Significance
Factors stemming from the tumor microenvironment can modulate drug resistance. Therefore, rational approaches to design efficacious targeted therapies are needed. Here, we show that fibroblasts reduce lapatinib sensitivity in a subset of HER2+ breast cancer cells via paracrine signaling that can be reversed by targeting downstream survival programs such as MTOR and antiapoptotic proteins (BCL-XL and MCL-1). Our finding that fibroblasts derived from either primary tumor or metastatic sites limit lapatinib response has important implications for developing treatment strategies that can restore sensitivity in multiple drug-resistant microenvironments. Due to the diversity of microenvironmental factors between these sites, targeting downstream survival programs instead of individual factors released by fibroblasts represents a promising strategy to combat microenvironment complexity.
Keywords: fibroblasts, tumor microenvironment, cell–cell interactions, drug resistance, breast cancer
Abstract
Despite the implementation of multiple HER2-targeted therapies, patients with advanced HER2+ breast cancer ultimately develop drug resistance. Stromal fibroblasts represent an abundant cell type in the tumor microenvironment and have been linked to poor outcomes and drug resistance. Here, we show that fibroblasts counteract the cytotoxic effects of HER2 kinase-targeted therapy in a subset of HER2+ breast cancer cell lines and allow cancer cells to proliferate in the presence of the HER2 kinase inhibitor lapatinib. Fibroblasts from primary breast tumors, normal breast tissue, and lung tissue have similar protective effects on tumor cells via paracrine factors. This fibroblast-mediated reduction in drug sensitivity involves increased expression of antiapoptotic proteins and sustained activation of the PI3K/AKT/MTOR pathway, despite inhibition of the HER2 and the RAS-ERK pathways in tumor cells. HER2 therapy sensitivity is restored in the fibroblast cocultures by combination treatment with inhibitors of MTOR or the antiapoptotic proteins BCL-XL and MCL-1. Expression of activated AKT in tumor cells recapitulates the effects of fibroblasts resulting in sustained MTOR signaling and poor lapatinib response. Lapatinib sensitivity was not altered by fibroblasts in tumor cells that exhibited sustained MTOR signaling due to a strong gain-of-function PI3KCA mutation. These findings indicate that in addition to tumor cell-intrinsic mechanisms that cause constitutive PI3K/AKT/MTOR pathway activation, secreted factors from fibroblasts can maintain this pathway in the context of HER2 inhibition. Our integrated proteomic–phenotypic approach presents a strategy for the discovery of protective mechanisms in fibroblast-rich tumors and the design of rational combination therapies to restore drug sensitivity.
HER2-overexpressing (HER2+) breast tumors account for ∼20% of all breast cancer cases and multiple therapies have been developed for the early and metastatic setting (1). In the metastatic setting, treatment options include HER2-targeted antibodies (naked or linked to a cytotoxic agent) or small molecules targeting the HER2-signaling axis (e.g., lapatinib and neratinib) (2). Most patients develop resistance to these agents. Therefore, it is critical to better understand the mechanisms that mediate tumor cell resistance to these therapies in order to develop more effective strategies for treatment of metastatic recurrent HER2+ breast cancer. Multiple mechanisms of HER2 therapy resistance have been proposed, including genetic truncation of the HER2 receptor (3), HER2 receptor mutations (4), compensatory HER3 signaling (5), activation of bypass signaling [e.g., PI3K (6–8), CyclinE (9), and CDK4/6 (10)], mesenchyme transition (11), and metabolic reprogramming (12, 13). In addition to these tumor-intrinsic resistance mechanisms, factors present in the tumor microenvironment can influence drug sensitivity (14). However, the contribution of these microenvironmental factors on HER2 therapy resistance remains poorly understood.
Stromal fibroblasts are abundant in the breast tumor microenvironment and tumor–fibroblast interactions can occur via direct cell–cell contact or through secretion of soluble factors that can activate multiple signaling pathways in tumor cells (15). Juxtracrine and paracrine interactions have been reported to affect sensitivity of breast cancer cells to a variety of therapies, including chemotherapy (16–18), endocrine therapy (19), and HER2 therapy (16, 20, 21). Identifying effective ways to overcome the protective effects of fibroblasts is a major challenge and need, with several different strategies being proposed based on analysis of the fibroblast secretome and signaling in tumor cells; these include blocking IL-6 and IL-8 (18) produced by fibroblasts or targeting pathways activated by fibroblasts in tumor cells (e.g., JAK/STAT and EGFR) (16, 19). Approaches that systematically measure fibroblast-mediated changes across multiple signaling pathways are necessary to develop rational therapies and overcome fibroblast-mediated resistance.
Using a panel of HER2+ breast cancer cell lines, we characterized cell growth dynamics under lapatinib treatment and found that coculture with multiple different fibroblast cell lines or primary fibroblast populations protect cancer cells from the cytotoxic effects of lapatinib. The fibroblast-mediated reduction in drug sensitivity was specific to HER2 kinase inhibitors, since treatment with the HER2-targeted antibody drug conjugate T-DM1 and paclitaxel did not alter drug responsiveness. Conditioned medium from the fibroblasts was sufficient to confer lapatinib resistance; specifically, these protective effects were mediated by secreted factors that were greater than 3 kDa. Interestingly, resistance was associated with sustained MTOR signaling activity and increased antiapoptotic protein levels in the presence of lapatinib, whereas RAS-ERK pathway inhibition was not significantly affected by fibroblast coculture. Drug sensitivity could be restored by addition of MTOR, BCL-XL, or MCL-1 inhibitors. These findings indicate that fibroblasts in the tumor microenvironment can maintain AKT/PI3K/MTOR pathway activity under conditions of HER2 inhibition and thus contribute to therapeutic response in tumors that lack mutations that constitutively activate this pathway and suggest a rational combination drug strategy to restore sensitivity.
Results
Fibroblasts Reduce Lapatinib Sensitivity in a Subset of HER2+ Breast Cancer Cell Lines In Vitro.
