Abstract
Background
Cryptosporidiosis, an enteric protozoon, causes substantial morbidity and mortality associated with diarrhea in children <2 years old in low- to middle-income countries. There is no vaccine and treatments are inadequate. A piperazine-based compound, MMV665917, has in vitro and in vivo efficacy against Cryptosporidium parvum. In this study, we evaluated the efficacy of MMV665917 in gnotobiotic piglets experimentally infected with Cryptosporidium hominis, the species responsible for >75% of diarrhea reported in these children.
Methods
Gnotobiotic piglets were orally challenged with C hominis oocysts, and oral treatment with MMV665917 was commenced 3 days after challenge. Oocyst excretion and diarrhea severity were observed daily, and mucosal colonization and lesions were recorded after necropsy.
Results
MMV665917 significantly reduced fecal oocyst excretion, parasite colonization and damage to the intestinal mucosa, and peak diarrheal symptoms, compared with infected untreated controls. A dose of 20 mg/kg twice daily for 7 days was more effective than 10 mg/kg. There were no signs of organ toxicity at either dose, but 20 mg/kg was associated with slightly elevated blood cholesterol and monocytes at euthanasia.
Conclusions
These results demonstrate the effectiveness of this drug against C hominis. Piperazine-derivative MMV665917 may potentially be used to treat human cryptosporidiosis; however, further investigations are required.
Keywords: animal model, Cryptosporidium hominis, gnotobiotic pigs, MMV665917
The efficacy of piperazine-derivative MMV665917 was previously reported in vitro and in vivo against Cryptosporidium parvum in acute (calves) and chronic (mice) infection models, respectively. Here, we report the drug’s efficacy against Cryptosporidium hominis using the piglet acute diarrhea model.
Cryptosporidiosis, an enteric parasite infection of humans and animals with a worldwide distribution, is a serious cause of diarrhea in children <2 years old in lower- and middle-income countries [1, 2]. Cryptosporidium hominis (~75%) and Cryptosporidium parvum (~20%) are the major species found in humans, followed by Cryptosporidium meleagridis and Cryptosporidium ubiquitum [3]. Although diarrhea caused by Cryptosporidium is acute and self-limiting within 2 weeks in healthy individuals, it can become chronic and life-threatening in young children with malnutrition and in immunocompromised individuals [4]. The therapeutic options for cryptosporidiosis are currently limited to 1 US Food and Drug Administration (FDA)-approved drug, nitazoxanide (NTZ) [5, 6]. Nitazoxanide shortens the duration of diarrhea and parasite excretion in immunocompetent children but is ineffective in immunocompromised patients [7] and in malnourished children [8]. Therefore, more effective drugs to treat cryptosporidiosis in populations at risk for poor outcomes are urgently needed.
Many Cryptosporidium drug candidates have been identified, but only a few appear to be effective and safe when further tested. The piperazine-derivative MMV665917 was identified through screening the Medicines for Malaria Venture “Malaria Box”, an open access collection of 400 compounds with activity against the erythrocyte stages of Plasmodium falciparum [9, 10]. MMV665917 has a high selectivity and potency in vitro for Cryptosporidium species including several C parvum strains and C hominis. MMV665917 rapidly reduces C parvum numbers in an in vitro assay analogous to a classical bacterial time-kill curve, whereas NTZ appears to inhibit C parvum growth without eliminating parasites [11]. Consistent with a potentially parasiticidal mode of action [11], MMV665917 was curative in both chronic and acute mouse models of infection. Finally, MMV665917 treatment significantly reduced parasite excretion and alleviated the severity of diarrhea against C parvum infection in the calf model [12].
Having recently tested several compounds in our gnotobiotic (GB) piglet acute diarrhea model against C hominis [13, 14], we show here that MMV665917 appears to be one of the most promising in terms of efficacy and minimal toxicity. The drug significantly reduced C hominis oocysts excretion in feces, reduced mucosal damage and parasite colonization, and reduced peak diarrheal symptoms. At a higher dose, mild serum biochemical and hematological abnormalities were observed but with no evidence of organ toxicity. Overall, these studies help validate MMV665917 as a potential treatment against C hominis, the main cause of cryptosporidiosis in humans.
METHODS
Cryptosporidium hominis Oocysts and Gnotobiotic Piglets
We have maintained the C hominis strain TU502, originally from a Ugandan diarrheic child, for over a decade in GB piglets [15]. TU502 oocysts were purified from gut contents and feces, as described previously [16]. Twenty-eight GB piglets, derived from 4 litters by Cesarean section, were maintained inside sterile isolators as approved by the Tufts University Institutional Animal Care and Use Committee. Piglets were fed 3 times daily with a total of 500–700 mL/day of human infant milk replacer (Similac Advance Ready to Feed Infant Formula, Abbott Nutrition). Piglets were monitored at least 3 times each day. Healthy piglets, selected 2 days after birth, were assigned to experimental groups.
