Abstract
Laminin is a well-defined component of the airway basement membrane (BM). Efficient binding of laminin via multiple interactions is important for nontypeable Haemophilus influenzae (NTHi) colonization in the airway mucosa. In this study, we identified elongation factor thermo-unstable (EF-Tu), l-lactate dehydrogenase (LDH), protein D (PD), and peptidoglycan-associated lipoprotein P6 as novel laminin-binding proteins (Lbps) of NTHi. In parallel with other well-studied Lbps (protein 4 [P4], protein E [PE], protein F [PF], and Haemophilus adhesion and penetration protein [Hap]), EF-Tu, LDH, PD, and P6 exhibited interactions with laminin, and mediated NTHi laminin-dependent adherence to pulmonary epithelial cell lines. More importantly, the NTHi laminin interactome consisting of the well-studied and novel Lbps recognized laminin LG domains from the subunit α chains of laminin-111 and -332, the latter isoform of which is the main laminin in the airway BM. The NTHi interactome mainly targeted multiple heparin-binding domains of laminin. In conclusion, the NTHi interactome exhibited a high plasticity of interactions with different laminin isoforms via multiple heparin-binding sites.
Novel laminin-binding proteins of Haemophilus influenzae were identified as part of a global laminin interactome. A high plasticity of interactions was observed with different laminin isoforms, probably by bacterial targeting of multiple heparin-binding domains present in all laminin isoforms.
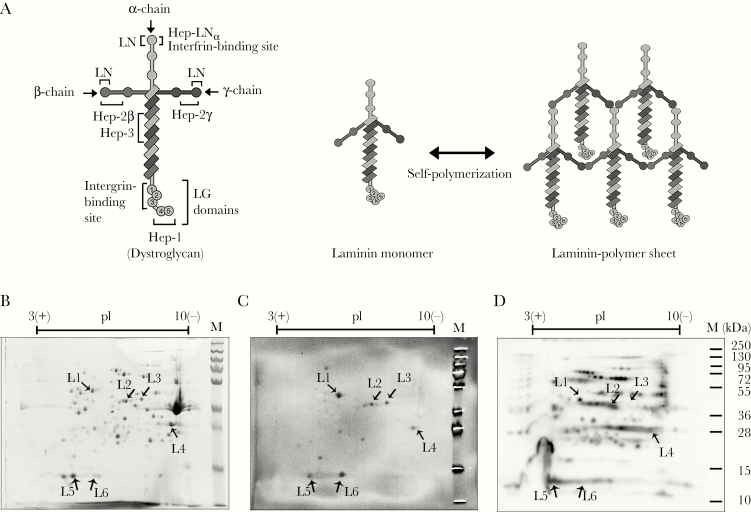
Laminins are heterotrimeric glycoproteins composed of subunit α, β, and γ chains (Figure 1A) [1]. Sixteen distinct laminin isoforms (molecular weight ~400 to 800 kDa) have been reported in humans, as a result of a selected combination of five different α chains (α1–α5), three β chains (β1–β3), and three γ chains (γ1–γ3) [1]. Laminin exhibits tissue specificities and displays an array of functions. The macromolecule is one of the major components of the basement membrane (BM). It is secreted from epithelial cells into the BM and self-assembles into laminin-polymer sheets [2]. In the BM, these laminin polymers are in complex with other extracellular matrix (ECM) components, and they form a supramolecular anchorage platform for epithelial cell polarization, migration, and proliferation and tissue structural scaffolding [1, 2].
Figure 1.
Screening of total surface-associated proteins of nontypeable Haemophilusinfluenzae (NTHi) involved in laminin binding. (A) Schematic representation of the laminin molecule. The left panel shows the general architecture of the laminin molecule. The glycoprotein (~800 kilodalton [kDa]) consists of trimeric polypeptides, namely, one α chain (~400 kDa), one β chain (~200 kDa), and one γ chain (~200 kDa) [1]. The coiled-coil domain at the C-terminal end of β and γ chains, and the adjacent portion of the α chain assemble together via disulfide bonds, resulting in a cross-shaped structure of laminin. The C-terminus of the α chain forms the end tips of the laminin long arm that carries a globular domain (LG). The LG domain is composed of five different subdomains, LG1–5. Two binding sites for epithelial cell integrin receptors are mapped at the LN domain and LG1–3 of α chain. Previously reported five heparin-binding sites indicated as Hep-1 (LG4–5), Hep-2β, Hep-2γ, Hep-3, and Hep-LNα are shown [20–22]. The right panel shows the formation of the laminin network that is formed through polymerization. Laminin self-assembles via the LN domain located at the N-terminal end of the short arm of each subunit chain [2]. The laminin sheets in the basement membrane are generally polymers of different laminin isoforms. (B–D) Immunoblotting assay for the identification of H. influenze novel laminin-binding proteins (Lbps) from the NTHi 3655 outer membrane (OM) fraction. (B) Separation of the OM fraction from NTHi 3655 on a two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (2D-SDS-PAGE) and visualized by Coomassie blue staining. (C) Far-Western blotting of NTHi 3655 OM fraction with Engelbreth-Holm-Swarm murine sarcoma laminin-111 (lamininmur) after separation on 2D-SDS-PAGE. Specific signals of laminin-binding spots were determined by comparing with control blots that were incubated with antibodies only but without laminin (blot not shown). Of note, the immunoassays were verified by the coidentification of P4, a well-studied H. influenzae Lbp [9], despite the lack of identification of protein E (PE), protein F (PF), and Haemophilus adhesion and penetration protein (Hap). This could be attributed to the nature of native conformation of PE that requires a protein dimer for interaction with extracellular matrix (ECM) proteins [13, 23], the relatively limited laminin binding of PF by Western blotting [9], and/or the high molecular weight of Hap to be analyzed on 2D-SDS-PAGE [9, 24]. (D) Western blotting detection of biotinylated surface proteins from NTHi 3655 OM fraction (2D-SDS PAGE separated) with horseradish peroxidase-conjugated streptavidin. Arrows labeled with L1–L6 in B–D corresponding to the protein spots that showed the laminin-binding signals in C. M, molecular weight protein marker in kDa.
Viral infections or mechanical abrasion in the airways result in epithelial cell layer damage and denudation of the airway BM [3, 4]. This ultimately exposes the BM-embedded laminin molecules to airway pathogens including nontypeable Haemophilus influenzae (NTHi). Nontypeable H. influenzae is a Gram-negative, human-restricted pathogen that commonly causes mucosal infections in the upper and lower airways, including exacerbations in patients with chronic obstructive pulmonary disease (COPD) [5, 6]. It is notable that ECM remodeling with increased laminin deposition occurs in the airway of COPD patients [5, 7].
Efficient binding of ECM components via multiple interactions is crucial for bacterial colonization in the airway mucosa [3, 4]. Nontypeable H. influenzae produces several surface proteins, namely, lipoprotein E (P4), Haemophilus adhesion and penetration protein (Hap), protein E (PE), and protein F (PF), to hijack host laminin molecules for efficient airway colonization [8–11]. However, despite deletion of these proteins, residual binding of NTHi to laminin remains. In this study, we report a global survey of previously unidentified NTHi laminin receptors, and we describe the pathogen’s interactions with different laminin isoforms via multiple heparin-binding sites. Our findings provide useful knowledge regarding the multiple interaction strategies adopted by NTHi for maximal pathogenesis.
