Abstract
Background
Although seasonal variation of home blood pressure (BP) has been reported to be higher in winter, seasonal difference in home BP (HBP) and its association with target organ damage (TOD) remains unclear.
Methods
This is a cross-sectional study using the dataset from the Japan Morning Surge-Home Blood Pressure (J-HOP) study to assess seasonal differences in HBP, prevalence of masked hypertension, and association of HBP with TOD. The J-HOP study is a nationwide, multicenter prospective study whose participants with cardiovascular risks underwent morning and evening HBP measurements for a 14-day period in 71 institutions throughout Japan. Urine albumin–creatinine ratio (UACR) and serum-B-type natriuretic peptide (BNP) were obtained at enrollment.
Results
Among 4,267 participants (mean age, 64.9 ± 10.9 years; 46.9% male; 91.4% hypertensives), 1,060, 979, 1,224, and 1,004 participants were enrolled in spring, summer, autumn, and winter, respectively. Morning and evening home systolic/diastolic BP levels, and prevalence of masked hypertension (office BP <140/90 mm Hg and HBP ≥135/85 mm Hg) were significantly lower in summer than other seasons after adjustment for covariates. When we assessed the interaction between BP parameters and each season for an association with TOD, we found the association between morning home diastolic BP and each of UACR and BNP was stronger in winter than other seasons (both P for interaction <0.05).
Conclusions
In this study, we revealed that the prevalence of masked hypertension was higher in other seasons than in summer and found a notable association between morning home diastolic BP and TOD in winter.
Keywords: blood pressure, home blood pressure, hypertension, masked hypertension, seasonal variation, target organ damage
The incidence of cardiovascular disease (CVD) events has been reported to be higher in winter than summer.1–3 Increased blood pressure (BP) induced by vasoconstriction due to exposure to cold temperature during winter, has been considered as one of various mechanisms in the increased CVD incidence.3
Home BP (HBP) measurement has been well accepted as a useful tool in the management of hypertension. It has been reported that HBP has stronger relationship with target organ damage (TOD) and cardiovascular outcomes than office BP.4–7 Although international guidelines recommend that HBP measurement be conducted both in morning and evening,8,9 elevated morning HBP is more strongly associated with TOD and cardiovascular events than evening HBP.7,10,11 However, these previous findings have not taken seasonal variations into account.
Several studies have demonstrated seasonal variation in HBP—namely, HBP was found to be higher in winter than summer.12–14 Therefore, the prevalence of masked hypertension (i.e., elevated HBP despite normal office BP) may also increase in winter. Moreover, when the exposure to cold temperature in winter overlaps with elevated BP in morning, this morning period is subject to worse conditions for certain organs. Thus, elevated morning HBP in winter may pose a higher cardiovascular risk than elevated morning HBP in other seasons. Although one previous study reported that the difference between HBP levels in summer and winter was correlated with CVD incidence,15 the results of that study do not answer our above-stated research questions. Therefore, we hypothesized that the association between HBP and TOD would be amplified in winter and in morning.
To test this hypothesis, we used the dataset of the Japan Morning Surge-Home Blood Pressure (J-HOP) study, a nationwide, multicenter prospective study of outpatients in clinical practice. J-HOP study participants conducted HBP measurements in morning and evening and measured their own urine albumin-to-creatinine ratio (UACR) and serum B-type natriuretic peptide (BNP) as markers of TOD. In this study, we analyzed the database of J-HOP study to identify potential seasonal differences in each of HBP, the prevalence of masked hypertension, and the association of HBP with TOD.
METHODS
Study design
The present study is a cross-sectional observational study and post hoc analysis of the J-HOP study. Details of the J-HOP study rationale, design, and procedures have been published.6 Briefly, 4,310 patients with a history or risk factors of CVD were recruited in 71 institutions throughout Japan between 2005 and 2012 (Supplementary Figure S1 online). Participants measured their own HBP on 14 consecutive days and then were followed-up for CVD events. The present study aimed to assess the seasonal variation of HBP measured at study enrollment and its association with TOD. The Institutional Review Board of Jichi Medical University School of Medicine approved the methods, and all of the patients provided written informed consent to participate and to have their data published.