Cryptosporidium hominis Challenge and MMV665917 Treatment
MMV665917 was suspended as 10 mg/mL in 1% Hypromellose (hydroxypropyl methyl-cellulose [HPMC]; H-3785, Sigma-Aldrich, St. Louis, MO) in water [12]. A total of 1–5 × 106 oocysts of C hominis was administered orally 2 days after birth. Three days later, at the onset of diarrhea, MMV665917 suspension was given orally twice daily for 7 days: 7 piglets received 20 mg/kg per dose; 3 piglets received 10 mg/kg per dose; and 8 piglets received no treatment. Of the 10 uninfected piglets, 4 received 20 mg/kg twice daily; 2 piglets received 10 mg/kg twice daily; 2 received 4 mL of 1% HPMC (vehicle); and 2 received neither treatment nor vehicle.
Clinical Observations and Sample Processing
Diarrheal symptoms were monitored twice per day, and severity was scored from 0 to 4 (0–no diarrhea; 1–brown or gray colored, soft stool, mild diarrhea; 2–brown to yellow colored, mucoid, small volumetric stool, mild to moderate diarrhea; 3–yellow colored, mucoid to watery, medium volumetric stool, moderate diarrhea; 4–yellow to white colored, watery, high volumetric stool, severe diarrhea) [13, 14]. Diarrheal scores were generated independently by 2 laboratory personnel and averaged. Piglets were weighed once per day throughout the study. Rectal swabs were collected daily and processed for microscopic oocyst counts and Cryptosporidium deoxyribonucleic acid (DNA) [13, 14, 17]. In brief, for oocyst counting, fecal suspensions were mounted on welled glass slides (diameter 7 mm) to ensure the same areas for all smears and stained by the modified Kenyon acid-fast method [14, 17]. Oocysts were counted in 30 fields under ×1000 magnification. Oocysts were counted independently by 2 laboratory personnel and averaged. Plasma samples were collected on day 0, 3, and 6 after the onset of drug treatment.
MMV665917 concentration was measured in plasma samples by liquid chromatography-tandem mass spectrometry (LC/MS/MS) using MMV665917 compound spiked into control plasma as a standard. Gut content samples collected from cecum and spiral colon at euthanasia were homogenized by vortexing in phosphate-buffered saline (0.1 g/mL) in a polypropylene tube. An internal standard (enalapril, LKT Laboratories) was added and protein precipitated with HCl/acetonitrile. The supernatant was added to a fresh tube, dried, resuspended in water/acetonitrile (10:90), and analyzed by LC/MS/MS.
Quantitative Real-Time Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (PCR) for Cryptosporidium 18S ribosomal ribonucleic acid gene was performed [13, 14] in triplicate for each sample. In brief, DNA in fecal samples was extracted and stored at –20°C until PCR. The primers (forward 5’-GCT TTA GAC GGT AGG GTA TTG G-3’; reverse 5’-CTT AGA TGT GGT AGC CGT TTC T-3’) and the probe (5’6-FAM/TTA GGG TTC /ZEN/GAT TCC GGA GAG GGA /3IABkFQ) were premixed at 5 µM (primers) and 2.5 µM (probe). Each 10-µL PCR reaction consisted of 5 µL PrimeTime Gene Expression Master Mix (Integrated DNA Technologies, Inc.), 1 µL of the premixed primers/probe; 2 µL sample DNA, and 2 µL H2O. Forty cycles of PCR were performed under the following conditions: polymerase activation at 95°C for 20 seconds, and amplification at 95°C for 1 second followed by 60°C for 20 seconds. Known C hominis DNA standard was prepared from TU502 oocysts counted by hemocytometer. The DNA amounts equivalent to Cryptosporidium oocysts were calculated from cycle threshold of samples using the equation of standard curve.
Microscopic Examination of Tissues at Euthanasia
Piglets were euthanized 2 days after MMV665917 treatment ended. At euthanasia, blood was collected and sent to Tufts Clinical Pathology Laboratory for hematology and serum biochemical analysis. At necropsy, the small and large intestines, stomach, liver, gall bladder, pancreas, kidney, lung, spleen, and brain were fixed in 10% neutral-buffered formalin, paraffin-embedded, and stained using hematoxylin and eosin at Tufts Cummings Histology laboratory. All microscopy was performed by a board certified veterinary pathologist (G. B.) blinded to the infection status and to the treatment(s). Non-gastrointestinal tissues were examined for the presence/absence of lesions. Gastrointestinal sections were scored for C hominis colonization [14, 18] and mucosal lesions severity [13].
Statistical Analyses
The data of oocyst excretions, Cryptosporidium DNA, and diarrhea scores among groups were compared by 1-way analysis of variance (ANOVA) with Dunn’s multiple comparisons test and Mann-Whitney U test. The (1) scores of mucosal lesion and (2) colonization among groups were compared by Mann-Whitney U test. Alteration of serum biochemical and hematological data in piglets was analyzed by 1-way ANOVA with Tukey’s multiple comparisons test. The Holm-Sidak method was used for correction of multiple comparison. We used GraphPad Prism 7.03 software (GraphPad Software, San Diego, CA) to perform graphing and statistical analyses; *, P < .05, **, P < .01, ***, P < .001, and ****, P < .0001 indicated levels of significance, as shown in the figures.