MATERIAL AND METHODS
Bacterial Strains and Human Airway Cell Lines
Nontypeable H. influenzae 3655 and isogenic mutants, NTHi clinical isolates, Escherichia coli DH5α (Stratagene, Santa Clara, CA), and BL21 (DE3) (Novagen, Darmstadt, Germany) were cultured as indicated (Supplementary Table S1). The human pulmonary cell lines (alveolar epithelial cells A549 [ATCC CCL-185], pharynx-derived Detroit-562 [ATCC CCL-138], and bronchial epithelial cells [NCI-H292]) were maintained as described previously [9].
Biotinylation of Bacterial Surface Proteins
Bacteria (1 × 1010 colony-forming unit [CFU]) from mid-log phase were labeled with 200 μM of sulfosuccinimidyl-6-[biotin-amido]hexanoate (EZ-LinkTM Sulfo-NHS-LC-Biotin) (Thermo Scientific, Rockford, IL) according to Voss et al [12]. Biotinylated bacterial pellets were washed with phosphate-buffered saline (PBS) prior to outer membrane protein (OMP) extraction and analysis.
Two-Dimensional Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis and Western Blotting
Bacterial OMP extracts were prepared [13] and then treated with 60 mL of ice-cold 100 mM Na2CO3 (pH 11) to remove cytoplasmic and periplasmic contaminants [14]. Membrane fractions were pelleted by ultracentrifugation (150 000 ×g at 4oC for 1 hour). Thereafter, OMPs (100 μg) were separated by two-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (2D-SDS-PAGE) followed by far-Western blotting [13]. For detection of laminin-binding proteins (Lbps), membranes were incubated with Engelbreth-Holm-Swarm murine laminin-111 (denoted as lamininmur hereafter) (5 μg/mL) (Sigma, St. Louis, MO) and detected with rabbit antimouse laminin-111 polyclonal antibodies (pAb) (Sigma) and horseradish peroxidase (HRP)-conjugated swine antirabbit pAb (Dako, Glostrup, Denmark). For detection of biotinylated proteins, membranes were incubated with HRP-streptavidin (Bio-Rad, Hercules, CA).
Recombinant Protein Expression and Antibody Production
Open reading frames (ORFs) of bacterial proteins (Table 1) were amplified from NTHi 3655 genomic deoxyribonucleic acid (DNA) by polymerase chain reaction with specific primers (Supplementary Table S2). Amplicons were digested with indicated restriction enzymes and cloned into pET26(b)+ (Novagen) for recombinant protein expression in E. coli BL21 (DE3). His-tagged proteins were purified as described previously [13]. Rabbit pAb directed against l-lactate dehydrogenase (LDH) were generated by immunization of rabbit with the peptide “CPTPGARYRDMHSGM” (GenScript, Piscataway, NJ). Rabbit anti-PD and anti-elongation factor thermo-unstable (EF-Tu) pAbs, and mouse anti-P6 monoclonal antibodies (clone-7F3) were prepared as described previously [15–17]. For production of His-tagged laminin LGs, ORFs of LG domains were amplified from pCMV6 carrying complementary DNA inserts of human laminin α1 (LAMA1) or α3 (LAMA3) (OriGene, Rockville, MD) with specific primers (Supplementary Table S2). Amplicons were cloned into pHLSec for protein expression in human embryo kidney cells (HEK293T), and proteins were purified as described previously [18].
Table 1.
List of Lamininmur-Binding Protein Spots Identified From the Outer Membrane Fraction by LC-MS/MS Analysis
| Protein Spot No. | Protein Description and Function | Gene | GenBank Accession Numbera | Molecular Weight (Da)b | pIb | Score | Sequence Coverage (%) |
|---|---|---|---|---|---|---|---|
| L1c | Elongation factor thermal unstable (EF-Tu) | tufA d | EDJ92442 | 43297.31 | 5.26 | 113 | 25 |
| L2c | Protein D, glycerophosphoryl diester phosphodiesterase (PD) | hpd e | EDJ92229 | 41873.98 | 6.51 | 153 | 29 |
| L3c | l-lactate dehydrogenase (LDH) | ldh e | EDJ92617 | 41873.32 | 6.55 | 221 | 36 |
| L4 | Lipoprotein E, NADP phosphatase (P4) | hel | EDJ92235 | 30501.34 | 8.98 | 316 | 29 |
| L5c | Peptidoglycan-associated outer membrane lipoprotein P6 (P6) | hp6 e | EDJ93375 | 16107.91 | 6.09 | 210 | 38 |
| L6c | 30S ribosomal protein S6 (Rib30s) | rpaF | EDJ92478 | 14484.32 | 5.46 | 192 | 58 |
Abbreviations: LC-MS/MS, liquid chromatography-tandem mass spectrometry; NTHi, nontypeable Haemophilus influenzae; ORF, open reading frame.
aAccession number indicated is based upon the NTHi 3655 annotated genome database.
bMolecular weight and pI of each protein is predicted using Protparam tool (https://web.expasy.org/protparam/).
cFull-length ORFs were cloned into pET26(b)+ for expression of His-tagged recombinant proteins in Escherichia coli BL21 (DE3) followed by purification.
eAlternative gene names for hpd, ldh, and hp6 are glpQ, lctD, and pal, respectively.
Enzyme-Linked Immunosorbent Assay
Purified proteins (50 nM) in coating buffer (100 mM NaH2CO3, pH 8.3) were immobilized on Polysorp plates (Nunc, Roskilde, Denmark). Thereafter, lamininmur was added, and bound laminin was detected with rabbit antimouse laminin pAb and HRP-swine anti-rabbit pAb. In a cell enzyme-linked immunosorbent assay (ELISA), confluent epithelial cells in 96-well plates were fixed with 4% formaldehyde and incubated with His-tagged recombinant proteins. Proteins adhered to the cells were detected with an HRP-rabbit anti-6×His pAb (AbCam, Cambridge, UK).
Direct Protein-Binding Assay
Bacterial binding to soluble ligands was assayed by flow cytometry or a radioligand method as described previously [9, 13]. Bacteria (1 × 107 CFU) were incubated with 25 nM ligand (flow cytometry) or 300 kpm radioligand in PBS-1% bovine serum albumin for 1 hour at 37oC, washed, and pelleted. For flow cytometry, bacteria-bound lamininmur was detected with rabbit antimouse laminin pAb and fluorescein isothiocyanate-conjugated-swine antirabbit pAb (Bio-Rad) on a BD FACS Verse (BD Biosciences, San Jose, CA). For the method with radiolabeled ligands, proteins were first labeled with 0.05 M 125Iodine (PerkinElmer, Waltham, MA) per mol protein by chloramine-T [13]. Bacteria with bound ligands were pelleted and measured in a Tri-Carb B3110TR Liquid Scintillation Counter (PerkinElmer). For direct adherence to polymerized ligands, laminins (2 μg/cm2) immobilized in 24-wells were incubated with bacteria (1 × 105 CFU) for 1 hour at 37oC and washed with PBS to remove unbound bacteria. Laminin-bound bacteria were retrieved with 0.1% Triton X-100 and plated for quantification of adherent bacteria.
Adherence Assay
Nontypeable H. influenzae adherence to epithelial cell lines was assayed as described previously [9]. In brief, monolayer epithelial cells grown to confluency in 24-wells were infected with bacteria at a multiplicity of infection of 100 at 37oC in medium without fetal calf serum for 1 hour. Unbound bacteria were removed by washing with PBS. Epithelial cells were detached and lysed by glass beads, and cell lysates were plated on chocolate agar for overnight incubation and CFU enumeration.
Statistical Analyses
We used GraphPad Prism 7 (GraphPad Software, San Diego, CA) for statistical analysis. One- or two-way analysis of variance (ANOVA) were used as indicated. Differences were considered statistically significant at P ≤ .05.