BP measurements
We assessed office BP and self-measured HBP. Three office BP readings were taken at 15-s intervals on two different occasions within 2 months, and the mean of these six readings was used as the patient’s office BP value. Office BP was recorded on two different occasions: before and after the self-measured HBP. Self-measured HBP was performed according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH2014), which were applied during the J-HOP study’s period.16 Three HBP readings were taken at 15-s intervals with patients in a seated position in both morning (within 1 h of waking and before taking antihypertensive medications) and evening (before going to bed) for 14 consecutive days. The first day’s HBP measurements were excluded, and the averages of the remaining HBP readings (mean ± SD, 11.2 ± 2.7 days) were used to calculate mean HBP levels. The office and HBP values were measured using the same validated, automatic, and oscillometric device (HEM-5001; Omron Healthcare, Kyoto, Japan). To avoid reporting bias, BP data were automatically stored in the memory of the device and were downloaded to a computer by a physician or nurse during office visits. Moreover, we also assessed masked morning/evening hypertension and white-coat morning/evening hypertension in each season, which were defined based on the Japanese hypertension guidelines that were in effect during the study period: masked morning/evening hypertension, office BP <140/90 mm Hg and morning or evening HBP ≥135/85 mm Hg, respectively; white-coat morning/evening hypertension, office BP ≥140/90 mm Hg and morning or evening HBP <135/85 mm Hg, respectively.17
Other assessments and seasonal factors
Medical history was obtained by medical records and direct interviews. The diagnosis of diabetes mellitus was defined as a self-reported history, diabetic medication use, fasting or non-fasting blood glucose level ≥126 mg/dl, or ≥200 mg/dl, respectively. Chronic kidney disease was defined as the presence of proteinuria or estimated glomerular filtration rate <60 ml/min/1.73 m2.18 Prevalent CVD included diagnosed angina pectoris, myocardial infarction, and cerebrovascular disease at enrollment. In addition, the methods used to measure biomarker assays are described in Supplementary Materials online. Blood and spot urine samples were collected in the morning in a fasting state at study enrollment. The seasons were defined as follows: spring ranged from March 1 to May 31; summer, June 1–August 31; autumn, September 1–November 30; and winter, December 1–the last day of next year’s February.19 Participants were classified according to whether their first day of HBP measurements occurred in each season. Subsequently, because Japan spans a wide latitude, various environmental factors, such as temperature, differ from region to region. Hence, we also assessed seasonal variations in BP parameters and their associations with TOD in each region. We divided the participating institutions into four regions: Northern (N = 341); Eastern (N = 1,742); Central (N = 1,144); and Western (N = 1,040), as shown in Supplementary Figure S1 online.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 (SPSS Inc., Chicago, IL) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical interface for R (The R Foundation for Statistical Computing, Vienna, Austria).17 Data are shown as the mean ± SD (continuous variables) or as percentages (categorical variables). Normally distributed continuous data were compared using Student’s t-test, and categorical data were assessed using the Chi-squared test. In addition, multiple comparisons were performed using analysis of variance and using Bonferroni correction. To assess the independent effect of each season on BP levels, we performed multivariable linear regression analysis defined summer as reference. Covariates included traditional risk factors such as age, sex, body mass index, current smoking, alcohol consumption, diabetes, dyslipidemia, chronic kidney disease, antihypertensive medication use, and pre-existing CVD. The models of morning or evening home systolic or diastolic BP (SBP/DBP) also included office SBP or DBP, respectively. To determine the independent effects of each season on the presence of masked and white-coat hypertension, multivariable logistic regression analysis was performed after adjusting for traditional risk factors. We performed multivariable linear regression analysis for the association between BP levels and TOD in each season. Because the distributions of the UACR and BNP were highly skewed, they were log-transformed for the analysis. In the model of office BP, covariates included traditional risk factors, and office SBP/DBP were added to the model of morning or evening home SBP/DBP, respectively. An analysis of interaction was performed to detect the difference in the association between BP parameters and log-transformed UACR or BNP between a targeted season and the other seasons using multivariable linear regression. The levels of statistical significance were established at a two-sided P value <0.05 for all tests.
RESULTS
Patients’ characteristics
The 4,267 participants were used for the present analysis from the J-HOP study. In whole participants, the mean age was 64.9 ± 10.9 years, and 46.9% were men, and 91.4% were hypertensive patients (Supplementary Table S1 online). Table 1 lists the participants’ characteristics in each season. Most of the host factors, such as sex, prevalence of diabetes, chronic kidney disease, and antihypertensive medication use, did not differ among seasons. Additionally, log-transformed UACR was lower in summer than autumn and winter. Log-transformed BNP was higher in summer than spring and winter.
Table 1.
