Significance
Pesticides and other toxic substances (e.g. lead ammunition, veterinary drugs) have caused the decline of many animal populations, highlighting the serious threat that wildlife poisoning poses for biodiversity conservation. While compelling evidence demonstrates the harmful effects of poisoning on individuals, its impact on populations rests mostly on indirect evidence. This study directly links poison-induced individual mortality to the population decline of a threatened vertebrate, the red kite (Milvus milvus), across hundreds of locations in Spain, offering strong proof of poisoning contributing to countrywide population decline of a once-abundant vertebrate. A greater understanding of the population-level effects of wildlife poisoning may strengthen effective actions against the use of toxic substances harmful for wildlife.
Keywords: wildlife poisoning, population dynamics, sentinel species, on-ground monitoring, diclofenac
Abstract
Toxicants such as organochlorine insecticides, lead ammunition, and veterinary drugs have caused severe wildlife poisoning, pushing the populations of several apex species to the edge of extinction. These prime cases epitomize the serious threat that wildlife poisoning poses to biodiversity. Much of the evidence on population effects of wildlife poisoning rests on assessments conducted at an individual level, from which population-level effects are inferred. Contrastingly, we demonstrate a straightforward relationship between poison-induced individual mortality and population changes in the threatened red kite (Milvus milvus). By linking field data of 1,075 poisoned red kites to changes in occupancy and abundance across 274 sites (10 × 10-km squares) over a 20-y time frame, we show a clear relationship between red kite poisoning and the decline of its breeding population in Spain, including local extinctions. Our results further support the species listing as endangered, after a breeding population decline of 31% to 43% in two decades of this once-abundant raptor. Given that poisoning threatens the global populations of more than 2,600 animal species worldwide, a greater understanding of its population-level effects may aid biodiversity conservation through increased regulatory control of chemical substances. Our results illustrate the great potential of long-term and large-scale on-ground monitoring to assist in this task.
Even though populations are a major target for both ecological risk assessments of toxicants (1, 2) and conservation management actions (3), evidence demonstrating straightforward relationships between the effects of toxic substances at individual and population levels remains scarce, especially in vertebrates (4). The paramount cases of DDT (dichlorodiphenyltrichloroethane) and other organochlorine pesticides (1, 5), lead ammunition (6), and diclofenac (7, 8) exemplify how most evidence of poisoning impacts at the population level is retrieved (SI Appendix, Appendix S1). In brief, scattered data of toxic effects from molecular to individual levels are gathered, and inferences on the relationship to observed population declines are determined through, for example, computational methods (4). For instance, strong evidence that identified diclofenac as the major cause of massive mortalities of Gyps vultures across the Indian subcontinent (7, 8) was linked with observed widespread population declines directly through deductive reasoning (7, 8) and the use of demographic simulations (9).
Inferring the effects of toxic substances at a population level from individual responses is not straightforward, as individual parameters known to be affected by toxicants (e.g., survival, fecundity) do not always correlate with population changes (2). Indeed, certain numbers of individuals can be removed from a population (e.g., poisoned) without necessarily leading to its decline due to such processes as density-dependent productivity or immigration, which may compensate for toxic effects at an individual level (1). As a result, forecasting the fate of populations affected by toxicants is a challenging task (10). Alternatively, the use of real-world population changes (e.g., through observed population growth rates), which already incorporate the complexity of population dynamics, has emerged as an ecologically sound option for assessing how toxic effects at the individual level translate into population-level impacts (2, 11). However, collecting field data at individual and population levels simultaneously is extremely costly and time-consuming, particularly at large spatiotemporal scales (10, 11). As a result, the available evidence linking individual responses to toxicants and observed population changes is limited to a few studies at local scales (e.g., one geographic location) and/or over short periods (12, 13). These spatiotemporal limitations, which in turn restrict sampling replication, weaken the strength of available evidence, and preclude drawing sound conclusions about the impact of toxicants on animal populations (11, 14).
Counteracting spatiotemporal limitations, we link field data of toxicant-induced individual mortality and population changes in the threatened red kite (Milvus milvus) across hundreds of localities in a 20-y time frame. To do so, we take advantage of two long-term and large-scale monitoring schemes: 1) Spanish national surveys of the presence and abundance of the species (∼3,500 10 × 10-km squares) in 1994 (15) and 2014 (16) and 2) data on wildlife poisoning events across Spain compiled by the ANTÍDOTO program (17) over the same period (n = 18,500 dead animals and 4,175 baits). The Spanish breeding population of red kite has decreased over the last 20 y, and poisoning, either intentional or accidental, has been suggested as one of the main causes behind this decline (16).