RESULTS
Identification of Novel Nontypeable Haemophilus influenzae Laminin-Binding Proteins
Nontypeable H. influenzae expresses multiple surface proteins including P4, PE, PF, and Hap for interactions with host laminin. In spite of this, residual laminin binding was observed in the NTHi Δhpe, Δhpf, Δhel, and Δhap mutants, suggesting the possibility of additional unidentified Lbps. Therefore, we separated OMP fractions of NTHi 3655 by 2D-SDS-PAGE and probed with lamininmur. The murine laminin-111 (heterotrimer of α1-β1-γ1 chains) has been widely used as a representative laminin model in several studies of host pathogen interactions [19, 20]. Six protein spots labeled as L1–L6 in Figure 1B and C (~12 to ~55 kDa) bound lamininmur. Proteins L1–L5, but not L6, were detected by streptavidin, indicating that they were biotinylated at the surface of NTHi (Figure 1D). This further suggested the surface topology of L1–L5. The identity of L1–L6 was determined by protein sequencing and is summarized in Table 1. Nontypeable H. influenzae protein corresponded to the following: L1, EF-Tu; L2, glycerophosphoryl diester phosphodiesterase (GlpQ/PD); L3, LDH; L4, the known Lbp P4 [9]; L5, peptidoglycan-associated lipoprotein P6 (P6); and L6, ribosomal protein 30s (Rib30s).
EF-Tu, LDH, PD, and P6 Contribute to Laminin Binding by Nontypeable Haemophilus influenzae
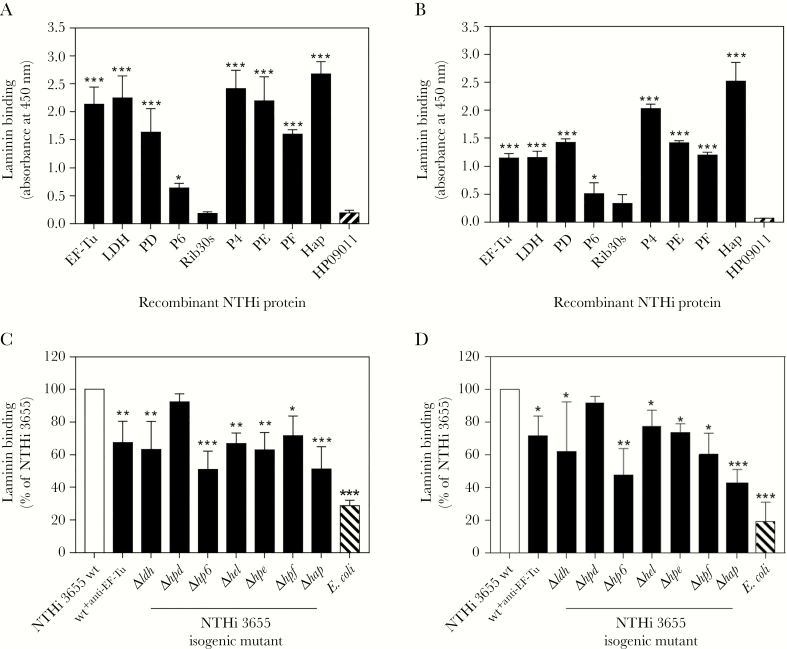
The authenticity of ligand-binding signals outlined in Figure 1C was determined by evaluating the laminin interaction of recombinant NTHi proteins, ie, EF-TuM1-K394, LDHM1-L381, PDS19-K364, P6S21-Y153, and Rib30sM1-E125 by ELISA. In addition, recombinant proteins of established Lbps (P4G22-K274, PE22-160, PF12-293, and HapE523-L1036) were included for comparison and as positive controls. We found that EF-Tu, PD, LDH, and P6 significantly (P ≤ .05) bound to both soluble (Figure 2A) and immobilized lamininmur (Figure 2B) compared to the negative control protein HP09011. Among all the Lbps tested, P6 and Hap showed the lowest and highest binding to lamininmur, respectively. In contrast, Rib30s exhibited negligible interaction and was therefore disregarded as an Lbp. Our data thus verified the detected EF-Tu, PD, LDH, and P6 (Figure 1) as previously unidentified Lbps.
Figure 2.
Interaction of nontypeable Haemophilus influenzae (NTHi) novel laminin-binding proteins (Lbps) (elongation factor thermal unstable [EF-Tu], l-lactate dehydrogenase [LDH], protein D [PD], and lipoprotein P6 [P6]) with laminin as determined by enzyme-linked immunosorbent assay and a direct binding assay. (A) Binding of soluble laminin (10 nM) to immobilized His-tagged NTHi Lbps (EF-TuM1-K394, LDHM1-L381, PDS19-K364, P6S21-Y153, Rib30sM1-E125, P4G22-K274, PE22-160, PF12-293, and HapE523-L1036). Laminin binding was detected with rabbit antimouse laminin and horseradish peroxidase (HRP)-swine antirabbit polyclonal antibodies (pAbs). (B) Relative binding capacities of NTHi Lbps (as listed in A) (10 nM) to immobilized laminin. In this assay, binding of NTHi proteins to immobilized laminin were detected with an HRP-conjugated anti-His pAb. In A and B, His-tagged NTHi protein HP09011 (GenBank accession number EDJ93055) that does not bind laminin was included as negative control [9]. (C) Direct ligand-binding assay of NTHi 3655 wild-type (wt), NTHi 3655+anti-EF-Tu, and Lbp-deficient mutants with soluble laminin as analyzed by flow cytometry. The laminin deposition on wt and knockout mutants was detected with rabbit antimouse laminin pAb and fluorescein isothiocyanate (FITC)-conjugated swine anti-rabbit pAb. The laminin binding of NTHi 3655+anti-EF-Tu was separately analyzed with rat antimouse laminin β1 pAb (AbCam) and FITC-conjugated goat antirat immunoglobulin G pAb (AbCam). Escherichia coli Top10 that did not bind laminin was included as negative control [9]. Data are presented as percentage of bacterial binding to laminin relatively to the NTHi 3655 wt that was set as 100%. (D) Adherence of NTHi 3655 wt, NTHi 3655+anti-EF-Tu, and Lbp-deficient mutants to immobilized laminin as assayed by a colony counting method. Data are presented as percentage of bacteria colony-forming units retrieved from adhesion to immobilized laminin relatively to the NTHi 3655 wt that was set as 100%. In A–D, all data represent mean values of three independent experiments and standard deviations are indicated by error bars. Differences between recombinant Lbps and control protein HP09011 in A and B, and between wt and mutants or NTHi 3655+anti-EF-Tu in C and D, were calculated by one-way ANOVA. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001.
