Significance
These findings indicate the important role of IL-38 in the inihibition of neurotensin-stimulated activation of microglia and the resulting release of proinflammatory molecules. Moreover, the reduced expression of IL-38 in the amygdala indicates that it may not be sufficient to prevent inflammation, and that its administration could serve as a novel treatment for children with autism spectrum disorder.
Keywords: autism, amygdala, IL-38, inflammation, microglia
Abstract
Autism spectrum disorder (ASD) is characterized by impaired social interactions and communication. The pathogenesis of ASD is not known, but it involves activation of microglia. We had shown that the peptide neurotensin (NT) is increased in the serum of children with ASD and stimulates cultured adult human microglia to secrete the proinflammatory molecules IL-1β and CXCL8. This process is inhibited by the cytokine IL-37. Another cytokine, IL-38, has been reported to have antiinflammatory actions. In this report, we show that pretreatment of cultured adult human microglia with recombinant IL-38 (aa3-152, 1–100 ng/mL) inhibits (P < 0.0001) NT-stimulated (10 nM) secretion of IL-1β (at 1 ng/mL) and CXCL8 (at 100 ng/mL). In fact, IL-38 (aa3-152, 1 ng/mL) is more potent than IL-37 (100 ng/mL). Here, we report that pretreatment with IL-38 (100 ng/mL) of embryonic microglia (HMC3), in which secretion of IL-1β was undetectable, inhibits secretion of CXCL8 (P = 0.004). Gene expression of IL-38 and its receptor IL-36R are decreased (P = 0.001 and P = 0.04, respectively) in amygdala from patients with ASD (n = 8) compared to non-ASD controls (n = 8), obtained from the University of Maryland NeuroBioBank. IL-38 is increased (P = 0.03) in the serum of children with ASD. These findings indicate an important role for IL-38 in the inhibition of activation of human microglia, thus supporting its development as a treatment approach for ASD.
Autism spectrum disorder (ASD) is characterized by impaired social interactions and communication, as well as stereotypic behaviors (1, 2), affecting 1 in 54 children in the United States (3). ASD may involve inflammation of the brain (4, 5) as evidenced by activation of microglia that secrete the proinflammatory cytokine IL-1β and the chemokine CXCL8 (6–8). These mediators are increased in the serum, cerebrospinal fluid, and brain of many patients with ASD (9–11). We recently reported significantly increased gene expression of additional proinflammatory molecules IL-18, its receptor IL-18R (12), and microRNA-155 (miR-155) (13) in the amygdala of children with ASD (14–16).
Previously we reported that the peptide neurotensin (NT) (17) is increased in the serum of patients with ASD (18, 19) and that NT can stimulate cultured adult human microglia to secrete IL-1β and CXCL8 (8). Recently, we reported that IL-37, a member of the IL-1 family of cytokines with antiinflammatory actions (20–24), can inhibit cultured human adult microglia stimulated by NT (12).
IL-38, like IL-37, also belongs to the IL-1 family and has been reported to have antiinflammatory activity (25, 26). IL-38 exists intracellularly as a precursor full-length form called IL-38 (aa1-152) and must be cleaved at the N terminus before it is secreted extracellularly as an active forms (27) that are presently known (28).
The main receptor for IL-38 is IL-36R, with the cofactor IL1-R9 and IL-1 receptor accessory protein-like 1 (IL-1RAPL1) also involved in the inhibitory action of IL-38 (29).
In this report, we compared the effect of the recombinant active forms of IL-38 (aa2-152, aa5-152, and aa3-152) to that of IL-37 using both cultured adult and embryonic human microglia stimulated by NT. We also investigated gene expression of IL-38 and its main receptor IL-36R in amygdala that regulate behavior (14, 15) and microglia express receptors for NT (30).
Results
IL-38 Inhibits Secretion of IL-1β from Adult Human Microglia Stimulated by Neurotensin.
Human microglia were pretreated with human recombinant IL-37 (100 ng/mL) and three human recombinant IL-38 analogs (aa2-152, aa5-152, aa3-152, 100 ng/mL) for 24 h and then were stimulated with NT (10 nΜ) for 24 h to determine the secretion of IL-1β and CXCL8. The IL-38 analogs aa2-152 and aa5-152 were equally potent to IL-37b inhibiting IL-1β and CXCL8 secretion from microglia by about 30% (Fig. 1). However, IL-38 aa3-152 inbibited (P < 0.0001) IL-1β secretion by 100% (Fig. 1).