Characteristics of the 4,267 study participants according to season assignment
| Descriptive variables (participants, N) | Spring (N = 1,060) | Summer (N = 979) | Autumn (N = 1,224) | Winter (N = 1,004) | P for trend |
|---|---|---|---|---|---|
| Age, years | 65.6 ± 10.7 | 64.5 ± 11.0 | 65.1 ± 11.3 | 64.2 ± 10.6* | 0.018 |
| Male, % | 45.3 | 45.2 | 47.1 | 50.0 | 0.023 |
| BMI, kg/m2 | 24.4 ± 3.5 | 24.3 ± 3.3 | 24.2 ± 3.6 | 24.3 ± 3.5 | 0.781 |
| Current smoking, % | 11.8 | 11.3 | 13.3 | 12.5 | 0.369 |
| Hypertension, % | 93.2 | 87.6* | 91.8† | 92.7† | 0.559 |
| Use of antihypertensive medication, % | 79.3 | 77.4 | 79.7 | 79.7 | 0.527 |
| Number of classes of antihypertensive medication, N | 1.60 ± 1.18 | 1.52 ± 1.20 | 1.54 ± 1.14 | 1.57 ± 1.17 | 0.862 |
| Diabetes mellitus, % | 23.2 | 25.2 | 25.7 | 23.2 | 0.869 |
| CKD, % | 21.9 | 23.0 | 24.7 | 20.8 | 0.875 |
| Statin treatment, % | 20.5 | 25.6* | 25.0 | 23.5 | 0.126 |
| Pre-existing CVD, % | 10.0 | 14.1* | 13.0 | 13.9* | 0.018 |
| Serum and urine biomarkers | |||||
| Fasting glucose, mg/dl | 107.0 ± 28.6 | 107.3 ± 27.6 | 107.5 ± 27.5 | 108.2 ± 26.6 | 0.025 |
| Total cholesterol, mg/dl | 203.2 ± 32.1 | 199.5 ± 33.2 | 201.8 ± 33.3 | 205.4 ± 33.5† | 0.105 |
| HDL cholesterol, mg/dl | 57.5 ± 15.3 | 55.8 ± 14.7 | 57.6 ± 15.3† | 59.3 ± 15.6* ,†,‡ | <0.001 |
| UACR, mg/g Cr | 13.2 (7.1, 29.1) | 12.3 (6.8, 25.7) | 13.8 (7.4, 35.2) | 13.4 (7.5, 32.8) | |
| Log UACRa | 1.12 (0.85, 1.46) | 1.09 (0.83, 1.41) | 1.14 (0.87, 1.55)† | 1.13 (0.88, 1.52)† | 0.029 |
| BNP, pg/ml | 18.6 (9.0, 36.9) | 19.5 (9.4, 43.2) | 19.3 (9.6, 39.6) | 17.3 (8.9, 34.7) | |
| Log BNPa | 1.27 (0.95, 1.57) | 1.29 (0.97, 1.64)* | 1.29 (0.98, 1.60) | 1.24 (0.95, 1.54)† | 0.301 |
| Office and home BP parameters | |||||
| Office SBP, mm Hg | 142.5 ± 18.0 | 139.4 ± 18.5* | 141.0 ± 18.3 | 141.6 ± 18.5 | 0.757 |
| Office DBP, mm Hg | 81.6 ± 11.3 | 80.8 ± 11.3 | 82.1 ± 11.4 | 82.4 ± 11.6 | 0.020 |
| Morning home SBP, mm Hg | 139.4 ± 15.5 | 134.1 ± 15.6* | 139.3 ± 16.1† | 140.3 ± 15.5† | <0.001 |
| Morning home DBP, mm Hg | 79.2 ± 9.8 | 77.3 ± 10.0* | 79.5 ± 10.2† | 80.3 ± 9.8† | <0.001 |
| Evening home SBP, mm Hg | 131.9 ± 14.9 | 126.7 ± 14.1* | 130.4 ± 15.2† | 131.3 ± 15.2† | 0.551 |
| Evening home DBP, mm Hg | 73.2 ± 9.4 | 71.0 ± 9.4* | 72.9 ± 10.0† | 73.5 ± 9.7† | 0.058 |
Data are expressed as mean ± SD or percentage. UACR and BNP are expressed as median (interquartile range). Pre-existing CVD includes angina pectoris, acute myocardial infarction, and stroke. Statistical significance was assessed using analysis of variance for parametric and categorical data, and Bonferroni correction for multiple comparisons. P values for trend in this table were calculated by Jonckheere–Terpstra’s test for continuous data and Cochran–Armitage trend test for categorical data. Statistical significance was defined as P < 0.05; statistical significant in seasonal differences: *vs. Spring, †vs. summer, and ‡vs. autumn. Abbreviations: BMI, body mass index; BNP, b-type natriuretic peptide; BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high-density lipoprotein; SBP, systolic blood pressure; UACR, urine albumin creatinine ratio.
aMultiple comparisons and P values for trend were calculated in log-transformed UACR and BNP.
Seasonal BP variation
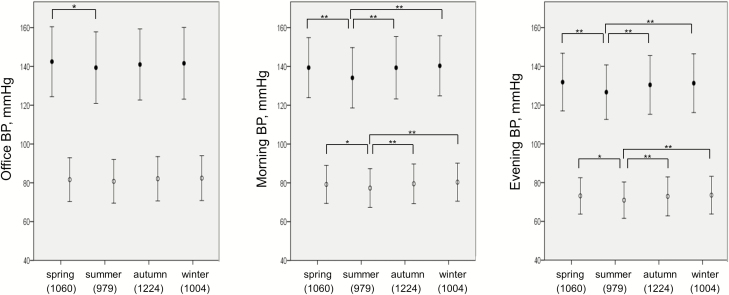
Figure 1 demonstrates the seasonal variation of office, morning, and evening HBP. Office SBP was lower in summer only compared with spring, and office DBP exhibited no significant difference in each season. Whereas, morning home SBP and DBP were significantly lower in summer compared with other seasons. Evening home SBP and DBP were also significantly lower in summer than the other seasons. These BP values are also shown in Table 1. Table 2 shows the multivariable linear regression analysis of each BP parameter adjusted for host factors. Because both office and HBP levels tended to be lower in summer than the other seasons, we defined summer as a reference. Both morning and evening home SBP/DBP were also higher in other seasons than in summer after adjusting for host factors and office SBP/DBP, and the estimate value was higher in winter than other seasons in both morning and evening home SBP/DBP. Additionally, in each divided region except the Northern one, morning and evening HBP levels also tended to be lower in summer than in the other seasons (Supplementary Table S2 online).