RESULTS
Oocyst Excretion
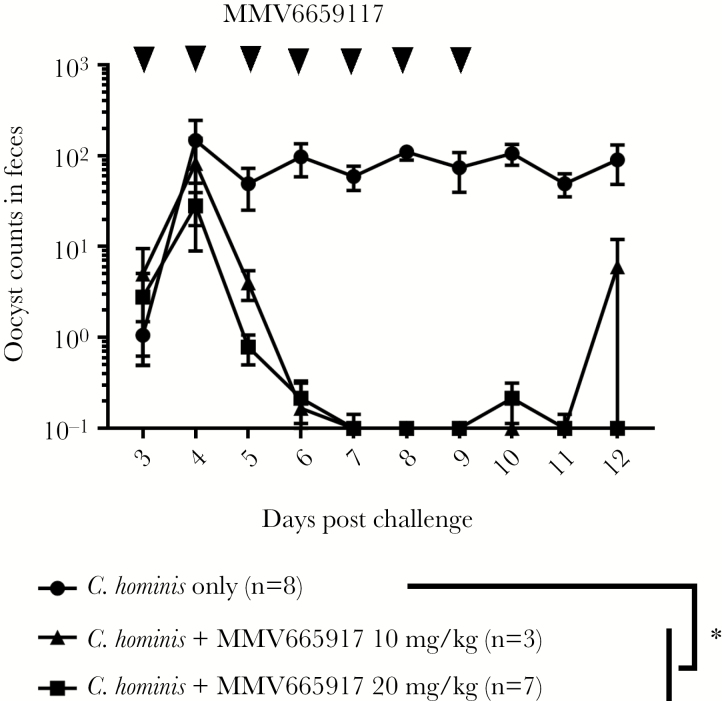
Gnotobiotic piglets are the only animal (apart from humans) to develop acute diarrhea and mucosal lesions due to the human pathogen C hominis. Therefore, piglets are especially suited to test new drugs against C hominis. We quantified oocyst excretion, Cryptosporidium DNA, intensity of diarrhea, and body weight changes to evaluate the piperazine-derivative MMV665917 against cryptosporidiosis. Figure 1 shows that MMV665917-treatment of C hominis-infected piglets significantly reduced fecal oocyst excretion at 20 mg/kg and 10 mg/kg compared with nontreated controls over time (Figure 1). The higher dose of 20 mg/kg MMV665917 reduced oocyst excretion to levels at or below the limits of detection (ie, 1 oocyst per 30 fields) during the 7 days of treatment and for 2 additional days after the end of treatment (Figure 1). In contrast, 1 of 3 piglets that received the lower dose of 10 mg/kg MMV665917 re-shed oocysts in feces within 2 days of treatment cessation (Figure 1).
Figure 1.
Fecal excretion of Cryptosporidium hominis oocysts after challenge. Piglets were inoculated orally with C hominis oocysts 2 days after birth and were treated with MMV665917 3 days postchallenge. Rectal swabs were processed for oocyst measurement. The plots represent mean ± standard error of the mean. The data of oocyst excretions between groups were compared by Dunn’s multiple comparisons test. *, P < .05. Cryptosporidium hominis-infected group (n = 8, closed circle [●]); C hominis-infected and MMV665917 (20 mg/kg)-treated group (n = 7, closed square ■); Cryptosporidium hominis-infected and MMV665917 (10 mg/kg)-treated group (n = 3, closed triangle [▲]).
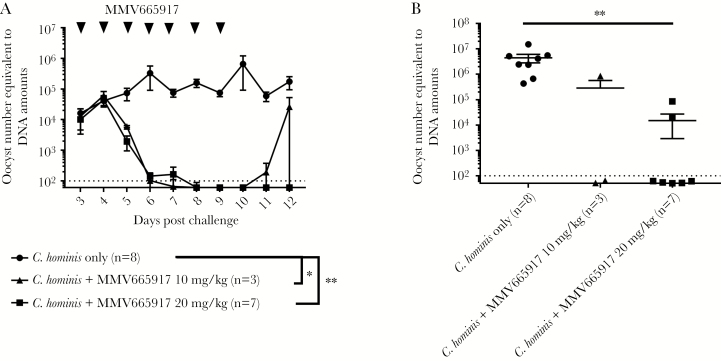
Likewise, both doses of MMV665917 significantly reduced Cryptosporidium DNA in feces over time while piglets were alive and in gut contents at euthanasia (Figure 2B). In 7 of 7 piglets, the higher dose of 20 mg/kg reduced C hominis DNA in feces to levels at or below the limits of detection (ie, DNA amount equivalent to 102C hominis oocysts) during the 7 days of treatment and the 2 days posttreatment (Figure 2A). At euthanasia, gut contents from 2 of 7 piglets treated with a 20 mg/kg dose of MMV665917 tested positive for Cryptosporidium DNA at very low levels (equivalent to 5 × 104 and 9 × 105 oocysts per 100 mg), whereas 5 of 7 piglets remained below the limits of detection (Figure 2B). Likewise, 1 of 3 piglets treated with 10 mg/kg MMV665917 had detectable C hominis DNA in feces within 2 days of treatment cessation (Figure 2A).
