Significance
Malaria kills ∼0.4 million people a year. Malaria parasite infection triggers complex immune responses that may control the infection or result in severe disease. The molecular mechanisms underlying host–parasite interaction and immune regulation are not completely understood. We perform a genome-wide genetic screen during early Plasmodium yoelii infection in mice and identify a large number of host and parasite genes/loci for future studies of the complex host–parasite interactions. We next investigate the functions of one of these host genes that encodes an E3 ubiquitin ligase (MARCH1). We show that MARCH1 regulates type I interferon signaling, T cell activation, and IFN-γ production during malaria infections. MARCH1 is a key molecule in immune responses and a potential target for immunotherapies.
Keywords: Plasmodium, host–parasite interaction, innate response, interferons
Abstract
Malaria infection induces complex and diverse immune responses. To elucidate the mechanisms underlying host–parasite interaction, we performed a genetic screen during early (24 h) Plasmodium yoelii infection in mice and identified a large number of interacting host and parasite genes/loci after transspecies expression quantitative trait locus (Ts-eQTL) analysis. We next investigated a host E3 ubiquitin ligase gene (March1) that was clustered with interferon (IFN)-stimulated genes (ISGs) based on the similarity of the genome-wide pattern of logarithm of the odds (LOD) scores (GPLS). March1 inhibits MAVS/STING/TRIF-induced type I IFN (IFN-I) signaling in vitro and in vivo. However, in malaria-infected hosts, deficiency of March1 reduces IFN-I production by activating inhibitors such as SOCS1, USP18, and TRIM24 and by altering immune cell populations. March1 deficiency increases CD86+DC (dendritic cell) populations and levels of IFN-γ and interleukin 10 (IL-10) at day 4 post infection, leading to improved host survival. T cell depletion reduces IFN-γ level and reverse the protective effects of March1 deficiency, which can also be achieved by antibody neutralization of IFN-γ. This study reveals functions of MARCH1 (membrane-associated ring-CH–type finger 1) in innate immune responses and provides potential avenues for activating antimalaria immunity and enhancing vaccine efficacy.
Interferons (IFNs) play important roles in host responses to malaria infections. IFN-γ is a central cytokine in controlling malaria infection; however, persistent high levels of IFN-γ can exacerbate the severity of malaria disease (1, 2). Type I IFN (IFN-I; IFN-α and IFN-β) also plays contrasting roles in malaria infections (3). An early spike of IFN-I is protective against blood stages in some Plasmodium yoelii models (4, 5) or against liver stages of Plasmodium berghei (6). Daily intraperitoneal injections of a recombinant human IFN-α prevent death by cerebral malaria (7). IFN-I can also substantially inhibit CD4+T-bet+T cell-derived IFN-γ production and suppress CD4+T cell-mediated parasite control during infections of lethal P. berghei ANKA or nonlethal Plasmodium chabaudi (8). In a follow-up study, IFN-I signaling was shown to directly affect conventional dendritic cell (cDC) function and limit the ability of cDCs to prime IFN-γ–producing type 1 T regulatory (Tr1) cells (9). AT-rich motifs from Plasmodium falciparum were reported to induce IFN-I signaling involving STING, TBK1, and IRF3/IRF7, and mice without IRF3, IRF7, TBK1, or TNFR1 were resistant to otherwise lethal cerebral malaria (10). More recently, IFN-I was shown to suppress innate immune cell function and IFN-γ production by parasite-specific CD4+T cells as well as to promote the development of interleukin 10 (IL-10)–producing Tr1 cells (11–13). IFNAR1 deficiency accelerated humoral immune responses and parasite control by boosting ICOS signaling during nonlethal blood-stage infections (13). Additionally, IFN-I and IFN-γ were involved in cDC death during malaria infection (14). Therefore, IFN-I can also down-regulate immune responses in some malaria infections. Appropriate levels and timing of IFN responses are critical for successful control of malaria infection.
Membrane-associated ring-CH–type finger 1 (MARCH1) is a member of the MARCH family of membrane-bound E3 ubiquitin ligases expressed mostly by DCs and B cells (15–17). MARCH1 has been shown to ubiquitinate CD86 and major histocompatibility complex class II (MHC-II) proteins for degradation to down-regulate adaptive immunity (15, 18–21), and a transmembrane (TM) region of CD83 can enhance MHC-II and CD86 expression by blocking MHC-II association with MARCH1 (22). MARCH1 may influence CD28, CTLA4, and PD-L1 signaling by regulating relative protein levels of CD80/CD86 on antigen-presenting cells (APCs). MARCH1 can be induced by IL-10 after TLR4 and CD40 signaling (23, 24). DCs express MARCH1 and produce IFN-I after stimulation; treatment of monocyte-derived macrophages (Macs) with IFN-I enhanced endogenous expression of MARCH1 and MARCH2 (25). However, whether MARCH1 can regulate IFN-I response remains unknown. Here we show that MARCH1 interacts with STING, MAVS, and various regulators of IFN-I signaling pathways to regulate IFN responses. Deficiency of MARCH1 significantly reduces serum IFN-I levels day 1 postinfection (pi) with P. yoelii N67 or YM parasites but increases CD86+DC cell populations and levels of IFN-γ and IL-10 day 4 pi, leading to improved host survival. This study discovers functions of MARCH1 in regulating IFN-I response and consequently antimalaria immunity.