Figure 1.
Office and home blood pressure levels in each season. Office and home BP were compared for each season, using analysis of variance and Bonferroni correction for multiple comparisons. Statistical significance was defined as P < 0.05, and black and white points in this figure indicate SBP and DBP levels, respectively. *P < 0.05, **P < 0.001, error bar represents 1 − SD. In this figure, BP, DBP, SBP, and SD indicate blood pressure, diastolic blood pressure, systolic blood pressure, and standard deviation, respectively.
Table 2.
Multivariable linear regression analysis for office and home BP in the 4,267 study participants
| Dependent variables | Office SBP | Morning home SBP | Evening home SBP | |||
|---|---|---|---|---|---|---|
| Seasons | B (95% CI) | P | B (95% CI) | P | B (95% CI) | P |
| Spring | 2.86 (1.28–4.44) | < 0.001 | 3.73 (2.56–4.91) | <0.001 | 3.98 (2.84–5.11) | <0.001 |
| Summer | Ref. | Ref. | Ref. | |||
| Autumn | 1.56 (0.03–3.09) | 0.046 | 4.31 (3.18–5.45) | <0.001 | 3.03 (1.94–4.13) | <0.001 |
| Winter | 2.32 (0.72–3.92) | 0.005 | 5.39 (4.20–6.57) | <0.001 | 4.06 (2.92–5.21) | <0.001 |
| R 2 | 0.024 | 0.280 | 0.250 | |||
| Dependent variables | Office DBP | Morning home DBP | Evening home DBP | |||
| Seasons | B (95% CI) | P | B (95% CI) | P | B (95% CI) | P |
| Spring | 1.13 (0.22–2.04) | 0.015 | 1.49 (0.84–2.19) | <0.001 | 1.88 (1.22–2.54) | <0.001 |
| Summer | Ref. | Ref. | Ref. | |||
| Autumn | 1.56 (0.69–2.44) | <0.001 | 1.53 (0.91–2.16) | <0.001 | 1.43 (0.79–2.07) | <0.001 |
| Winter | 1.37 (0.45–2.29) | 0.003 | 2.02 (1.36–2.67) | <0.001 | 1.88 (1.21–2.55) | <0.001 |
| R 2 | 0.170 | 0.452 | 0.391 |
All analyses were adjusted for host factors (age, sex, body mass index, prevalent CVD, current smoking, alcohol consumption, diabetes, dyslipidemia, chronic kidney disease, and use of antihypertensive medications). Additionally, office SBP or office DBP was included in analysis of morning and evening home SBP or DBP, respectively. These analyses were assessed by using multivariate linear regression analysis. B and R2 indicate the nonstandardized coefficient and coefficient of determination, respectively. Abbreviations: BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
The prevalence of masked hypertension and white-coat hypertension in each season
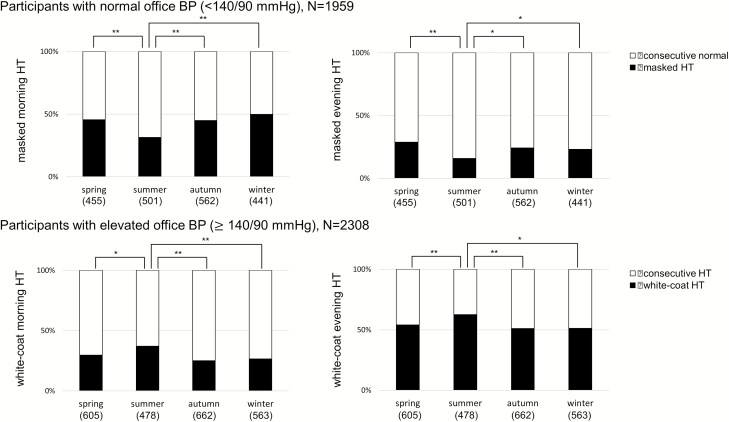
Figure 2 shows seasonal variations in the prevalence of masked hypertension in participants with well-controlled office BP (<140/90 mm Hg, N = 1,959) and the prevalence of white-coat hypertension in participants with elevated office BP (≥140/90 mm Hg, N = 2,308). The prevalence of masked morning and evening hypertension was significantly lower in summer than in the other seasons (all P < 0.05). Moreover, approximately 50% of patients with well-controlled had masked hypertension defined by morning HBP. In contrast, the prevalence of white-coat hypertension defined by either morning or evening HBP was significantly higher in summer than the other seasons (all P < 0.05). When summer was defined as a reference, the other seasons were associated with a significantly higher risk of the presence of masked hypertension after adjustment for host factors, irrespective of whether the assessment was made using morning or evening HBP (Table 3). Moreover, the other seasons were associated with a lower risk of the presence of white-coat morning and evening hypertension compared with summer as a reference (Table 3).
Figure 2.
