At birth, an infant’s brain is packed with roughly 100 billion neurons—some 15% more than it will have as an adult. As we learn and grow, our experiences strengthen the circuits that prove most relevant while the others weaken and fade.
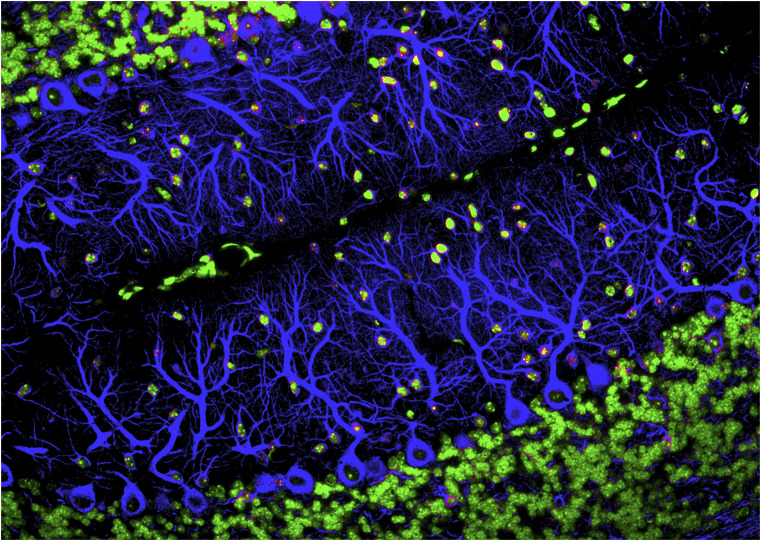
Nerve cells in the cerebellum called purkinje cells (blue) are among the brain cells that undergo synaptic pruning as we age. Researchers are starting to recognize how pruning gone awry in children and teenagers could lay the foundation for neurological disorders. Image credit: Science Source/NIGMS/Yinghua Ma/Timothy Vartanian/Cornell University.
“One extreme view of this would be that you start out wired up for every possible contingency,” says Jeff Lichtman, a neuroscientist at Harvard University in Cambridge, MA. Over time, a large percentage of those wires are permanently disconnected, says Lichtman. “What you're left with is a narrower nervous system,” he explains. “But it’s tuned exactly to the world you found yourself in.”
The process of elimination is key to forming a healthy, adaptive brain. Researchers have documented waves of neuronal cell death and the dramatic reduction of neurons’ connecting axon fibers early in neural development. But synapses, the fixed points where one cell’s axon exchanges signals with another cell, continue to be selectively removed at least through adolescence in humans, refining a coarse neural map into mature circuits.
With improved imaging techniques and molecular tools, researchers are now exploring why synaptic pruning—the targeted elimination of functional synapses—happens and how it works. The amount and timing of neural activity are central to determining which synapses get reinforced and retained, and which get weaker—which flags them for destruction. Elements of the immune system appear to be essential to carrying out the elimination process. But researchers are also coming to recognize how pruning gone awry in children and teenagers could lay the foundation for neurological disorders, such as schizophrenia or autism. One possibility is that these are diseases of the wiring diagram—what Lichtman terms “connectopathies.” It may also be the case that some of the same pruning mechanisms that normally help refine brain wiring early in life contribute to later pathological synapse loss in dementia and other neurodegenerative disorders. If so, the pruning machinery could be a therapeutic target.
Selective Sculpting
Since the 1970s, neuroscientists have known that synaptic density in the brain changes with age. Peter Huttenlocher, a pediatric neurologist at the University of Chicago, IL, painstakingly counted synapses in electron micrographs of postmortem human brains, from a newborn to a 90-year-old. In 1979, he showed that synaptic density in the human cerebral cortex increases rapidly after birth, peaking at 1 to 2 years of age, at about 50% above adult levels (1). It drops sharply during adolescence then stabilizes in adulthood, with a slight possible decline late in life.
In 1983, psychiatrist Irwin Feinberg, then at the University of California at San Francisco, described the reduction in density as synaptic “pruning” (2). Much like pruning a rosebush, the removal of weaker structures reallocates resources to those remaining, allowing them to grow stronger and more stable, explains Carla Shatz, a neurobiologist at Stanford University in Palo Alto, CA.
At first, it might seem inefficient to create an excess of connections only to remove many of them later, says Alison Barth, a neuroscientist at Carnegie Mellon University in Pittsburgh, PA. In fact, computational biology suggests that selective pruning optimizes the brain’s circuits. In 2015, Barth and her colleagues used simulated neural networks to look at how synapse removal shapes network structure and function, comparing different rates and timing of elimination (3). Patterns like those measured in mouse and human brains—an initial period of rapid, aggressive elimination, followed by a slower decline—improved the capacity of the resulting network to carry information. “Networks that are constructed through overabundance and then pruning are much more robust and efficient than networks that are constructed through other means,” Barth says. “Evolution has selected for [these] properties of network construction,” a process she calls “incredibly beautiful.”