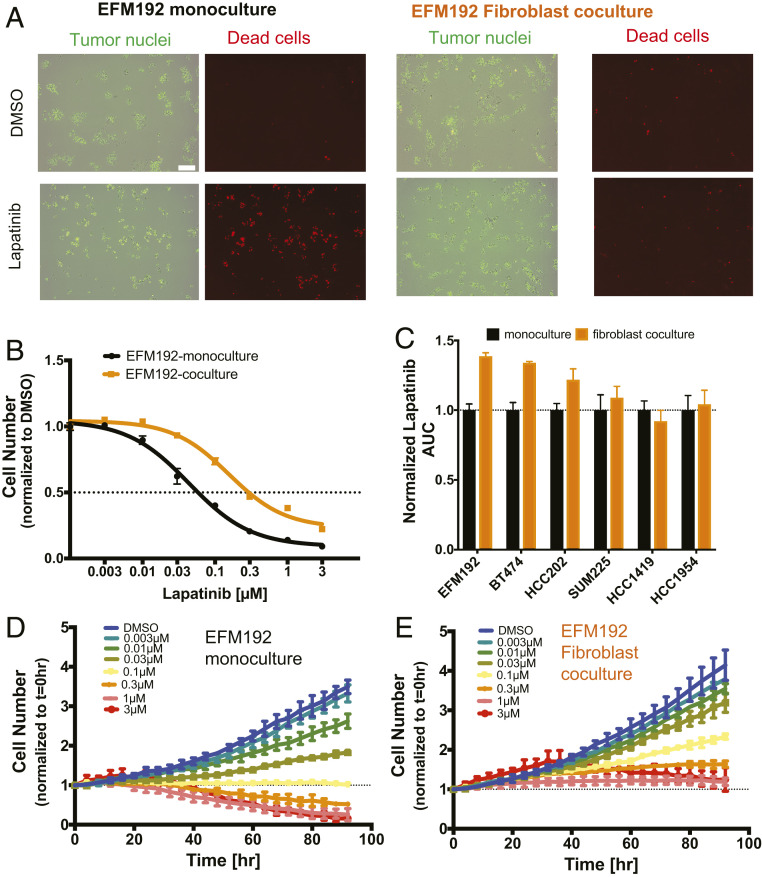
To measure the responsiveness of HER2+ breast cancer cells to HER2-targeted therapy under conditions in which fibroblasts directly interact with tumor cells, we developed an imaging-based dynamic coculture assay by tagging tumor cells with the nuclear marker H2B-GFP (Fig. 1A). This allowed us to simultaneously monitor the effects of fibroblasts on baseline tumor cell growth rate, as well as the specific effects of lapatinib on tumor cells with or without fibroblast coculture (Fig. 1A). H2B-GFP expressing tumor cells exhibited a similar response to lapatinib compared to wild-type tumor cells (SI Appendix, Fig. S1A). We employed a panel of six HER2+ breast cancer cell lines with a range of lapatinib sensitivities (SI Appendix, Fig. S1B) and estrogen receptor expression (SI Appendix, Table S1). Direct coculture with AR22 fibroblasts, which are derived from human breast tissue, resulted in decreased lapatinib sensitivity in three cell lines (EFM192, BT474, HCC202) as indicated by a 20 to 40% increase (P = 0.002 to 0.02, Fig. 1C) in the area under the curve (AUC) compared to monoculture. The drug response of the other three cell lines (SUM225, HCC1419 and HCC1954) was not altered by fibroblast coculture. Although lapatinib reduced tumor cell numbers in the presence of fibroblasts across all HER2+ cell lines in a dose-dependent manner, the fibroblast-protected cell lines displayed a fivefold higher IC50 when cultured with fibroblasts (Fig. 1B and SI Appendix, Fig. S1B).
Fig. 1.
Fibroblasts limit lapatinib response in a subset of HER2+ breast cancer cell lines in vitro. (A) H2B-GFP (green) expressing EFM192 tumor cells cocultured with AR22 fibroblasts for 96 h under control (dimethyl sulfoxide [DMSO]) and lapatinib (1 μM) treatment. Representative images from three biological replicates of monoculture and coculture of viable (green only) and dead (orange) tumor cells (red objects: ethidium bromide staining). (Scale bar, 200 μm.) (B) Cells were incubated with increasing drug concentrations for 96 h and the number of tumor cells was assayed in monoculture (black) and AR22 coculture (orange). Data are representative of three independent experiments and error bars are SD for three replicate wells. (C) Lapatinib AUC values. Data are derived from three independent experiments and error bars are SEM for three biological replicates. (D and E) Change in viable EFM192 tumor cell numbers over time at increasing lapatinib concentrations in monoculture and coculture with AR22 fibroblasts. Data are representative of three independent experiments and error bars correspond to SD for n = 3 replicate wells.
We investigated whether the reduced drug sensitivity of the EFM192, BT474, and HCC202 cell lines arises from differences in tumor cell growth rate or cell viability under AR22 fibroblast coculture conditions (Fig. 1 D and E and SI Appendix, Fig. S2). In the absence of lapatinib treatment, tumor cell numbers increased by twofold (HCC1419) to sevenfold (HCC1954) over 96 h (SI Appendix, Fig. S2). Fibroblast coculture did not change the baseline growth rate of any HER2+ breast cancer cell line (Fig. 1 D and E and SI Appendix, Fig. S2). Treatment with increasing concentrations of lapatinib in monoculture resulted in heterogeneous responses among the cell lines, ranging from growth inhibitory (HCC202 and HCC1954) to cytostatic (HCC1419), to cytotoxic (EFM192, BT474, and SUM225). Thus, the cell lines that were unaffected by fibroblast coculture were not intrinsically less sensitive to lapatinib. The presence of fibroblasts dampened the lapatinib responses in the three fibroblast-protected cell lines, diminishing the growth inhibitory effect in HCC202 and shifting the cytotoxic response to a cytostatic one in EFM192 and BT474 cells. For the three fibroblast-insensitive cell lines (SUM225, HCC1419, and HCC1954) we found that fibroblast coculture did not alter tumor cell response to lapatinib. These tumor cell line growth results under fibroblast coculture conditions agree with our independent measurements of lapatinib sensitivity using AUC (Fig. 1C). Analysis of drug response using a growth rate inhibition metric (22) that corrects for differences in cell proliferation, also confirmed the differential effects of fibroblasts in lapatinib drug sensitivity (SI Appendix, Fig. S3A).
We also investigated the effects of AR22 fibroblasts on responsiveness to neratinib (23) and afatinib (24), two FDA-approved HER2 kinase inhibitors that are currently in the clinic. Treatment with these inhibitors elicited a dose-dependent reduction in cell numbers in three breast cancer cell lines (EFM192, HCC202, and HCC1954) (SI Appendix, Fig. S3 B and C). Fibroblast coculture significantly decreased the sensitivity of EFM192 and HCC202 cells to neratinib and afatinib compared to monoculture, while no difference was observed for the fibroblast-insensitive cell line HCC1954. On the contrary, response to the FDA-approved HER2 antibody drug conjugate T-DM1 was not altered by the presence of fibroblasts in the fibroblast-protected cell lines EFM192 and BT474 (SI Appendix, Fig. S4A). Fibroblast viability was not altered by T-DM1 (SI Appendix, Fig. S4B). Cancer cell sensitivity to the tubulin-targeted chemotherapy paclitaxel (SI Appendix, Fig. S4C) was also not influenced by fibroblasts. These findings are consistent with a previous coculture screen that compared kinase inhibitors to chemotherapeutics (25), and an in vivo xenograft study that compared the pathologic response between lapatinib and T-DM1 (25). Taken together, these results highlight that direct coculture with AR22 fibroblasts reduces sensitivity to HER2 kinase-targeted therapy in a subset of HER2+ breast cancer cell lines, causing a shift from a growth-inhibited/cytotoxic phenotype to a sustained growth/cytostatic phenotype.
Diverse Fibroblast Lines Confer Protective Effects that Are Mediated Via Secreted Factors.