We further assessed the direct role of EF-Tu, LDH, PD, and P6 in NTHi interaction with laminin. The laminin-binding activity between NTHi 3655 wild-type (wt) and the isogenic mutants Δldh, Δhpd, and Δhp6 was compared by a direct ligand binding assay. Because the genetic deletion of essential genes including EF-Tu is lethal to NTHi [15, 25], an NTHi phenotype with deficient surface EF-Tu (NTHi 3655+anti-EF-Tu) was “alternatively” generated by excessive saturation of intact bacteria with an anti-EF-Tu pAb. Mutants Δhel, Δhpe, Δhpf, and Δhap that are partially attenuated in laminin binding were used as internal controls [9]. It is interesting to note that, when compared with the NTHi 3655 wt, P6 deletion caused a major reduction in binding to both soluble and immobilized lamininmur by 49.2% and 47.6 %, respectively, and this was in parallel with the Hap-deficient NTHi 3655Δhap (45.5% and 42.0%) (Figure 2C and D). Nontypeable H. influenzae 3655Δldh and NTHi 3655+anti-EF-Tu showed partial decrement in binding to both forms of lamininmur. However, NTHi 3655Δhpd did not exhibit significant impairment in lamininmur binding. The results indicated that EF-Tu, LDH, and P6 significantly contributed to the NTHi-laminin interaction. Therefore, we grouped EF-Tu, LDH, PD, and P6 together with P4, PE, PF, and Hap as the “NTHi laminin interactome.” Our data also unveiled the variable binding capacities and different roles of surface-associated proteins composing the NTHi laminin interactome in bacterial binding to laminin.
EF-Tu, LDH, and P6 but Not PD Facilitate Nontypeable Haemophilus influenzae Adherence to Airway Epithelial Cells by Targeting Cellular Laminin
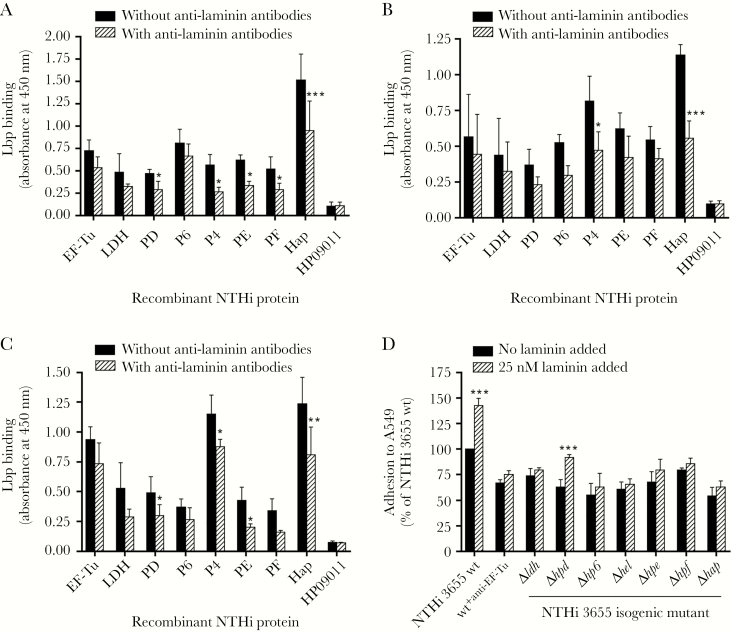
Because laminin in the BM provides an anchorage platform for epithelial cell attachment via interaction of LG domain with cellular integrin receptors, we further investigated whether EF-Tu, LDH, P6, and PD could promote NTHi adhesion by targeting cell-associated laminin as previously described for P4, PF, PE, and Hap [9]. We found that, just like P4, PE, PF, and Hap, the recombinant EF-Tu, LDH, PD, and P6 bound to the panel of airway cell lines in a whole-cell ELISA, whereas the negative control protein HP09011 did not (Figure 3A–C). However, the interaction was reduced after preincubation of cell lines with antilaminin antibodies. This suggested that the attachment of EF-Tu, PD, P6, and LDH to the cell lines involved interactions with cell-associated laminin.
Figure 3.
Haemophilus elongation factor thermal unstable (EF-Tu), l-lactate dehydrogenase (LDH), protein D (PD), and lipoprotein P6 (P6) promote nontypeable Haemophilus influenzae (NTHi) adhesion to pulmonary epithelial cells by targeting laminin as a bridging molecule. (A–C) Direct interactions of recombinant NTHi laminin-binding proteins (Lbps) (EF-TuM1-K394, LDHM1-L381, PDS19-K364, P6S21-Y153, Rib30sM1-E125, P4G22-K274, PE22-160, PF12-293, and HapE523-L1036) (10 nM) to a panel of fixed pulmonary epithelial cell lines immobilized on 96-well plates: A549 (A), Detroit 562 (B), and NCI-H292 (C) as measured by cell enzyme-linked immunosorbent assay. HP09011 was included as negative control. Recombinant EF-Tu, LDH, PD, and P6 bound to the cell lines to the similar degree as the well-established NTHi Lbps (protein 4 [P4], protein E [PE], protein F [PF], and Haemophilus adhesion and penetration protein [Hap]), whereas the negative control protein HP09011 did not. Preincubation of cell lines with antilaminin antibodies (equal mixture of antilaminin-111 polyclonal antibodies (Sigma) and antilaminin-332 monoclonal antibodies [AbCam]) as previously described [9] reduced NTHi Lbps binding compared to without preincubation with antilaminin antibodies. Of note, the presence of cellular laminin in A549, Detroit 562, and NCI-H292 was confirmed (data not shown) as previously described before performing protein-cell binding assays [9]. (D) Adhesion assay with NTHi 3655 wild-type (wt), isogenic mutants and NTHi 3655+anti-EF-Tu to the alveolar epithelial cell line A549 upon supplementation of soluble laminin as analyzed by a colony counting method. Data are presented as percentage of bacterial adhesion to cells relatively to the NTHi 3655 wt without preincubation with lamininmur that was set as 100%. Bacterial preincubation with laminin promoted NTHi 3655 wt and NTHi 3655Δhpd adhesion, but not to other Lbp-deletion mutants and NTHi 3655+anti-EF-Tu. All data in A–D represent mean values of three independent experiments, and error bars indicate standard deviations. Statistical differences between preincubation with and without antilaminin antibodies in A–C, or between preincubation with or without laminin in D, were calculated by a two-way ANOVA. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001.
Denuded BM as a result of microbial infection or inflammation during acute COPD may expose lung laminins to airway pathogens [3, 5, 26]. Degradation of laminin polymers by bacteria or neutrophil proteases may resolve the laminin molecules from the ECM scaffold [26–28]. Thus, we hypothesized that the NTHi laminin interactome could pick up pericellular cell-free laminin to the bacterial surface for subsequent adherence to the airway epithelial cell layer. We compared the adherence of NTHi 3655 wt and Lbp-deficient mutants in the presence or absence of supplemented lamininmur. Adherence of NTHi 3655 wt to A549 cells increased after preincubation of bacteria with lamininmur (25 nM) (Figure 3D). Reduced adherence to A549 was observed with NTHi 3655Δhpd, Δhp6, and Δldh and NTHi 3655+anti-EF-Tu, as well as mutants NTHi 3655Δhel, Δhpe, Δhpf, and Δhap that are defective in cell adherence as previously reported [9]. Preincubation with lamininmur only promoted A549 adherence of NTHi 3655Δhpd but not to any other Lbp-deletion mutants or NTHi 3655+anti-EF-Tu. Thus, in addition to P4, PE, PF, and Hap, Haemophilus EF-Tu, LDH, P6, and PD were able to manipulate both cell-associated and cell-free laminin for enhanced bacterial adherence to epithelial cells.
The Haemophilus Laminin Interactome Has Unique Interactions With LG Domains of Human Laminin α1 and α3B
Laminin-111 expression is upregulated during embryonic lung development, but it is progressively reduced with low expression in the adult lung [1, 29–32]. It is notable that the laminin-111 of murine and human origins share high protein sequence homology (76.1%/85.9%, 92.8%/96.8%, and 92.6%/96.5% of identity/similarity for α1, β1 and γ1 chains, respectively). On the other hand, laminin-3B32 (α3B-β3-γ2) is the main laminin isoform expressed at the BM of the epithelial cell layer in adult lung [1, 29–32]. Hence, we were interested in defining whether the NTHi laminin interactome could also bind the human laminin-111 and 332. In this study, we focused on the LG domains of α1 and α3B (denoted as α3 hereafter) chains based on the previous finding that LG4–5 domains of lamininmur are also the binding sites for PE and PF [8, 19].
