The introduction of antiretroviral therapy (ART) in the 1990s has turned the once fatal condition of HIV-1/AIDS into a chronic illness. However, it has failed to fully eradicate the virus, which remains in a latent state in a small number of CD4+ T cells in individuals on ART. One promising therapeutic strategy called “shock and kill” utilizes small-molecule latency-reversing agents (LRAs) to “shock” the virus out of latency, resulting in the “killing” of previously latent, now virus-producing cells by way of viral cytopathic effects or immune cytolytic mechanisms. While many LRAs are able to reverse viral latency of in vitro models of HIV-1 latency, most have little to no effect in resting CD4+ T cells from HIV+ patients. In PNAS, Timmons et al. (1) demonstrate that latency reversal for many LRAs depends on heat shock factor 1 (HSF1), a mediator of the stress response known to induce HIV-1 transcription. Their work not only sheds light on latency-reversing mechanisms but also explains the discrepancies in LRA activity between HIV-1 latency models based on immortalized vs. primary cells.
The central bottleneck in studying HIV-1 latency is the lack of methods to home in on the elusive population of latently infected cells in HIV+ patients. Consequently, surrogate models in immortalized immune cell lines were developed to allow for mechanistic studies of HIV-1 latency. Their known proviral sequence, uniform integration site, and convenient scalability make cell lines, such as Jurkat-Latent (J-Lats), a pillar of molecular latency studies (2, 3). However, they do have limitations, which also extend to in vitro primary T cell models, in recapitulating latent infection in vivo (4). It remains the gold standard to validate any results obtained in J-Lats or primary T cell models in resting T cells from HIV+ patients on ART.
Nevertheless, HIV-1 model systems have been useful for identifying LRAs and understanding molecular mechanisms that lead to viral transcription. In general terms, the provirus is reactivated when cellular transcription factors, such as nuclear factor-κB (NF-κB), bind the transcriptionally silenced long terminal repeat (LTR) and recruit a central transcription coactivator, the positive transcription elongation factor (P-TEFb), to potentiate viral transcription. P-TEFb, which consists of the Cyclin T1 (CycT1) regulatory unit bound to Cyclin-dependent kinase 9, is crucial for viral transcription elongation. Since the discovery of the virus, more than 50 different LRAs have be identified to induce HIV-1 transcription (5). They can be roughly divided in two categories: one that mimics T cell activation such as protein kinase C (PKC) agonists and second mitochondria-derived activator of caspase (SMAC) mimetics; the other that targets the chromatin state of the integrated provirus, including epigenetic modulators such as histone deacetylase (HDAC) and bromodomain and extraterminal domain protein inhibitors.
Beyond T cell activation and epigenetic pathways, cellular homeostatic programs also intersect with the control of viral latency (6–8). As the master regulator of the heat shock response, HSF1 maintains proteostasis by up-regulating the expression of heat shock proteins (HSPs), chaperones that help fold or degrade proteins, in response to elevated temperatures, toxic chemicals, and other cellular stress factors. Previous studies have shown that elevated temperatures can reactivate HIV-1 in cell lines (6, 7). HSF1 is further known to bind the LTR and recruit P-TEFb for effective transcription elongation (7).
Timmons et al. (1) report the surprising observation that many LRAs, including PKC agonists and HDAC inhibitors, act through the heat shock response and involve HSF1. The authors first observed robust latency reversal when they treated their home-built primary CD4+ T cell model of latency with proteasome inhibitors but not with thiol-modifying compounds. This observation suggested that proteasome inhibitors activate HSF1 through the accumulation of denatured proteins, independent of thiol modification. Moreover, the authors examined whether known LRAs such as PMA/I (phorbol 12-myristate 13-acetate and ionomycin), panobinostat, bryostatin, and bortezomib induce expression of HSP70, a direct target of HSF1 signaling. All of them induced expression of HSP70, thus implicating HSF1 broadly in latency reversal. To validate the broad connection between latency reversal and HSF1, they confirmed by chromatin immunoprecipitation that both HSF1 and its active phosphorylated form bind the HIV-1 LTR in J-Lats in response to PMA/I, consistent with previous studies (9).