Prevalence of masked hypertension and white-coat hypertension in each season. The upper part of this figure shows the comparison of the prevalence of masked HT between seasons in 1,959 participants who had well-controlled office BP (<140/90 mm Hg). The lower part of this figure shows the comparison of the prevalence of white-coat HT between seasons in 2,308 participants who had elevated office BP (≥140/90 mm Hg). These comparisons were analyzed using analysis of variance and Bonferroni correction for multiple comparisons. Masked morning and evening HT were defined as elevated morning or evening home BP ≥ 135/85 mm Hg in patients with well-controlled office BP. White-coat HT was defined as morning or evening home BP <135/85 mm Hg in patients with elevated office BP. These definitions were based on the 2014 Japanese guidelines. *P < 0.05, **P < 0.001. In this figure, BP and HT indicate blood pressure and hypertension, respectively.
Table 3.
Multivariable logistic regression analysis of the prevalence of masked hypertension and white-coat hypertension in each season
| Participants with normal office BP (<140/90 mm Hg), N = 1,959 | ||||||
|---|---|---|---|---|---|---|
| Masked morning HT | Masked evening HT | |||||
| Event/number | Odds ratio (95% CI) | P | Event/number | Odds ratio (95% CI) | P | |
| Spring | 213/455 | 1.90 (1.45–2.50) | <0.001 | 132/455 | 2.06 (1.48–2.87) | <0.001 |
| Summer | 155/501 | Ref. | 84/501 | Ref. | ||
| Autumn | 247/562 | 1.78 (1.38–2.31) | <0.001 | 140/562 | 1.56 (1.13–2.16) | 0.007 |
| Winter | 226/441 | 2.36 (1.79–3.10) | <0.001 | 111/441 | 1.76 (1.26–2.47) | 0.001 |
| Participants with elevated office BP (≥140/90 mm Hg), N = 2,308 | ||||||
| White-coat morning HT | White-coat evening HT | |||||
| Event/number | Odds ratio (95% CI) | P | Event/number | Odds ratio (95% CI) | P | |
| Spring | 174/605 | 0.68 (0.52–0.88) | <0.001 | 304/605 | 0.61 (0.47–0.78) | <0.001 |
| Summer | 181/478 | Ref. | 296/478 | Ref | ||
| Autumn | 177/662 | 0.62 (0.48–0.80) | <0.001 | 335/662 | 0.60 (0.46–0.77) | <0.001 |
| Winter | 143/563 | 0.55 (0.42–0.72) | <0.001 | 283/563 | 0.56 (0.43–0.73) | <0.001 |
Masked morning or evening HT was defined as office BP <140/90 mm Hg and masked morning or evening home BP ≥135/85 mm Hg; white-coat HT was defined as office BP >140/90 mm Hg and morning or evening home BP <135/85 mm Hg. The traditional host factors (age, sex, body mass index, prevalent CVD, current smoking, alcohol consumption, diabetes, dyslipidemia, chronic kidney disease, and use of antihypertensive medications) were adjusted by using multivariate logistic regression analysis. Abbreviations: BP, blood pressure; CI, confidence interval; CVD, cardiovascular disease; HT, hypertension.
Association between BP and TOD according to season
Table 4 shows the results of the multivariate linear regression analysis of BP parameters for log-transformed UACR and BNP adjusted for host factors in winter and other seasons. Although all BP parameters were associated with log-transformed UACR in winter, there was an interaction between office, morning, or evening home DBP and log-transformed UACR according to winter and other seasons (all P for interaction <0.05). There were no interactions between BP parameters and log-transformed UACR in the spring, summer, and autumn models (Supplementary Table S3 online). According to BNP, in winter, there was a significant association between morning home DBP and log-transformed BNP, but this association was not observed in the other seasons (Table 4 and Supplementary Table S4 online). There was an interaction between morning home DBP and log-transformed BNP according to winter and other seasons (P for interaction = 0.011). Although morning home SBP was significantly associated with log-transformed BNP in each season, the interaction between morning home SBP and log-transformed BNP was observed only in the model of autumn (P for interaction = 0.049) (Supplementary Table S4 online).
Table 4.