Fig. 1.
IL-38 inhibits secretion of IL-1β (Left) and CXCL8 (Right) from adult human microglia stimulated by neurotensin. Human microglia cells were pretreated with IL-37 (100 ng/mL) and three IL-38 analogs (aa2-152, aa5-152, aa3-152, 100 ng/mL) for 24 h and then were stimulated with NT (10 nM) for 24 h to determine the secretion of IL-1β by specific ELISAs. All conditions were performed in triplicates for each dataset and repeated twice (n = 2).
Dose–Response of IL-38 on Secretion of IL-1β and CXCL8 from Adult Human Microglia Stimulated by Neurotensin.
Human microglia cells were pretreated with IL-38 (aa3-152, 1–100 ng/mL) for 24 h and then were stimulated with NT (10 nΜ) for 24 h to determine the secretion of IL-1β and CXCL8. All conditions were performed in triplicates for each dataset and repeated twice (n = 2). IL-38 aa3-152 inhibited (P < 0.0001) secretion of IL-1β by almost 90% even at 1 ng/mL (Fig. 2).
Fig. 2.
Dose–response of IL-38 on secretion of IL-1β (Left) and CXCL8 (Right) from adult human microglia stimulated by neurotensin. Human microglia cells were pretreated with IL-38 (aa3-152, 1–100 ng/mL) for 24 h and then were stimulated with NT (10 nM) for 24 h to determine the secretion of IL-1β by specific ELISAs. All conditions were performed in triplicates for each dataset and repeated twice (n = 2).
Effect of IL-38 on Secretion of IL-1β and CXCL8 on Secretion from Embryonic Human Microglia Stimulated by Neurotensin.
Embryonic microglia (HMC3) were pretreated with human recombinant IL-37b (100 ng/mL) and the three human recombinant IL-38 analogs (aa2-152, aa5-152, aa3-152, 100 ng/mL) for 24 h and then were stimulated with NT (10 nΜ) for 24 h to determine the secretion of IL-1β and CXCL8. Embryonic microglia (HMC3) do not secrete IL-1β (31), but secretion of CXCL8 was inhibited (P = 0.004) by all IL-38 analogs equally (100 ng/mL) (Fig. 3).
Fig. 3.
Effect of IL-38 on secretion of CXCL8 from neonatal human microglia stimulated by neurotensin. Human microglia cells were pretreated with IL-38 (aa3-152, 1–100 ng/mL) for 24 h and then were stimulated with NT (10 nM) for 24 h to determine the secretion of CXCL8 by specific ELISAs. All conditions were performed in triplicates for each dataset and repeated twice (n = 2).
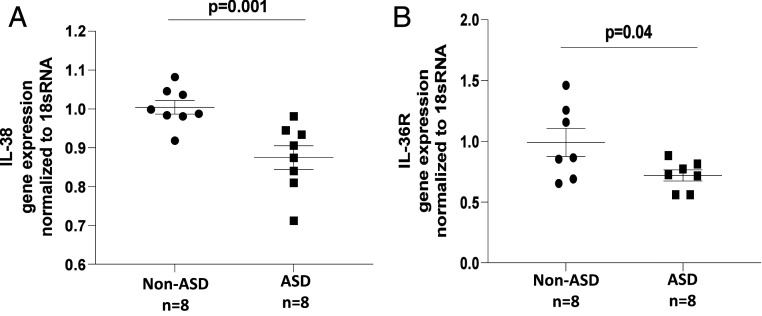
Gene Expression of IL-38 and IL-36R Is Decreased in the Amygdala of Children with ASD.
Gene expression levels of (i) IL-38 and (ii) the IL-38 receptor IL-36R in the amygdala of ASD and non-ASD subjects were measured by qRT-PCR. Gene expression was normalized to 18S rRNA control gene. Gene expression of both IL-38 (P = 0.001) and IL-36R (P = 0.04) was reduced in children with ASD (Fig. 4).