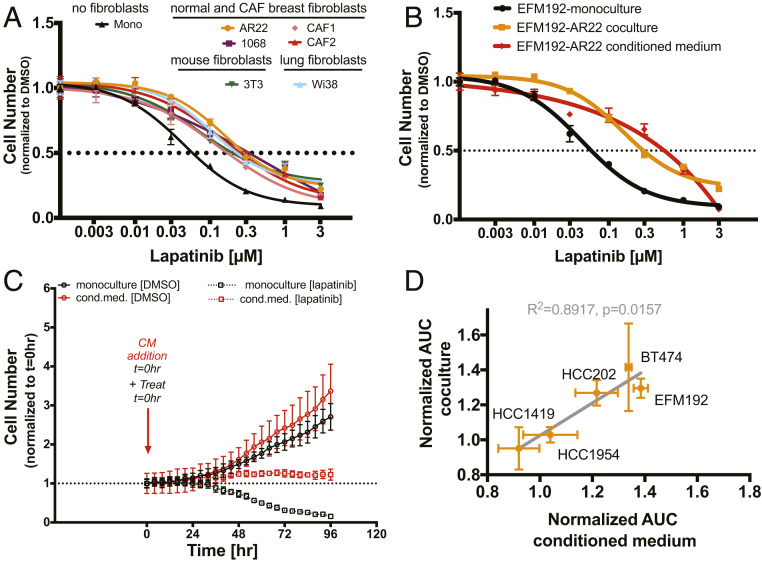
Given previous reports that fibroblasts from different tissues influence drug responses (17), we assessed the effects of distinct fibroblast cell lines on lapatinib sensitivity. Our panel included a line of murine fibroblasts (3T3), primary human breast fibroblasts from normal breast (AR22 and CCD1068) or tumor tissue (CAF1 and CAF2), and human fibroblasts from the lung, an organ where HER2+ breast tumors commonly metastasize. All fibroblasts tested were able to protect EFM192 cells, inducing a three- to sixfold increase in lapatinib IC50 (Fig. 2A). To test whether direct cell contact is required, we examined the effects of fibroblast-conditioned medium on lapatinib sensitivity. Fibroblast-conditioned medium phenocopied the protective effects of direct coculture, as measured by assaying viable cell numbers after 96 h of treatment (Fig. 2B) and characterizing tumor cell growth dynamics in response to lapatinib (Fig. 2C). Conditioned medium from two different fibroblast cell lines (AR22 and CCD1068) resulted in a similar increase in lapatinib AUC (31% vs. 35% P = 0.02 and P = 0.009, SI Appendix, Fig. S5A) providing further supportive evidence of the paracrine nature of this protective effect. Using a 3-kDa filter (26), we found that the conditioned medium fraction above 3 kDa blocked the lapatinib-induced effects on tumor cell numbers and that the <3-kDa fraction did not affect sensitivity, showing that small molecules secreted by fibroblasts do not alter lapatinib sensitivity (SI Appendix, Fig. S5B). Next, we analyzed the cytokines and growth factors present in the conditioned medium from these two fibroblast cell lines and found a similar secretion profile (SI Appendix, Fig. S5C). Specifically, 25 factors representing different ligand families, including interleukins, FGF, VEGF, WNT, and proteases, were detected at higher levels compared to control medium (SI Appendix, Fig. S5C). Finally, we analyzed the effects of conditioned medium in the panel of the HER2+ breast cancer cell lines and found a strong correlation (P = 0.016, R2 = 0.89, Fig. 2D) between lapatinib sensitivity under conditioned medium and direct fibroblast coculture conditions. These results demonstrate that fibroblasts from distinct tissue microenvironments can confer protective effects via paracrine tumor–fibroblast signaling.
Fig. 2.
Diverse fibroblast cell lines reduce lapatinib sensitivity that is mediated via secreted factors. (A) Human fibroblasts (primary lung: Wi38; primary normal breast: 1068; primary cancer-associated fibroblasts: CAF1 and CAF2; immortalized normal breast: AR22; and murine fibroblasts: 3T3) reduce lapatinib sensitivity. Representative dose–response data from at least two biological replicates for each tumor–fibroblast pair. Error bars are SD for three replicate wells. (B) Conditioned medium from normal breast fibroblasts (AR22) reduces lapatinib sensitivity. Representative experiment for three biological replicates. Error bars are SD for three replicate wells. (C) Treatment with AR22-conditioned medium confers protection from lapatinib-induced cell death. Results are representative of three biological replicates. Error bars are SD for three replicate wells. (D) Cancer cell lines that are desensitized to lapatinib in coculture (increase in normalized AUC >1) are also desensitized by conditioned medium (Pearson R2 = 0.8917, P = 0.02). Error bars are SEM for three biological replicates.
Fibroblast Coculture Results in Sustained MTOR Signaling in Tumor Cells Despite Blockade of the EGFR/HER2 Axis.
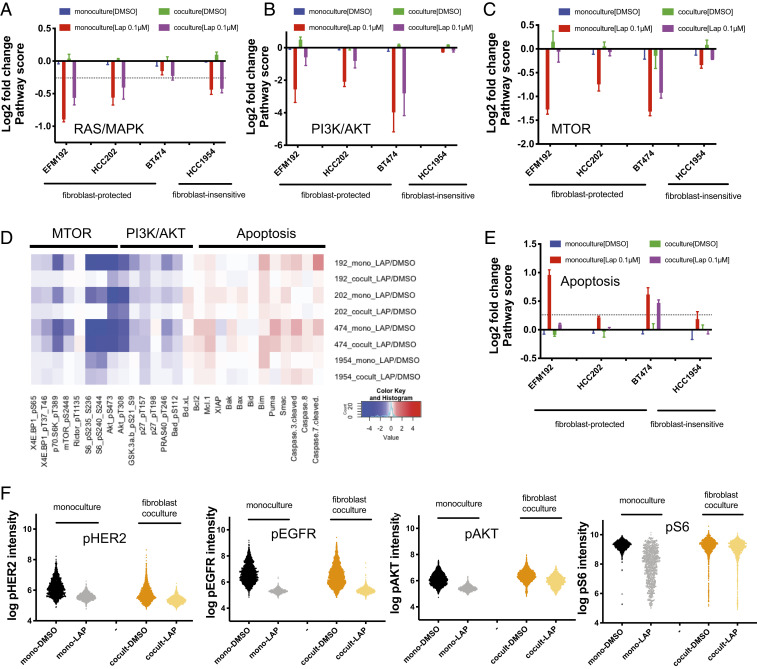
Given that fibroblasts secrete many factors that could contribute to lapatinib resistance, we were interested in investigating whether specific pathways downstream of HER2 were differentially affected by paracrine signaling with fibroblasts in order to define the critical pathways responsible for resistance. To examine this, we measured protein and phosphoprotein levels under monoculture and coculture conditions using reverse phase protein arrays (RPPA). We characterized protein level changes and pathway activity in nine signaling pathways and their protein members (27). These pathways included receptor tyrosine kinases (RTKs), the HER2-activated pathways PI3K/AKT and RAS/MAPK, downstream pathways (cell cycle, MTOR, and apoptosis), DNA damage, and hormone A and hormone B signaling. To physically separate the fibroblasts from the tumor cells, we used Transwell filters and analyzed tumor cell protein lysates. Protein measurements were performed in three fibroblast-protected (EFM192, HCC202, and BT474) and one fibroblast-insensitive (HCC1954) HER2+ breast cancer cell lines. In the absence of drug treatment, the protein levels of the direct lapatinib targets phospho-EGFRY1173 and phospho-HER2Y1248 were not significantly altered by AR22 fibroblast Transwell coculture (SI Appendix, Fig. S6A, average change across all cell lines: +10% for phospho-EGFR, P = 0.38 and −6% for phospho-HER2, P = 0.63). Treatment with lapatinib (0.1 μΜ) for 48 h resulted in effective blockade of these two drug targets under both monoculture and coculture conditions for all cell lines (SI Appendix, Fig. S6A, average change across all cell lines: 57% inhibition of phospho-EGFR, P < 0.001 and 86% inhibition in phospho-HER2, P < 0.001).
