: blood pressure, epidemiology, hypertension, organ damage, risk factors, self-report
There is an ongoing need for pragmatic and efficient approaches to refining our assessment of hypertension associated risks in clinical practice. Despite some limitations with respect to precision, self- or interviewer-administrated questionnaires are known to be accurate and useful for determining hypertension status and other health-related factors in practice as well as in epidemiological research. In prior studies that have evaluated whether use of self-reported hypertension is a reliable method for diagnosing hypertension,1–5 the observed agreement of self-reported hypertension with medical record- or examination-based hypertension has varied between 69% and 88%. Importantly, the specificity of self-reported hypertension has generally been reported to be high, despite the sensitivity being low with a high level of between-study variation.
Hypertension-mediated organ damage (HMOD) increases the risk of overt cardiovascular disease (CVD) considerably.6–9 A limited number of prior studies have demonstrated that objectively defined early onset of hypertension (i.e., based on repeated, objective measurements) is strongly associated with increased risk of HMOD and CVD death, whereas late onset hypertension is not.10–12 However, no studies have compared the agreement between self-reported and objectively defined hypertension onset age, or their relation with HMOD. As blood pressure (BP) data spanning decades are not usually available in regular clinical practice, physicians often need to rely on self-report to determine the age of hypertension onset. It is therefore important to investigate the association between self-reported age of hypertension onset and HMOD to understand if self-reported hypertension onset age can be used for improving risk assessment in patients with hypertension.
In this study, we studied a sample of 2,649 Coronary Artery Risk Development in Young Adults (CARDIA) study participants who underwent repeated BP measurements and determination of left ventricular hypertrophy (LVH), left ventricular diastolic dysfunction (LVDD), coronary calcification, and albuminuria. The participants also reported their age at the time of hypertension diagnosis. Our focus was to determine if individuals with self-reported early onset hypertension, a pragmatically feasible assessment in clinical practice, have an increased risk for HMOD compared with individuals with self-reported late onset hypertension. In addition, we aimed to assess the agreement between self-reported and objectively defined hypertension onset age.
METHODS
Study population
We included participants from the multicenter CARDIA study. The original CARDIA cohort involved 5,115 participants, recruited between 1985 and 1986, with mean age at baseline (Year 0) 25 ± 4 years (range 18–30). The participants were selected evenly by sex, race, education, and age groups across all 4 study centers in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Details of the CARDIA study protocol have been reported previously.13 Follow-up exams were conducted 2, 5, 7, 10, 15, 20, and 25 years after baseline. For the current study, we included individuals who participated in the Year 25 exam of the CARDIA study in 2010–2011 (n = 3,499). The final study sample included 2,649 individuals, after exclusion of participants with missing covariate or outcome data (n = 819), or information on self-reported hypertension status (n = 41). The CARDIA study was approved by institutional committees in each participating center and all participants provided informed consent.
Data acquisition
BP was measured 3 times at all follow-up exams between 1985–1986 and 2010–2011 as previously described.12,14 In order to attain comparable BP values, the oscillometric values obtained during the last 2 exams were calibrated to sphygmomanometer values with a previously introduced formula.15 At the Year 25 exam, measurements for echocardiographic left ventricular mass and pulse wave recordings for early and late phase peak velocities, coronary artery calcification score and urine albumin–creatinine ratio were conducted on all participants. Standardized protocols across all study centers were used for echocardiographic measurements, performed with a 2-dimensionally guided M-mode and Doppler echocardiography. All echocardiograms were analyzed centrally by trained echocardiographic sonographers and left ventricular mass along with pulse wave Doppler recordings of peak velocity flow in early and late diastole was calculated from the echocardiograms.16 We defined left ventricular mass index as left ventricular mass divided by body surface area [0.007184 × weight (kg)0.425 × height (cm)0.725]. Urinary creatinine and albumin were measured from single, untimed spot urine samples, which were centrally assayed by standard procedures.17 Coronary artery calcification score was measured using a cardiac multidetector computed tomography and Agatston score was calculated for each as previously described.18
Use of antihypertensive medication and smoking status was collected with self-administrated questionnaires. Use of medications was also documented according to any medications brought on examination site. History of preeclampsia or high BP during pregnancy without other symptoms of preeclampsia was self-reported by women at all follow-up visits. Serum glucose, total cholesterol, and high-density lipoprotein cholesterol were quantified from fasting samples.13,19