Fig. 4.
Decreased gene expression levels of IL-38 and IL-36R in the amygdala of children with ASD. Gene expression levels of IL-38 (A) and IL-36R (B) in the amygdala of ASD and non-ASD subjects were measured by qRT-PCR. Gene expression was normalized to 18S rRNA control gene. Measurements were repeated three times each.
IL-38 Is Increased in the Serum of Children with ASD.
IL-38 levels are increased (P = 0.033) in the serum of children with ASD compared to non-ASD controls (Fig. 5).
Fig. 5.
Increased IL-38 serum concentration in children with ASD. Symbols represent individual data points: The long horizontal lines represent the mean, and the shorter ones show the SD for each group.
Discussion
In this report, we show that IL-38 can inhibit NT-stimulated secretion of proinflammatory molecules from cultured human adult (IL-1β and CXCL8) and embryonic (CXCL8) microglia. Embryonic microglia do not secrete IL-1β (31). We chose to use human embryonic microglia (32) because unlike adult microglia, they may better reflect how microglia from children with ASD would respond (33). IL-38 is known to inhibit immune processes (34), but its effect on human microglia or its gene expression in the brain had not been investigated until now.
This study shows that IL-38 is a more potent inhibitor than IL-37, which we had previously reported to be able to inhibit NT-stimulated gene expression and secretion of IL-1β and CXCL8 from cultured human adult microglia (12).
In this report, we show decreased gene expression of IL-38 and IL-36R in amygdala of children with ASD. Previously, we had reported that gene expression of IL-37 is increased in the brain of children with ASD (12). The reason for these seemingly opposite findings is not clear. We speculate that the increase in IL-37 may serve to compensate for the decreased expression of IL-38.
An additional finding in this report is that of increased serum levels of IL-38 in about 30% of children with ASD, as compared to non-ASD controls. It is interesting to speculate that children with higher serum IL-38 had fewer ASD-related symptoms. If such cooccurrence were true, it would imply that peripeheral IL-38 could cross the blood–brain barrier and partially correct any IL-38 deficinecy in the amygdala.
Amygdala are responsible for social behavior (14, 15), and dysfunctional neuronal connectivity in the amygdala has been associated with ASD (35). Schumann and coworkers had shown that brains of children with ASD have an increased number of neurons and greater dendritic density in the basal amygdala than age-matched non-ASD controls (36). They also reported that enlargement of the amygdala in toddlers with ASD related to severity of behavior (37). Their findings are consistent with decreased synaptic pruning by microglia when they are in their activated proinflammatory phase (M2). This data are also consistent with our hypothesis that focal inflammation in the amygdala could contribute to the pathogenesis of ASD (Fig. 6) (38).
Fig. 6.
(A) Diagrammatic representation of the proposed role of IL-38 in autism. Gene expression of IL-38 is decreased (violet letters) in the amygdala, which regulates behavior of autistic children, and IL-38 inhibits release of proinflammatory molecules from human microglia (green box and line), suggesting that administering IL-38 could be a way to address some aspects of autism. (B) Diagrammatic representation of the proposed interactions and sites of action of IL-38. Neurotensin from an unidentified source (brain or gut) stimulates microglia in the amygdala to secrete the proinflammatory molecules IL-1beta and CXCL8, which contribute to focal inflammation and disrupt neuronal connectivity. This process could be inhibited by IL-38 (green line), the gene expression of which, and possibly of its receptor IL-36R, is reduced in amygdala of children with ASD, thus permitting inflammation.
It is not clear what stimulates inflammation in the amygdala. Increased neurotensin could be one reason for the inflammation because (i) it stimulates embryonic microglia and (ii) is increased in the serum of most children with ASD (18, 19). Other stimuli of microglia activation could include mast cell-derived molecules (39), mycotoxins (40), or gut-derived neurotoxins (41) such as propionic acid (42).