Figure 2.
Quantification of Cryptosporidium deoxyribonucleic acid (DNA) in feces and gut contents: (A) Cryptosporidium DNA in feces after dosing with MMV665917, and (B) Cryptosporidium DNA in gut contents. Piglets were inoculated orally with Cryptosporidium hominis oocysts 2 days after birth and were treated with MMV665917 3 days postchallenge. Rectal swabs were processed for Cryptosporidium DNA measurement. Plot A represents mean ± standard error of the mean (SEM), and a scatter dot plot is shown in B (mean ± SEM). The data of Cryptosporidium DNA between groups were compared by Dunn’s multiple comparisons test (*, P < .05; **, P < .01). Cryptosporidium hominis-infected group (n = 8, closed circle [●]); C hominis-infected and MMV665917 (20 mg/kg)-treated group (n = 7, closed square [■]); C hominis-infected and MMV665917 (10 mg/kg)-treated group (n = 3, closed triangle [▲]).
Overall, these results indicate that 7 days of twice-daily treatment with MMV665917 best reduces C hominis oocyst shedding and DNA from feces, although a minority of piglets still had low amounts of Cryptosporidium DNA detectable in the intestinal tracts and 1 piglet treated with 10 mg/kg MMV665917 had rebounded excretion of oocysts after treatment was stopped.
Clinical Observations
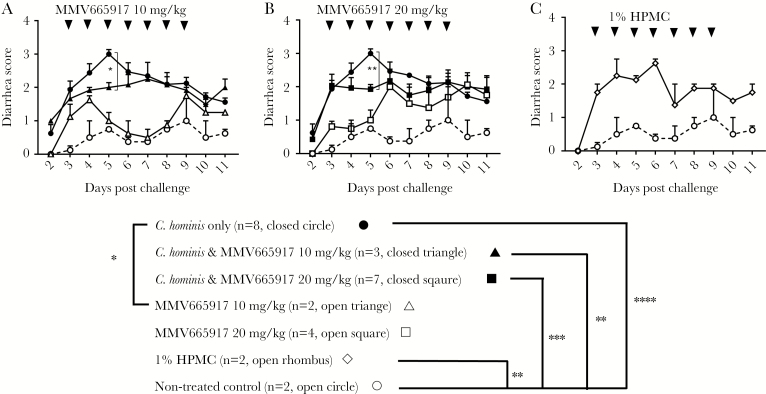
As expected, C hominis infection caused moderate to severe diarrhea (score >2) that peaked on day 5 postchallenge, compared with uninfected controls (score 0 to 1) (Figure 3A and B). MMV665917 treatment significantly alleviated the peak of diarrhea induced by C hominis although those piglets still developed mild to moderate diarrhea (Figure 3A and B), and diarrhea scores stabilized at a score of 2 (2.0 ± 0.11 and 2.0 ± 0.09 with 20 mg/kg and 10 mg/kg, respectively). Further testing in noninfected piglets determined that 1% HPMC as a vehicle contributed to diarrhea in the absence of MMV6617 (Figure 3C). Untreated control piglets had no or mild diarrhea throughout the study. Mild diarrhea, which may be caused by the diet, is occasionally observed on untreated control GB piglets. Overall, these results suggest that MMV665917 reduces peak severity of diarrhea due to C hominis, but that the vehicle 1% HPMC contributes to diarrhea by other mechanisms, perhaps by acting as an osmotic agent.
Figure 3.
Daily diarrhea scores observed in piglets after oocyst challenge and treatments: (A) MMV665917 20 mg/kg; (B) MMV665917 10 mg/kg; (C) 1% hydroxypropyl methyl-cellulose (HPMC). Piglets were inoculated orally with Cryptosporidium hominis oocysts 2 days after birth. The clinical signs of cryptosporidiosis were monitored daily, and diarrheal symptom were scored as described in Methods. The plots represent mean ± standard error of the mean. The data of diarrhea score between groups were compared by Dunn’s multiple comparisons test and Mann-Whitney test on each day: *, P < .05; **, P < .01; ***, P < .001; ****, P < .0001. Cryptosporidium hominis-infected group (n = 8, closed circle [●]); C hominis-infected and MMV665917 (20 mg/kg)-treated group (n = 7, closed square [■]); MMV665917 (20 mg/kg)-treated group (n = 4, open square [□]); C hominis-infected and MMV665917 (10 mg/kg)-treated group (n = 3, closed triangle [▲]); MMV665917 (10 mg/kg)-treated group (n = 2, open triangle [△]); 1% HPMC-treated group (n = 2, open rhombus [◊]); and nontreated control (n = 2, open circle [○]).
All piglets in all groups were weighed daily. The piglets infected with C hominis have lower growth rate than the noninfected piglets as observed in previous studies; however, the difference was not statistically significant across all groups (data not shown).