Treatment with lapatinib resulted in effective inhibition of the RTK pathway across all cell lines (average inhibition >60%, P < 0.004) for both monoculture and coculture conditions (SI Appendix, Fig. S6B). The cell cycle pathway was also inhibited across all cell lines with fibroblast coculture slightly attenuating the average percent inhibition from 20 to 13% (SI Appendix, Fig. S6C). RAS/MAPK signaling was effectively inhibited by lapatinib across all cell lines and coculture with fibroblasts did not attenuate inhibition (Fig. 3A: average inhibition >30%, P = 0.024). In contrast, while PI3K/AKT signaling was effectively inhibited under monoculture conditions for the three fibroblast-protected cell lines (Fig. 3B: average inhibition >80%, P = 0.004), fibroblasts strongly attenuated the extent of lapatinib pathway inhibition by more than 30% for EFM192 and HCC202 cells and by 8% for BT474 cells. Similarly, MTOR signaling was largely unaffected by lapatinib treatment in the fibroblast cocultures for EFM192 and HCC202 (Fig. 3C), while a 13% attenuation in MTOR pathway was measured for BT474.
Fig. 3.
Paracrine coculture with fibroblasts results in sustained MTOR signaling in tumor cells. (A–D) Pathway score changes in EFM192, HCC202, BT474, and HCC1954 induced by lapatinib (0.1 μM) treatment in monoculture and coculture conditions. Values are average log2-transformed ratios of each sample normalized to the dimethyl sulfoxide (DMSO) monoculture control in each cell line for at least two biological replicates. Error bars are SEM. (D) Heatmap of protein expression changes for MTOR/PI3K/Apoptotic pathway members. Red color indicates a protein increase, while blue indicates protein decrease for the average log2-transformed ratios. (E) Apoptosis pathway scores visualized in the same way as A–C. (F) Analysis of phospho-HER2 (Tyr1248), phospho-EGFR (Tyr1068), phospho-AKT (Ser473), and phospho-S6 (Ser235/236) protein expression changes in direct EFM192-AR22 cocultures using immunofluorescence. Cells were dosed with 0.1 μM of lapatinib for 48 h. Data are representative of two biological replicates.
Interestingly, PI3K/AKT and MTOR were not effectively inhibited by lapatinib in the fibroblast-insensitive HCC1954 cells, suggesting that PI3K/AKT and MTOR activation in these cells is HER2 independent. We also examined the extent of inhibition of individual phosphoproteins in the PI3K/AKT/MTOR pathway, such as phospho-AKTS473, phospho-4EBP1S65, phospho-70S6KT389, and phospho-MTORS2448 (Fig. 3D). Under monoculture, these proteins were more strongly inhibited in the fibroblast-protected cell lines EFM192, HCC202, and BT474 (average inhibition 51%, P = 0.005) compared to the fibroblast-insensitive HCC1954 cell line (average inhibition 10%, P = 0.06). Paracrine coculture with fibroblasts rescued this inhibition in the fibroblast-protected cells by 10 to 58% compared to only 2 to 8% for the fibroblast-insensitive HCC1954 cells. Notably, coculture resulted in dramatic rescue of phospho-MTORS2448 inhibition in EFM192, HCC202, and BT474, which resulted effectively in MTOR signaling staying “on” (no inhibition in EFM192 and HCC202 and 25% inhibition in BT474). Fibroblast coculture differentially affects the MTOR and PI3K/AKT pathways, indicating that secreted factors from fibroblasts activate MTOR and PI3K/AKT independent of HER2.
Lapatinib did not significantly alter the DNA damage response pathway (SI Appendix, Fig. S6D), while heterogeneous responses were observed for the apoptosis (Fig. 3E) and hormone A/B pathways (SI Appendix, Fig. S6 E and F). EFM192 and BT474 exhibited a stronger induction in apoptotic pathway score after lapatinib treatment of monocultures compared to cocultures, which is consistent with the cell growth measurements showing cell death at lapatinib concentrations higher than 0.1 μΜ (SI Appendix, Fig. S2). In these two cell lines, fibroblasts rescued the increase in apoptotic pathway score by 87% in EFM192 and by 15% in BT474. To further explore the heterogeneous response of these pathways to lapatinib, we analyzed the protein level changes for individual pathway members (Fig. 3D). Because coculture induced large differences in the apoptotic pathway score, we first examined individual antiapoptotic protein level changes in the fibroblast-protected cell lines. Lapatinib treatment increased MCL-1 levels in EFM192 and BT474, and BCL-XL in HCC202 under both monoculture and coculture conditions. On the contrary, proapoptotic proteins (BIM, PUMA, and SMAC) were increased by lapatinib only under monoculture conditions, showing that signaling from fibroblasts prevents the lapatinib-induced elevation in proapoptotic proteins and converts cytotoxic responses to cytostatic.
To independently examine the effects of lapatinib on the AKT/MTOR pathway, we also performed immunofluorescence (28) under conditions of direct coculture in the fibroblast-protected EFM192 and the fibroblast-insensitive HCC1954 cells. Consistent with the paracrine coculture results in the Transwell filters, phospho-S6S235/S236 was not inhibited by lapatinib treatment in direct fibroblast coculture in EFM192 cells, despite effective blockade of phospho-EGFRT1068 and phospho-HER2T1248 (Fig. 3F and SI Appendix, Fig. S7 A and B). Direct fibroblast coculture reduced the extent of phospho-AKT S473 inhibition in EFM192 (Fig. 3F), while phospho-AKT/S6 protein levels were not altered by fibroblast coculture in the fibroblast-insensitive HCC1954 cells (SI Appendix, Fig. S7 C–F). These results show that fibroblast-induced effects on HER2 inhibition involve fibroblast secretion of prosurvival factors that activate AKT/MTOR signaling in tumor cells and prevent induction of proapoptotic signaling.
Expression of Constitutively Activated AKT in Tumor Cells Results in Sustained MTOR Signaling and Reduced Lapatinib Sensitivity.