Definitions and subgrouping
Self-reported hypertension onset age was determined using a self-administered questionnaire at the Year 25 exam from replies to the following questions: “Has a doctor or nurse ever said that you have high blood pressure or hypertension?” and “At what age were you first told this?” Objectively defined hypertension onset age was determined as BP ≥140/90 mm Hg or use of antihypertensive agents at 2 consecutively attended exams (at 0, 2, 5, 7, 10, 15, 20, or 25 years of follow-up). When hypertension onset was based on 2 consecutive exams, the age at the first examination on which the criteria for hypertension were met was considered as the age of hypertension onset, consistent with previous studies.11,12,20 We also used an alternative definition for objectively defined hypertension onset age which required high BP (≥140/90 mm Hg) or use of antihypertensive agents at only one exam. We formed subgroups based on the participants’ self-reported age at hypertension onset (<35 years, 35–44 years, ≥45 years, or no hypertension).12 We defined albuminuria as urine albumin–creatinine ratio >30 mg/g21 and coronary calcification as Agatston score ≥100.22 The presence of LVH was defined as left ventricular mass index >115 g/m2 in men and left ventricular mass index >95 g/m2 in women.23 For LVDD, we used the cutoff values for peak velocity flow ratio between early and late diastole as >2.0 or <0.8.24 We defined diabetes as use of antihyperglycemic medication or detected serum fasting glucose of ≥7 mmol/l.
Statistical analyses
We examined the participants’ characteristics at the Year 25 exam in the whole study sample and by subgroups based on self-reported hypertension onset age. We also compared the baseline (Year 0) characteristics between participants who were included in the study sample and those who were excluded to evaluate the potential for selection bias. We examined the prevalence of HMODs according to hypertension onset age in the whole study sample and additionally by each race–sex group. We used 1-way analysis of variance for continuous variables and chi-squared test for categorial variables to compare the characteristics between the groups. Urine albumin–creatinine ratio was log-transformed for analyses due to skewed distribution. We examined the relation between hypertension onset age and presence of HMOD in a case–control (presence of HMOD vs. no HMOD) study setting. Participants who did not report having hypertension were considered as the referent group. We used univariable and multivariable logistic regression models to study the relation between self-reported age of hypertension onset groups and HMOD. We also assessed the linear trend between age of hypertension onset strata and HMOD by entering the strata in the models as a continuous variable. We included conventional HMOD risk factors, i.e., age, sex, race, total serum cholesterol, high-density lipoprotein cholesterol, body mass index, diabetes, smoking status, use of antihypertensive medication, and systolic BP as covariates in the multivariable-adjusted analyses. Covariate and HMOD data were drawn from the Year 25 exam. We assessed the agreement between self-reported and objectively defined hypertension using weighted kappa coefficients. The kappa weights were constructed, and the weighted kappa coefficients were computed according to the standard settings of SAS software version 9.4. We performed a sensitivity analysis on the agreement between self-reported and objectively defined hypertension using an alternative definition of hypertension onset. In these analyses, objectively defined hypertension onset was based on high BP (BP ≥140/90 mm Hg or use of antihypertensive medication) on a single exam, instead of on 2 consecutive exams. We also performed a subgroup analysis to determine the agreement between self-reported and objectively defined hypertension onset age among individuals without antihypertensive medication. We performed all statistical analyses with SAS version 9.4 (SAS Institute, Cary, NC). We considered 2-sided P values <0.05 as statistically significant.
RESULTS
The participants’ characteristics at the Year 25 exam in subgroups by self-reported hypertension onset age are shown in Table 1. The mean age of the study sample was 50 ± 4 years (range 43–55 years), 57% were women, and 48% were black. Participants were more likely to be women and have diabetes in the early onset than in the late onset hypertension group. There were more black participants in the early than in the late onset group. Of the participants with hypertension onset <35 years, 27% self-reported history of having preeclampsia and 42% of having high BP during pregnancy. In all individuals with hypertension, the corresponding prevalence rates were 16% and 19%, respectively. The baseline characteristics of the study sample and the excluded participants were largely similar (Supplementary Table S1 online). Overall, the mean level and prevalence of LVH, LVDD, coronary calcification, and albuminuria measured at the Year 25 exam statistically significantly differed between the subgroups of hypertension onset age (P < 0.01 for all, Table 2). The prevalence of all HMODs was highest in the group with reported hypertension onset under 35 years of age (Table 2). The prevalence of HMODs by hypertension onset age in race–sex subgroups is reported in Supplementary Table S2 online.