Histopathology
Microscopic examination was performed in a blinded fashion for presence/absence of lesions, lesion types, and scoring according to a previously established severity scale [13]. As expected, all piglets infected with C hominis showed mild to moderate villus blunting and fusion and mild to moderately increased lamina proprial and intraepithelial lymphocytes. Likewise, all piglets infected with C hominis showed mild to moderate epithelial cell loss, epithelial attenuation and erosion, individual single-cell necrosis, and mild to moderately increased lymphocytes in the lamina propria and intraepithelial locations. Occasionally, C hominis-infected piglets also developed crypt abscesses.
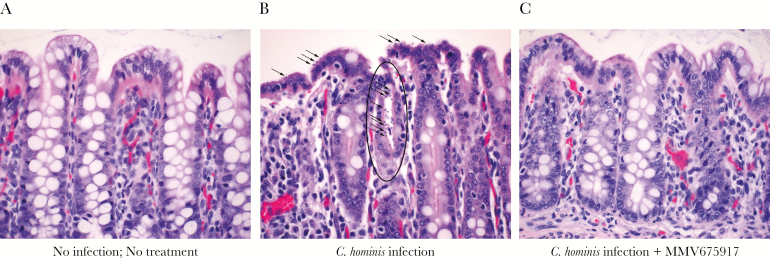
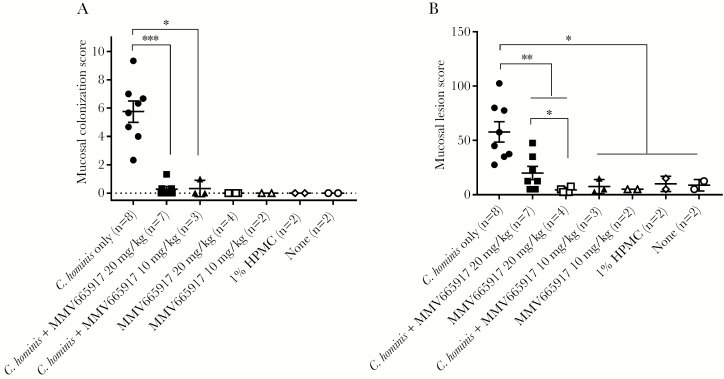
Representative examples of C hominis are shown in Figure 4. Cryptosporidium hominis was rarely detected in the small intestine (data not shown) and frequently detected in cecum (data not shown) and spiral colon of challenged piglets (Figure 4B) but not in normal, noninfected controls (Figure 4A) or in MMV665917-treated piglets (Figure 4C). As expected from oocyst counts, and fecal PCR, MMV665917 significantly reduced colonization (Figure 5A) and mucosal lesion (Figure 5B) scores compared with piglets that only received C hominis. Low to no colonization was observed in all other groups: MMV665917, vehicle (1% HPMC), or none. Infected piglets treated with 20 mg/kg MMV665917 had higher lesion scores than the piglets treated with 10 mg/kg, but this may reflect biological or experimental variation because the group sizes were not balanced. Overall, the microscopy results showed that MMV665917 significantly decreased colonization and partially ameliorated C hominis-induced mucosal damage and inflammation.
Figure 4.
Microscopic examination of hematoxylin and eosin-stained sections magnified ×40 from the spiral colon. (A) No infection, No treatment; (B) Cryptosporidium hominis infection only; and (C) C hominis and MMV665917 20 mg/kg. Spiral colon is colonized by C hominis at the surface epithelium (arrows) and in a colonic gland (oval) (B), whereas no parasite forms are found in a C hominis-infected piglet that was treated with MMV665917 (C).
Figure 5.
Mucosal colonization and lesion scores in piglets. (A) Colonization scores; (B) lesion scores. Mucosal colonization was scored from 0 to 5 (0 = no infection detected; 1 = 1%–20% epithelial surface infected; 2 = 21%–40% surface infected; 3 = 41%–60% surface infected; 4 = 61%–80% surface infected; 5 = 81%–100% surface infected) in 3 fields from each anatomic site: duodenum, jejunum, ileum, cecum, and spiral colon and then averaged. For each piglet, the final score is a sum of the averages (A). Mucosal lesion scores were generated for each site for changes in villi, epithelial cells, and inflammation. The following scores were used: normal = 0, equivocal = 2.5, mild = 5, moderate = 10, marked = 15. The final lesion score was a sum of scores from duodenum, jejunum, ileum, cecum, and colon for each piglet. The scatter plot (A and B) represents mean ± standard error of the mean. A Mann-Whitney test was conducted using GraphPad Prism 7.03: *, P < .05; **, p<.01; ***, P < .001. Cryptosporidium hominis-infected group (n = 8, closed circle [●]); C hominis-infected and MMV665917 (20 mg/kg)-treated group (n = 7, closed square [■]); MMV665917 (20 mg/kg)-treated group (n = 4, open square [□]); C hominis-infected and MMV665917 (10 mg/kg)-treated group (n = 3, closed triangle [▲]; MMV665917 (10 mg/kg)-treated group (n = 2, open triangle [△]); 1% hydroxypropyl methyl-cellulose (HPMC)-treated group (n = 2, open rhombus [◊]); and nontreated control (n = 2, open circle [○]).