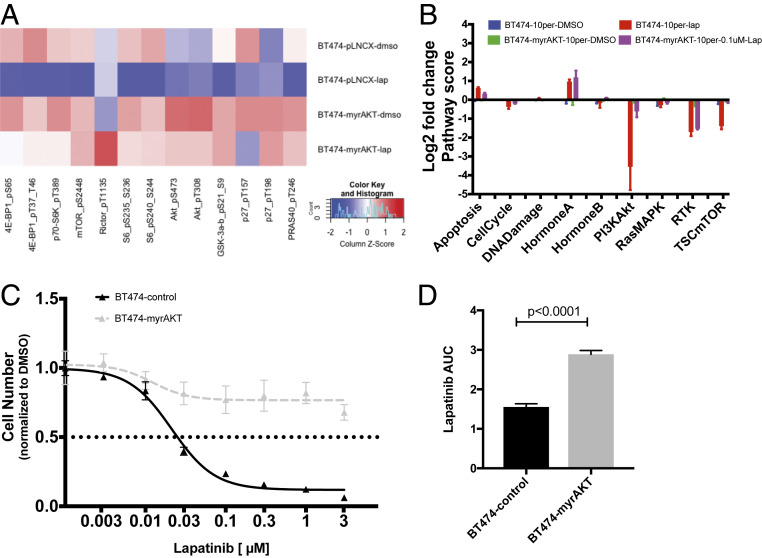
To further examine the contribution of the AKT/MTOR pathway in altering lapatinib sensitivity in fibroblast-protected HER2+ tumor cells, we overexpressed myristoylated AKT, a constitutively active form of AKT (29), in one of the fibroblast-protected cell lines, BT474 cells. Compared to the control cells, myrAKT-overexpressing cells exhibited an increase of 356% and 430% in phospho-AKTS473 and phospho-AKTT308, respectively (Fig. 4A), while MTOR pathway targets, such as phospho-MTORS2448 and phospho-S6S235/236, increased only slightly by 4% and 13%, respectively. Treatment of control and myrAKT cells with lapatinib resulted in effective inhibition of the HER2-signaling axis as demonstrated by a similar extent of phospho-HER2 and phospho-EGFR down-regulation (SI Appendix, Fig. S8A: 60% decrease in phospho-EGFR and 90% decrease in phospho-HER2) and RTK pathway scores (Fig. 4B: 69% compared to 65% inhibition). However, myrAKT significantly reduced the level of lapatinib-induced inhibition of the PI3K/AKT and MTOR pathways (PI3K/AKT: 91% vs. 35%, MTOR 62% vs. 10%). This attenuated inhibition of the PI3K/AKT/MTOR pathway in response to lapatinib was also reflected at the individual phosphoprotein levels (Fig. 4A). Specifically, the percent inhibition decreased from 82 to 31% in phospho-AKTS473, 93 to 20% in phospho-S6S235/236, and 47 to 0% in phospho-MTORS2448 in control compared to myrAKT cells. We next measured the sensitivity of the BT474 control and myrAKT cells to lapatinib in monoculture. We found that the BT474-myrAKT cells were less sensitive to lapatinib, with a 86% increase in lapatinib AUC (P < 0.001) compared to the control cells (Fig. 4 C and D). Immunofluorescence analysis of AKT/MTOR protein expression confirmed the protein array results, with the myrAKT cells exhibiting sustained AKT/MTOR signaling despite inhibition of phospho-EGFRT1068 and phospho-HER2T1248 levels (SI Appendix, Fig. S8 B–E). Hence, these results show that sustained AKT/MTOR signaling is sufficient to alter lapatinib sensitivity.
Fig. 4.
Overexpression of AKT results in sustained MTOR signaling despite lapatinib treatment and reduces lapatinib sensitivity. (A) Heatmap of absolute protein expression levels of MTOR and PI3K/AKT pathway members in control BT474 cells (pLNCX) and AKT-overexpressing BT474 (myrAKT) under no-treatment (dimethyl sulfoxide [DMSO]) and lapatinib-treatment (0.1 μM) conditions. Data are average median-normalized values for at least two biological replicates. Red indicates protein increase compared to median, while blue indicates protein decrease. (B) Pathway score changes due to lapatinib treatment in control and AKT-overexpressing BT474 cells. (C) Lapatinib dose–response for control and myrAKT BT474 cells. Error bars are SD for three replicate wells and results are representative of three biological replicates. (D) Lapatinib AUC values were quantified and compared between the control and AKT-overexpressing BT474 cells. Error bars represent SEM from three biological replicates.
Targeting MTOR and Antiapoptotic Proteins Eliminates Fibroblast-Protected Tumor Cells.
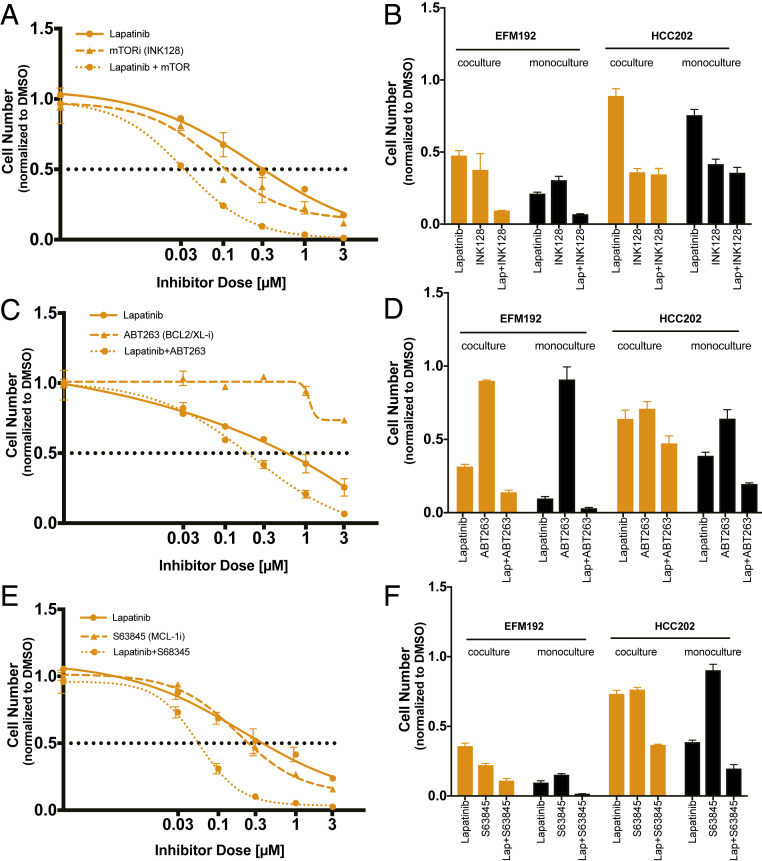
We next investigated whether inhibiting MTOR would resensitize the fibroblast-protected cancer cell lines EFM192 and HCC202 to HER2-targeted therapy. Cells were treated with single-agent or combination treatment of lapatinib + INK128 (dual MTORC1/2 inhibitor) (30) or lapatinib + everolimus (MTORC1 specific inhibitor) (30) under both monoculture and coculture conditions (Fig. 5 A and B and SI Appendix, Fig. S9 A–C). Single-agent treatment with INK128 resulted in larger decreases in cell number compared to everolimus. Under coculture conditions either MTOR inhibitor, when combined with lapatinib, was effective in reducing cell numbers in both cell lines to lower levels compared to lapatinib single-agent treatment (Fig. 5B and SI Appendix, Fig. S9B). Combination treatment resulted in complete eradication of the fibroblast-protected EFM192 cancer cells (94% and 99% reduction in viable cell numbers at the maximum dose for everolimus and INK128, respectively). HCC202 cell numbers under coculture were more effectively reduced by combining INK128 with lapatinib, resulting in a 77% reduction in viable cells, compared to a 46% reduction for everolimus. This disparity in rescue ability of INK128 compared to everolimus is consistent with the inability of everolimus to block both the MTORC2 complex and phospho-4EBP1 sites (30). Under monoculture conditions, combination treatment of MTOR inhibitors with lapatinib also resulted in lower viable cell numbers compared to either treatment alone with a larger extent of sensitization in EFM192 compared to HCC202 cells (Fig. 5B and SI Appendix, Fig. S9B). This reduction of viable cell numbers by MTOR inhibition in monoculture is expected because MTOR represents a central prosurvival pathway in tumor cells.