Table 1.
Study sample characteristics at Year 25
| Self-reported HTN onset age | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All | <35 y | 35–44 y | ≥45 y | No HTN | P value |
| N | 2,649 | 194 | 297 | 340 | 1,818 | |
| Age, years (SD) | 50.1 (3.6) | 49.8 (3.7) | 49.0 (3.7) | 51.8 (2.7) | 50.0 (3.6) | <0.001 |
| No. women (%) | 1,509 (57.0) | 122 (62.9) | 175 (58.9) | 191 (56.2) | 1,021 (56.2) | 0.28 |
| Black (%) | 1,262 (47.6) | 139 (71.7) | 216 (72.7) | 201 (59.1) | 706 (38.8) | <0.001 |
| BMI, kg/m2 (SD) | 25.2 (5.5) | 27.8 (6.1) | 27.8 (5.8) | 27.0 (5.8) | 24.2 (5.0) | <0.001 |
| Current smoker (%) | 440 (16.6) | 34 (17.5) | 57 (19.2) | 72 (21.2) | 277 (15.2) | 0.028 |
| Diabetes (%) | 236 (8.9) | 43 (22.2) | 54 (18.2) | 52 (15.3) | 87 (4.8) | <0.001 |
| Cholesterol, mmol/l (SD) | 5.0 (0.9) | 4.9 (1.1) | 4.8 (0.9) | 4.9 (1.0) | 5.1 (0.9) | <0.001 |
| HDL, mmol/l (SD) | 1.5 (0.5) | 1.5 (0.5) | 1.4 (0.4) | 1.5 (0.4) | 1.6 (0.5) | <0.001 |
| Systolic blood pressure, mm Hg (SD) | 118 (15.3) | 126 (16.5) | 126 (16.4) | 126 (16.8) | 115 (13.3) | <0.001 |
| Diastolic blood pressure, mm Hg (SD) | 73.7 (10.8) | 79.2 (12.0) | 79.4 (11.0) | 78.4 (10.9) | 71.3 (9.8) | <0.001 |
| Use of antihypertensive medication (%) | 677 (25.6) | 150 (77.3) | 248 (83.5) | 254 (74.7) | 25 (1.4) | <0.001 |
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; HTN, hypertension; SD, standard deviation.
Table 2.
Prevalence of hypertension-mediated organ damage by self-reported hypertension onset age
| Self-reported HTN onset age | ||||||
|---|---|---|---|---|---|---|
| All | <35 y | 35–44 y | ≥45 y | No HTN | P value | |
| N | 2,649 | 194 | 297 | 340 | 1,818 | |
| LVMI, g/m2 (SD) | 85 (21.3) | 94 (25.6) | 91 (25.1) | 90 (21.4) | 82 (19.4) | <0.001 |
| LVH, n (%) | 437 (16.5) | 66 (34.0) | 65 (21.9) | 75 (22.1) | 231 (12.7) | <0.001 |
| E/A ratio (SD) | 1.3 (0.4) | 1.17 (0.3) | 1.24 (0.4) | 1.20 (0.3) | 1.35 (0.4) | <0.001 |
| LVDD, n (%) | 234 (8.8) | 29 (15.0) | 27 (9.1) | 40 (11.8) | 138 (7.6) | 0.001 |
| CAC-score, AU (SD) | 40 (206) | 140 (542) | 60 (288) | 57 (168) | 23 (103) | <0.001 |
| Coronary calcification, n (%) | 230 (8.7) | 40 (20.6) | 34 (11.5) | 49 (14.4) | 107 (5.9) | <0.001 |
| UACR, median (Q1–Q3) | 4.8 (3.3–8.4) | 6.6 (4.0–16.5) | 6.2 (4.0–12.9) | 5.4 (3.6–9.7) | 4.4 (3.1–7.2) | <0.001 |
| Albuminuria, n (%) | 159 (6.0) | 26 (13.4) | 38 (12.8) | 28 (8.2) | 67 (3.7) | <0.001 |
Abbreviations: AU, Agatston units; CAC-score, coronary artery calcification score; E/A ratio, ratio between E wave peak velocity flow in early diastole and A wave peak velocity flow in late diastole; HTN, hypertension; LVDD, left ventricular diastolic dysfunction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; Q1, lower quartile; Q3, upper quartile; SD, standard deviation; UACR, urine albumin/creatinine ratio.