Next, the authors turned to KRIBB11 [N(2)-(1H-indazole-5-yl)-N(6)-methyl-3-nitropyridine-2,6-diamine], a small-molecule inhibitor of HSF1-dependent recruitment of P-TEFb to promoters of HSPs (10). The anticipation was that if HSF1 is broadly involved in latency reversal, a wide spectrum of LRAs should be inhibited by KRIBB11 treatment. Indeed, latency-reversing effects of PMA/I, stress activators bis(2-hydroxybenzylidene)acetone (HBB2) and bortezemib, HDAC inhibitors panobinostat and romidepsin, and PKC agonist bryostatin were suppressed by the addition of KRIBB11 in J-Lat cells (Fig. 1). This was not explained by off-target effects of KRIBB11 on NF-κB or HDAC function.
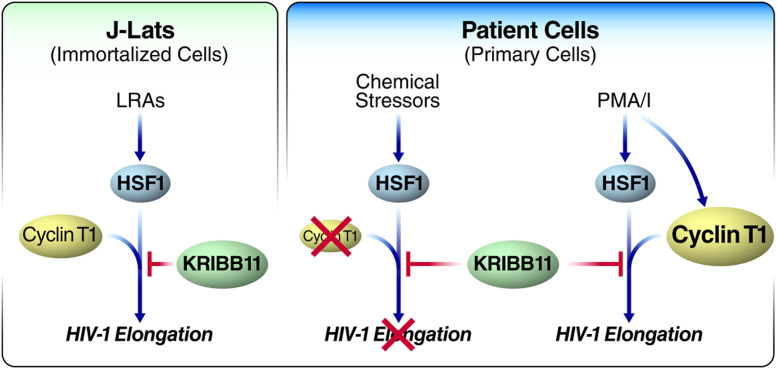
Fig. 1.
KRIBB11 reveals differences in latency reversal in immortalized vs. primary T cells. In immortalized J-Lat cells, KRIBB11 can block the latency-reversing abilities of most LRAs (bryostatin, panobinostat, romidepsin, HBB2, bortezomib, and PMA/I) by inhibiting the recruitment of CycT1, which partners with HSF1 to induce HIV-1 transcription elongation. In patient cells, chemical stressors (disulfiram, HBB2, bortezomib, tricyclic bis[cyano enone] [TBE-31], and Shikonin) known to activate HSF1 are unable to reverse HIV-1 latency, perhaps due to low levels of CycT1 in primary cells compared with J-Lats. By contrast, PMA/I reactivates HIV-1 by increasing the expression of CycT1. As a result, KRIBB11 is able to inhibit PMA/I’s LRA function.
The response was different in primary T cells from HIV+ patients. Remarkably, KRIBB11 did not block the viral reactivation induced by a number of classic LRAs, indicating that something fundamental was missing. Similarly, the induction of stress pathways by small molecules or an increase in temperature was unable to reverse latency of primary cells and did not induce the production of viral particles measured by a quantitative viral outgrowth assay. The exception was treatment with PMA/I, a strong T cell receptor mimic that effectively activates HIV-1 transcription but was inhibited by KRIBB11 in patient-derived primary T cells (Fig. 1). When the authors performed droplet digital PCR to measure different transcript lengths among HIV-1–specific transcripts in cells from HIV+ patients, they found that PMA/I-induced transcription of 5′ elongated HIV-1 transcripts was significantly reduced by KRIBB11 while the overall quantity of transcripts remained unchanged. This finding pointed to a unique involvement of P-TEFb in PMA/I-induced latency reversal but not in reversal induced by other LRAs in primary T cells.
A key determinant of P-TEFb activity in cell lines vs. T cells from HIV+ patients is the well-known discrepancy in the level of CycT1 present (11). Western blots confirmed higher amounts of CycT1 protein in J-Lats than in CD4+ T cells from healthy and HIV+ patients. The authors concluded that KRIBB11 reduced HSF1-mediated transcription elongation of HIV-1 induced by PMA/I via P-TEFb recruitment to the LTR. This would explain why KRIBB11 blocks LRAs in cell lines but not in T cells from HIV+ patients. How does PMA/I treatment effectively engage HSF1 for latency reversal in patients’ resting T cells, despite low CycT1 levels? Indeed, CycT1 levels increased when the authors treated primary resting T cells with PMA/I, supporting the model that low CycT1 levels in primary T cells interfere with the effective involvement of HSF1 in HIV-1 reactivation unless artificially boosted (Fig. 1).
In PNAS, Timmons et al. demonstrate that latency reversal for many LRAs depends on heat shock factor 1 (HSF1), a mediator of the stress response known to induce HIV-1 transcription.