To assess the role of poisoning on the observed changes in the distribution and abundance of breeding red kites in 274 10 × 10-km squares between 1994 and 2014 (Fig. 1), we included the observed data on individual poisoning of red kites and other animal species (e.g., dogs, raptors; Materials and Methods and SI Appendix, Table S1) in each square as explanatory variables in generalized linear models (GLMs). Poisoning was defined here as individual intoxication, mostly by baits illegally used to kill wildlife but also by other toxic compounds used for pest and predator control, as well as anthropogenic pollutants such as lead. Land use changes known to determine the habitat suitability for red kites were also taken into consideration (18).
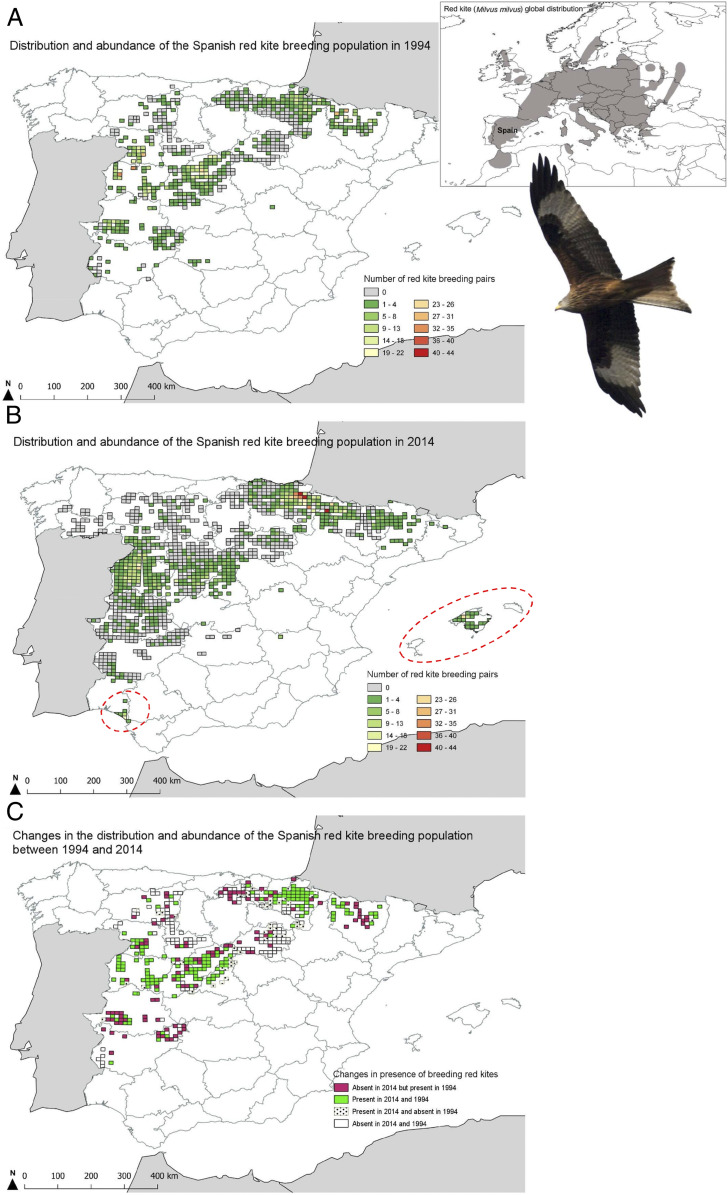
Fig. 1.
Distribution and abundance of the red kite breeding population per 10 × 10-km square in Spain. Data were obtained from the species censuses in 1994 (15) (A) and 2014 (16) (B). Changes in occupancy and abundance per square were obtained by combining both censuses (C). Red kite populations in the Balearic Islands and Doñana (within dashed circles) were excluded from the analyses (see Materials and Methods). The global distribution of the species according to the IUCN (24) is shown in the map at the top right. (Red kite image credit: Public Domain Pictures/George Hodan.)
Results and Discussion
A total of 1,075 red kites were registered as poisoned in mainland Spain, including 657 confirmed through toxicologic analyses and 418 suspected but without toxicological confirmation, in the 1995 to 2013 between-census period (Materials and Methods and SI Appendix, Table S2). Aldicarb and carbofuran were detected in >82% of the poisoned red kites analyzed (i.e., 316 and 228 individuals, respectively) (SI Appendix, Table S2). Despite being banned in Europe in 2003 and 2007, respectively, these two insecticides are still being used illegally to kill wildlife (17).