Coefficient values (95% confidence interval) of blood pressure parameters for UACR and BNP of winter vs. other seasons
| BP parameters | UACR | BNP | ||
|---|---|---|---|---|
| Winter (N = 1,004) | Other seasons (N = 3,263) | Winter (N = 1,004) | Other seasons (N = 3,263) | |
| Office SBP, 10 mm Hg | 0.047† (0.030, 0.064) | 0.056† (0.046, 0.065) | 0.002 (−0.012, 0.015) | 0.014† (0.007, 0.022) |
| P int = 0.233 | P int = 0.095 | |||
| Office DBP, 10 mm Hg | 0.078† (0.048, 0.108) | 0.052† (0.035, 0.068) | −0.014 (−0.037, 0.010) | −0.024† (−0.037, −0.010) |
| P int = 0.039 | P int = 0.343 | |||
| Morning home SBP, 10 mm Hg | 0.068† (0.045, 0.092) | 0.066† (0.053, 0.078) | 0.046† (0.028, 0.065) | 0.028† (0.018, 0.038) |
| P int = 0.270 | P int = 0.445 | |||
| Morning home DBP, 10 mm Hg | 0.092† (0.051, 0.133) | 0.052† (0.029, 0.075) | 0.044† (0.012, 0.076) | 0.004 (−0.019, 0.020) |
| P int = 0.008 | P int = 0.011 | |||
| Evening home SBP, 10 mm Hg | 0.046† (0.023, 0.070) | 0.057† (0.045, 0.070) | 0.013 (−0.005, 0.032) | 0.014* (0.003, 0.024) |
| P int = 0.143 | P int = 0.798 | |||
| Evening home DBP, 10 mm Hg | 0.057† (0.016, 0.097) | 0.042† (0.019, 0.064) | −0.007 (−0.038, 0.025) | −0.026† (−0.045, −0.007) |
| P int = 0.044 | P int = 0.076 |
This table shows the nonstandardized coefficient (95% confidence interval) of each BP parameter for log-transformed UACR and BNP in multivariable linear regression models in the subjects in winter (N = 1,004) vs. other seasons (N = 3,263). The models for office BPs included traditional host factors (age, sex, body mass index, prevalent CVD, current smoking, alcohol consumption, diabetes, dyslipidemia, chronic kidney disease, and use of antihypertensive medications). The model for morning or evening home BPs included traditional host factors and office SBP or DBP. The values of P for interaction were calculated from the analysis model in all participants (N = 4,267), which included each BP parameter, winter (vs. other seasons), the interaction between each BP parameter and winter (vs. other seasons), and traditional host factors. Abbreviations: BNP, b-type natriuretic peptide; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; UACR, urine albumin creatinine ratio. Pint = P for interaction.
*P < 0.05.
† P < 0.01.
In addition, the P value for interaction in the multivariate linear regression models of morning home DBP with log-transformed UACR was significant, and with log-transformed BNP, the P value was marginally significant in the comparison between winter and other seasons even after adjustment using Bonferroni’s correction (P for interaction between morning home DBP and winter for log-transformed UACR, P for interaction = 0.048; for log-transformed BNP, P for interaction = 0.066).
The results of the multivariate linear regression analysis of each BP parameter for log-transformed UACR and BNP in winter vs. other seasons were also assessed in each divided region. The coefficient values of morning home DBP for both log-transformed UACR and BNP tended to be higher in winter than in the other seasons. However, there were no significant interactions between each BP parameter and both log-transformed UACR and BNP in the regional models (Supplementary Tables S5 and S6 online).
Discussion
The summary of the results in this study is as follows. (i) Morning and evening HBP were lower in summer than in the other seasons after adjustment for host factors. (ii) The prevalence of masked hypertension was significantly lower in summer than in the other seasons even after adjusting for host factors. (iii) There was an interaction between morning home DBP and TODs such as UACR and BNP according to winter and other seasons after adjustment for host factors and its BP level. To the best of our knowledge, this is the first study to observe that the relationship between HBP and TOD was higher specifically in winter.
Seasonal variation of morning HBP
In the present study, defined summer as reference, the coefficient of morning home SBP in winter was higher than that of either office or evening home SBP in winter. Iwahori et al. reported a cohort study assessing the same subjects, morning and evening HBP were lower in summer and higher in winter,14 but the participants had relatively low cardiovascular risks, and more than half of the participants had not received hypertensive treatment. However, most of our participants were hypertensives (91.4%) and had one or more cardiovascular risks. Although our study was a cross-sectional study, our findings suggested that morning HBP was also lower in summer and higher in winter even in participants with relatively high cardiovascular risk in a practice setting.
Sympathetic nervous and renin–angiotensin systems are activated in morning, which is generally considered the reason of elevated BP in morning.20,21 Moreover, morning HBP level is associated with outside temperature,22 and lowering temperature activates sympathetic nervous systems with BP elevation.23 As a result, morning HBP may be strongly affected by the cold temperature in winter.
Masked and white-coat hypertension in each season
In the present study, the prevalence of masked hypertension was significantly higher in other seasons than in summer. Masked hypertension is recognized as an important CVD risk.24–26 We revealed that masked hypertension diagnosed by morning HBP was a risk factor for stroke in clinical practice.26 Moreover, approximately 50% of our patients with well-controlled office BP had uncontrolled morning HBP in winter. This winter increase in the proportion of patients with masked hypertension may be associated with the increased winter mortality from CVD. Subsequently, the prevalence of white-coat hypertension was higher in summer than other seasons in the present study. Especially in summer, referring only to office BP level, it is difficult for physicians to adjust the dose of antihypertensives, which may lead to adverse events due to hypotension.
Winter increase in the association between HBP and TOD
In our multivariable analysis with each season defined as a reference, relationships between morning home DBP and TODs such as UACR or BNP were noted in winter. To the best of our knowledge, this is the first study to demonstrate a seasonal difference in the relationship between HBP and TOD. We previously reported that morning HBP had a greater correlation with TOD than evening HBP.7 The findings of the current study provide adding information that there was a seasonal variation about the association between morning HBP level and TOD.
In our participants, office, morning, and evening home DBP were more strongly associated with UACR in winter compared with other seasons. However, the relationship of all BP parameters with UACR did not exhibit any seasonal changes in spring, summer, or autumn. These results indicated that BP parameters in winter might be a more important marker for renal dysfunction than that in other seasons. Though UACR has been reported to increase in winter in diabetes,27 in our study the elevated BP levels in winter seemed to have a stronger effect on UACR than that in other seasons. Moreover, increases in proteinuria relative to UACR are widely recognized as important risk factors for progression to renal failure.28,29 Based on our findings, increased BP levels especially in winter may contribute to the progression of chronic kidney disease.