Similar patterns of excess connectivity followed by refinement show up throughout the nervous system, including the visual system, cerebellum, and neuromuscular junctions. In the 1980s, Shatz and her colleagues performed a series of seminal experiments in the visual system of cats. In the weeks before birth, a cat’s retinal cells send their axons to a brain structure called the lateral geniculate nucleus (LGN), where they branch diffusely and form synapses on many parts of the structure. Over time, most of those branches and synapses are pruned away as a select few grow elaborate arbors in a single region, laying out a map of visual space on the LGN (4, 5). But when the researchers blocked neural activity, the pruning failed. Without neural activity to trigger or guide pruning, the branches remained diffuse and overlapping, resulting in a jumbled visual map (6, 7).
“We showed that there was both a functional and structural basis for this activity-dependent synaptic remodeling,” Shatz says. Simultaneously, “the other synapses that were made in the right places became bigger and more beautiful and stronger.”
Surprising Role
With clear evidence that synaptic activity is what guides proper pruning, researchers’ attention turned to uncovering the cellular mechanisms that might regulate the remodeling. Cornelius Gross, a neurobiologist at the European Molecular Biology Laboratory in Rome, Italy, noted that mouse neurons upregulate a signaling molecule called fractalkine during synaptic maturation. Fractalkine signals to the brain’s resident immune cells, microglia, which are perhaps best known for their role in engulfing tagged pathogens and cellular debris during an immune response. “That gave us a clue,” Gross says. “The microglia might be doing something with the synapses.”
In a 2011 study, his team spotted synaptic material inside microglia, suggesting that the cells might play an active role in pruning synapses. Plus, disrupting fractalkine communication between microglia and neurons in an otherwise healthy mouse left brain circuits immature into adulthood, implicating microglia-mediated synaptic pruning as a critical step in refining the circuitry (8).
Just a few years earlier, neuroscientist Beth Stevens, then a postdoctoral fellow at Stanford University, had unexpectedly uncovered a similar role for a group of immune molecules, called complement proteins, in synaptic pruning on retinal axons (9). It came as a bit of a surprise. “This was a time where people weren’t really thinking about [innate] immune molecules doing these types of things in the healthy developing brain,” Stevens says.
In the immune system, complement proteins are thought to tag cell membranes, telling microglia what to engulf. So, Stevens suspected that there might be a link to the emerging role of microglia in synaptic remodeling. In 2012, her team at Boston Children’s Hospital, MA, found that, indeed, in the newborn mouse visual system, microglia can engulf synapses in the LGN in a process mediated by both complement and neuronal activity. And similar to Gross’ results with fractalkine, blocking those complement signals disrupted the developing visual circuits (10). Stevens and her colleagues proposed that complement molecules may tag low-activity synapses, marking them for microglial destruction.
It’s still not entirely clear what role the microglia play, however. With time-lapse imaging, Gross’ team recently watched microglia “nibbling” on presynaptic structures of live neurons in culture, a process called trogocytosis that immune cells use to sample, or even kill, pathogens and diseased cells (11, 12). But, he says, they didn’t find compelling evidence that the microglia actually engulf or prune full synapses. “They seem to nibble, but the nibble doesn't necessarily mean they’re eliminating. So, I think the jury's out. I don't think we know what they do there yet,” Gross says.