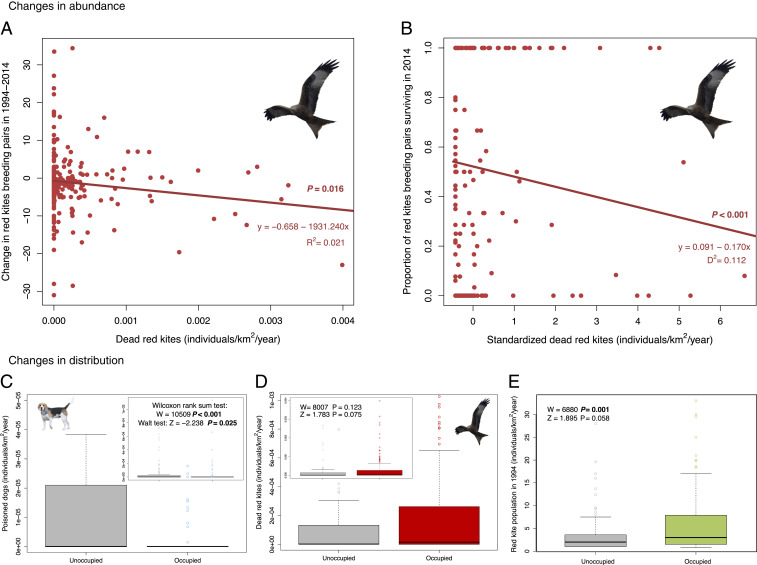
Poison-driven red kite mortality (both suspected and confirmed) showed a negative impact on red kite abundance (Fig. 2 A and B). Our results show a decrease in the number of breeding pairs as the number of poisoned red kites increased in a 10 × 10-km square, highlighting a pernicious effect of poisoning on the species population. This result is further supported by the negative relationship between the number of poisoned dogs, considered a good indicator of poison incidence (19) (see below), and changes in the breeding population of red kite (SI Appendix, Table S3). In contrast, while the presence of poisoned dogs (presumably by illegally poisoned baits) also negatively influenced red kite occupancy (Fig. 2C), the opposite effect was observed for poisoned red kites—that is, a greater probability of occupancy in sites with a higher number of poisoned kites (Fig. 2D). This apparently counterintuitive result may be due to the presence of dead red kites, and thus the probability of locating them is greater at locations with larger breeding populations. Red kite population size—the number of breeding pairs in 1994 and the mean number of breeding pairs in 1994 and 2014—also explains changes in occupancy and abundance, respectively (Fig. 2E and SI Appendix, Table S3). Locations with larger breeding populations seem to be more resistant to local extinction despite suffering high kite mortality, including poisoning, perhaps due to density-dependent or immigration processes. Indeed, red kite populations can expand to some extent even when exposed to toxic substances, as has been observed for some reintroduced or isolated populations (20, 21).
Fig. 2.
Main factors explaining changes in red kite occupancy and abundance in 1994 to 2014. (A) When raw data were considered, the density of dead red kites (both confirmed and suspected as poisoned) negatively correlated with the observed changes in the number of breeding pairs per 10 × 10-km square (n = 274). Although this linear relationship weakened after removing the influential point, x = 0.004 (i.e., P > 0.05), this point had no effect in the best model, further supporting the observed negative trend. (B) Dead red kites explaining the variation in the proportion of breeding pairs surviving in 2014 when included in the final best model, while keeping the remaining variables constant at their mean values; that is, locations with greater numbers of dead kites had lower proportions of surviving pairs. (C–E) In agreement with the occupancy models for breeding kites, raw data showed that locations where red kite breeding pairs disappeared between 1994 and 2014 (n = 107) had more dogs confirmed as poisoned (C), fewer dead red kites (both confirmed and suspected as poisoned) (D), and a smaller breeding population (E) in 1994. Wilcoxon tests show differences for the raw data. Wald tests for each variable once included in the final best model are provided for comparisons. Significant P values (<0.05) are in bold type. Additional details are provided in Materials and Methods and SI Appendix, Table S3. (Red kite image and dog image credit: Public Domain Pictures/George Hodan and Pixabay/AlbanyColley.)
Our results are in accordance with existing demographic models on the impact of poison on the species (13, 22) and support previous works suggesting a marked impact of poisoning on small and isolated populations (21, 23), while concurring that poisoning represents a major global threat for the species (24). Furthermore, we detected local extinctions in 107 10 × 10-km squares, underscoring the important impact of poisoning on red kite distribution and abundance. Therefore, in the absence of effective measures to eradicate or minimize poisoning, further local extinctions may occur. Along with poison, our models showed a positive association of irrigated crops on red kite presence (SI Appendix, Table S3) that could be due to irrigated farmlands harboring a more heterogeneous landscape with trees for breeding and open areas for foraging. This association is especially strong in the main species strongholds found in northwestern Spain (25). The negative impact of urban and forested areas on the species abundance (SI Appendix, Table S3) would be in agreement with the decreased availability of such heterogeneous farming landscapes in highly urbanized or forested 10 × 10-km squares.