Fig. 5.
Fibroblast-protected tumor cells can be eliminated by targeting downstream survival programs. (A, C, and E) Single and combination dose–response curves for lapatinib and (A) MTOR inhibitor (INK128), or (B) BCL-2/XL inhibitor (ABT263), or (C) MCL-1 inhibitor (S63845) in EFM192 coculture with AR22. Inhibitors are dosed at a 3:1 lapatinib:INK128 ratio and 1:1 lapatinib:ABT263 or S63845 ratio. Maximum lapatinib doses are 3 μM for every combination. Error bars are SD for three replicate wells and results are representative of three biological replicates. (B, D, and F) Comparison of normalized viable cell numbers for EFM192 and HCC202 fibroblast-protected cell lines for single-agent treatment and combination for monoculture and coculture. Error bars are SD for three replicate wells and results are representative of three biological replicates.
Given the increase in antiapoptotic protein levels in lapatinib-treated tumor cells under coculture conditions (Fig. 3E), we hypothesized that antiapoptotic proteins present an actionable vulnerability to restore lapatinib sensitivity. We tested the effects of a dual BCL-2/BCL-XL inhibitor (ABT-263) or a MCL-1 (S63845) inhibitor that are currently in clinical trials (31). Single-agent treatment with ABT-263 did not reduce cell numbers effectively in either cell line under monoculture or coculture conditions (minimum viable cell numbers 71% for HCC202 coculture) (Fig. 5 C and D). Combination treatment of ABT263 with lapatinib resulted in almost complete killing of EFM192 cells in coculture (93% reduction in viable cells at maximum dose) and a significant reduction in HCC202 cell numbers (53% reduction) (SI Appendix, Fig. S9D). Targeting BCL-2/BCL-XL with ABT-263 in combination with lapatinib was also effective under monoculture conditions for both cell lines (Fig. 5D and SI Appendix, Fig. S9D). Single-agent treatment with S63845 resulted in a greater dose-dependent reduction of cell numbers in EFM192 compared to HCC202 under both monoculture and coculture conditions (Fig. 5 E and F and SI Appendix, Fig. S9E). Targeting MCL-1 in combination with lapatinib resulted in further decreases in cell numbers across multiple drug concentrations (Fig. 5E and SI Appendix, Fig. S9E). MCL-1 and lapatinib combination treatment reduced the fraction of EFM192 viable cells to 10% at concentration as low as 0.3 μΜ, while HCC202 cell numbers were reduced to 23% at the maximum dose under coculture conditions. These results suggest that inhibiting MTOR, BCL-2/BCL-XL, or MCL-1 in combination with lapatinib is an effective approach to sensitize fibroblast-protected cells to HER2-targeted therapy.
Discussion
Resistance to HER2-targeted therapies represents a major challenge, as the majority of advanced HER2+ breast cancer patients eventually exhibit disease progression (32, 33). In this work, we present an integrated phenotypic–proteomic approach to systematically investigate how fibroblasts modulate tumor cell response to HER2 therapy. We found that fibroblasts protect a subset of HER2+ breast cancer cells from lapatinib-induced cell death via paracrine signaling, while the response to paclitaxel or the HER2-conjugated chemotherapeutic T-DM1 is not influenced by the presence of fibroblasts. Specifically, fibroblast-secreted factors sustain prosurvival signaling despite effective blockade of the HER2-signaling axis. Combination treatment with lapatinib together with either an MTOR, BCL-2/XL, or MCL-1 inhibitor restored lapatinib drug sensitivity and thus such drug combinations represent a rational approach to sensitize fibroblast-protected HER2+ breast tumors to HER2-targeted therapy.
Previous preclinical (7, 34) and clinical studies (35, 36) that investigated tumor cell autonomous mechanisms of HER2 therapy resistance have provided evidence that activation of PI3K/AKT signaling via PTEN copy number loss or PI3K mutation is associated with poor response to therapy. Our results demonstrate that sustained PI3K/AKT or MTOR signaling through fibroblast-secreted factors represents an additional resistance mechanism in tumors that do not carry genomic alterations in the PI3K/AKT pathway. We found that the HER2+ cell line HCC1954 which carries a strong gain-of-function PI3KCA mutation (H1047R) exhibited sustained MTOR signaling after lapatinib treatment, and fibroblast coculture did not alter sensitivity to lapatinib. On the contrary, the BT474 cells that carry a weaker gain-of-function PI3KCA mutation (K111N) (37), were protected by fibroblasts and exhibited a significant reduction in AKT/MTOR signaling after lapatinib treatment. A detailed understanding of how the PI3K/AKT/MTOR pathway is activated by different genomic alterations is necessary to predict which tumors utilize this pathway to limit HER2 therapy efficacy. Taken together, these results suggest that prosurvival fibroblast signals are not required to sustain MTOR signaling when the PI3K/AKT pathway is activated by strong gain-of-function mutations. In the clinic, a subset of patients that lack mutations in the PI3K/AKT pathway (35, 36) exhibit resistance to HER2-targeted therapy, raising the possibility that factors from the tumor microenvironment may contribute to the survival of residual tumor cells in fibroblast-rich tumors.
MTOR has been proposed as a promising target for restoring drug sensitivity in breast cancers that are HER2 therapy resistant (33, 38). Preclinical evidence includes studies using in vitro cell lines with differential lapatinib (39, 40) or trastuzumab (6, 39, 41) sensitivity, tumor progression in transgenic animals (42, 43), and drug efficacy studies in cell line xenografts (6, 42, 44) and patient-derived xenografts (44, 45). In vivo combination studies of MTOR inhibitors with lapatinib (44) or trastuzumab (6, 42, 45) resulted in effective tumor growth inhibition or tumor regression. Clinical trials investigating trastuzumab and everolimus (46) or sirolimus (47) combination therapies have shown that a subset of patients derived survival benefit from this combination therapy. These patients exhibited activation of the PI3K/MTOR pathway that was assessed either by PTEN copy alterations or high expression of pS6 (48). Our findings suggest that the efficacy of HER2 and MTOR inhibitor combinations may at least in part be due to inhibition of fibroblast-mediated sustained MTOR signaling; thus, together these results highlight the potential for MTOR inhibitors to block both autonomous and microenvironment-mediated cancer vulnerabilities.