Results
Screening Host–Parasite Genetic Interactions Identifies MARCH1 as an IFN-I Regulator.
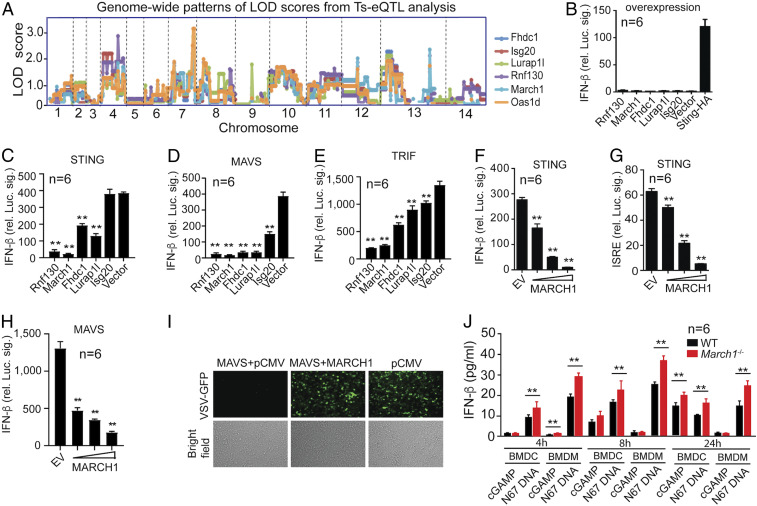
Previously, we developed a genetic approach, transspecies expression quantitative trait locus (Ts-eQTL) analysis, to cluster and predict gene functions using messenger RNA (mRNA) from spleens day 4 pi with P. yoelii 17XNL, N67, and their 24 progeny (26, 27). In the analyses, host genes that were linked to parasite genetic markers with LOD (logarithm of the odds) scores ≥2.0 or ≥3.0 were selected and clustered based on the similarity of their genome-wide pattern of LOD scores (GPLS). Genes that function in the same or related pathways were found to have similar GPLSs and were clustered together, which can be used to predict functions of unknown genes. Many negative regulators of IFN-I response were found, likely due to sampling mRNA relatively late (day 4 pi) after malaria infection. In order to search for potential receptors in IFN-I signaling, here we performed Ts-eQTL analysis using new mRNA samples collected from spleen tissues during early infection (24 h pi) (28) and again identified a large number of gene clusters based on GPLSs (Dataset S1A). Among the clusters were those containing Toll-like receptors (TLR2, 3, 4, 7, 8, 9, 11, 12, and 13) and DDX58 (RIG-I), suggesting activation of various innate immune responses. Among the significantly linked host genes are 13 G protein-coupled receptors, 40 olfactory receptors, and 37 solute carriers that may play important roles in interaction with parasite molecules. The identification of RIG-I and the olfactory receptors that were not found in our previous Ts-eQTL analysis using mRNA from day 4 infection suggests that the 24-h dataset has good potential for identifying receptors of immune signaling after malaria infection. To more systematically investigate the differential responses at 24 h and day 4 pi, we performed Gene Ontology (GO) term enrichment analysis of the genes in the GPLS clusters with LOD scores ≥2.0 (Dataset S1A) and the similar gene list based on day 4 mRNA samples (data s5 in ref. 27). GO terms such as G protein-coupled receptor activity, olfactory receptor activity, and signal transducer activity were significantly (false discovery rate, q value < 0.05; Fisher’s exact test) enriched in the 24-h gene list, whereas diverse activities such as glucose-6-phosphate dehydrogenase activity, response to oxidative stress, and cytokine activity were significantly (q value < 0.05) enriched in the day 4 genes (Dataset S1B). This analysis also significantly (LOD score ≥ 3.0) links 1,587 host genes to 108 parasite microsatellite markers (Dataset S2). These results suggest that many receptor activities are captured in the 24-h dataset, providing important leads for identifying receptors or sensors in recognizing parasite molecules. However, the large numbers of host genes and parasite genetic loci preclude functional analyses of all of the linked genes at this time. Here we focus on the March1 gene in the k11.3 cluster because it was clustered with two IFN-stimulated genes (Isg20 and Oas1d) having very similar GPLSs (Fig. 1A), suggesting a potential function in regulating IFN response. Additionally, MARCH1 is an E3 ubiquitin ligase that is known to regulate adaptive immune response (15, 18–21). Therefore, MARCH1 is likely to act as a critical regulator bridging host innate IFN-I, T cell, and antibody responses.
Fig. 1.
Identification of MARCH1 as a regulator of IFN-I signaling. (A) A cluster of six genes, including the known IFN-stimulated genes Isg20 and Oas1d, with a similar genome-wide pattern of LOD scores. (B) Ectopic expression of individual genes in 293T cells does not induce IFN-β expression as measured through luciferase activity driven by the IFN-β promoter. (C–E) March1, Rnf130, Fhdc1, Lurap1l, and Isg20 (except Isg20 for STING) significantly inhibit STING-, MAVS-, or TRIF-stimulated IFN-β production. (F and G) Dose–response of luciferase signals driven by the IFN-β (F) or ISRE (G) promoter after cotransfection with STING and MARCH1 (50, 100, and 200 ng plasmid). (H) Dose–response of luciferase signals driven by the IFN-β promoter after cotransfection with MAVS and MARCH1 (50, 100, and 200 ng plasmid). (B–H) Significance compared with those of vector controls. EV, empty vector. (I) Reversal of MAVS-mediated inhibition of VSV replication by MARCH1. (J) Higher IFN-β protein in culture supernatants in bone marrow-derived DCs or macrophages from March1−/− than those of WT mice after stimulation with cGAMP or parasite DNA. IFN-β was measured using an enzyme-linked immunosorbent assay (ELISA). Mann–Whitney U test, error bars represent mean and SD comparing individual pairs of experiments (n = 6): **P < 0.01. Each experiment was independently repeated.