Our results provide straightforward evidence linking the effects of poisoning on individuals (i.e., mortality) with the population trends of a wild species using field data at a fine spatiotemporal scale across hundreds of locations. The response of red kite populations to poisons suggests a strong potential for this species to act as a sentinel of toxic environmental risk in natural ecosystems (26). Interestingly, the density of dogs confirmed as poisoned per square kilometer and per year best predicted the observed changes in occupancy of the red kite breeding population (Fig. 2C and SI Appendix, Table S3). Dog owners are expected to actively look for their missing pets, thereby increasing the detectability of the poisoning cases affecting them. Considering the low detectability of wildlife poisoning events (17, 27), poisoned domestic animals emerge as a reliable index of the true incidence of toxic substances on wildlife (19) (SI Appendix, section S2).
Overall, considering the low detectability of wildlife poisoning (17, 27) and the difficulty of gathering population data over large areas (10), our results highlight poisoning as a major driver of countrywide population decline in a threatened vertebrate. Changes in distribution and abundance are major criteria used to evaluate the conservation status of a species (28). Thus, our findings further support the listing of the species as “endangered” in Spain, where the number of red kite breeding pairs has decreased by at least 31% in 20 y (16), with poisoning a seemingly major and persistent cause of this reduction (16, 28). Poisoning in all its different forms (e.g., poaching, pest and predator control, anthropogenic pollutants) threatens the global populations of at least 2,602 animal species, representing ∼3.5% of the 73,488 animal species and ∼3.5% of the 50,816 vertebrate species included on the International Union for Conservation of Nature (IUCN) Red List (29) (SI Appendix, Table S4). Poisoning threatens ∼756 species (8.4%) of the 9,013 vertebrate species listed under threatened categories (i.e., “Critically Endangered,” “Endangered,” and “Vulnerable”). Two hundred forty-five species of raptors and carnivores (28.5%) are also threatened by poisoning, including almost one-half of the species listed as “Critically Endangered,” “Endangered,” or “Vulnerable” (SI Appendix, Table S4).
Surprisingly, although ample evidence highlights the pernicious effects of poisoning on individuals (1, 4–8), defining the impact of toxicants at high levels of biological organization (e.g., populations) remains a challenging task, resting mostly on the use of inferential methods (4). As a result, we lack straightforward evidence linking field data on the impact of toxic compounds at individual and population levels at a detailed spatiotemporal extent large enough to further support a widespread (rather than local) consistent effect (11). Direct evidence such as that provided here on the effect of poisoning on the population of a wild species at fine spatiotemporal scales across hundreds of locations strengthens the bridge between population dynamics and conservation biology (10), providing further support for taking effective actions against the use of toxic compounds pernicious to wildlife (e.g., lead ammunition, rodenticides) (4, 11). Such evidence may help improve the regulatory processes of chemical substances, including postapproval adaptation (11, 30). In this context, our data demonstrate the strong potential of existing monitoring schemes, such as the ANTÍDOTO program in Spain (17) and the SAGIR monitoring in France (27), to provide straightforward evidence of the impact of toxic substances across species over large spatiotemporal scales (11).
Materials and Methods
Study Species and Study Area.
The red kite (M. milvus) is an endemic raptor of the western Palearctic (Fig. 1), globally listed as “Near Threatened” (24). The European population, estimated as 25,200 to 33,400 breeding pairs, currently accounting for >95% of the global species population, has decreased by ∼30% over the last three generations (34.5 y), mainly due to declines within its core breeding areas in Germany, France, and Spain (26). Spain (our study area) holds roughly 2,000 breeding pairs and an estimated 50,000 wintering individuals (16), representing one of the world’s main breeding strongholds and wintering areas for red kites (31, 32). Nonetheless, the Spanish red kite wintering and breeding populations have experienced sharp decreases (7 to 19% and 31 to 43%, respectively) over the last two decades (15, 16, 33). Although the species trend in the last decade could be considered globally stable (16, 33), in Spain the red kite is legally considered “Endangered,” meaning that the species probability of survival is low as long as the primary threats to the population remain in place (34).