Unadjusted odds of HMODs were highest in the group with self-reported hypertension onset at <35 years of age (Table 3). Compared with participants who did not report having hypertension, participants with hypertension onset at <35 years had unadjusted odds ratios of 3.54 (95% confidence interval (95% CI), 2.55–4.92), 2.14 (95% CI, 1.39–3.29), 4.15 (95% CI, 2.79–6.19), and 4.05 (95% CI, 2.50–6.54) for LVH, LVDD, coronary calcification, and albuminuria, respectively. After adjusting for HMOD risk factors apart from systolic BP, the odds of HMOD remained statistically significant in participants with onset at <35 years of age (P < 0.01 for all). After additional adjustment for systolic BP, only hypertension onset at <35 years was statistically significantly associated with LVH, LVDD, and coronary calcification or albuminuria, except for LVDD, for which the association with hypertension onset at ≥45 years retained statistical significance (Table 3).
Table 3.
Odd ratios of hypertension-mediated organ damage according to self-reported hypertension onset age
| LVH | LVDD | Coronary calcification | Albuminuria | |||||
|---|---|---|---|---|---|---|---|---|
| Self-reported HTN onset age | n/N | OR (95% CI) | n/N | OR (95% CI) | n/N | OR (95% CI) | n/N | OR (95% CI) |
| Unadjusted model | ||||||||
| <35 | 66/194 | 3.54 (2.55–4.92)* | 29/194 | 2.14 (1.39–3.29)* | 40/194 | 4.15 (2.79–6.19)* | 26/194 | 4.05 (2.50–6.54)* |
| 35–44 | 65/297 | 1.93 (1.42–2.62)* | 27/297 | 1.22 (0.79–1.88) | 34/297 | 2.07 (1.38–3.11)* | 38/297 | 3.83 (2.52–5.83)* |
| ≥45 | 75/340 | 1.94 (1.45–2.60)* | 40/340 | 1.62 (1.12–2.36)‡ | 49/340 | 2.69 (1.88–3.86)* | 28/340 | 2.35 (1.49–3.71)* |
| No HTN | 231/1,818 | 1.00 | 138/1,818 | 1.00 | 107/1,818 | 1.00 | 67/1,818 | 1.00 |
| Multivariable-adjusted model | ||||||||
| <35 | 66/194 | 3.25 (2.09–5.06)* | 29/194 | 2.24 (1.26–3.97)† | 40/194 | 3.22 (1.71–6.04)* | 26/194 | 2.54 (1.29–5.01)† |
| 35–44 | 65/297 | 1.84 (1.18–2.87)† | 27/297 | 1.35 (0.75–2.45) | 34/297 | 1.55 (0.80–3.02) | 38/297 | 2.62 (1.37–5.01)† |
| ≥45 | 75/340 | 1.78 (1.19–2.66)† | 40/340 | 1.75 (1.05–2.93)‡ | 49/340 | 1.17 (0.63–2.15) | 28/340 | 1.59 (0.84–2.99) |
| No HTN | 231/1,818 | 1.00 | 138/1,818 | 1.00 | 107/1,818 | 1.00 | 67/1,818 | 1.00 |
| Multivariable + SBP-adjusted model | ||||||||
| <35 | 66/194 | 2.38 (1.51–3.76)* | 29/194 | 2.32 (1.28–4.18)† | 40/194 | 2.87 (1.50–5.47)† | 26/194 | 1.62 (0.81–3.26) |
| 35–44 | 65/297 | 1.30 (0.82–2.07) | 27/297 | 1.40 (0.76–2.58) | 34/297 | 1.38 (0.69–2.73) | 38/297 | 1.62 (0.82–3.18) |
| ≥45 | 75/340 | 1.31 (0.86–1.97) | 40/340 | 1.81 (1.06–3.08)‡ | 49/340 | 1.06 (0.57–1.96) | 28/340 | 1.04 (0.54–1.98) |
| No HTN | 231/1,818 | 1.00 | 138/1,818 | 1.00 | 107/1,818 | 1.00 | 67/1,818 | 1.00 |
Abbreviations: CI, confidence interval; HDL-cholesterol, high-density lipoprotein cholesterol; HTN, hypertension; LVDD, left ventricular diastolic dysfunction; LVH, Left ventricular hypertrophy; n/N indicates number of individuals with organ damage/number of individuals in category; OR, odds ratio; SBP, systolic blood pressure. Multivariable-adjusted model is adjusted for age, sex, race, diabetes, body mass index, total serum cholesterol, HDL-cholesterol, smoking status, and use of antihypertensive medication. In the multivariable-adjusted models, the P for trend in odds ratios was 0.15, 0.17, 0.18, and 0.47 for LVH, LVDD, coronary calcification, and albuminuria, respectively. The corresponding P values were 0.96, 0.17, 0.11, and 0.75 for the multivariable + SBP-adjusted models, respectively.