MARCH1 Inhibits IFN-I Signaling Pathways In Vitro and In Vivo.
We used cell line experiments to initially confirm the observations of Ts-eQTL analysis based on mRNA levels in response to malaria infection. Expression of the five genes (Rnf130, March1, Fhdc1, Lurap1l, and Isg20) in the k11.3 cluster in 293T cells did not stimulate luciferase gene expression driven by the IFN-β promoter (Fig. 1B). However, four of the five genes (excluding Isg20) significantly inhibited STING-driven signaling, and all five genes suppressed MAVS- and TRIF-induced IFN-I responses in vitro (Fig. 1 C–E). March1 and Rnf130, both encoding E3 ubiquitin ligases, had the most significant inhibitory effects on STING/MAVS/TRIF-induced IFN-I responses. Luciferase signals driven by IFN-β or the ISRE promoter after transfecting increasing amounts of March1 plasmid showed a dose-dependent inhibition of STING-induced signaling by MARCH1 (Fig. 1 F and G). Similarly, ectopic MARCH1 expression reduced MAVS-stimulated IFN-β signaling in a dose-dependent manner (Fig. 1H) and reversed inhibition of vesicular stomatitis virus (VSV) replication by MAVS-mediated IFN-I response in 293T cells (Fig. 1I). Bone marrow-derived Macs (BMDMs) and dendritic cells (BMDCs) from March1 knockout (KO, March1−/−) mice produced significantly higher levels of IFN-β than the cells from wild-type (WT) mice 4, 8, and 24 h after parasite genomic DNA (pDNA) stimulation (Fig. 1J). For cGAMP stimulation, IFN-β level was significantly increased in the March1−/− BMDMs at 4 h and in the March1−/− BMDC culture at 24 h. Significantly higher mRNA levels of Ifn-β, Isg15, and Mx1 genes were observed in the March1−/− cells compared with WT cells after stimulation of BMDM cells with ISD, cGAMP, or pDNA for 6 h (SI Appendix, Fig. S1 A–C). These results show that March1 is an inhibitor of DNA/RNA-induced IFN-I signaling pathways in primary cells.
To investigate the roles of MARCH1 in regulating IFN-I responses in vivo, we injected poly(I:C) and cGAMP (200 μg each) into WT and March1−/− mice to stimulate TLR3- and STING-mediated IFN-I signaling pathways, respectively, and measured serum IFN-α and IFN-β 6 h after injection. Significantly higher levels of IFN-α and IFN-β were observed in the March1−/− mice than WT mice in both cases (Fig. 2 A and B). The results are consistent with those of in vitro observations, suggesting that MARCH1 is an inhibitor of DNA/RNA-induced IFN-I signaling pathways.
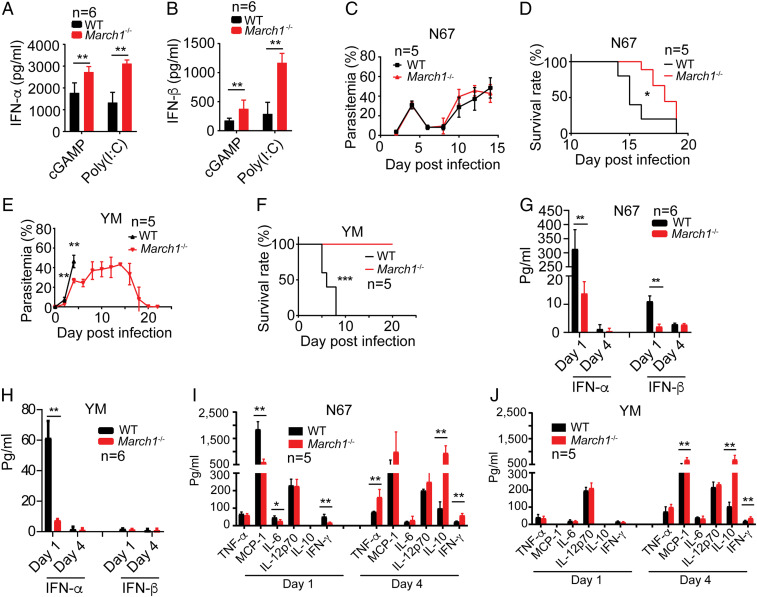
Fig. 2.
MARCH1 deficiency reduces early IFN responses and improves host survival after malaria infections. (A and B) Serum levels of IFN-α (A) and IFN-β (B), measured using ELISA, 6 h after stimulation of WT and March1−/− mice with poly(I:C) or cGAMP (200 μg, i.v.). (C–F) Parasitemia (C and E) and host survival (D and F) after infection with N67 (C and D) or YM (E and F) parasites. Parasitemia was counted by microscopic examination of Giemsa-stained thin blood smears. (G and H) Serum levels of IFN-α and IFN-β in WT or March1−/− mice day 1 and day 4 pi with N67 or YM parasite. (I and J) Serum inflammatory cytokine and chemokine levels in WT or March1−/− mice day 1 and day 4 pi with N67 (I) or YM (J). All cytokines or chemokines were measured using ELISA. Mann–Whitney U test, error bars represent mean and SD comparing individual pairs (n = 5 or 6 replicates): **P < 0.01. For D and F, log-rank test: *P < 0.05, ***P < 0.001. Each experiment was independently repeated.