Tumor cells interact with multiple cell types in the breast stroma, including lymphocytes and fibroblasts, that could differentially regulate therapeutic efficacy. A high tumor-infiltrating lymphocyte score has been associated with improved response to HER2-targeted therapy in the clinic (49) and in an immunocompetent mouse model, lapatinib treatment induced a Stat1-dependent cytokine release that enhanced antitumor immune effects (50). On the other hand, fibroblasts have been shown to regulate breast tumor cell metabolism (51) and reduce sensitivity to estrogen therapy (52). However, in our coculture studies the fibroblast-protective effects were mediated by the conditioned medium fraction with a molecular weight larger than 3 kDa, suggesting that metabolites are not sufficient to reduce lapatinib sensitivity (SI Appendix, Fig. S5B). Extracellular matrix deposition by fibroblasts has been previously associated with resistance to chemotherapy and lapatinib and could be reversed by targeting JAK/STAT signaling or hyaluronic acid (16). Studies employing stimulation with single cytokines (e.g., FGF, HGF, IL-6, IL-8, and NRG1β) have also demonstrated that these secreted factors can induce resistance to chemotherapeutics (18), lapatinib (53), and other tyrosine kinase inhibitors (21). The multifactorial nature of the fibroblast-secreted factors (SI Appendix, Fig. S5C) makes it challenging to block fibroblast-mediated resistance; hence we decided to focus on downstream signaling pathways that were maintained in the fibroblast-protected state. Inhibiting MTOR restored lapatinib sensitivity in the fibroblast-protected tumor cells (Fig. 5). Previous studies have shown that MTOR signaling can be activated by different cytokines [e.g., IL-6 (54), NRG1β (53)] and matrix proteins (e.g., hyaluronic acid via JAK/STAT signaling) (16); thus MTOR presents a convergence node to microenvironment-mediated resistance. To identify predictive biomarkers of drug sensitivity, new experimental systems are needed that incorporate the tumor stroma and have improved predictive capabilities of preclinical in vivo and clinical responses (55).
The prosurvival phenotype conferred by fibroblast coculture (Fig. 3 A–E) was also associated with an increase in antiapoptotic protein expression that could be exploited with a combination treatment of lapatinib and a BCL-2/XL or MCL-1 inhibitor. This represents an alternative therapeutic strategy to targeting vulnerabilities associated with the fibroblast-protected state that can be mediated via multiple fibroblast-secreted factors. Importantly, the BCL-2 family network is downstream of multiple signaling pathways that have been implicated in HER2 therapy resistance (PI3K/AKT, RAS/MAPK, JNK, and RTK) and can be activated by these fibroblast-secreted factors. Analysis of tumor cell survival in breast cancer in vivo xenografts treated with lapatinib, showed that tumor cells that were in direct contact with basement membrane exhibited up-regulation of the BCL-2 antiapoptotic protein33. BCL-XL- or MCL-1-targeted therapy could be personalized depending on the BCL-2 family expression status of the HER2+ tumor using a proteomic approach that we previously developed in ovarian cancer (56). The HER2 and antiapoptotic combination treatment results show effective reduction in viable cell numbers and are consistent with previous preclinical reports in HER2+ breast cancer models (25, 57). Our systems approach of profiling multiple signaling pathways combined with new dynamic drug response monitoring, uncovered the differential effects of fibroblasts on PI3K/AKT vs. RAS/MAPK pathways and provided insights into mechanisms of tumor–fibroblast communication. Furthermore, this profiling approach can help address the in vitro to in vivo gap of response to anticancer treatments, while also allowing the study of therapeutic effects on fibroblasts.
Understanding how tumor cells integrate signals from the tumor microenvironment is critical for uncovering mechanisms of therapy resistance. The ability of fibroblasts derived from primary tumor and metastatic sites to alter lapatinib response has important implications for developing treatment strategies that can restore sensitivity in multiple drug-resistant microenvironments. Due to the diversity of microenvironmental factors between these sites, targeting downstream survival programs instead of individual factors released by fibroblasts represents a promising treatment strategy to combat microenvironment complexity. Our integrated approach of drug response profiling with proteomic analysis of multiple signaling pathways allows for rational combination therapy design to target fibroblast-protected tumor cells.
Materials and Methods
Cell Lines, Drug Response Assays, and Protein Expression Analysis.
Breast cancer cell lines (EFM192, BT474, HCC202, HCC1419, SUM225, and HCC1954) were a gift from Dennis Slamon, University of California Los Angeles, Los Angeles, CA; fibroblast cell lines (CCD1068, Wi38, and 3T3) were purchased from ATCC; and primary fibroblasts (AR22, CAF1, and CAF2) were derived from normal or breast cancer tissue (SI Appendix, Supplementary Methods). Cells were grown in the appropriate medium and drug dose–response assays were conducted in 96-black/clear well plates (SI Appendix, Supplementary Methods). Cyclic immunofluorescence experiments were also performed in 96-black/clear well plates, and protein lysate samples were prepared using 6-well plates and Transwell filters.
Statistical Analysis.
Reported values are mean ± SEM unless otherwise stated. Statistical analyses were performed in Prism. The level of statistical significance is marked by asterisks in the figures and we considered P values below 0.05 significant. For the pathway inhibition analysis, two-tailed one-sample t tests were performed.
Materials and Data Availability.
Requests for reagents and code should be directed to the corresponding author. RPPA data are available on Figshare at (https://figshare.com/articles/RPPA_data/12199835/1).
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute (R00CA222554 to I.K.Z.; U01CA217842 to G.B.M., CA166672 to MD Anderson Cancer Center [MDACC] RPPA core, Breast SPORE 1P50CA168504 to Dana-Farber/Harvard Cancer Center), the Department of Defense (W81XWH-14-1-0222 to I.K.Z.), the Breast Cancer Research Foundation (BCRF-18-110 to G.B.M. and 18-021 to J.S.B.), the Susan G. Komen Foundation (SAC110052 to G.B.M.), and NCICA16672 to the MDACC RPPA core. We thank the Nikon Imaging Center and the Institute for Chemistry and Cell Biology-Longwood Screening Facility at Harvard Medical School for providing access to instruments, Dr. Angelica Martinez-Gakidis for scientific editing, Dr. Yiling Lu for RPPA studies, Ms. Ashka Patel and Ms. Lynda Chichester for help with the primary tumor tissues, Dr. Jonathan Kelber for helpful discussions, and Dr. David Livingston for AR22 fibroblasts.
Footnotes
Competing interest statement: D.A.D. is on the Academic Advisory Board of Oncology, Analytics, Inc, and consults for Novartis. G.B.M. receives support or acts as consultant for AstraZeneca, ImmunoMET, Ionis, Lilly, PDX Pharmaceuticals, Signalchem Lifesciences, Symphogen, and Tarveda. J.S.B. consults for Agios and Effector Pharmaceuticals.
Data deposition: RPPA data are available on Figshare at https://figshare.com/articles/RPPA_data/12199835/1.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000648117/-/DCSupplemental.