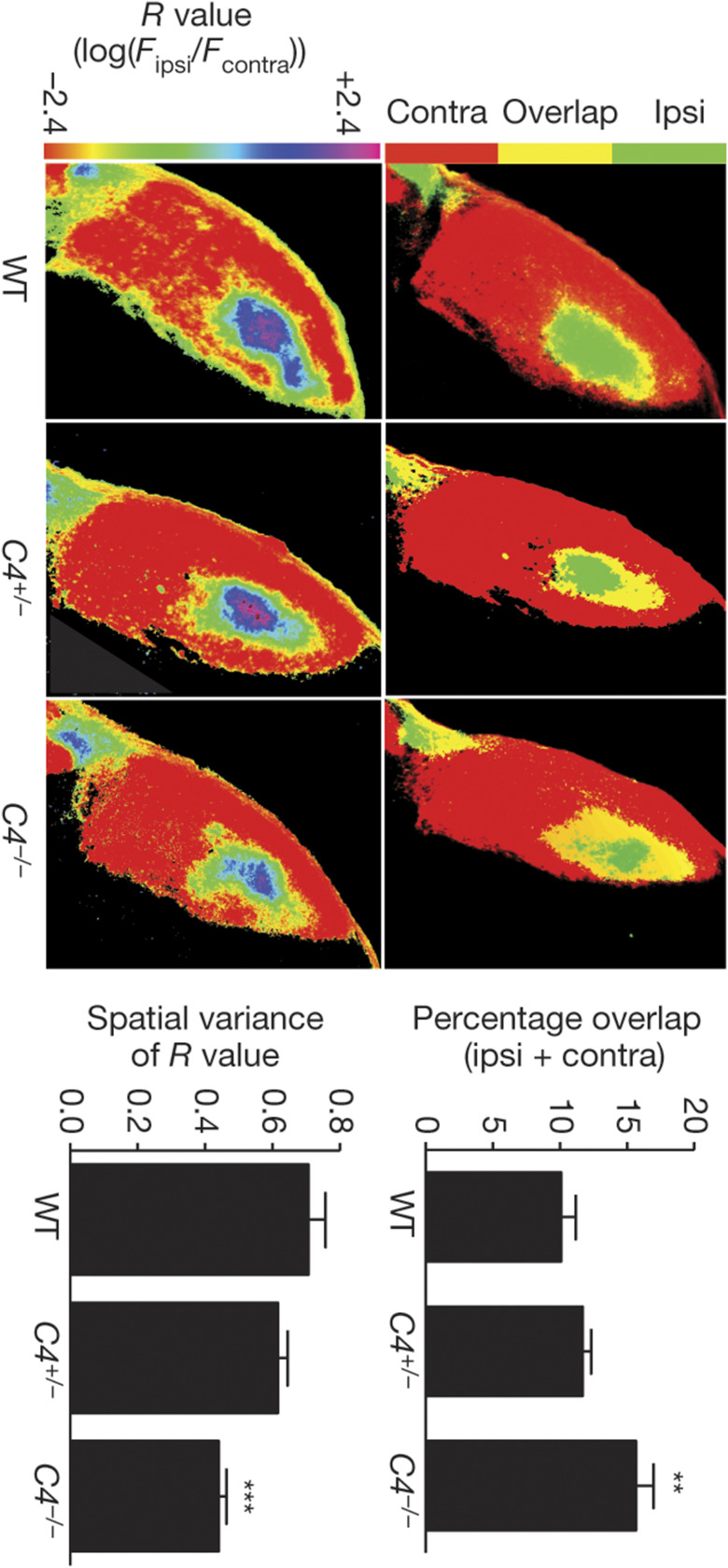
Complement signaling plays an important role in synaptic refinement in the visual system. Neuronal projections from both eyes overlap in the brain (yellow) more in mice with one (C4+/−) or no (C4−/−) copies of the complement component 4 gene compared with normal mice (WT), leading to a jumbled map of visual space. contra, contralateral projection; ipsi, ipsilateral projection. Reproduced by permission of ref. 13: Springer Nature, Nature, copyright 2016.
Altered Circuits
As early as the 1980s, Feinberg and others suggested that aberrant synaptic elimination—either too much or too little—could contribute to the altered circuits seen in neurodevelopmental disorders. Researchers started to investigate whether dysregulation of normal pruning mechanisms could lead to faulty wiring being laid down early in life.
Brains of individuals with schizophrenia, for example, have fewer synapses than normal. Some researchers have hypothesized that excess synaptic pruning could trigger the disease—likely during the active period of synapse elimination in adolescence, which coincides with the typical onset of schizophrenia. In contrast, human and animal studies of autism suggest that a deficit of pruning may be what leads to the overabundance of synaptic connections seen in that disorder. In both conditions, recent studies are beginning to implicate the same molecular signals active during neural development, including the complement system and microglia.
Genome-wide association studies are also hinting at links between genetic risk factors related to pruning and the mechanisms of neurological disorders. In 2016, Stevens and her Harvard University colleague Steven McCarroll linked certain variants of the human gene for complement component 4 (C4) to an increased risk of schizophrenia, making C4 the first gene linked to a specific mechanism underlying the disease (13). Gene variants that boosted C4 expression the most were associated with higher schizophrenia risk, suggesting that high levels of the protein could promote excess pruning.
Supporting this interpretation, psychiatrist Roy Perlis and his colleagues at Massachusetts General Hospital in Boston last year reported higher-than-normal synaptic elimination in cells derived from patients with schizophrenia, providing direct evidence of dysregulated synaptic remodeling in this disease (14). The team also correlated the C4 gene variants McCarroll’s team had identified with increased engulfment of synapses by microglia-like cells generated from the patients.