Malaria Infection Reverses the Effects of MARCH1 Deficiency on IFN-I Signaling.
To investigate the role of MARCH1 during malaria infection, we infected WT and March1−/− mice with N67 and YM parasites that stimulate different levels of IFN-I response. N67, but not YM, stimulates a strong early IFN-I response in WT C57BL/6 mice (4, 5). March1−/− mice had significantly better survival rates than WT mice; however, only YM-infected March1−/− mice had significantly lower parasitemia at day 3 to 4 pi (Fig. 2 C–F). Previously, we showed that a peak of IFN-I level 24 h pi was associated with parasitemia control in N67 infections (4). To our surprise, March1−/− mice infected with YM and N67 had significantly lower day 1 serum IFN-α levels than those of WT mice (Fig. 2 G and H). Although March1−/− mice infected with N67 had significantly lower day 1 serum IFN-β than those of WT mice, both WT and March1−/− mice infected with YM produced very little IFN-β. Low levels of IFN-α and IFN-β were detected in mice infected with N67 or YM day 4 pi. These results show that malaria infection inhibits IFN-I response in the absence of March1. The results also suggest that in March1−/− mice, mechanisms other than early IFN-I production such as activation of DC and T cells may play important roles in controlling host survival, particularly in YM infection.
We next measured serum levels of TNF-α, MCP-1, IL-6, IL-12p70, IL-10, and IFN-γ day 1 and day 4 pi for potential factors contributing to the improved host survival rates. The N67-infected March1−/− mice had significantly lower levels of MCP-1, IL-6, and IFN-γ day 1 pi but significantly higher TNF-α, IL-10, and IFN-γ levels day 4 pi than the infected WT mice (Fig. 2I). In mice infected with YM parasite, no or low levels of TNF-α, MCP-1, IL-6, IL-10, and IFN-γ day 1 pi but significantly higher levels of MCP-1, IL-10, and IFN-γ were observed in the infected March1−/− mice on day 4 pi (Fig. 2J). These results show that, in addition to affecting early IFN-I production, March1 deficiency also increases production of IFN-γ, IL-10, and other cytokines/chemokines at later phases of malaria parasite infection. March1 deficiency also leads to expansion of white pulp in uninfected and malaria-infected spleens (SI Appendix, Fig. S1 D and E). Interestingly, the spleens from WT mice, but not in the March1−/− mice, had large hemozoin pigment deposits in the red pulp day 4 pi (arrows, SI Appendix, Fig. S1E).
MARCH1 Regulates Expression or Phosphorylation of Multiple Proteins in IFN-I Signaling Pathways.
We next investigated whether MARCH1 can affect expression of key proteins in IFN-I signaling pathways after parasite infection in vivo. Proteins from spleen homogenates of uninfected and infected WT or March1−/− mice were detected upon Western blotting using protein-specific antibodies. Key proteins known to function in IFN-I signaling pathways such as MAVS, STING, and TRAF3 were expressed at higher levels in uninfected March1−/− than in uninfected WT mice (Fig. 3A). The protein levels for MAVS, STING, and TRAF3 in the March1−/− mice had a pattern of an increased level in uninfected, decreased on day 1 pi, and increased again on day 4 pi with both parasites (Fig. 3 A and B). We next divided the signals of key proteins in IFN-I response from day 1 and day 4 infected mice by those of day 0 (uninfected) to adjust for the differences in basal protein levels of the uninfected March1−/− and WT mice. We then divided the resulting values from March1−/− mice by those of WT mice and showed a greater degree of decreases in the levels of MAVS, STING, and TRAF3 in March1−/− than WT mice day 1 pi with N67 or YM (Fig. 3 C and D), which could partially explain the reduced IFN-I production in March1−/− mice day 1 pi. March1 deficiency had limited effects on protein levels of TBK1 and IRF3 (Fig. 3 A–D). TBK1 phosphorylation was increased in N67-infected WT mice day 1 pi but was not detectable or was expressed at low levels in uninfected or other infected mice. The results show that MARCH1 deficiency increases STING and MAVS expression in uninfected mice; however, malaria infection can inhibit the expression of both proteins day 1 pi. The elevated pTBK1 level in day 1 N67-infected WT mice is consistent with the increased serum IFN-I in these mice.