Pharmacokinetics
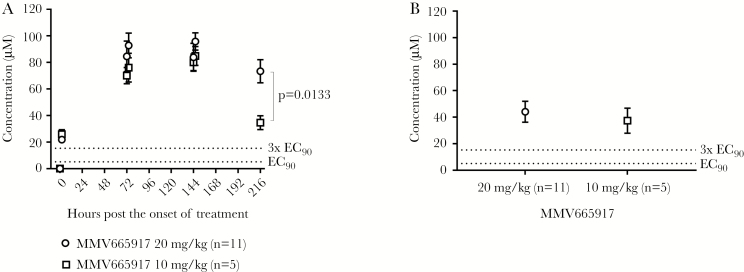
MMV665917 was dosed at 12-hour intervals for 7 days, and plasma concentrations were determined at 2 hours and 12 hours postdose on days 0, 3, 6, and 9 after the onset of drug treatment (Figure 6). At all timepoints, the measured plasma concentrations exceeded 3× EC90 (90% maximal effective concentration; 15.0 µM in the presence of 10% serum) (Figure 6A), which was the concentration previously found to be necessary to maximize the rate of parasite elimination in vitro [11]. In general, 20 mg/kg yielded slightly higher plasma concentrations than 10 mg/kg, but the differences were not significant. More specifically, within 2 hours of administration, plasma concentrations of MMV665917 were 26.0 ± 3.4 µM and 21.8 ± 1.3 µM at 10 mg/kg and 20 mg/kg, respectively. On day 3, plasma concentrations were 70.0 ± 6.1 µM and 84.6 ± 11.5 µM at 10 mg/kg and 20 mg/kg, respectively. On day 6, plasma concentrations were 80.0 ± 6.3 µM and 91.6 ± 7.9 µM at 10 mg/kg and 20 mg/kg, respectively. On day 9 (60 hours after treatment ended), plasma concentrations declined: 34.7 ± 5.2 µM at 10 mg/kg dose vs 68.7 ± 8.2 µM at 20 mg/kg. These results indicate that plasma concentrations increase rapidly and maintain a steady state with twice-daily dosing. MMV665917 was measured in gut contents after euthanasia. In the gut contents, measured 60 hours after treatment ended, concentrations were still higher than the EC90 37.5 ± 4.2 µM at 10 mg/kg dose and 44.1 ± 2.4 µM at 20 mg/kg dose (Figure 6B). This suggests that high MMV665917 exposure is quite prolonged in an active form in the gastrointestinal tract and thus this dosing regimen may be providing the maximal therapeutic effect possible for the compound. Although C hominis-infected piglets showed higher plasma concentration of MMV665917 than the noninfected piglets, there was no statistical significance (data not shown).
Figure 6.
MMV665917 concentration in plasma and gut contents: (A) MMV665917 concentration in plasma after dosing with MMV665917 20 mg/kg or 10 mg/kg dose and (B) MMV665917 concentration in gut contents. Piglets were inoculated orally with Cryptosporidium hominis oocysts 2 days after birth and dosing with MMV665917 began 3 days postchallenge. Plasma samples were collected at 0 hour (before 1st dose), 2 hours (post 1st dose), 72 hours (12 hours post 6th dose), 74 hours (2 hours post 7th dose), 144 hours (12 hours post 12th dose), 146 hours (2 hours post 13th dose), and 216 hours (60 hours post 14th dose). Gut contents were also collected at 216 hours (60 hours post 14th dose). Unpaired t test was conducted using GraphPad Prism 7.03. EC90 = 5.0 (2.5 to 13.3) μM.
Blood Analysis
Thirty-five analytes were evaluated in serum and blood sampled at euthanasia. Cryptosporidium hominis infection altered few blood biochemical and hematological parameters (Supplement 1). The majority were not affected. In TU502-infected piglets, total protein was significantly higher than those of uninfected piglets. These changes are consistent with diarrhea induced by C hominis infection as previously reported, likely reflecting mild dehydration [13].
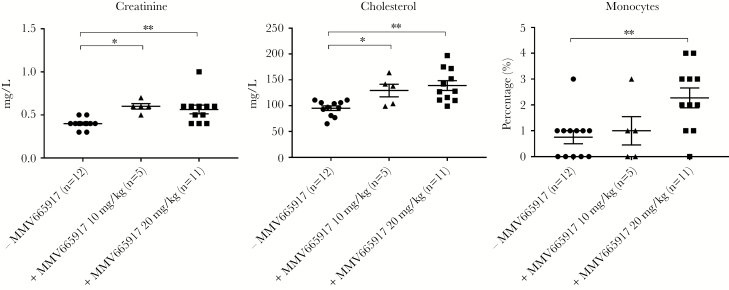
Piglets treated with MMV665917 had differences in 3 parameters: serum creatinine, cholesterol, and circulating monocytes in blood (Figure 7). These results suggest that energy metabolism, cholesterol metabolism, and monocyte release or migration may be altered by MMV665917. However, an additional study is required, because many piglets were infected with C hominis and all received the vehicle. Thus, we cannot fully assess independent effects of MMV665917.