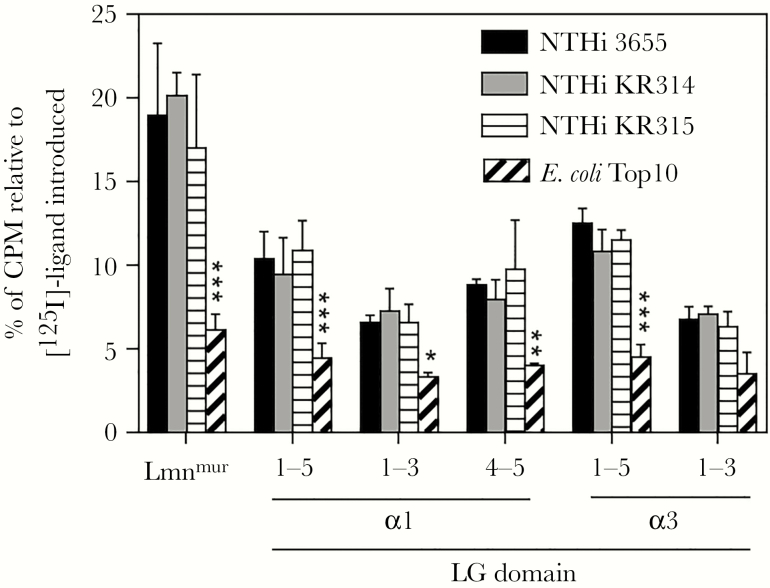
To assess the interaction of bacteria with LG domains, full-length (LG1–5) and truncated LG fragments (LG1–3 and LG4–5) of human LAMA1 and LAMA3 were recombinantly expressed, radiolabeled, and subjected to a direct binding assay with intact bacteria. Of note, LG4–5 of the α3 chain was excluded from the analysis due to low yield recombinant protein produced. For α1 and α3 chains, NTHi 3655 and clinical isolates KR314 and KR315 showed significant (P ≤ .001) binding to LG1–5, which was higher than their binding to the respective LG1–3, compared to control E. coli (Figure 4). Moreover, NTHi binding to α1-derived LG4–5 was comparable with the full-length LG.
Figure 4.
Relative binding affinities of nontypeable Haemophilus influenzae (NTHi) strains to variable LG domains of laminin α1 and α3 chains as determined by a radioligand direct binding assay. Multiple strains of NTHi (NTHi 3655, and clinical strains of NTHi KR314 and KR315) were incubated with radiolabeled lamininmur and recombinant LG1–5, LG1–3, and LG4–5. Escherichia coli Top10 and lamininmur were included as negative and positive controls, respectively. Data are presented as percentage of radioactivity of ligands retained on bacteria after washing relatively to the radioactivity of the total ligand introduced that was set as 100%. Data represent mean values of three independent experiments, and error bars indicate standard deviations. Statistically significant differences between NTHi and the E. coli control for each ligand were calculated by two-way ANOVA. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001. CPM, count per minute; 125I, 125Iodine; Lmnmur, lamininmur.
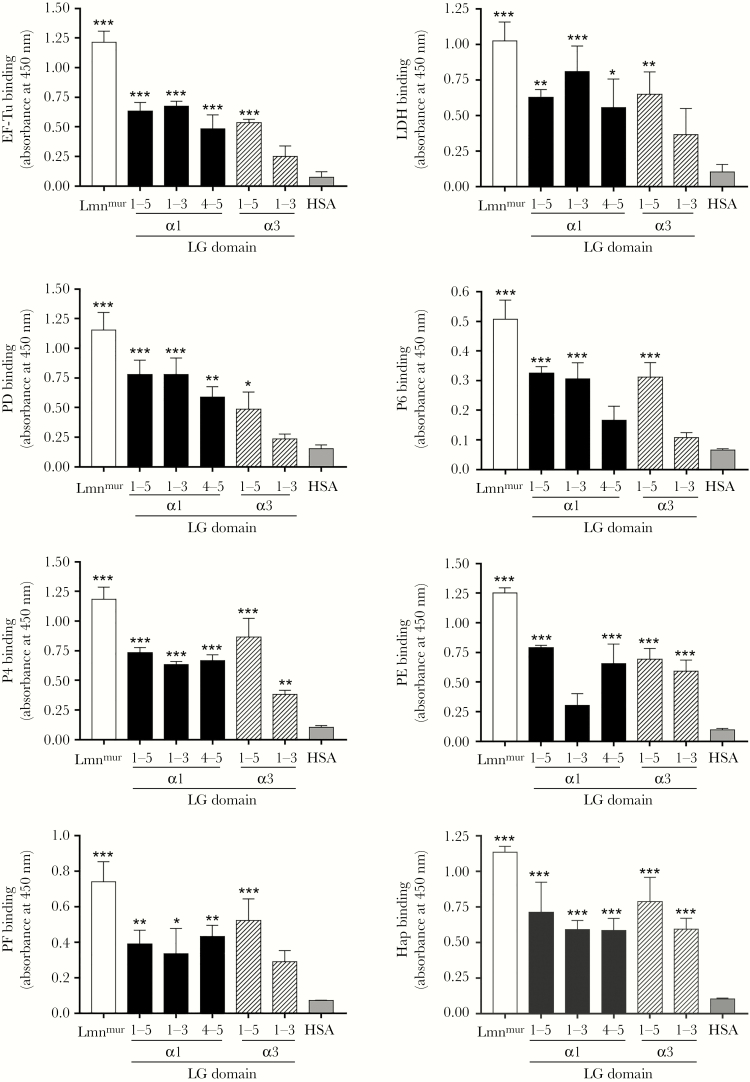
When the LG was tested with recombinant bacterial proteins on ELISA, all NTHi Lbps showed binding to LG1–5 derived from α1 and α3 chains compared to the negative control (Figure 5). However, binding activities against α1-related LG fragments were heterogeneous among the Lbps. Recombinant EF-Tu, LDH, and PD exerted higher binding to LG1–3 than LG4–5, whereas PE and PF reversely bound LG4–5 better than LG1–3. Recombinant P4, P6, and Hap had similar binding capacity to both LG1–3 and LG4–5. It is interesting to note that, for LG domains derived from the α3 chain, all NTHi Lbps consistently displayed lower binding to LG1–3 compared to the LG1–5, in concordance with the data of Figure 4. This indicated that in the α3 chain, LG1–3 was not involved in bacterial or Lbps binding to LG1–5. Finally, however, binding to LG1–5 by intact bacteria or Lbps was only partial, relative to binding to the whole lamininmur molecule (Figure 4 and 5). These observations suggested alternative binding sites for NTHi on the laminin molecule in addition to the LG domain. Taken together, our data indicate that the NTHi laminin interactome recognizes laminin LG domains of human origin but with unique interactions for different isoforms of laminin.
Figure 5.