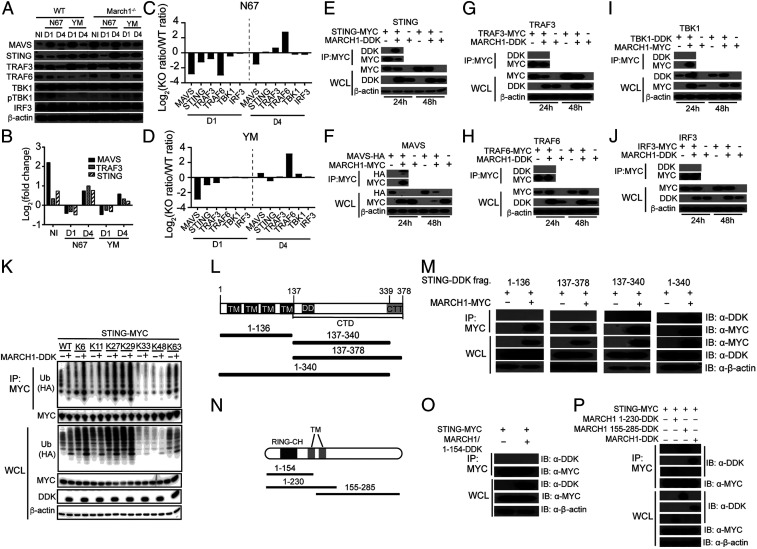
Fig. 3.
MARCH1 interacts with and regulates key molecules in IFN-I signaling pathways. (A) Western blotting analysis of proteins from spleens of uninfected (NI) or day 1 (D1) or day 4 (D4) infected WT and March1−/− mice. Antibodies against host proteins are as indicated (Left). pTBK1 was detected using an anti-phosphorylation antibody. β-Actin was used as protein loading control. (B) Log2(fold change) of protein expression (March1−/− over WT) scanned from Western blot signals in A after adjusting for protein loading. (C and D) Plots of ratios of scanned protein signals from March1−/− mice over those of WT mice in A after adjusting for protein levels from uninfected mice and protein loading. Signals of key proteins in IFN-I response from day 1 and day 4 infected mice were first divided by those of day 0 (uninfected) to adjust for the differences in basal protein levels of the uninfected March1−/− and WT mice. (E–J) Western blots of immunoprecipitated proteins (Top two panels) and total protein expression (Bottom three panels) from 293T cells transfected with plasmids containing molecules as indicated. Anti-MYC, -HA, or -DDK antibodies were used to pull down and detect proteins as indicated. WCL, whole-cell lysate. (K) STING ubiquitination in the presence or absence of MARCH1. 293T cells were transfected with STING-MYC and ubiquitin-HA molecules having a specific available lysine (K) site plus or minus MARCH1-DDK. STING-MYC was pulled down (immunoprecipitated) using anti-MYC antibody. Ubiquitination levels of STING were detected using an anti-HA antibody for HA-tagged ubiquitin. (L) Diagrams of STING deletion constructs, indicating the numbers of amino acids in each construct. (M) Co-IP to detect binding of STING with MARCH1 fragments as shown in L. (N) Diagrams of MARCH1 deletion constructs, indicating the numbers of amino acids in each construct. (O and P) Co-IP to detect binding of STING with MARCH1 fragments as shown in M. IB, immunoblotting.
To determine whether MARCH1 interacts with key molecules in IFN-I signaling pathways, we transfected 293T cells with plasmids expressing DDK (DYKDDDDK or Flag), MYC, or hemagglutinin (HA)-tagged molecules and performed coimmunoprecipitation (co-IP) experiments. MARCH1 interacted with STING and MAVS (pulled down by anti-MYC antibodies) but not with TRAF3, TRAF6, TBK1, or IRF3 (Fig. 3 E–J). Consistent with the observations of increased protein level in uninfected March1−/− mice, overexpression of MARCH1 reduced STING, MAVS, and TRAF3 protein levels (Fig. 3 E–G) but increased TRAF6 and TBK1 protein expression (Fig. 3 H and I). Conversely, TRAF6 and TBK1 also increased MARCH1 protein level in vitro. No change in the protein level of IRF3 was observed after expression of MARCH1 (Fig. 3J). These results show that MARCH1 can regulate expression of several molecules in IFN-I response through direct (STING and MAVS) or indirect interactions, and malaria parasite infection can reduce the expression of these proteins day 1 pi.
To investigate the potential mechanism of reduced STING protein level in the presence of MARCH1, we evaluated STING ubiquitination with or without the expression of MARCH1 after transfection of 293T cells with plasmids containing Sting and/or March1. The presence of MARCH1 increased STING ubiquitination through K6, K11, K27, and K29 linkages (Fig. 3K). Although little is known about the function of K6 ubiquitination, K11 and K29 ubiquitination have been implicated in proteasomal degradation of target proteins (29, 30), which may partially explain the increased STING protein in the uninfected March1−/− mice (Fig. 3A) and the reduced STING in cells transfected with a plasmid carrying the March1 gene (Fig. 3E). Additionally, K27 ubiquitination of STING has been reported to recruit and activate TBK1 (31). The results suggest that MARCH1 may mediate STING ubiquitination to regulate its protein level and signaling.
Transmembrane Domains of MARCH1 Interact and Colocalize with STING.