Red kites are opportunistic raptors that feed on a wide range of food resources, from small prey such as rodents, passerines, and young rabbits to all types of meat remains, including organic waste at dumps and carcasses of any size from large ungulates to reptiles, birds, and rodents (24). This makes the species highly susceptible to different toxic compounds, including those legally administered for pest control and deliberately poisoned baits aimed at illegal killing of predators (26, 35–37). Illegal poison use is currently considered among the main threats for the species (24) and has been suggested to be a major factor behind red kite population declines in southwestern Europe (35). Mortality caused by various toxic substances is considered a factor in delaying the expansion of the Scottish reintroduced population of red kites (22), in contrast with reintroduced populations in England (20). Despite the reported high mortality caused by toxicants, these two populations are expanding, however (20, 22). In contrast, extensive anticoagulant rodenticide use in agrarian landscapes in northwestern Spain has been identified as a major driver of short-term population declines at the regional level (38). For instance, high mortality rates and demographic modeling support illegal poison use as a major cause of red kite population declines in small isolated populations in Doñana National Park and the Balearic Islands (13, 21, 23). The suggested major role of poisoning as a driver of large-scale and long-term declines of this species remains unproven, however.
Red Kite Breeding Population.
Data on the distribution and abundance of breeding red kites in mainland Spain were obtained from two national censuses performed in 1994 and 2014, the longest monitoring period available to date (15, 16). The census methodology is described in detail elsewhere (15, 39). In brief, breeding kites were located within 10 × 10-km UTM (Universal Transverse Mercator) squares in March to July of each monitoring year by trained volunteers through both car surveys at low speed (≤40 km/h) to obtain the individuals observed per km and an active search of breeding territories from vantage observation points or intensive nest searching. Data obtained by both methods were converted to population estimates using the equations shown in refs. 15 and 39.
Data on the presence and abundance of breeding red kites in 1994 were obtained for a total of 2,990 10 × 10-km squares (i.e., ∼300,000 km2; 60% of the country’s area). The species presence was confirmed in 386 squares (i.e., 38,600 km2), with an estimated total breeding population of 3,333 to 4,044 pairs (Fig. 1A) (15). In 2014, the species census covered 1,400 10 × 10-km squares (i.e., 140,000 km2), and the presence of red kites was reported in 554 squares, with an estimated population of 2,312 to 2,440 breeding pairs (Fig. 1B) (16). After correction for potential overestimation of breeding pairs detected in the last census, the Spanish breeding population was estimated as ∼2,000 breeding pairs, representing nearly a 40% decrease in its distribution over the two decades (15, 16). Data on red kite presence from both censuses were combined to obtain a response variable of the change in occupancy of breeding red kites per 10 × 10-km square in the 20-y period considered (n = 274; Fig. 1C and SI Appendix, Table S1). Those squares with breeding pairs in both censuses (i.e., 1994 and 2014) were codified as presences (value of 1; n = 167). Squares with breeding kites in 1994 but not in 2014 were considered as absences (or losses, codified as 0; n = 107). Changes in the abundance of breeding pairs among censuses were codified as a variable with two vectors: “number of succeeds,” the number of breeding pairs estimated per 10 × 10-km square in 2014, and “number of failures,” the number of breeding pairs that disappeared between 1994 and 2014 (40). Those squares with no change or an increase in the abundance of breeding pairs in the considered period were assigned 0 failures.
We excluded data from Baleares Islands and Doñana from the analyses, because these populations are geographically isolated from the rest of the Spanish red kite population and seem to present different habitat preferences (20). Nonetheless, both populations are known to have been heavily impacted by poisoning (13, 21, 23, 41).
Wildlife Poisoning Data and Other Environmental Variables.
To assess the potential influence of wildlife poisoning on the observed changes in distribution and abundance of the breeding population of red kite, we used a large and exhaustive database of confirmed or suspected cases of wildlife poisoning compiled over more than 3 decades in Spain by WWF Spain and Spanish Ornithological Society (SEO/BirdLife) (17). Most of these data were gathered by survey programs coordinated by governments of autonomous regions in Spain, and toxicologic analyses were performed at public and private ecotoxicology laboratories designated by the competent authorities. We considered 12 variables related to the incidence of poisoning in the fauna in general and in raptors and kites in particular (SI Appendix, Table S1). According to the low detectability of wildlife poisoning (estimated in 5 to 15% of the total cases) (17, 27), we also considered the number of poisoned dogs per square kilometer and year as a potentially more accurate index of the actual incidence of wildlife poisoning (19). Considering the red kite trophic ecology (i.e., feeding on small carcasses and meat remains) (24), we also calculated the density of poisoned baits as a proxy for the incidence of poisoning in natural ecosystems. Poison-related variables were calculated in two ways: considering all the episodes registered in the database in the period between censuses (i.e., 1995 to 2013; n = 18,500 animals and 4,175 baits) and considering only those episodes in which the presence of a toxic compound susceptible of poisoning wildlife was confirmed (n = 9,562 animals and 3,257 baits). All these variables were initially considered in the models.