IL-38 exists intracellularly as a precursor full-length form (aa1-152), which must be cleaved at the N terminus before it is secreted extracellualry as an active form (27), like other members of the IL-1 family (27). The processing sites have not been identified. Vassili Kalabokis (Bio-techne) synthesized the most plausible active analogs used in the present study: (i) IL-38 aa3-152, (ii) aa2-152, and (iii) aa5-152, and we show that the aa5-152 fragment shows the most inhibitory activity
Both the cultured human adult and embryonic microglia used in this study were immortalized and may not reflect the behavior of primary microglia that are difficult to derive and keep in culture. Nevertheless, we had previously reported that immortalized and human adult microgliagave comparable results (8). Unfortunately, no information on the severity of symptoms or the presence of any concurrent diseases was available for the children with ASD. A follow-up study using brain samples from a different brain bank where such information is available would be very useful.
Conclusion
Since the prevalence of ASD has been projected to increase from 1 in 54 children presently to 1 in 40 children in the United States by 2025 (43), it is even more urgent to develop effective therapies. The present study indicates the important role of IL-38 in the inhibition of activation of microglia, thus supporting its development as a treatment for autism spectrum disorder.
Materials
Human recombinant IL-37, Isoform b, N terminus: Val46 and IL-38 analogs (aa2-152, aa5-152, and aa3-152) were synthesized and donated by R&D Systems (Minneapolis, MN). Human recombinant IL-37, Isoform b (Val46-Asp218) was from from R&D Systems, catalog no. 7585-IL. DNA sequences encoding IL-38 analogs (aa2-152, aa5-152, and aa3-152) were expresed in Escherichia coli at R&D Systems. The recombinant IL-38 proteins were purified by standard chromatographic methods to greater than 95% purity and the N-termini were confirmed by N-terminal sequencing analysis. Endotoxin level of all IL-38 variants was less than 0.10 EU per 1 μg of the protein by the limulus amebocyte lysate method (R&D Systems).
NT and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich. Taqman gene expression primers were purchased from Applied Biosystems. Enzyme-linked immunosorbent assay (ELISA) kits for IL-38, IL-1β ,and CXCL8 were purchased from R&D Systems.
Methods
Human Serum Samples.
Blood (1 mL) was obtained on empty stomach or at least 2 h from a meal because NT is known to increase postprandially (44). Blood was obtained between 9 and 11 AM to avoid any diurnal variations, which has been reported to affect even allergic responses (45, 46). Blood was collected in serum separator vacutainer tubes (BD Biosciences). One blood sample was collected in ethylene diamine tetraacetic acid containing tubes in order to separate the plasma for later use. The second blood sample was allowed to clot at room temperature for about 15–30 min, and then was centrifuged at 1,000–2,000 × g for 10 min at 4 °C. The upper clear fraction (serum) was carefully removed and aliquoted (0.5 mL per tube) into clean plastic capped tubes. All ASD and non-ASD control blood samples were labeled with a code number, age, and sex and were stored at −80 °C. They were later shipped on dry ice to Tufts University for further analysis.
Institutional review board review approval and informed consent were not required for this study because the samples had been previously obtained for other purposes and were provided without any identifiers.
Serum IL-38 Measurement.
IL-38 (DY9110-05) levels in serum samples from children with ASD were quantified by using commercially available ELISA kits (R&D Systems) as per manufacturer’s instructions. For all experiments the minimum detectable level by ELISA was 5 pg/mL.
Human Brain Samples.
Postmortem human brain tissues of deceased Caucasian male children (3–14 y old) with ASD (n = 8) and non-ASD (n = 8) were obtained from the NIH NeuroBioBank at the University of Maryland, Baltimore, MD (https://neurobiobank.nih.gov; application approved October 30, 2015; Biospecimen availability confirmed). Samples were obtained from Amygdala. The only inclusion criteria used were males, 1–12 y of age, who had died in car accidents. Unfortunately, there is no available information of how diagnosis of ASD was reached, what the level of cognitive or functional level was before death, or the presence of any comorbidities. Controls were selected without any known brain disease or trauma and were matched to the subjects with ASD to the extent possible.
Frozen brain tissues were sectioned (30 µm thickness) using a Cryostat. Brain areas were available from the same subjects, which allowed direct comparisons of outcome measures between regions within the same subjects. Samples were provided from males only because ASD is four times more common in males than females and to avoid any additional gender and hormonal variabilities. The deceased children whose brain samples were analyzed were unrelated to those whose serum was obtained.