“In this case, the genetics are telling us that the microglia are really part of the disease mechanism.”
—Beth Stevens
Late-Life Pruning?
Some of the same immune mechanisms have also been implicated in neurodegenerative disease, raising questions about whether developmental pruning machinery may be reactivated by reduced synaptic activity or other disease processes. One hypothesis, Stevens says, is that these mechanisms are dysregulated by a challenge such as disease, injury, or aging. Aberrant activation of the developmental pruning mechanism could follow.
Many Alzheimer’s risk genes are also expressed or enriched in microglia, Stevens notes. “In this case, the genetics are telling us that the microglia are really part of the disease mechanism,” she says. What’s less clear, says Shatz, is “what molecules at the synapse are being regulated by neural activity—and whether these molecules are needed to protect the synapse from being removed or are part of a punishment system that is killing off those synapses that do not have activity.”
To that end, Shatz and others are exploring another family of immune molecules called major histocompatibility complex class I (MHC-I), which can regulate neural plasticity and synaptic activity. An MHC-I receptor called paired immunoglobulin-like receptor B, or PirB, is important for synaptic pruning during development. But Shatz was intrigued by the fact that the molecule is expressed throughout life.
To see whether it might play a part in disease, Shatz’s group knocked out the PirB gene in a mouse model of Alzheimer’s disease. “And to our amazement, we found that the mice were protected from memory loss even though they had…the same high levels of plaques and the bad beta-amyloid and everything as regular Alzheimer’s mice,” Shatz says.
They went on to show, in 2013, that PirB binds to the soluble form of beta-amyloid, which accumulates in neurons of Alzheimer’s patients (15). That binding leads the neuron to destroy the actin cytoskeleton in synaptic structures, providing a possible mechanism for amyloid-triggered destabilization of synapses, which may then mark them for removal. Shatz hypothesizes that a shift in the balance from strengthening toward weakening of synapses may occur early on in disease and could serve as a biomarker, enabling intervention sooner than is currently possible. Stevens and others have also found, in experiments with mouse models of Alzheimer’s, that blocking complement signaling mitigated synapse loss as well as some of the animals’ behavioral and cognitive deficits.
Still, understanding how the pieces fit together in the context of the whole brain has been challenging. Much of the in vivo work has been done in the relatively well-understood and easily accessible visual system. But it’s possible—even likely—that there are distinct mechanisms and molecules at play in different brain regions and at different times in development.
What’s more, it’s not clear how pathological synapse loss relates to the highly regulated pruning events seen during development, Shatz says. For example, synapse loss in disease could result from the death of cells or axons rather than from low activity. But the molecular studies are providing a roadmap that may lead toward better understanding—and ultimately treatment—of neurological disorders.
“Now that we know what to look for,” Stevens says, “we have the ability…to look in human tissue, human samples like [cerebrospinal fluid], to see if there are changes in these pathways in the human brain, especially early in the courses of these diseases.”
References
- 1.Huttenlocher P. R., Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Res. 163, 195–205 (1979). [DOI] [PubMed] [Google Scholar]
- 2.Feinberg I., Schizophrenia: Caused by a fault in programmed synaptic elimination during adolescence? J. Psychiatr. Res. 17, 319–334 (1982-1983). [DOI] [PubMed] [Google Scholar]
- 3.Navlakha S., Barth A. L., Bar-Joseph Z., Decreasing-rate pruning optimizes the construction of efficient and robust distributed networks. PLOS Comput. Biol. 11, e1004347 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shatz C. J., Kirkwood P. A., Prenatal development of functional connections in the cat’s retinogeniculate pathway. J. Neurosci. 4, 1378–1397 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sretavan D., Shatz C. J., Prenatal development of individual retinogeniculate axons during the period of segregation. Nature 308, 845–848 (1984). [DOI] [PubMed] [Google Scholar]
- 6.Sretavan D. W., Shatz C. J., Stryker M. P., Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336, 468–471 (1988). [DOI] [PubMed] [Google Scholar]
- 7.Shatz C. J., Competitive interactions between retinal ganglion cells during prenatal development. J. Neurobiol. 21, 197–211 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Paolicelli R. C., et al. , Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Stevens B., et al. , The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Schafer D. P., et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dance A., Core concept: Cells nibble one another via the under-appreciated process of trogocytosis. Proc. Natl. Acad. Sci. USA 116, 17608–17610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhard L., et al. , Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 9, 1228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekar A. et al.; Schizophrenia Working Group of the Psychiatric Genomics Consortium , Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellgren C. M., et al. , Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 22, 374–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T., et al. , Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science 341, 1399–1404 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]