To account for other factors able to influence the observed changes in the red kite breeding population, we considered several environmental factors previously identified as important to determine habitat suitability for the species in Spain. Seoane et al. (18) highlighted the importance of different land uses (e.g., cropland, forest, pasture), topographic variables (e.g., elevation, slope), and climatic variables (e.g., temperature) on the distribution and abundance of red kites. Among these variables considered important for the red kite in 1994, we incorporated into our analyses those susceptible of meaningful and noticeable changes in 1994 to 2014. We used CORINE Land Cover information from 1990 and 2012 (42) to calculate the percent change in crop, forest, and pasture lands, as well as urban surfaces, per 10 × 10-km square among the considered red kite censuses (SI Appendix, Table S1). We assumed that the remaining variables considered important for explaining habitat suitability for the red kite (e.g., climate, topography) had not changed noticeably in the period analyzed.
We considered the number of breeding pairs of red kite estimated per 10 × 10-km square in 1994 and the geometric mean of the breeding pairs estimated in 1994 and 2014 as a way to better determine red kite abundance during the considered period (SI Appendix, Table S1). Squares with a higher breeding population could be more resilient to species threats such as poisoning and land use change and thereby have a higher probability of remaining occupied. Red kite population sizes were used also to control the potential effect of population size on the number of poisoned kites recorded per square. The species population size was included in the predictive models in two ways, as an independent explanatory variable and as a divisor of the number of poisoned red kites in each square, to obtain an explanatory variable of poisoned kites per breeding pair (SI Appendix, Table S1).
Analysis.
We used GLMs with binomial error distributions and “logit” links to explain the observed changes in the distribution and abundance of breeding red kites between 1994 and 2014. From the significantly correlated explanatory variables (i.e., those with a Spearman correlation P < 0.05), we included in the models the one with the greater ecological significance or, if not easily discriminated, the variable with best results in a univariate GLM (e.g., lower corrected Akaike information criterion [AICc]) (43).
We used multimodel selection to choose the best models (43). We randomly selected 70% of the data to train the models and the remaining 30% to test the results. We used the training dataset to generate a set of models with all possible combinations of the considered explanatory variables. We tested both linear and quadratic responses, as well as interactions between pairs of variables. The resulting models were ranked by lower AICc and higher relative weight (ωm), which indicates the probability that a model was the best one among all the candidate models evaluated. From these ranked models, we selected a set of best models comprising those whose weights added to 0.95 (Σωm = 0.95). From the set of best models, we depleted the redundant ones, that is, those including the same variables of other more explicative models (i.e., with lower AICc and higher ωm) but with some additional variable that complicates the model without improving its predictive ability. Once filtered, model weights were recalculated to sum to 1. The models resulting from this selection process were combined into a single model considered the final best model (43, 44). The coefficients of the explanatory variables included in the final best model were calculated by weighting each variable coefficient by the weight of the model in which the variable was included.
The relative contribution of each variable to the final best model (ωi) was calculated by summing the weights of the models (ωm) within the set of best models (Σωm = 0.95) that included the considered variable. The most important variables were those with the highest relative weight (ωi = Σωm). We expected that our approach of selecting noncorrelated variables to include in the models would minimize multicollineality, thereby minimizing the issues associated with pondered models (44). Nonetheless, to obtain additional information on the performance of the explanatory variables, we also calculated Z values and P values using the Wald test (SI Appendix, Table S3) for each variable included in the final best model. All statistical analyses were performed in R (45).
Global Assessment of Poisoning as a Species Threat.
We searched the IUCN Red List (29) for animal species threatened by poisoning at global scale according to the IUCN criteria for the inclusion of threats. We considered a species to be threatened by poisoning when threat category “5.1.2: Unintentional Effects (species is not the target)”, “5.1.3: Persecution/Control,” or “9.3.3: Herbicides and Pesticides” was recorded in the IUCN’s assessment of the species. We restricted our search to only global assessments of animal species (i.e., kingdom Animalia). We also considered these figures for vertebrate species (i.e., phylum Chordata) and for raptors (i.e., orders Accipitriformes, Cathartiformes, Falconiformes, and Strigiformes) and carnivores (i.e., order Carnivora), as predators are either frequently prosecuted through illegal poisoning or affected by secondary poisoning (e.g., consuming poisoned prey, such as rodents) (1, 4–8, 27, 35–38, 46, 47). The main results of this search are described in detail in SI Appendix, Table S4.
Data Availability.
Data on red kite populations are available in the census publications (15, 16). Additional details on this, as well as access to the ANTÍDOTO database on wildlife poisoning, are available on request from WWF Spain or SEO/BirdLife.