Figure 7.
Alteration of serum biochemical and hematological data in piglets after MMV665917 treatment. Serum biochemical and hematological parameters were analyzed from blood samples of euthanized piglets. The scatter plots represent mean ± standard error of the mean. Tukey’s multiple comparisons test was conducted among 3 groups using GraphPad Prism 7.03. *, P < .05; **, P < .01. −MMV665917 (n = 12) is sum of Cryptosporidium hominis-infected group (n = 8), 1% hydroxypropyl methyl-cellulose-treated group (n = 2), and nontreated control (n = 2); +MMV665917 10 mg/kg (n = 5) is sum of C hominis-infected and MMV665917 (10 mg/kg)-treated group (n = 3) and MMV665917 (10 mg/kg)-treated group (n = 2); +MMV665917 20 mg/kg (n = 11) is sum of C hominis-infected and MMV665917 (20 mg/kg)-treated group (n = 7) and MMV665917 (20 mg/kg)-treated group (n = 4).
Tissue Toxicity
Tissue toxicity of MMV665917 was investigated microscopically in a blinded fashion by a board-certified veterinary pathologist. No significant microscopic lesions were observed in any of the following organs: lungs, liver, gall bladder, pancreas, spleen, kidneys, brain stem, cerebellum, hypothalamus, thalamus, hippocampus, pineal gland, cerebral cortex, or choroid plexus.
DISCUSSION
Cryptosporidiosis is a major cause of morbidity and mortality due to enteric disease in children <2 years old in developing countries [1, 2, 19]. Currently, there are no effective control measures for this at-risk population. Therefore, the search for an effective treatment remains a priority.
We previously established and optimized the piglet model for C hominis (the species found in three quarters of cases of human cryptosporidiosis), using the FDA-approved drugs NTZ and azithromycin, and several experimental drugs including bumped kinase inhibitors targeting the calcium-dependent protein kinase of C parvum [13, 14].
The piperazine-derivative MMV665917 is an especially promising anticryptosporidial drug lead. Unlike NTZ and many other anticryptosporidial lead compounds, eg, clofazimine, MMV665917 rapidly eliminates parasites in in vitro time-kill assays, suggesting that it is parasiticidal [11]. Furthermore, although leads such as clofazimine that inhibit Cryptosporidium growth without themselves eliminating parasites are often efficacious in mouse models of acute C parvum infection, these compounds lack efficacy in a NOD SCID gamma mouse model of chronic cryptosporidiosis [11]. MMV665917, on the other hand, is curative in both acute and chronic mouse models [11]. Furthermore, it was recently found to reduce both diarrhea and parasite shedding in a neonatal calf model of C parvum infection [12]. In this study, we demonstrate its effectiveness against C hominis, the species most commonly found in humans. Our results demonstrated that 1 week of twice-daily dosing with MMV665917 reduced the following: (1) oocyst excretion in feces, (2) parasite DNA in feces and gut contents, (3) mucosal colonization, and (4) mucosal damage. Both 10 mg/kg and 20 mg/kg doses were efficacious with a slight improvement at the highest dose given. However, neither dose fully eliminated parasites, because 1 of 3 piglets treated with 10 mg/kg started excreting oocysts within 3 days after treatment ended. In addition, 2 of 7 piglets that received 20 mg/kg still had detectable DNA in the cecum and colon, which, although neither animal relapsed during the limited follow-up period, presumably reflects the ongoing presence of viable parasites. After C hominis challenge, and once oocyst excretion in feces commence, the surface and floor inside the isolator are heavily contaminated potentially re-exposing the piglet to reinfection. Therefore, it is possible that the GB piglets ingest the residual oocysts in the isolator and then the infection is resumed once the concentration of MMV665917 is decreased under the effective concentration.
MMV665917 eliminated the severity of diarrhea attributed to cryptosporidiosis; however, treated piglets still had some evidence of diarrhea due to the vehicle (1% HPMC). The average volumes of 1% HPMC for MMV665917 10 mg/kg and 20 mg/kg doses were 1.8 mL and 3.6 mL, and the volume of 1% HPMC only in control was 4 mL. The piglets with 1.8 mL of 1% HPMC (MMV665917 10 mg/kg dose) had milder diarrhea than those with 3.6 mL (MMV665917 20 mg/kg dose) and 4 mL of 1% HPMC. These observations suggest a dose-dependent, positive correlation between HPMC concentration and diarrhea severity. Hydroxypropyl methyl-cellulose (CAS No. 9004-65-3) is approved by the FDA for use as a food additive and as an inactive ingredient in numerous oral, ophthalmic, and topical nasal drug product preparations [20]. Hydroxypropyl methyl-cellulose is a modified cellulose dietary fiber that generates a viscous solution that may reduce contact between food and digestive enzymes or interfere with diffusion of nutrients to the absorptive surface [21]. Different vehicles may improve clinical efficacy of MMV665917 for treatment of C hominis infection in this piglet model. Because drug formulation may significantly alter exposure time and concentration along the intestinal epithelium, additional mouse pharmacokinetic and efficacy studies may be warranted to identify a more suitable vehicle.