*P < 0.001.
† P < 0.01.
‡ P < 0.05.
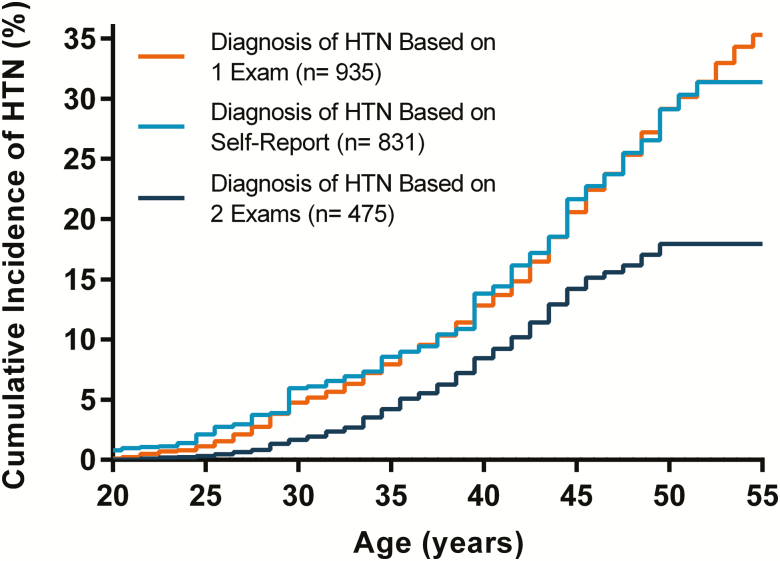
The cumulative incidence of self-reported and objectively defined hypertension onset by age is visualized in Figure 1. During follow-up, 17.9% and 31.4% of the participants developed objectively defined and self-reported hypertension, respectively. However, when the diagnosis of objectively defined hypertension was based on high BP or use of antihypertensive medication on a single exam, 35.3% of the participants developed hypertension. The level of agreement between self-reported and objectively defined hypertension onset age groups is presented in Table 4. When the diagnosis of objective hypertension was based on 2 exams, the sensitivity and specificity of self-reported hypertension were 95% and 83%, respectively. In contrast, when the diagnosis was based on 1 exam, the sensitivity and specificity were 79% and 95%, respectively. The overall agreement between hypertension onset age groups by self-report and hypertension onset age groups based on either 1 or 2 exams was 78.8% and 77.7%, with kappa coefficients of 0.66 (95% CI 0.63–0.68) and 0.48 (95% CI 0.44–0.51), respectively. The agreement was lower among individuals who were not using antihypertensive medication (Supplementary Table S3 online).
Figure 1.
Cumulative incidence of hypertension onset based on self-reported and objectively defined onset age. Self-reported hypertension onset age was based on a self-administered questionnaire at Year 25 exam. Objective hypertension onset age was based on measured BP ≥140/90 mm Hg or use of antihypertensive medication at either 1 or 2 consecutively attended exams between 1985 and 2011. N = 2,649. Abbreviations: BP, blood pressure; HTN, hypertension.
Table 4.