We next investigated the domains of STING and MARCH1 that mediated protein interaction. The STING molecule has four TM domains: an N-terminal domain, a C-terminal domain (CTD; amino acids 138 to 379) that contains the dimerization domain (DD), and the carboxyl-terminal tail (CTT; amino acids 340 to 379) (32). We made four expression constructs containing the N-terminal TM domains (amino acids 1 to 136), the CTD domain with (amino acids 137 to 378) or without (amino acids 137 to 340) the CTT domain, and a protein missing the CTT domain (amino acids 1 to 340) only (Fig. 3L). We performed co-IP pull-down experiments using the individual truncated constructs and showed that MARCH1 could be pulled down by the construct with almost the full length of STING (amino acids 1 to 340 missing the CTT) only (Fig. 3M). MARCH1 has an N-terminal RING-CH domain and two TMs (Fig. 3N). We also investigated which MARCH1 domain contributed to the binding to STING. Similarly, we made three MARCH1 deletion constructs, amino acids 1 to 154-DDK, amino acids 1 to 230-DDK, and amino acids 155 to 285-DDK (Fig. 3N). Co-IP pull-down experiments showed interactions of amino acids 1 to 230 and amino acids 155 to 285 MARCH1 peptides with STING but not the N-terminal segment of amino acids 1 to 154, showing the importance of amino acids 154 to 230 in the interaction with STING (Fig. 3 O and P). The results suggest a critical role of the two transmembrane domains (amino acids 155 to 175 and 197 to 217) in its interaction with STING. The TM domains of MARCH1 were reported to mediate interaction with CD86 (33).
Malaria Parasite Infection Affects IFN Signaling in the Absence of March1.
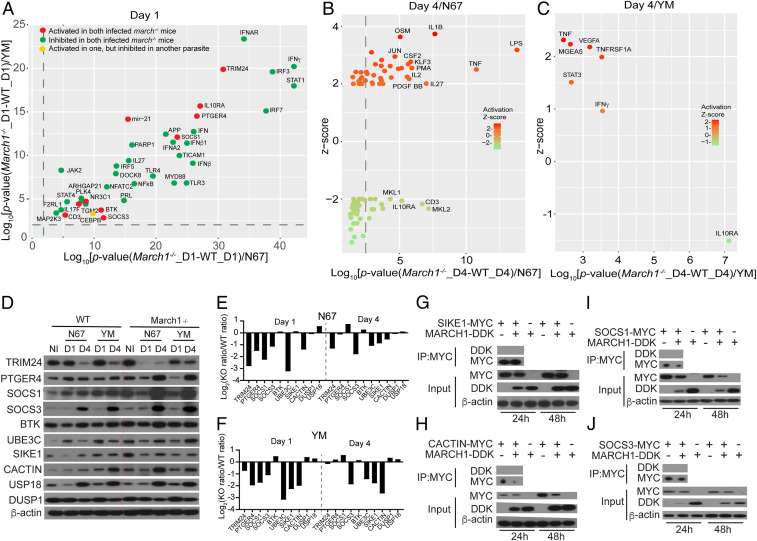
To further dissect the mechanism of the reduced IFN-I level in infected March1−/− mice, we isolated RNA from mouse spleens day 1 and day 4 pi (three mice for each parasite) of infected WT and March1−/− mice and hybridized to the Illumina Mouse-Ref8 v2 microarray. After quality control evaluation, quantile normalization, and ANOVA analysis of gene expression levels between different treatment groups, we used a twofold cutoff to generate gene lists that were further analyzed using Ingenuity Pathway Analysis (IPA; QIAGEN; https://www.qiagenbioinformatics.com/products/ingenuity-pathway-analysis). The analysis of upstream regulators examines how many known targets of each transcription regulator are present in the dataset, and compares their direction of change with what is expected from the literature in order to predict potentially relevant transcriptional regulators (34). Significantly (P < 0.05) activated (z score ≥ 2.0) or inhibited (z score ≤ −2.0) upstream regulators after malaria parasite infection were identified (Dataset S3). In March1−/− mice infected with N67 or YM parasite day 1 pi, the top inhibited molecules were key molecules in the IFN-I and IFN-γ signaling pathways such as TLR3, TLR4, MyD88, IRF3, IRF7, TRIF (TICAM1), IFNAR, and STAT1 as well as IFN-β and IFN-γ (green dots, Fig. 4A). These results are consistent with the observations of reduced serum levels of IFN-α/β and IFN-γ in the N67-infected March1−/− mice (only IFN-α in YM-infected mice) day 1 pi (Fig. 2 G and H). On the other hand, several upstream regulators (red dots, Fig. 4A) including IL-10RA, PTGER4(EP4), TRIM24, SOCS1, SOCS3, and microRNA 21 (mir21) were activated on day 1 in March1−/− mice infected with N67 and YM, many of which are known to negatively regulate IFN responses (for example, SOCS1/3, TRIM24, USP18, and mir21) (5, 35–37). On day 4 pi, fewer upstream regulators were significantly changed in the infected March1−/− mice, particularly in mice infected with YM parasites (Fig. 4 B and C and Dataset S3). TNF-α was the only molecule significantly activated by both YM and N67 in day 4 infected March1−/− mice. These activated upstream regulators may down-regulate IFN-I signaling pathways, leading to the reduced serum IFN-I levels observed in infected March1−/− mice.
Fig. 4.