RNA Isolation from Brain Tissue.
Total RNA was extracted from frozen brain tissue specimens from ASD and non-ASD subjects using the mirVana miRNA Isolation Kit (Ambion, Life Technologies) after frozen tissue section homogenization. Reverse transcription (RT) was performed with 500–1,000 ng of total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Life Technologies). RNA purity (A260/280 ratios) was calculated, and RNA integrity numbers (RIN) for all samples were reported by the brain bank.
Real-Time qRT-PCR.
qRT-PCR was performed using Taqman gene expression assays (Applied Biosystems) to assess the gene expression of IL-38 (Hs00544661_m1) and IL-36R (Hs00543916_m1). All qPCR studies were conducted using inventoried Taqman gene expression probes from Invitrogen, which were validated by the vendor and publicly available. Samples were run for 45 cycles using the Applied Biosystems 7300 Real-Time PCR System. Normalization of gene expression to 18SrRNA (4310893E) and comparison of gene expression between groups was calculated according to the 2 -∆∆Ct method by Schmittgen (47).
For all messenger RNA studies, tissue samples were included if RIN is above 5.0. However, due to evidence showing that RIN values are not always the most accurate predictors of RNA quality in human postmortem brain samples (48), postmortem interval and pH measures were used as indicators of tissue quality, as these factors have been reported to correlate with protein levels (49). Furthermore, cause of death was used as an additional indicator of tissue integrity (50).
Human Microglia Cell Cultures.
The immortalized human microglia-SV40 cell line derived from primary human microglia was purchased from Applied Biological Materials Inc. (ABM Inc.) and was cultured in Prigrow III medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin in type I collagen-coated T25-flasks (ABM Inc.). Microglia-SV40 maintain their phenotype and proliferation rates for over 10 passages, during which all experiments were performed using multiple microglia thaws and subcultured cells. Experiments were carried out in type I collagen-coated plates (BD PureCoat ECM Mimetic Cultureware Collagen I peptide plates, Becton Dickinson) (51). Cell viability was determined by Trypan blue (0.4%) exclusion.
The HMC3 cell line was isolated from primary mixed cultures of human spinal cord and cortical cells derived from 8- to 12-wk-old embryos. The HMC3 cells were immortalized through transfection with SV40 large T antigen, were purchased from American Type Culture Collection (ATCC) and were cultured in EMEM (ATCC 30–2003) medium supplemented with 10% FBS (ATCC 30–2020) and 1% penicillin/streptomycin in Corning T-75 flasks. HMC3 microglia cells maintain their phenotype and proliferation rates for over 10 passages, during which all experiments were performed using multiple microglia thaws and subcultured cells. Experiments were carried out in clear polystyrene plates (Corning). Cell viability was determined by Trypan blue (0.4%) exclusion.
The cultured microglia were not used after 10 passages in order to avoid the possibility they may change their phenotype while in culture.
IL-1β and CXCL8 Secretion from Human Adult and Embryonic Microglia.
SV40 microglia (0.5 ×105 cells per well) were seeded in 12-well, type I collagen or poly-l-lysine-coated plates (Becton Dickinson) and HMC3 microglia were seeded in 12-well plates (Corning) for 24 h. Pretreatment with human rIL-38 (100 ng/mL) for 24 h and then stimulation with NT (10 nM) or LPS (10 ng/mL) (Sigma-Aldrich) for 24 h was carried out. Supernatant fluids were collected, and IL-1β (DY201) and CXCL8 (DY208) secretion from human microglia-conditioned culture medium was quantified by using commercially available ELISA kits (R&D Systems) as per manufacturer’s instructions. For all experiments, the control cells were treated with equal volume of culture medium and the minimum detectable level for all mediators by ELISA was 5 pg/mL.
Statistical Analysis.