Supplementary Material
Acknowledgments
Hundreds of volunteers and personnel from Spanish autonomous governments (Comunidades Autonomas [CCAA]) participated in the national census of red kites, organized by SEO/Birdlife, with financial support from the Royal Society for the Protection of Birds, the Spanish Ministry of Environment (SME), and CCAA. The database of poisoned animals was compiled by CCAA and SME and coordinated by WWF Spain and SEO/Birdlife through the ANTÍDOTO program. This work was funded by WWF Spain under project UCLM-UCTR170245, and by the BBVA Foundation (“Ayudas Fundación BBVA a Equipos de Investigación Científica 2018”) through the TÓXICO project.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922355117/-/DCSupplemental.
References
- 1.Walker C. H., Sibly R. M., Hopkin S. P., Peakall D. B., Principles of Ecotoxicology, (CRC Press, 2012). [Google Scholar]
- 2.Forbes V. E., Calow P., Population growth rate as a basis for ecological risk assessment of toxic chemicals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 1299–1306 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soulé M. E., Viable Populations for Conservation, (Cambridge University Press, 1987). [Google Scholar]
- 4.Köhler H. R., Triebskorn R., Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 341, 759–765 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Newton I., Population Ecology of Raptors, (T & AD Poyser, 1979). [Google Scholar]
- 6.Finkelstein M. E. et al., Lead poisoning and the deceptive recovery of the critically endangered California condor. Proc. Natl. Acad. Sci. U.S.A. 109, 11449–11454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oaks J. L. et al., Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427, 630–633 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Shultz S. et al., Diclofenac poisoning is widespread in declining vulture populations across the Indian subcontinent. Proc. Biol. Sci. 271 (suppl. 6), S458–S460 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green R. E. et al., Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 41, 793–800 (2004). [Google Scholar]
- 10.Oro D., Grand challenges in population dynamics. Front. Ecol. Evol. 1, 2 (2013). [Google Scholar]
- 11.Macini F., Woodcock B. A., Isaac N. J. B., Agrochemicals in the wild: Identifying links between pesticide use and declines of nontarget organisms. Curr. Opin. Environ. Sci. Health 11, 53–58 (2019). [Google Scholar]
- 12.Triebskorn R. et al., Establishing causality between pollution and effects at different levels of biological organization: The VALIMAR project. Hum. Ecol. Risk Assess. 9, 171–194 (2003). [Google Scholar]
- 13.Tenan S., Adrover J., Muñoz Navarro A., Sergio F., Tavecchia G., Demographic consequences of poison-related mortality in a threatened bird of prey. PLoS One 7, e49187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fedak K. M., Bernal A., Capshaw Z. A., Gross S., Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 12, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viñuela J., Martí R., Ruíz A., The Red Kite in Spain, Monograph 6 [in Spanish] (SEO/BirdLife, 1999).
- 16.Molina B., The Red Kite in Spain, III, National Census: Wintering and Breeding Population in 2014 and Census Methodology [in Spanish] (SEO/BirdLife, 2015).
- 17.Cano C., De la Bodega D., Ayerza P., Mínguez E., Poisoning in Spain: Evolution of Wildlife Poisoning, 1992–2013 [in Spanish] (WWF Spain & SEO/BirdLife, Spanish, 2016).
- 18.Seoane J., Viñuela J., Díaz-Delgado R., Bustamante J., The effects of land use and climate on red kite distribution in the Iberian Peninsula. Biol. Conserv. 111, 401–414 (2003). [Google Scholar]
- 19.Martínez-Haro M. et al., Relationship of the toxicity of pesticide formulations and their commercial restrictions with the frequency of animal poisonings. Ecotoxicol. Environ. Saf. 69, 396–402 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Molenaar F. M. et al., Poisoning of reintroduced red kites (Milvus milvus) in England. Eur. J. Wildl. Res. 63, 94 (2017). [Google Scholar]
- 21.Tavecchia G., Adrover J., Navarro A. M., Pradel R., Modelling mortality causes in longitudinal data in the presence of tag loss: Application to raptor poisoning and electrocution. J. Appl. Ecol. 49, 297–305 (2012). [Google Scholar]
- 22.Smart J. et al., Illegal killing slows population recovery of a re-introduced raptor of high conservation concern: The red kite, Milvus milvus. Biol. Conserv. 143, 1278–1286 (2010). [Google Scholar]
- 23.Sergio F. et al., Preservation of wide-ranging top predators by site-protection: Black and red kites in Doñana National Park. Biol. Conserv. 125, 11–21 (2005). [Google Scholar]
- 24.BirdLife International , Milvus milvus. The IUCN Red List of Threatened Species 2018: e.T22695072A131877336. https://www.iucnredlist.org/species/22695072/131877336. Accessed March 3, 2019.