References
- 1.Gingras I., Gebhart G., de Azambuja E., Piccart-Gebhart M., HER2-positive breast cancer is lost in translation: Time for patient-centered research. Nat. Rev. Clin. Oncol. 14, 669–681 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Oh D. Y., Bang Y. J., HER2-targeted therapies–A role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Scaltriti M. et al., Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl. Cancer Inst. 99, 628–638 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Xu X. et al., HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2+ breast cancer. Clin. Cancer Res. 23, 5123–5134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sergina N. V. et al., Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445, 437–441 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien N. A. et al., Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin. Cancer Res. 20, 3507–3520 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Rexer B. N., Chanthaphaychith S., Dahlman K., Arteaga C. L., Direct inhibition of PI3K in combination with dual HER2 inhibitors is required for optimal antitumor activity in HER2+ breast cancer cells. Breast Cancer Res. 16, R9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni J. et al., Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat. Med. 22, 723–726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaltriti M. et al., Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc. Natl. Acad. Sci. U.S.A. 108, 3761–3766 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goel S. et al., Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29, 255–269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hangauer M. J. et al., Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deblois G. et al., ERRα mediates metabolic adaptations driving lapatinib resistance in breast cancer. Nat. Commun. 7, 12156 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruprecht B. et al., Lapatinib resistance in breast cancer cells is accompanied by phosphorylation-mediated reprogramming of glycolysis. Cancer Res. 77, 1842–1853 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Place A. E., Jin Huh S., Polyak K., The microenvironment in breast cancer progression: Biology and implications for treatment. Breast Cancer Res. 13, 227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houthuijzen J. M., Jonkers J., Cancer-associated fibroblasts as key regulators of the breast cancer tumor microenvironment. Cancer Metastasis Rev. 37, 577–597 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Marusyk A. et al., Spatial proximity to fibroblasts impacts molecular features and therapeutic sensitivity of breast cancer cells influencing clinical outcomes. Cancer Res. 76, 6495–6506 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landry B. D. et al., Tumor-stroma interactions differentially alter drug sensitivity based on the origin of stromal cells. Mol. Syst. Biol. 14, e8322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su S. et al., CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Brechbuhl H. M. et al., Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin. Cancer Res. 23, 1710–1721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straussman R. et al., Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487, 500–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson T. R. et al., Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487, 505–509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafner M., Niepel M., Chung M., Sorger P. K., Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat. Methods 13, 521–527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paranjpe R. et al., Neratinib in HER2-positive breast cancer patients. Ann. Pharmacother. 53, 612–620 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Harbeck N. et al.; LUX-Breast 1 study group , Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): An open-label, randomised, phase 3 trial. Lancet Oncol. 17, 357–366 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Zoeller J. J., Bronson R. T., Selfors L. M., Mills G. B., Brugge J. S., Niche-localized tumor cells are protected from HER2-targeted therapy via upregulation of an anti-apoptotic program in vivo. NPJ Breast Cancer 3, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W. et al., Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell 165, 1092–1105 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akbani R. et al., A pan-cancer proteomic perspective on the Cancer Genome Atlas. Nat. Commun. 5, 3887 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J. R., Fallahi-Sichani M., Sorger P. K., Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat. Commun. 6, 8390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmelzle T. et al., Functional role and oncogene-regulated expression of the BH3-only factor Bmf in mammary epithelial anoikis and morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 104, 3787–3792 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilar E., Perez-Garcia J., Tabernero J., Pushing the envelope in the mTOR pathway: The second generation of inhibitors. Mol. Cancer Ther. 10, 395–403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight T., Luedtke D., Edwards H., Taub J. W., Ge Y., A delicate balance–The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 162, 250–261 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Pernas S., Tolaney S. M., HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 11, 1758835919833519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernieri C. et al., Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: Current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol. Hematol. 139, 53–66 (2019). [DOI] [PubMed] [Google Scholar]
- 34.O’Brien N. A. et al., Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol. Cancer Ther. 9, 1489–1502 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Chandarlapaty S. et al., Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res. 18, 6784–6791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rimawi M. F. et al., Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res. Treat. 167, 731–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gymnopoulos M., Elsliger M. A., Vogt P. K., Rare cancer-specific mutations in PIK3CA show gain of function. Proc. Natl. Acad. Sci. U.S.A. 104, 5569–5574 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Margariti N., Fox S. B., Bottini A., Generali D., “Overcoming breast cancer drug resistance with mTOR inhibitors”. Could it be a myth or a real possibility in the short-term future? Breast Cancer Res. Treat. 128, 599–606 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Brady S. W., Zhang J., Tsai M. H., Yu D., PI3K-independent mTOR activation promotes lapatinib resistance and IAP expression that can be effectively reversed by mTOR and Hsp90 inhibition. Cancer Biol. Ther. 16, 402–411 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin D. N., Ruiz-Saenz A., Gulizia N., Moasser M. M., Chemical probing of HER2-amplified cancer cells identifies TORC2 as a particularly effective secondary target for combination with lapatinib. Oncotarget 6, 41123–41133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gayle S. S., Arnold S. L., O’Regan R. M., Nahta R., Pharmacologic inhibition of mTOR improves lapatinib sensitivity in HER2-overexpressing breast cancer cells with primary trastuzumab resistance. Anticancer. Agents Med. Chem. 12, 151–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller T. W. et al., Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin. Cancer Res. 15, 7266–7276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosley J. D., Poirier J. T., Seachrist D. D., Landis M. D., Keri R. A., Rapamycin inhibits multiple stages of c-Neu/ErbB2 induced tumor progression in a transgenic mouse model of HER2-positive breast cancer. Mol. Cancer Ther. 6, 2188–2197 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-García C. et al., Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy. Clin. Cancer Res. 18, 2603–2612 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Hsu P. Y. et al., Dual mTOR kinase inhibitor MLN0128 sensitizes HR+/HER2+ breast cancer patient-derived xenografts to trastuzumab or fulvestrant. Clin. Cancer Res. 24, 395–406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andre F. et al., Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-Overexpressing metastatic breast cancers: Combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J. Clin. Oncol. 34, 2115–2124 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Acevedo-Gadea C. et al., Sirolimus and trastuzumab combination therapy for HER2-positive metastatic breast cancer after progression on prior trastuzumab therapy. Breast Cancer Res. Treat. 150, 157–167 (2015). [DOI] [PubMed] [Google Scholar]
- 48.André F. et al., Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 15, 580–591 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Dieci M. V. et al., Integrated evaluation of PAM50 subtypes and immune modulation of pCR in HER2-positive breast cancer patients treated with chemotherapy and HER2-targeted agents in the CherLOB trial. Ann. Oncol. 27, 1867–1873 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Hannesdóttir L. et al., Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur. J. Immunol. 43, 2718–2729 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Avagliano A. et al., Metabolic reprogramming of cancer associated fibroblasts: The slavery of stromal fibroblasts. BioMed Res. Int. 2018, 6075403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Outschoorn U. E. et al., Understanding the metabolic basis of drug resistance: Therapeutic induction of the warburg effect kills cancer cells. Cell Cycle 10, 2521–2528 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson S. S. et al., Microenvironment-mediated mechanisms of resistance to HER2 inhibitors differ between HER2+ breast cancer subtypes. Cell Syst. 6, 329–342.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinno J. et al., Interleukin-6 influences stress-signalling by reducing the expression of the mTOR-inhibitor REDD1 in a STAT3-dependent manner. Cell. Signal. 28, 907–916 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Valkenburg K. C., de Groot A. E., Pienta K. J., Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zervantonakis I. K. et al., Systems analysis of apoptotic priming in ovarian cancer identifies vulnerabilities and predictors of drug response. Nat. Commun. 8, 365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merino D. et al., Synergistic action of the MCL-1 inhibitor S63845 with current therapies in preclinical models of triple-negative and HER2-amplified breast cancer. Sci. Transl. Med. 9, eaam7049 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.