Relative interactions of the recombinant nontypeable Haemophilus influenzae (NTHi) interactome with LG domains of laminin α1 and α3 chains as analyzed by enzyme-linked immunosorbent assay. Immobilized recombinant LG fragments of laminin α1 (LG1–5, LG1–3, and LG4–5) and laminin α3 (LG1–5 and LG1–3) were incubated with NTHi laminin-binding proteins (Lbps) (10 nM) (EF-TuM1-K394, LDHM1-L381, PDS19-K364, P6S21-Y153, P4G22-K274, PE22-160, PF12-293, and HapE523-L1036). Binding of NTHi proteins to the LG fragments were analyzed with Lbp-specific rabbit polyclonal antibody (pAb) (anti-EF-Tu [15], anti-LDH, anti-PD [16], anti-P4 [9], anti-PE [9], anti-PF [13], and anti-Hap [9]) and mouse anti-P6 monoclonal antibody (clone-7F3) [17], followed by horseradish peroxidase-conjugated swine antirabbit pAb or rabbit antimouse pAb as secondary antibodies. Lamininmur and human serum albumin (HSA) were included as positive and negative controls, respectively. Data represent mean values of three independent experiments, and error bars indicate standard deviations. Statistical differences between the binding of laminin or LG fragments, and HSA by each Lbp were calculated by one-way ANOVA. *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001. Lmnmur, lamininmur.
The Nontypeable Haemophilus influenzae Laminin Interactome Targets Multiple Heparin-Binding Sites on Laminin
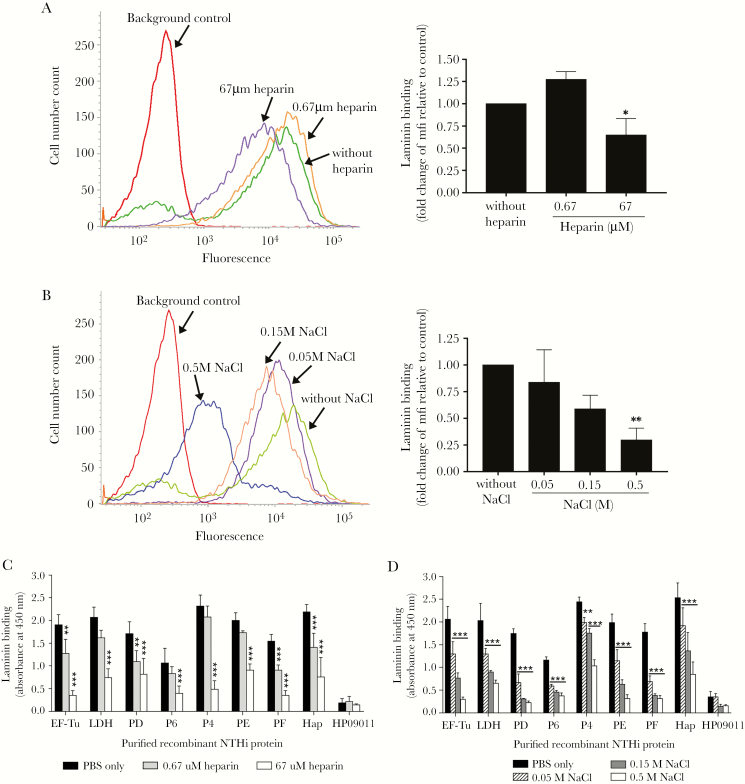
There are five heparin-binding domains located on the α, β, and γ chains of laminin (Figure 1A) [20–22]. The major heparin-binding domain Hep-1 is located on the LG4–5 of the α chain, which is also the binding site for PE and PF [8, 19]. However, because the binding capacity of bacteria or Lbps to LG1–5 was partial relative to lamininmur (Figure 4 and 5), we hypothesized the plausibility of additional binding sites for the pathogen on the laminin molecule. To date, little is known about the total laminin-binding characteristics of NTHi. To predict the common NTHi binding sites on laminin, a direct bacterial ligand-binding assay was done in the presence of heparin and NaCl. Binding of NTHi 3655 wt to lamininmur was gradually reduced when lamininmur (25 nM) was preincubated with increasing concentrations of heparin (0.67–67 μM) (Figure 6A) or in the presence NaCl (0.05–0.5 M) (Figure 6B).
Figure 6.
Characterization of heparin inhibition and ionic interaction in laminin binding of nontypeable Haemophilus influenzae (NTHi). (A and B) Direct protein-binding assay of NTHi 3655 with lamininmur in the presence of heparin and NaCl as measured by flow cytometry. (A) Binding of NTHi 3655 with lamininmur (25 nM) that had been preincubated with various concentrations of heparin (0.67 and 67 μM). (B) Interaction of NTHi 3655 with lamininmur (25 nM) in the presence of increasing concentrations of NaCl (0.05–0.5 M). Impaired bacterial binding to laminin was observed in the presence of 67 μM heparin or NaCl at ≥50 mM. Left panels in A and B show representative histograms of three independent experiments, and to the right fold change of mean fluorescence intensity (mfi) of laminin binding in the presence of heparin or NaCl relative to the control without inhibitors is shown. (C and D) Inhibition of the laminin-NTHi laminin-binding proteins (Lbps) interaction by heparin and NaCl as analyzed by enzyme-linked immunosorbent assay. Immobilized recombinant NTHi Lbps (EF-TuM1-K394, LDHM1-L381, PDS19-K364, P6S21-Y153, Rib30sM1-E125, P4G22-K274, PE22-160, PF12-293, and HapE523-L1036) were loaded with the following: (C) lamininmur (10 nM) that was preincubated with heparin (0.67 and 67 μM) or (D) lamininmur (10 nM) in the presence of NaCl (0.05–0.5 M). Heparin at ≥0.67 μM or NaCl at ≥50 mM disrupted the interaction of laminin with all NTHi Lbps. HP09011 was included as negative control. In A–D, data represent mean values of three independent experiments and error bars indicate standard deviations. Statistically significant differences in laminin binding between conditions with and without heparin or NaCl were calculated by one-way (for A and B) and two-way ANOVA (for C and D). In D, horizontal bars indicate similar significance of statistical differences within group data, when compared with the control without additional NaCl (phosphate-buffered saline only). *, P ≤ .05; **, P ≤ .01; and ***, P ≤ .001.
We also evaluated the inhibitory effects of heparin and NaCl on NTHi Lbps. Recombinant PE and PF were included as positive controls. Preincubation of lamininmur with increasing concentrations of heparin (0.67–67 μM) or addition of NaCl (0.05–0.5 M) suppressed the binding of all Lbps to lamininmur in a dose-dependent manner (Figure 6C and D). This implied that the heparin-treated lamininmur was not accessible to bacterial Lbps thus precluding the bacterial binding. Results were consistent with the data obtained in Figure 6A and B. In conclusion, our findings suggested that the common binding sites of the NTHi laminin interactome on laminin overlap with multiple heparin-binding domains that are also ionic-interaction dependent.
DISCUSSION
In this study, global screening of NTHi Lbps from the bacterial OM proteome resulted in the identification of EF-Tu, LDH, PD, and P6 as novel Lbps. We presented experimental evidence that supports the important role of the novel Lbps in laminin binding of NTHi. Although PD and P6 are known for their surface topology [33, 34], detection of EF-Tu and LDH by streptavidin as a results of surface biotinylation revealed their cell surface topology. This is in good agreement with other well-described microbial Lbps that are surface associated and thus accessible to host proteins (Figure 1) [3, 4]. In parallel with the well-studied Lbps, EF-Tu, LDH, PD, and P6 exhibited positive interactions with both soluble and immobilized (polymerized) laminin and thus mediated laminin-dependent adherence to airway epithelial cells (Figure 2 and 3). Nontypeable H. influenzae 3655 with masked surface-EF-Tu or genetic deletion of LDH and P6 were attenuated in laminin binding and hence unable to absorb cell-free and cell-associated laminin for adherence (Figure 2 and 3). The nonattenuated laminin binding of NTHi 3655Δhpd could be attributed to the upregulated expression of other Lbps as compensation for the loss of proteins involved in laminin deposition. This is supported by experiments with Bacteroides fragilis; deletion of the TonB-dependent fibronectin-binding protein (fnbp) caused enhanced fibronectin binding by another fnbp [35].