Timmons et al. (1) broaden the role of HSF1 in HIV-1 transcription and highlight its previously unappreciated mechanistic involvement in many LRAs. The study also underscores the importance of sufficient levels of CycT1 in order for LRAs to effectively reverse latency in people living with HIV-1. The finding that PMA/I treatment reverses latency by boosting CycT1 production is encouraging as it indicates that cotreatment with LRAs and CycT1-enhancing agents could have clinical promise. The efficiency of latency reversal is known to increase when PKC agonists such as PMA synergize with other LRAs. The main reason is thought to be an increase in NF-κB mobilization (12). This explanation might have to be reconsidered to include the possibility that increasing levels of CycT1, alone or in combination with NF-κB recruitment, as the main reason why PMA potentiates other LRAs.
The broad involvement of HSF1 in latency reversal opens an avenue for targeting it with small-molecule drugs as part of a shock and kill strategy. However, whether this finding in immortalized cells translates to primary T cells remains unclear. Compounds that induce chemical stress and HSF1 expression failed to stimulate significant HIV-1 expression in resting CD4+ T cells from HIV+ patients (1). While the results presented indicate that the lack of P-TEFb in resting primary T cells is the culprit (Fig. 1), future studies should exclude the possibility that HSF1 is uniquely mobilized in immortalized cells and explore new strategies for more robust mobilization of HSF1 and P-TEFb in primary cells (13).
An interesting aspect of this study is the spotlight it shines on the relationship between heat and HIV-1. Timmons et al. (1) attempted to reactivate HIV-1 in HIV+ patient T cells with the mitogen phytohemagglutinin and an elevated temperature of 39 °C to no avail. Previous studies have found that HIV-1 is able to replicate faster and more efficiently in primary CD4+ T cells at 39.5 °C and that elevated temperatures, often in combination with other T cell-activating stimuli, are sufficient for latency reversal in latently infected cell lines and peripheral blood mononucleated cells (6, 7). In fact, increased temperatures have been shown to facilitate HIV-1 infection by enhancing the fluidity of cellular and viral membranes (6). Both elevated environmental and physiological fever temperatures can impact HIV-1 replication and transmission (14). The finding that apart from its physiological impact, the heat shock response is a central part of the transcriptional control of the virus is important and underscores the potential of body and environmental temperature modulation as a possible asset for efficient treatments based on latency reversal in the future.
Acknowledgments
We thank John Carroll for graphics, Francoise Chanut for editorial assistance, and Lauren Weiser for administrative assistance. This work was supported by the National Institute of Drug Abuse (DP1DA038043) and the National Institute of Allergy and Infectious Diseases (R37AI083139).
Footnotes
The authors declare no competing interest.
See companion article, “HSF1 inhibition attenuates HIV-1 latency reversal mediated by several candidate LRAs in vitro and ex vivo,” 10.1073/pnas.1916290117.
References
- 1.Timmons A, et al. HSF1 inhibition attenuates HIV-1 latency reversal mediated by several candidate LRAs in vitro and ex vivo. Proc. Natl. Acad. Sci. U.S.A. 117, 15763–15771 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan A., Bisgrove D., Verdin E., HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22, 1868–1877 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan A., Defechereux P., Verdin E., The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 20, 1726–1738 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spina C. A., et al. , An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog. 9, e1003834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ait-Ammar A., et al. , Current status of latency reversing agents facing the heterogeneity of HIV-1 cellular and tissue reservoirs. Front. Microbiol. 10, 3060 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roesch F., et al. , Hyperthermia stimulates HIV-1 replication. PLoS Pathog. 8, e1002792 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan X. Y., et al. , Heat shock factor 1 mediates latent HIV reactivation. Sci. Rep. 6, 26294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallejo-Gracia A., et al. , FOXO1 promotes HIV latency by suppressing ER stress in T cells. Nat Microbiol., 10.1038/s41564-020-0742-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawat P., Mitra D., Cellular heat shock factor 1 positively regulates human immunodeficiency virus-1 gene expression and replication by two distinct pathways. Nucleic Acids Res. 39, 5879–5892 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon Y. J., et al. , KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J. Biol. Chem. 286, 1737–1747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang K., Sung T. L., Rice A. P., Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. J. Virol. 86, 3244–3252 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang G., Dandekar S., Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retroviruses 31, 4–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendillo M. L., et al. , HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150, 549–562 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talman A., Bolton S., Walson J. L., Interactions between HIV/AIDS and the environment: Toward a syndemic framework. Am. J. Public Health 103, 253–261 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]