CONCLUSIONS
We showed that the piperazine-derivative MMV665917 is an effective compound against C hominis by multiple molecular, histological, and clinical outcomes in the piglet model. Furthermore, no major organ toxicities were identified. Although we observed changes in 3 blood parameters at euthanasia (creatine, cholesterol, and circulating monocytes were elevated in treated piglets), it was not clear whether the effects were specific to MMV665917 or some other aspect of infection or vehicle administration. Additional investigations are still required to optimize the dose range, the frequency, duration, and to minimize possible adverse effects associated with the vehicle. Studies to determine MMV665917’s mechanism of action are also of high interest and may aid in optimization. Overall, we show results and the earlier reports on this drug suggest that this compound could potentially form a basis for an effective treatment against cryptosporidiosis for humans.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank John Feddo, and Brendon Davis, and Julia Dilo for technical assistance.
Financial support. This work was funded by the Bill & Melinda Gates Foundation award number OPP1132800.
Potential conflicts of interest. C. D. H. has 2 patents pending: US 62/140762 and PCT/US16/25284. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2. Sow SO, Muhsen K, Nasrin D, et al. The burden of Cryptosporidium diarrheal disease among children < 24 months of age in moderate/high mortality regions of sub-Saharan Africa and South Asia, utilizing data from the Global Enteric Multicenter Study (GEMS). PLoS Negl Trop Dis 2016; 10:e0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laurent F, Lacroix-Lamandé S. Innate immune responses play a key role in controlling infection of the intestinal epithelium by Cryptosporidium. Int J Parasitol 2017; 47:711–21. [DOI] [PubMed] [Google Scholar]
- 4. Abubakar II, Aliyu SH, Arumugam C, Hunter PR, Usman N. Prevention and treatment of cryptosporidiosis in immunocompromised patients. Cochrane Database of Systematic Reviews 2007. Art. No.: CD004932. doi:10.1002/14651858.CD004932.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong DK, Wong CJ, Gutierrez K. Severe cryptosporidiosis in a seven-year-old renal transplant recipient: case report and review of the literature. Pediatr Transplant 2007; 11:94–100. [DOI] [PubMed] [Google Scholar]
- 6. Rossignol JF. Cryptosporidium and Giardia: treatment options and prospects for new drugs. Exp Parasitol 2010; 124:45–53. [DOI] [PubMed] [Google Scholar]
- 7. Abubakar I, Aliyu SH, Arumugam C, Usman NK, Hunter PR. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br J Clin Pharmacol 2007; 63:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amadi B, Mwiya M, Musuku J, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360:1375–80. [DOI] [PubMed] [Google Scholar]
- 9. Van Voorhis WC, Adams JH, Adelfio R, et al. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog 2016; 12:e1005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bessoff K, Spangenberg T, Foderaro JE, Jumani RS, Ward GE, Huston CD. Identification of Cryptosporidium parvum active chemical series by repurposing the open access malaria box. Antimicrob Agents Chemother 2014; 58:2731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jumani RS, Bessoff K, Love MS, et al. A novel piperazine-based drug lead for Cryptosporidiosis from the medicines for malaria venture open-access malaria box. Antimicrob Agents Chemother 2018; 62:e01505–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stebbins E, Jumani RS, Klopfer C, et al. Clinical and microbiologic efficacy of the piperazine-based drug lead MMV665917 in the dairy calf cryptosporidiosis model. PLoS Negl Trop Dis 2018; 12:e0006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee S, Ginese M, Beamer G, et al. Therapeutic efficacy of bumped kinase inhibitor 1369 in the acute pig model of Cryptosporidium hominis. Antimicrob Agents Chemother 2018; 62:e00147–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee S, Harwood M, Girouard D, et al. The therapeutic efficacy of azithromycin and nitazoxanide in the acute pig model of Cryptosporidium hominis. PLoS One 2017; 12:e0185906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Akiyoshi DE, Feng X, Buckholt MA, Widmer G, Tzipori S. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect Immun 2002; 70:5670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chappell CL, Okhuysen PC, Langer-Curry R, et al. Cryptosporidium hominis: experimental challenge of healthy adults. Am J Trop Med Hyg 2006; 75:851–7. [PubMed] [Google Scholar]
- 17. Morada M, Lee S, Gunther-Cummins L, et al. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int J Parasitol 2016; 46:21–9. [DOI] [PubMed] [Google Scholar]
- 18. Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol 1994; 1:450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burdock GA. Safety assessment of hydroxypropyl methylcellulose as a food ingredient. Food Chem Toxicol 2007; 45:2341–51. [DOI] [PubMed] [Google Scholar]
- 21. Maki KC, Carson ML, Miller MP, et al. Hydroxypropylmethylcellulose and methylcellulose consumption reduce postprandial insulinemia in overweight and obese men and women. J Nutr 2008; 138:292–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.