Agreement between self-reported and objectively defined age of hypertension onset
| Objectively defined HTN onset age | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis based on high BP at 2 consecutive exams | Diagnosis based on high BP at 1 exam | |||||||
| Never, n (%) | <35 y, n (%) | 35–44, y n (%) | ≥45 y, n (%) | Never, n (%) | <35 y, n (%) | 35–44, y n (%) | ≥45 y, n (%) | |
| Self-reported HTN onset age | ||||||||
| Never, n (%) | 1,794 (67.7) | 3 (0.1) | 8 (0.3) | 13 (0.5) | 1,626 (61.4) | 42 (1.6) | 36 (1.4) | 114 (4.3) |
| <35 y, n (%) | 50 (1.9) | 65 (2.5) | 67 (2.5) | 12 (0.5) | 18 (0.7) | 88 (3.3) | 65 (2.5) | 23 (0.9) |
| 35–44 y, n (%) | 103 (3.9) | 20 (0.8) | 133 (5.0) | 41 (1.6) | 23 (0.9) | 40 (1.5) | 150 (5.7) | 84 (3.2) |
| ≥45 y, n (%) | 227 (8.6) | 5 (0.2) | 41 (1.6) | 67 (2.5) | 47 (1.8) | 21 (0.8) | 48 (1.8) | 224 (8.5) |
Abbreviations: BP, blood pressure; HTN, hypertension. Overall agreement between self-reported HTN onset age and objectively defined HTN onset age based on 2 exams was 77.7%, with weighted kappa of 0.48 (95% CI 0.44–0.51). Overall agreement between self-reported HTN onset age and objectively defined HTN onset age based on 1 exam was 78.8%, with weighted kappa of 0.66 (95% CI 0.63–0.68).
Discussion
In this study, we demonstrate that self-reported early onset hypertension (onset at <35 years) is strongly associated with increased odds of LVH, LVDD, coronary calcification, and albuminuria in midlife, whereas late onset hypertension is not. These associations appear to be similar to what has been previously reported for objectively defined early onset hypertension that was based on repeated BP measurements.12 The agreement between self-reported and objectively defined hypertension onset age groups ranged from moderate to substantial,25 depending on the definition of objectively defined hypertension. Given that HMOD is a strong predictor of CVD outcomes,6–9,26 our findings could have important clinical implications as self-reported hypertension onset age is feasible to assess in everyday clinical practice unlike many other BP indices that are used for measuring long-term BP exposure.
Several different indices have been previously used to assess the impact of long-term exposure to high BP levels, such as antecedent BP, cumulative BP, and BP trajectories.27–29 Assessment of hypertension onset age seems to add an alternative, and possibly advantageous, method for evaluating long-term BP exposure. Namely, the other previously mentioned indices require complex calculations and precise data on prior repeated BP measurements. These indices are therefore unlikely to be implemented into daily clinical practice. In the current study, we defined age of onset categories by 10-year age strata as previously. However, no standard definition for early onset hypertension exists.11,12,30 We observed that the prevalence of all HMODs differed between self-reported hypertension onset age subgroups (Table 2), even though the mean BP levels were similar across the subgroups at Year 25 exam (Table 1). This could be a result of the between-group differences in historical BP loads. We also observed that women with early onset hypertension were more likely to have history of preeclampsia or high BP during pregnancy, which might also in part explain our findings. In normal clinical settings, physicians will most likely experience challenges with gaining access to previous medical records that may lack consistently measured and documented BP data. Determining hypertension onset age by self-report, however, could be used as a pragmatically feasible method to add precision in the cardiovascular risk assessment of patients with hypertension. In addition, exposure to high BP in either early or later in life calls for different treatment approaches as current guidelines recommend different BP treatment thresholds and targets for older patients.9,26,31 Furthermore, given the previously described strong heritability and genetic underpinnings of early onset hypertension,11,20,30,32 hypertension onset age could be therefore used both as a familial trait when assessing an individual’s risk for hypertension and as a specific type of BP trait when estimating risk for CVD outcomes.
In previous studies, the specificity of self-reported hypertension, compared with objectively defined hypertension, has been over 90%. In contrast, sensitivity has varied between 49% and 87%.1–5 In this study, both specificity and sensitivity of self-reported hypertension were relatively high. We also observed that the agreement between self-reported and objective hypertension onset age, as assessed by the kappa statistic, was substantial when objective hypertension onset based on high BP at 1 exam. However, this agreement was only moderate when high BP on 2 consecutive exams was required for hypertension onset (Table 4 and Figure 1). The optimal definition of objectively defined hypertension onset therefore depends on whether the goal is to achieve maximal correlation between objectively defined hypertension and either self-reported hypertension onset or HMOD. In addition, the correlation between self-reported and objective hypertension onset age will always depend on the diagnostic accuracy of hypertension, the adequacy of patient education, and the clinical patient–physician interaction.