Activated and inhibited upstream regulators in March1−/− mice after infection with N67 or YM parasites. (A–C) mRNAs from spleens of uninfected and day 1 and day 4 infected WT and March1−/− mice were hybridized to Illumina mouse microarray and data were analyzed using IPA. (A) Correlation plots of log10 (P values) of common genes significantly activated (red dots; P < 0.05 and z scores ≥ 2.0) or inhibited (green dots; P < 0.05 and z scores ≤ −2.0) in infected March1−/− mice (compared with those of WT mice) day 1 pi with N67 or YM parasites. (B) Plot of upstream regulators significantly activated (z scores ≥ 2.0) or inhibited (z scores ≤ 2.0) day 4 pi with N67 parasite. (C) The same plot as in B for YM-infected mice. (D) Western blots of proteins from spleens of uninfected or day 1 or day 4 infected WT or March1−/− mice. Total-tissue lysates were separated on sodium dodecyl sulfate/polyacrylamide gels and detected using specific antibodies after blotting onto nitrocellulose membrane. Antibodies against host proteins are as indicated (Left). β-Actin was used as protein loading control. (E and F) Plots of ratios of scanned protein signals from March1−/− mice over those of WT mice in D after adjusting for protein levels from uninfected mice as in Fig. 3C. (G–J) Western blots of immunoprecipitated proteins (Top two panels) and total protein expression (Bottom three panels) from 293T cells transfected with plasmids containing genes encoding SIKE1 (G), CACTIN (H), SOCS1 (I), or SOCS3 (J), with or without March1. Antibodies used to pull down and to detect tagged protein expression are as labeled.
We next performed Western blotting to investigate protein levels of selected upstream regulators activated day 1 pi, including TRIM24, PTGER4, SOCS1, SOCS3, BTK, UBE3C, SIKE1, CACTIN, USP18, and DUSP1 in infected March1−/− and WT mice day 1 and day 4 pi. Again, we plotted the ratios of the signals from March1−/− mice over those of WT mice as in Fig. 3C and showed a slightly larger increase of USP18 in March1−/− mice than WT mice day 1 pi with both N67 and YM (Fig. 4 E and F). Other proteins with positive ratios on day 1 or day 4 pi included BTK, SIKE1, SOCS1, DUSP1, and PTGER4. These proteins could also contribute to the reduced IFN-I production in the infected March1−/− mice. In vitro coexpression of MARCH1 and SIKE1, CACTIN, SOCS1, or SOCS3 in 293T cells showed slight reduction of SIKE1, CACTIN, SOCS1, and SOCS3 proteins, although CACTIN reduction occurred only at 48 h (Fig. 4 G–J). The results are consistent with IPA showing activation of these upstream regulators in infected March1−/− mice. Interestingly, expression of SIKE1, SOCS1, and SOCS3 also reduced MARCH1 protein levels. No direct interaction of MARCH1 with CACTIN, SIKE1, SOCS1, or SOCS3 was detected by co-IP experiments, suggesting that MARCH1 may affect expression of these proteins indirectly. Together, these data show that MARCH1 can modulate IFN-I response by affecting multiple upstream regulators of IFN-I response.
MARCH1 Deficiency Affects Multiple Immune Cell Populations.
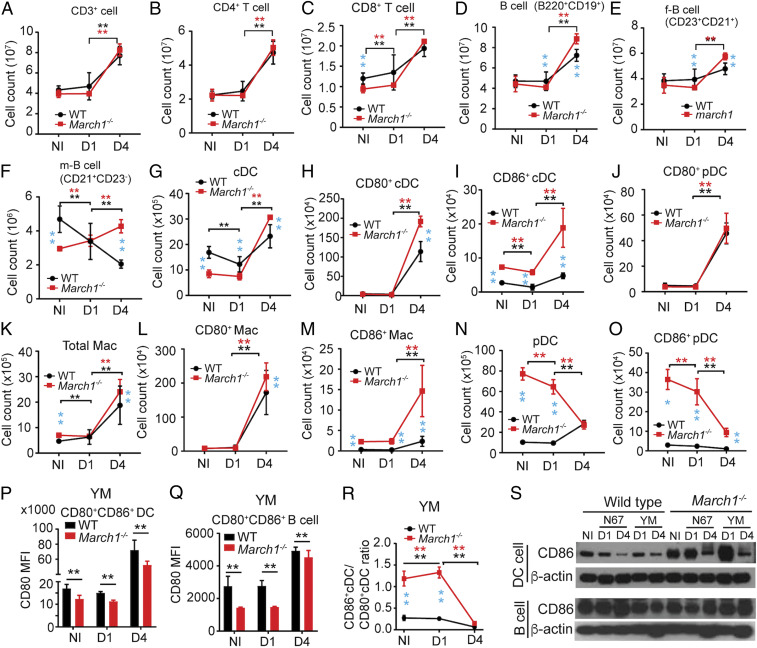
We also performed experiments to address two important issues associated with altered protein expression and improved survival of March1−/− mice. First, we investigated whether altered splenic cell populations also contribute to STING and MAVS protein levels after March1 KO and/or malaria parasite infection. Second, we dissected the mechanism protecting infected March1−/− mice in the absence of early IFN-I production. We counted the numbers and percentages of splenic immune cell populations in uninfected and day 1 and day 4 YM-infected WT and March1−/− mice to address the first question (Fig. 5 A–O and SI Appendix, Fig. S2). The numbers of T cells (including CD4+T and CD8+T cells), total B cells (including follicular and marginal zone B cells), DCs (including cDCs, CD80+DCs, CD86+cDCs, and CD80+pDCs [plasmacytoid DCs]), and Macs (including CD80+Macs and CD86+Macs) were significantly increased in infected WT (except marginal zone B cells) and March1−/− mice day 4 pi (Fig. 5 A–M). However, the numbers of pDCs and CD86+pDCs in the uninfected and day 1 infected March1−/− mice were significantly higher than those of WT mice but declined quickly on day 4 pi (Fig. 5 N and O). Similarly, the percentages of total T cells, CD4+T cells, total B cells, cDCs, CD80+cDCs, CD80+pDCs, total Macs, CD80+Macs, and CD86+Macs were significantly increased in the March1−/− and WT mice (except total B cells and cDCs in WT mice) (SI Appendix, Fig. S2 A–I). Interestingly, the percentages of CD8+T cells, follicular B cells, marginal zone B cells, CD86+DCs, pDCs, and CD86+pDCs declined day 4 in the March1−/− and WT mice (except CD86+cDCs and pDCs in WT mice) (SI Appendix, Fig. S2 J–O). Reduced CD80 expression levels were observed in the CD86+CD80+DCs and CD86+CD80+B cells of March1−/− mice (Fig. 5 P and Q). The ratios of CD86+cDCs/CD80+cDCs were significantly higher in noninfected and day 1 infected March1−/− mice than WT mice (Fig. 5R). Additionally, the percentages of D80+CD86+cDCs were significantly increased in day 1 and day 4 infected March1−/− than WT mice (SI Appendix, Fig. S2P). Intriguingly, the numbers of marginal zone B cells declined in WT mice but increased in the March1−/− mice after infection (Fig. 5F). Therefore, March1 KO increases pDC, CD86+cDC, and CD86+pDC populations in uninfected and day 1 infected mice, whereas malaria parasite infection increases T cell, B cell, cDC, and Mac populations (particularly CD80+ cells) in WT and March1−/− mice day 4 pi. Malaria infection also reduces pDC and CD86+pDC populations in March1−/− mice.