All conditions were performed in triplicate, and all experiments were repeated at least three times (n = 3). Results from cultured cells are presented as mean ± SD. Comparisons were made between control and stimulated cells using the unpaired, two-tailed, Student’s t test with significance of comparisons denoted by the horizontal lines and by *P < 0.05, **P < 0.001, and ***P < 0.0001 (52). Analysis of human brain samples are presented as a scattergram with symbols representing individual data points and the horizontal lines representing the mean for each group. Normality of distribution was checked with the Shapiro–Wilk’s test. Depending on whether data were normally distributed, comparison between the non-ASD and the ASD groups was performed using either paired t test or Wilcoxon matched-pairs signed rank test. Significance of comparisons is denoted by P < 0.05 (*), P < 0.001 (**), and P < 0.0001 (***). The analysis was performed by using the GraphPad Prism version 7.0 software (GraphPad Software).
Data Availability.
All data have been disclosed in the sections above.
Acknowledgments
We thank the NIH NeuroBioBank (https://neurobiobank.nih.gov/) for making the human brain samples available. We thank Dr. Charles Dinarello (University of Colorado Anschutz Medical Campus) and Dr. Vassili Kalabokis for useful discussions, and Dr. Vassili Kalabokis (Bio-techne Corporation) for providing IL-37 and IL-38. Finally, we thank the brain tissue NIH NeuroBiobank at the University of Maryland for the brain samples. This work was supported in part by an anonymous donation and a small grant form Bio-techne Corporation (to T.C.T.).
Footnotes
The authors declare no competing interest.
References
- 1.Fombonne E., Epidemiology of pervasive developmental disorders. Pediatr. Res. 65, 591–598 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Lai M. C., Lombardo M. V., Baron-Cohen S., Autism. Lancet 383, 896–910 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Maeener M., et al. , Prevalence of autism spectrum disorder among children aged 8 years–Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. Morb. Mortal. Wkly. Rep. 69, 1–12 (2020). Erratum in: Morb. Mortal. Wkly. Rep. 69, 103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theoharides T. C., Asadi S., Patel A. B., Focal brain inflammation and autism. J. Neuroinflammation 10, 46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Belle J. E. et al., Maternal inflammation contributes to brain overgrowth and autism-associated behaviors through altered redox signaling in stem and progenitor cells. Stem Cell Reports 3, 725–734 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S. et al., Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity-dependent genes in autism. Nat. Commun. 5, 5748 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama R., Ikegaya Y., Microglia in the pathogenesis of autism spectrum disorders. Neurosci. Res. 100, 1–5 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Patel A. B., Tsilioni I., Leeman S. E., Theoharides T. C., Neurotensin stimulates sortilin and mTOR in human microglia inhibitable by methoxyluteolin, a potential therapeutic target for autism. Proc. Natl. Acad. Sci. U.S.A. 113, E7049–E7058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman A. W. et al., Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 33, 195–201 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Li X. et al., Elevated immune response in the brain of autistic patients. J. Neuroimmunol. 207, 111–116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsilioni I., Taliou A., Francis K., Theoharides T. C., Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl. Psychiatry 5, e647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsilioni I. et al., IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin. Proc. Natl. Acad. Sci. U.S.A. 116, 21659–21665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almehmadi K. A., Tsilioni I., Theoharides T. C., Increased expression of miR-155p5 in amygdala of children with Autism Spectrum Disorder. Autism Res. 13, 18–23 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Bauman M. L., Kemper T. L., The neuropathology of the autism spectrum disorders: What have we learned? Novartis Found. Symp. 251, 112–122, discussion 122–128, 281–297 (2003). [PubMed] [Google Scholar]
- 15.Bliss-Moreau E., Moadab G., Santistevan A., Amaral D. G., The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav. Brain Res. 322, 123–137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Impellizzeri D. et al., Adelmidrol, a palmitoylethanolamide analogue, as a new pharmacological treatment for the management of acute and chronic inflammation. Biochem. Pharmacol. 119, 27–41 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Carraway R., Leeman S. E., The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 248, 6854–6861 (1973). [PubMed] [Google Scholar]
- 18.Angelidou A. et al., Neurotensin is increased in serum of young children with autistic disorder. J. Neuroinflammation 7, 48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsilioni I. et al., Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing Bull Terriers with a phenotype similar to autism. Transl. Psychiatry 4, e466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello C. A., The IL-1 family and inflammatory diseases. Clin. Exp. Rheumatol. 20 (5), S1–S13 (2002). [PubMed] [Google Scholar]