In contrast to EF-Tu and LDH, PD and P6 are well-studied NTHi virulence factors. Nontypeable H. influenzae P6 is a surface peptidoglycan-associated lipoprotein involved in maintaining the integrity of the cell envelope. The P6-deficient mutant of NTHi is highly fragile and sensitive to bactericidal substances compared to the wt counterpart [17, 33, 36]. This may explain the major reduction in laminin binding by NTHi 3655Δhp6 despite the relatively low binding by recombinant P6 (Figure 2). Protein D is essential for NTHi-induced OM in animal models [34]. It catalyzes the decoration of NTHi lipooligosaccharide (LOS) with phosphorylcholine, promotes bacterial internalization into monocytes, and impairs ciliary beating of epithelial cells [16, 34, 37]. In this study, we discovered the additional virulent role of P6 and PD as laminin hijackers. EF-Tu is an intracellular protein essential for protein synthesis, but it also moonlights as a surface protein important for bacterial pathogenesis [38, 39]. In other pathogens, EF-Tu interacts with several ECM proteins and complement regulator (ie, Factor H) for enhanced colonization [38–42]. In this study, we reported a moonlighting role of Haemophilus EF-Tu in laminin binding of NTHi. Moreover, we recently reported the surface exposure and antigenicity of Haemophilus EF-Tu [15]. In contrast to other pathogens, little is known about LDH in NTHi. Deletion of ldh caused Streptococcus pyogenes to lose cysteine proteinase SpeB activity, thus making the bacterium avirulent [43]. More importantly, LDH of Streptococcus suis type 2 and Lactobacillus rhamnosus was isolated as an extracellular protein and shown to interact with laminin and fibronectin [42, 44]. Although LDH of NTHi was reported to be upregulated during iron/hemin supplementation [45], our study further determined LDH as a novel Lbp, contributing to NTHi pathogenesis.
The human α3 chain of airway laminin-3B32 has 26.8% and 40.7% of protein sequence identity and similarity with the α1 chain, respectively (Supplementary Table S3). It is interesting to note that the NTHi laminin interactome exerted interaction plasticity to LG1–5 from both α1 and α3 chains despite their limited sequence homology. This could be partly attributed to the unique heparin-binding site on the LG4–5 of α1 and α3, as defined by LG fragments mapping (Figures 4 and 5) and heparin inhibition in the binding of α3-derived LG1–5 (Supplementary Figure S2) [1, 32]. In addition, LG1–3 in α1 but not α3 is also a binding target for NTHi on LG1–5 via interaction with EF-Tu, PD, and LDH (Figure 5). We further discovered the common binding sites of NTHi laminin interactome that involve multiple heparin-binding sites on the laminin molecule. This was deduced from the effective inhibition of heparin and NaCl to preclude the laminin binding by bacteria and Lbps (Figure 6). Moreover, LG1–5 fragment could only partially block the bacterial binding to laminin (Supplementary Figure S3). The heparin-binding motif sequence is defined as XBBXXBX, XBBXBX, XBBBXXBBBXXBBX, and TXXBXXTBXXXTBB (X, hydrophobic or uncharged amino acid; B, basic amino acid; and T, a turn) [32, 46–48]. Thus, it is positively charged and electrostatically interacts with the negatively charged heparin. The inclination of NTHi and its interactome towards the laminin heparin-binding sites could be partly attributed to the net negative charge of cell surface contributed by LOS and anionic surface proteins including some Lbps [49, 50]. We postulate that the ability of NTHi to target multiple heparin-binding sites will enable the pathogen to alternative interactions with laminin when the LG domains are occupied by epithelial cell basolateral receptors. Moreover, because the heparin-binding domains also exist in other laminin isoforms [1, 20], this will allow NTHi to interact with an extensive range of tissue-specific laminins and thus broader colonization niches in the airways.
CONCLUSIONS
In conclusion, this is the first report on the multiple interaction strategies of NTHi for maximum binding to the host laminin molecule. This involves expression of the NTHi laminin interactome consisting of at least 8 surface proteins that concurrently target multiple heparin-binding domains of laminin. Our findings shed new lights on the pathogenesis mechanism of NTHi in the manipulation of host ECM proteins that may be the impetus for future antimicrobial drug development.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the Clinical Microbiology laboratory at Labmedicine Skåne (Lund, Sweden) for providing clinical isolates.
Financial support. This work was supported by grants from Foundations of Anna and Edwin Berger (to K. R.), the Swedish Medical Research Council (Grant Number K2015-57X-03163-43-4; to K. R.), the Cancer Foundation at the University Hospital in Malmö (to K. R.), the Royal Physiographical Society (to Y.-C. S., F. J., and B. S.), the Skåne County Council’s Research and Development Foundation (to K. R.), and the Heart Lung Foundation (Grant Number 20180401; to K. R.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Aumailley M. The laminin family. Cell Adh Migr 2013; 7:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hohenester E, Yurchenco PD. Laminins in basement membrane assembly. Cell Adh Migr 2013; 7:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chagnot C, Listrat A, Astruc T, Desvaux M. Bacterial adhesion to animal tissues: protein determinants for recognition of extracellular matrix components. Cell Microbiol 2012; 14:1687–96. [DOI] [PubMed] [Google Scholar]
- 4. Singh B, Fleury C, Jalalvand F, Riesbeck K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev 2012; 36:1122–80. [DOI] [PubMed] [Google Scholar]
- 5. Su YC, Jalalvand F, Thegerström J, Riesbeck K. The interplay between immune response and bacterial infection in COPD: focus upon non-typeable Haemophilus influenzae. Front Immunol 2018; 9:2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jalalvand F, Riesbeck K. Update on non-typeable Haemophilus influenzae-mediated disease and vaccine development. Expert Rev Vaccines 2018; 17:503–12. [DOI] [PubMed] [Google Scholar]
- 7. Bidan CM, Veldsink AC, Meurs H, Gosens R. Airway and extracellular matrix mechanics in COPD. Front Physiol 2015; 6:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hallström T, Singh B, Resman F, Blom AM, Mörgelin M, Riesbeck K. Haemophilus influenzae protein E binds to the extracellular matrix by concurrently interacting with laminin and vitronectin. J Infect Dis 2011; 204:1065–74. [DOI] [PubMed] [Google Scholar]
- 9. Su YC, Mukherjee O, Singh B, et al. . Haemophilus influenzae P4 interacts with extracellular matrix proteins promoting adhesion and serum resistance. J Infect Dis 2016; 213:314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jalalvand F, Su YC, Mörgelin M, et al. . Haemophilus influenzae protein F mediates binding to laminin and human pulmonary epithelial cells. J Infect Dis 2013; 207:803–13. [DOI] [PubMed] [Google Scholar]
- 11. Fink DL, Green BA, St Geme JW 3rd. The Haemophilus influenzae hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect Immun 2002; 70:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voss BJ, Gaddy JA, McDonald WH, Cover TL. Analysis of surface-exposed outer membrane proteins in Helicobacter pylori. J Bacteriol 2014; 196:2455–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su YC, Jalalvand F, Mörgelin M, Blom AM, Singh B, Riesbeck K. Haemophilus influenzae acquires vitronectin via the ubiquitous Protein F to subvert host innate immunity. Mol Microbiol 2013; 87:1245–66. [DOI] [PubMed] [Google Scholar]