The strengths of this study include a large, diverse, prospective cohort with up to 25 years of follow-up and a high participation rate (68.4% of the original cohort took part in the Year 25 exam). Moreover, information on both self-reported and objectively defined hypertension onset age was available as the participants BP and medication use were serially recorded throughout the study. However, information about the initial source of self-reported hypertension diagnoses was not available in CARDIA study. Yet, self-administrative questionnaires were used which prevent potential social desirability bias.33,34 In addition, we lacked information on the precise duration or intensity of the participants’ antihypertensive treatment. However, we lacked information on the precise duration or intensity of the participants’ antihypertensive treatment. We aimed to minimize this effect by accounting use of antihypertensive medication at the Year 25 exam. The mean age of participants during HMOD assessment was 50 years, only 5 years over the lower age threshold of hypertension onset at ≥45 years, which could have some effect on our results. However, the models were adjusted for age at the Year 25 exam to account for this potential bias. Another caveat to the interpretation of our results is that the CARDIA study participants are likely to have increased awareness of their state of health and hypertension status due to regularly attended medical examinations. Our results may not therefore be fully generalizable to the population at large. Future research is therefore warranted to determine whether these findings apply to other similar or different study settings and populations.
In summary, our findings suggest that self-reported age of hypertension onset is a feasible method for assessing risk of HMOD in midlife. More research is warranted to clarify the value of self-reported hypertension onset age in CVD risk prediction in other populations.
Supplementary Material
Acknowledgments
We acknowledge the important contributions of the CARDIA participants and investigators. This study was conducted using CARDIA Research Materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the CARDIA study or the National Heart, Lung, and Blood Institute.
Funding
K. Suvila was supported by grants from the Aarne Koskelon Säätiö and Sydäntutkimussäätiö. T.J. Niiranen was funded by the Academy of Finland (grant no. 321351), the Urmas Pekkala Foundation, the Paavo Nurmen Säätiö, the Suomen Lääketieteen Säätiö, and the Emil Aaltosen Säätiö. S. Cheng was supported by National Institutes of Health grants R01-HL134168, R01-HL131532, R01-HL143227, and R01-HL142983. J.A.C. Lima was supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute, the Intramural Research Program of the National Institute on Aging, and an intra-agency agreement between National Institute on Aging and National Heart, Lung, and Blood Institute (AG0005).
DISCLOSURE
The authors declared no conflict of interest.
References
- 1. Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol 1997; 145:762–769. [DOI] [PubMed] [Google Scholar]
- 2. Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 2004; 57:1096–1103. [DOI] [PubMed] [Google Scholar]
- 3. Kislaya I, Tolonen H, Rodrigues AP, Barreto M, Gil AP, Gaio V, Namorado S, Santos AJ, Dias CM, Nunes B. Differential self-report error by socioeconomic status in hypertension and hypercholesterolemia: INSEF 2015 study. Eur J Public Health 2019; 29:273–278. [DOI] [PubMed] [Google Scholar]
- 4. Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol 2003; 56:148–154. [DOI] [PubMed] [Google Scholar]
- 5. Gonçalves VSS, Andrade KRC, Carvalho KMB, Silva MT, Pereira MG, Galvao TF. Accuracy of self-reported hypertension: a systematic review and meta-analysis. J Hypertens 2018; 36:970–978. [DOI] [PubMed] [Google Scholar]
- 6. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 7. Viazzi F, Leoncini G, Conti N, Tomolillo C, Giachero G, Vercelli M, Deferrari G, Pontremoli R. Combined effect of albuminuria and estimated glomerular filtration rate on cardiovascular events and all-cause mortality in uncomplicated hypertensive patients. J Hypertens 2010; 28:848–855. [DOI] [PubMed] [Google Scholar]
- 8. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 2007; 115:459–467. [DOI] [PubMed] [Google Scholar]
- 9. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 10. Buck C, Baker P, Bass M, Donner A. The prognosis of hypertension according to age at onset. Hypertension 1987; 9:204–208. [DOI] [PubMed] [Google Scholar]
- 11. Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Heritability and risks associated with early onset hypertension: multigenerational, prospective analysis in the Framingham Heart Study. BMJ 2017; 357:j1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suvila K, McCabe EL, Lehtonen A, Ebinger JE, Lima JAC, Cheng S, Niiranen TJ. Early onset hypertension is associated with hypertensive end-organ damage already by midlife. Hypertension 2019; 74:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988; 41:1105–1116. [DOI] [PubMed] [Google Scholar]