Our multivariable linear regression models revealed the differences in the interaction between morning home DBP and log-transformed BNP according to winter vs. other seasons. Exacerbations of heart failure increase in winter,30 and plasma natriuretic peptide which is related to cardiovascular outcomes has been reported to present a mild elevation in winter.31,32 Thus, these previous findings, when taken together with our present results, might explain the increased incidence of heart failure in winter. From our observation of a strong relationship between morning home DBP and BNP in winter, elevated morning HBP in winter might constitute a risk factor for the progression of hypertensive heart disease.
Study limitations
The strengths of this study include its recruitment from a nationwide and the inclusion of a large number of clinical practice patients with CVD risk factors. Moreover, we used the same validated device to measure HBP, applied standardized HBP measurement schedules, and had a high patient retention rate. However, this study also has some potential limitations. The most notable was that our observations were cross-sectional BP profiles of participants in each season—that is, we did not conduct HBP monitoring for the same subjects throughout the four seasons. Additionally, various environmental factors differ regionally in Japan, and these differences might affect the seasonal variation of BP. Thus, we also analyzed the seasonal variation of BP parameters in each region in Japan.
In the present study, seasonal variation of HBP was characterized as a fall in summer compared with other seasons. About half of patients with well-controlled office BP were actually diagnosed with masked morning hypertension in winter. Moreover, we revealed that the relationship between morning HBP and TOD was stronger in winter than in the other seasons. In conclusion, intensive management of hypertension using HBP measurements might be useful to prevent the progression of organ damages such as renal dysfunction and heart failure, especially in winter.
Funding
This study was financially supported in part by a grant from the 21st Century Center of Excellence Project run by Japan’s Ministry of Education, Culture, Sports, Science, and Technology (MEXT); a grant from Foundation for Development of the Community (Tochigi); a grant from Omron Healthcare Co., Ltd; a Grant-in-Aid for Scientific Research (B; 21390247) from The Ministry of Education, Culture, Sports, Science, and Technology of Japan, 2009 to 2013; and funds from the MEXT-supported program for the Strategic Research Foundation at Private Universities, 2011 to 2015 Cooperative Basic and Clinical Research on Circadian Medicine (S1101022) to K. Kario. The funding sponsors had no role in the study design or conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation of the article; or in the decision to submit the article for publication.
DISCLOSURE
Kario K. received research funding from Omron Healthcare Co., Fukuda Denshi, and A&D Co. The authors declared no conflict of interest.
Supplementary Material
Acknowledgments
We gratefully acknowledge the numerous study investigators, fellows, nurses, and research coordinators who participated in the J-HOP study at the various study sites.
References
- 1. Jakovljević D, Salomaa V, Sivenius J, Tamminen M, Sarti C, Salmi K, Kaarsalo E, Narva V, Immonen-Räihä P, Torppa J, Tuomilehto J. Seasonal variation in the occurrence of stroke in a Finnish adult population. The FINMONICA Stroke Register. Finnish Monitoring Trends and Determinants in Cardiovascular Disease. Stroke 1996; 27:1774–1779. [DOI] [PubMed] [Google Scholar]
- 2. The Eurowinter Group. Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. The Eurowinter Group. Lancet 1997; 349:1341–1346. [PubMed] [Google Scholar]
- 3. Yang L, Li L, Lewington S, Guo Y, Sherliker P, Bian Z, Collins R, Peto R, Liu Y, Yang R, Zhang Y, Li G, Liu S, Chen Z; China Kadoorie Biobank Study Collaboration Outdoor temperature, blood pressure, and cardiovascular disease mortality among 23 000 individuals with diagnosed cardiovascular diseases from China. Eur Heart J 2015; 36:1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension 2010; 55:1346–1351. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Saito I, Kushiro T, Teramukai S, Tomono Y, Okuda Y, Shimada K. Morning home blood pressure is a strong predictor of coronary artery disease: the HONEST study. J Am Coll Cardiol 2016; 67:1519–1527. [DOI] [PubMed] [Google Scholar]
- 6. Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J, Kario K; J-HOP Study Group Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the Japanese general practice population: the Japan Morning Surge-Home Blood Pressure Study. Hypertension 2016; 68:54–61. [DOI] [PubMed] [Google Scholar]
- 7. Hoshide S, Kario K, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J; J-HOP Study Group Association of morning and evening blood pressure at home with asymptomatic organ damage in the J-HOP Study. Am J Hypertens 2014; 27:939–947. [DOI] [PubMed] [Google Scholar]
- 8. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 9. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 10. Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111:1777–1783. [DOI] [PubMed] [Google Scholar]