- 14. Molloy MP, Herbert BR, Slade MB, et al. . Proteomic analysis of the Escherichia coli outer membrane. Eur J Biochem 2000; 267:2871–81. [DOI] [PubMed] [Google Scholar]
- 15. Thofte O, Su YC, Brant M, et al. . EF-Tu from non-typeable Haemophilus influenzae is an immunogenic surface-exposed protein targeted by bactericidal antibodies. Front Immunol 2018; 9:2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janson H, Carl n B, Cervin A, et al. . Effects on the ciliated epithelium of protein D-producing and -nonproducing nontypeable Haemophilus influenzae in nasopharyngeal tissue cultures. J Infect Dis 1999; 180:737–46. [DOI] [PubMed] [Google Scholar]
- 17. Murphy TF, Kirkham C, Lesse AJ. Construction of a mutant and characterization of the role of the vaccine antigen P6 in outer membrane integrity of nontypeable Haemophilus influenzae. Infect Immun 2006; 74:5169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr 2006; 62:1243–50. [DOI] [PubMed] [Google Scholar]
- 19. Su YC, Halang P, Fleury C, Jalalvand F, Mörgelin M, Riesbeck K. Haemophilus Protein F orthologs of pathogens infecting the airways: exploiting host laminin at heparin-binding sites for maximal adherence to epithelial cells. J Infect Dis 2017; 216:1303–7. [DOI] [PubMed] [Google Scholar]
- 20. Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol 2012; 28:523–53. [DOI] [PubMed] [Google Scholar]
- 21. Kouzi-Koliakos K, Koliakos GG, Tsilibary EC, Furcht LT, Charonis AS. Mapping of three major heparin-binding sites on laminin and identification of a novel heparin-binding site on the B1 chain. J Biol Chem 1989; 264:17971–8. [PubMed] [Google Scholar]
- 22. Skubitz AP, McCarthy JB, Charonis AS, Furcht LT. Localization of three distinct heparin-binding domains of laminin by monoclonal antibodies. J Biol Chem 1988; 263:4861–8. [PubMed] [Google Scholar]
- 23. Singh B, Al-Jubair T, Mörgelin M, Thunnissen MM, Riesbeck K. The unique structure of Haemophilus influenzae protein E reveals multiple binding sites for host factors. Infect Immun 2013; 81:801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Link AJ, Hays LG, Carmack EB, Yates JR 3rd. Identifying the major proteome components of Haemophilus influenzae type-strain NCTC 8143. Electrophoresis 1997; 18:1314–34. [DOI] [PubMed] [Google Scholar]
- 25. Mobegi FM, van Hijum SA, Burghout P, et al. . From microbial gene essentiality to novel antimicrobial drug targets. BMC Genomics 2014; 15:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3:726–33. [DOI] [PubMed] [Google Scholar]
- 27. Heck LW, Morihara K, Abrahamson DR. Degradation of soluble laminin and depletion of tissue-associated basement membrane laminin by Pseudomonas aeruginosa elastase and alkaline protease. Infect Immun 1986; 54:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heck LW, Blackburn WD, Irwin MH, Abrahamson DR. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol 1990; 136:1267–74. [PMC free article] [PubMed] [Google Scholar]
- 29. Thul PJ, Lindskog C. The human protein atlas: a spatial map of the human proteome. Protein Sci 2018; 27:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierce RA, Griffin GL, Mudd MS, et al. . Expression of laminin alpha3, alpha4, and alpha5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol 1998; 19:237–44. [DOI] [PubMed] [Google Scholar]
- 31. Miner JH, Patton BL, Lentz SI, et al. . The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol 1997; 137:685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rousselle P, Beck K. Laminin 332 processing impacts cellular behavior. Cell Adh Migr 2013; 7:122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michel LV, Snyder J, Schmidt R, et al. . Dual orientation of the outer membrane lipoprotein P6 of nontypeable Haemophilus influenzae. J Bacteriol 2013; 195:3252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson RW, McGillivary G, Denoël P, Poolman J, Bakaletz LO. Abrogation of nontypeable Haemophilus influenzae protein D function reduces phosphorylcholine decoration, adherence to airway epithelial cells, and fitness in a chinchilla model of otitis media. Vaccine 2011; 29:1211–21. [DOI] [PubMed] [Google Scholar]
- 35. Pauer H, Cavalcanti SN, Teixeira FL, et al. . Inactivation of a fibronectin-binding TonB-dependent protein increases adhesion properties of Bacteroides fragilis. J Med Microbiol 2013; 62:1524–30. [DOI] [PubMed] [Google Scholar]
- 36. Godlewska R, Wiśniewska K, Pietras Z, Jagusztyn-Krynicka EK. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol Lett 2009; 298:1–11. [DOI] [PubMed] [Google Scholar]
- 37. Ahrén IL, Janson H, Forsgren A, Riesbeck K. Protein D expression promotes the adherence and internalization of non-typeable Haemophilus influenzae into human monocytic cells. Microb Pathog 2001; 31:151–8. [DOI] [PubMed] [Google Scholar]
- 38. Henderson B, Martin A. Bacterial virulence in the moonlight: multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect Immun 2011; 79:3476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Widjaja M, Harvey KL, Hagemann L, et al. . Elongation factor Tu is a multifunctional and processed moonlighting protein. Sci Rep 2017; 7:11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kunert A, Losse J, Gruszin C, et al. . Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J Immunol 2007; 179:2979–88. [DOI] [PubMed] [Google Scholar]
- 41. Yu Y, Wang H, Wang J, et al. . Elongation factor thermo unstable (EF-Tu) moonlights as an adhesin on the surface of Mycoplasma hyopneumoniae by binding to fibronectin. Front Microbiol 2018; 9:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q, Liu H, Du D, et al. . Identification of novel laminin- and fibronectin-binding proteins by far-Western blot: capturing the adhesins of Streptococcus suis type 2. Front Cell Infect Microbiol 2015; 5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oehmcke-Hecht S, Nass LE, Wichura JB, Mikkat S, Kreikemeyer B, Fiedler T. Deletion of the L-lactate dehydrogenase gene ldh in Streptococcus pyogenes leads to a loss of SpeB activity and a hypovirulent phenotype. Front Microbiol 2017; 8:1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Aryantini NP, Kondoh D, Nishiyama K, et al. . et al. Anchorless cell surface proteins function as laminin-binding adhesins in Lactobacillus rhamnosus FSMM22. FEMS Microbiol Lett 2017; 364. doi:10.1093/femsle/fnx056 [DOI] [PubMed] [Google Scholar]
- 45. Whitby PW, Seale TW, VanWagoner TM, Morton DJ, Stull TL. The iron/heme regulated genes of Haemophilus influenzae: comparative transcriptional profiling as a tool to define the species core modulon. BMC Genomics 2009; 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishihara J, Ishihara A, Fukunaga K, et al. . Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat Commun 2018; 9:2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meneghetti MC, Hughes AJ, Rudd TR, et al. . Heparan sulfate and heparin interactions with proteins. J R Soc Interface 2015; 12:0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Andersson E, Rydengård V, Sonesson A, Mörgelin M, Björck L, Schmidtchen A. Antimicrobial activities of heparin-binding peptides. Eur J Biochem 2004; 271:1219–26. [DOI] [PubMed] [Google Scholar]
- 49. Beveridge TJ. Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol 1999; 181:4725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol 2010; 2:a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.