- 14. Ostchega Y, Nwankwo T, Sorlie PD, Wolz M, Zipf G. Assessing the validity of the Omron HEM-907XL oscillometric blood pressure measurement device in a National Survey environment. J Clin Hypertens (Greenwich) 2010; 12:22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobs DR Jr, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension 2012; 59:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, Stengel E, Sidney S, Lewis CE, Schreiner PJ, Shikany JM, Keck K, Merlo J, Gidding SS, Lima JA. Quality control and reproducibility in M-Mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography 2015; 32:1233–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murtaugh MA, Jacobs DR Jr, Yu X, Gross MD, Steffes M; Coronary Artery Risk Development in Young Adults Study Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol 2003; 158:676–686. [DOI] [PubMed] [Google Scholar]
- 18. Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA study. J Am Coll Cardiol 2007; 49:2013–2020. [DOI] [PubMed] [Google Scholar]
- 19. Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CI, Koepsell TD; CARDIA Study Oral contraceptive use and association with glucose, insulin, and diabetes in young adult women: the CARDIA Study. Coronary Artery Risk Development in Young Adults. Diabetes Care 2002; 25:1027–1032. [DOI] [PubMed] [Google Scholar]
- 20. Wang NY, Young JH, Meoni LA, Ford DE, Erlinger TP, Klag MJ. Blood pressure change and risk of hypertension associated with parental hypertension: the Johns Hopkins precursors study. Arch Intern Med 2008; 168:643–648. [DOI] [PubMed] [Google Scholar]
- 21. Wachtell K, Palmieri V, Olsen MH, Bella JN, Aalto T, Dahlöf B, Gerdts E, Wright JT Jr, Papademetriou V, Mogensen CE, Borch-Johnsen K, Ibsen H, Devereux RB. Urine albumin/creatinine ratio and echocardiographic left ventricular structure and function in hypertensive patients with electrocardiographic left ventricular hypertrophy: the LIFE study. Losartan Intervention for Endpoint Reduction. Am Heart J 2002; 143:319–326. [DOI] [PubMed] [Google Scholar]
- 22. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008; 358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 23. Proietti M, Marra AM, Tassone EJ, De Vuono S, Corrao S, Gobbi P, Perticone F, Corazza GR, Basili S, Lip GY, Violi F, Raparelli V; ARAPACIS Study Investigators ; GIS Group. Frequency of left ventricular hypertrophy in non-valvular atrial fibrillation. Am J Cardiol 2015; 116:877–882. [DOI] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 26. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2018; 71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 27. Lauer MS, Anderson KM, Levy D. Influence of contemporary versus 30-year blood pressure levels on left ventricular mass and geometry: the Framingham Heart Study. J Am Coll Cardiol 1991; 18:1287–1294. [DOI] [PubMed] [Google Scholar]
- 28. Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR Jr, Liu K, Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014; 311:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zemaitis P, Liu K, Jacobs DR Jr, Cushman M, Durazo-Arvizu R, Shoham D, Palmas W, Cooper R, Kramer H. Cumulative systolic BP and changes in urine albumin-to-creatinine ratios in nondiabetic participants of the multi-ethnic study of atherosclerosis. Clin J Am Soc Nephrol 2014; 9:1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Risk for hypertension crosses generations in the community: a multi-generational cohort study. Eur Heart J 2017; 38:2300–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benetos A, Bulpitt CJ, Petrovic M, Ungar A, Agabiti Rosei E, Cherubini A, Redon J, Grodzicki T, Dominiczak A, Strandberg T, Mancia G. An expert opinion from the European Society of Hypertension-European Union Geriatric Medicine Society working group on the management of hypertension in very old, frail subjects. Hypertension 2016; 67:820–825. [DOI] [PubMed] [Google Scholar]
- 32. Wilk JB, Djousse L, Arnett DK, Hunt SC, Province MA, Heiss G, Myers RH. Genome-wide linkage analyses for age at diagnosis of hypertension and early-onset hypertension in the HyperGEN study. Am J Hypertens 2004; 17:839–844. [DOI] [PubMed] [Google Scholar]
- 33. Okamoto K, Ohsuka K, Shiraishi T, Hukazawa E, Wakasugi S, Furuta K. Comparability of epidemiological information between self- and interviewer-administered questionnaires. J Clin Epidemiol 2002; 55:505–511. [DOI] [PubMed] [Google Scholar]
- 34. Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant 2013; 47:2025–2047. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.