- 25.González-Estébanez F. J., García-Tejero S., Mateo-Tomás P., Olea P. P., Effects of irrigation and landscape heterogeneity on butterfly diversity in Mediterranean farmlands. Agric. Ecosyst. Environ. 144, 262–270 (2011). [Google Scholar]
- 26.Márquez C., Vargas J. M., Villafuerte R., Fa J. E., Understanding the propensity of wild predators to illegal poison baiting. Anim. Conserv. 16, 118–129 (2015). [Google Scholar]
- 27.Berny P., Pesticides and the intoxication of wild animals. J. Vet. Pharmacol. Ther. 30, 93–100 (2007). [DOI] [PubMed] [Google Scholar]
- 28.International Union for Conservation of Nature Standards and Petitions Subcommittee , Guidelines for Using the IUCN Red List Categories and Criteria, Version 13. http://cmsdocs.s3.amazonaws.com/RedListGuidelines.pdf. Accessed 3 April 2020.
- 29.International Union for Conservation of Nature , The IUCN Red List of Threatened Species, Version 2018-2. https://www.iucnredlist.org/. Accessed 3 April 2020.
- 30.Krimsky S., The unsteady state and inertia of chemical regulation under the US Toxic Substances Control Act. PLoS Biol. 15, e2002404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knott J., Newbery P., Barov B., Species Action Plan for the Red Kite Milvus milvus in the European Union, (European Commission, 2009), p. 55. [Google Scholar]
- 32.Carter I., The Red Kite, (Arlequin Press, 2001). [Google Scholar]
- 33.Cardiel I. E., The Red Kite in Spain, II: National Census [in Spanish] (SEO/BirdLife, 2006).
- 34.Spanish Official Gazette (BOE). Spanish Royal Decree on Threatened Wild Species and Wild Species Under Special Protection. BOE-A-2011-3582 [in Spanish]. https://www.boe.es/buscar/pdf/2011/BOE-A-2011-3582-consolidado.pdf. Accessed 10 April 2020.
- 35.Villafuerte R., Viñuela J., Blanco J. C., Extensive predator persecution caused by population crash in a game species: The case of red kites and rabbits in Spain. Biol. Conserv. 84, 181–188 (1998). [Google Scholar]
- 36.Berny P., Gaillet J. R., Acute poisoning of red kites (Milvus milvus) in France: Data from the SAGIR network. J. Wildl. Dis. 44, 417–426 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Olea P. P. et al., Lack of scientific evidence and precautionary principle in massive release of rodenticides threatens biodiversity: Old lessons need new reflections. Environ. Conserv. 36, 1–4 (2009). [Google Scholar]
- 38.Mougeot F., García J. T., Viñuela J., “Breeding biology, behaviour, diet and conservation of the red kite (Milvus milvus), with particular emphasis on Mediterranean populations” in Ecology and Conservation of European Dwelling Forest Raptors and Owls, Zuberogoitia I., Martínez J. E., Eds. (Editorial Diputación Foral de Vizcaya, 2011), pp. 190–204. [Google Scholar]
- 39.Viñuela J., Road transects as a large-scale census method for raptors: The case of the red kite Milvus milvus in Spain. Bird Study 44, 155–165 (1997). [Google Scholar]
- 40.Crawley M. J., The R Book, (Imperial College London at Silwood Park, 2007). [Google Scholar]
- 41.Sergio F. et al., Protected areas under pressure: Decline, redistribution, local eradication and projected extinction of a threatened predator, the red kite, in Doñana National Park, Spain. Endanger. Species Res. 38, 189–204 (2019). [Google Scholar]
- 42.Copernicus, CORINE Land Cover European inventory, 1990 and 2012. https://land.copernicus.eu/pan-european/corine-land-cover. Accessed August 2, 2019.
- 43.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information Theoretical Approach, (Springer, 2002). [Google Scholar]
- 44.Cade B. S., Model averaging and muddled multimodel inferences. Ecology 96, 2370–2382 (2015). [DOI] [PubMed] [Google Scholar]
- 45.R Core Development Team , R: A Language and Environmental Statistical Computing, (R Foundation for Statistical Computing, 2015). [Google Scholar]
- 46.Mateo-Tomás P., Olea P. P., Sánchez-Barbudo I. S., Mateo R., Alleviating human–wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 49, 376–385 (2012). [Google Scholar]
- 47.Ogada D. L., The power of poison: Pesticide poisoning of Africa’s wildlife. Ann. N. Y. Acad. Sci. 1322, 1–20 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data on red kite populations are available in the census publications (15, 16). Additional details on this, as well as access to the ANTÍDOTO database on wildlife poisoning, are available on request from WWF Spain or SEO/BirdLife.