- 11. Asayama K, Ohkubo T, Kikuya M, Obara T, Metoki H, Inoue R, Hara A, Hirose T, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Prediction of stroke by home “morning” versus “evening” blood pressure values: the Ohasama study. Hypertension 2006; 48:737–743. [DOI] [PubMed] [Google Scholar]
- 12. Stergiou GS, Myrsilidi A, Kollias A, Destounis A, Roussias L, Kalogeropoulos P. Seasonal variation in meteorological parameters and office, ambulatory and home blood pressure: predicting factors and clinical implications. Hypertens Res 2015; 38:869–875. [DOI] [PubMed] [Google Scholar]
- 13. Hanazawa T, Asayama K, Watabe D, Hosaka M, Satoh M, Yasui D, Obara T, Inoue R, Metoki H, Kikuya M, Imai Y, Ohkubo T. Seasonal variation in self-measured home blood pressure among patients on antihypertensive medications: HOMED-BP study. Hypertens Res 2017; 40:284–290. [DOI] [PubMed] [Google Scholar]
- 14. Iwahori T, Miura K, Obayashi K, Ohkubo T, Nakajima H, Shiga T, Ueshima H. Seasonal variation in home blood pressure: findings from nationwide web-based monitoring in Japan. BMJ Open 2018; 8:e017351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanazawa T, Asayama K, Watabe D, Tanabe A, Satoh M, Inoue R, Hara A, Obara T, Kikuya M, Nomura K, Metoki H, Imai Y, Ohkubo T; HOMED‐BP (Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure) investigators Association between amplitude of seasonal variation in self‐measured home blood pressure and cardiovascular outcomes: HOMED‐BP Study. J Am Heart Assoc 2018; 7: e008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2014). Hypertens Res 2014; 37:253–253. [DOI] [PubMed] [Google Scholar]
- 17. Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 2013; 48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 19. Sheng CS, Cheng YB, Wei FF, Yang WY, Guo QH, Li FK, Huang QF, Thijs L, Staessen JA, Wang JG, Li Y. Diurnal blood pressure rhythmicity in relation to environmental and genetic cues in untreated referred patients. Hypertension 2017; 69:128–135. [DOI] [PubMed] [Google Scholar]
- 20. Kario K, Pickering TG, Hoshide S, Eguchi K, Ishikawa J, Morinari M, Hoshide Y, Shimada K. Morning blood pressure surge and hypertensive cerebrovascular disease. role of the alpha adrenergic sympathetic nervous system. Am J Hypertens 2004; 17:668–675. [DOI] [PubMed] [Google Scholar]
- 21. Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T. Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension 2002; 40:827–833. [DOI] [PubMed] [Google Scholar]
- 22. Murakami S, Otsuka K, Kono T, Soyama A, Umeda T, Yamamoto N, Morita H, Yamanaka G, Kitaura Y. Impact of outdoor temperature on prewaking morning surge and nocturnal decline in blood pressure in a Japanese population. Hypertens Res 2011; 34:70–73. [DOI] [PubMed] [Google Scholar]
- 23. Winnicki M, Canali C, Accurso V, Dorigatti F, Giovinazzo P, Palatini P. Relation of 24-hour ambulatory blood pressure and short-term blood pressure variability to seasonal changes in environmental temperature in stage I hypertensive subjects. Results of the Harvest Trial. Clin Exp Hypertens 1996; 18:995–1012. [DOI] [PubMed] [Google Scholar]
- 24. Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, Menard J, Mallion JM. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA 2004; 291:1342–1349. [DOI] [PubMed] [Google Scholar]
- 25. Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, Boggia J, Johansson JK, Ohkubo T, Tsuji I, Jula AM, Imai Y, Staessen JA; International Database on HOme blood pressure in relation to Cardiovascular Outcome (IDHOCO) Investigators Prognosis of white-coat and masked hypertension: international Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension 2014; 63:675–682. [DOI] [PubMed] [Google Scholar]
- 26. Fujiwara T, Yano Y, Hoshide S, Kanegae H, Kario K. Association of cardiovascular outcomes with masked hypertension defined by home blood pressure monitoring in a Japanese general practice population. JAMA Cardiol 2018; 3:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wada Y, Hamamoto Y, Ikeda H, Honjo S, Kawasaki Y, Mori K, Koshiyama H. Seasonal variations of urinary albumin creatinine ratio in Japanese subjects with Type 2 diabetes and early nephropathy. Diabet Med 2012; 29:506–508. [DOI] [PubMed] [Google Scholar]
- 28. Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int 2003; 63:1468–1474. [DOI] [PubMed] [Google Scholar]
- 29. Halbesma N, Kuiken DS, Brantsma AH, Bakker SJ, Wetzels JF, De Zeeuw D, De Jong PE, Gansevoort RT. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol 2006; 17:2582–2590. [DOI] [PubMed] [Google Scholar]
- 30. Stewart S, McIntyre K, Capewell S, McMurray JJ. Heart failure in a cold climate. Seasonal variation in heart failure-related morbidity and mortality. J Am Coll Cardiol 2002; 39:760–766. [DOI] [PubMed] [Google Scholar]
- 31. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004; 350:655–663. [DOI] [PubMed] [Google Scholar]
- 32. Khezri BS, Cederblad M, Helmersson-Karlqvist J, Karlsson B, Melhus H, Larsson A. Seasonal variability of NT-proBNP in Swedish primary care patients. Chronobiol Int 2017; 34:1473–1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.