- 21.Dinarello C. A., Simon A., van der Meer J. W., Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao M. et al., IL-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis. 9, 582 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalli G. et al., Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. U.S.A. 114, 2313–2318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinarello C. A., Bufler P., Interleukin-37. Semin. Immunol. 25, 466–468 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Xie L. et al., IL-38: A new player in inflammatory autoimmune disorders. Biomolecules 9, 345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercurio L. et al., IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment. Cell Death Dis. 9, 1104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwak A., Lee Y., Kim H., Kim S., Intracellular interleukin (IL)-1 family cytokine processing enzyme. Arch. Pharm. Res. 39, 1556–1564 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Henry C. M. et al., Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Rep. 14, 708–722 (2016). [DOI] [PubMed] [Google Scholar]
- 29.van de Veerdonk F. L., de Graaf D. M., Joosten L. A., Dinarello C. A., Biology of IL-38 and its role in disease. Immunol. Rev. 281, 191–196 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Martin S., Dicou E., Vincent J. P., Mazella J., Neurotensin and the neurotensin receptor-3 in microglial cells. J. Neurosci. Res. 81, 322–326 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Timmerman R., Burm S. M., Bajramovic J. J., An overview of in vitro methods to study microglia. Front. Cell. Neurosci. 12, 242 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dello Russo C. et al., The human microglial HMC3 cell line: Where do we stand? A systematic literature review. J. Neuroinflammation 15, 259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Careaga M., Murai T., Bauman M. D., Maternal immune activation and autism spectrum disorder: From rodents to nonhuman and human primates. Biol. Psychiatry 81, 391–401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W. D., Huang A. F., Role of interleukin-38 in chronic inflammatory diseases: A comprehensive review. Front. Immunol. 9, 1462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan J. T., Barger N., Amaral D. G., Schumann C. M., Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PLoS One 9, e110356 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avino T. A. et al., Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. U.S.A. 115, 3710–3715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumann C. M., Barnes C. C., Lord C., Courchesne E., Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol. Psychiatry 66, 942–949 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Theoharides T. C., Kavalioti M., Tsilioni I., Mast cells, stress, fear and autism spectrum disorder. Int. J. Mol. Sci. 20, 3611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theoharides T. C., Tsilioni I., Patel A. B., Doyle R., Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry 6, e844 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratnaseelan A. M., Tsilioni I., Theoharides T. C., Effects of mycotoxins on neuropsychiatric symptoms and immune processes. Clin. Ther. 40, 903–917 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Petra A. I. et al., Gut-microbiota-brain Axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin. Ther. 37, 984–995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacFabe D. F. et al., Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 176, 149–169 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Leigh J. P., Du J., Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 45, 4135–4139 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Gullo L., Pezzilli R., Tomassetti P., de Giorgio R., Plasma cholecystokinin and neurotensin after an ordinary meal in humans. A prolonged time study. Gastroenterol. Clin. Biol. 22, 25–28 (1998). [PubMed] [Google Scholar]
- 45.Nakamura Y. et al., Circadian regulation of allergic reactions by the mast cell clock in mice. J. Allergy Clin. Immunol. 133, 568–575 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Nakao A., Nakamura Y., Shibata S., The circadian clock functions as a potent regulator of allergic reaction. Allergy 70, 467–473 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Schmittgen T. D., Livak K. J., Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Sonntag K. C. et al., Limited predictability of postmortem human brain tissue quality by RNA integrity numbers. J. Neurochem. 138, 53–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fountoulakis M., Hardmeier R., Höger H., Lubec G., Postmortem changes in the level of brain proteins. Exp. Neurol. 167, 86–94 (2001). [DOI] [PubMed] [Google Scholar]
- 50.McCullumsmith R. E., Hammond J. H., Shan D., Meador-Woodruff J. H., Postmortem brain: An underutilized substrate for studying severe mental illness. Neuropsychopharmacology 39, 65–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsilioni I., Theoharides T. C., Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflammation 15, 239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel A. B., Theoharides T. C., Methoxyluteolin inhibits neuropeptide-stimulated proinflammatory mediator release via mTOR activation from human mast cells. J. Pharmacol. Exp. Ther. 361, 462–471 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data have been disclosed in the sections above.