Fig. 5.
Splenic immune cell counts from noninfected or infected WT or March1−/− mice after YM infection. (A–O) Different splenic T cells, B cells, DCs, and Macs were counted from noninfected and day 1 and day 4 infected WT or March1−/− mice. Cells were isolated from mouse spleens, labeled with cell type-specific antibodies, and counted using flow cytometry. Cell markers are as labeled, and antibodies are listed in Dataset S4. f-B cell, follicular B cell; m-B cell, marginal zone B cell. (P) Mean fluorescence intensity (MFI) of CD80 expression in splenic CD80+CD86+DC cells from NI and D1 and D4 YM-infected mice. (Q) MFI of CD80 expression in splenic CD80+CD86+B cells from NI and D1 and D4 YM-infected mice. (R) Ratios of splenic CD86+DCs over CD80+DCs from NI and D1 and D4 YM-infected mice. Mann–Whitney U test, error bars represent mean and SD comparing individual pairs (n = 6 mice for all experiments): **P < 0.01. Black asterisk, WT; red asterisk, March1−/−; light blue, comparing WT and March1−/− mice. (S) CD86 protein expression in purified B and DC cells isolated from uninfected or infected WT or March1−/− mice, detected on Western blots using protein-specific antibodies as indicated. β-Actin was used as protein loading control. Each experiment was independently repeated.
The changes in protein levels of MAVS, STING, and TRAF3 in the spleens of uninfected March1−/− mice can be potentially explained by changes in cell numbers of CD80+ and/or CD86+DCs. To better quantitate the relationship of protein level and cell count, we focused on changes in protein expression and DC and B cell counts in WT and March1−/− mice with and without YM infection. We performed Western blotting using lysates from DC and B cells purified from uninfected and YM-infected mice day 1 and day 4 pi. The results showed a pattern of increased protein levels of MAVS, STING, and TRAF3 in uninfected and day 4 infected splenic DCs of March1−/− mice, but decreased levels day 1 pi, compared with those of WT mice (SI Appendix, Fig. S3 A and B). The up–down–up pattern of protein levels was not observed for B cells (SI Appendix, Fig. S3 C and D). We next compared the ratios of the three proteins in DCs from uninfected and day 1 and day 4 YM-infected mice with the ratios of DC and Mac cell counts of March1−/− over WT mice (the same calculation as in Fig. 3C) to estimate the contributions of cell population changes to the protein levels. Similar patterns of changes in signals of STING, MAVS, and TRAF3 to those of total spleen extract (Fig. 3C) were observed in DC extract, but not B cells, on day 1 pi (SI Appendix, Fig. S3 E and F). These results suggest that the signals for these proteins in the whole-spleen lysates are mostly derived from DCs. Small negative values for pDC and Mac populations were obtained from day 1 March1−/− mice, suggesting that the cell populations may have limited contribution to the changes in protein level on day 1 pi. On day 4 pi, the dramatic decrease in pDC population likely contributed to the larger degree of reduction in protein levels in the March1−/− than WT infected mice (SI Appendix, Fig. S3 E and G). Therefore, MARCH1 may influence the expression of key proteins such as STING in IFN-I responses through direct ubiquitination for degradation and by altering the numbers of cell populations expressing the target proteins during malaria infections.
MARCH1 is known to be expressed in DC and B cells to regulate CD86 and/or MHC-II levels (18, 19, 23, 38). We next isolated T cells, B cells, DCs, neutrophils, and monocytes from uninfected and day 1 and day 4 YM-infected mice and performed qPCR to determine March1 mRNA levels in these cell populations. Our data showed similar increased expression of March1 transcripts in T cells, B cells, DCs, and neutrophils (except monocytes) at day 4 pi, with B cells having the highest level of March1 transcripts (SI Appendix, Fig. S3H). We also investigated how March1 KO and malaria parasite infection affected CD86 protein expression in these cells. March1 KO greatly increased CD86 expression in DCs, but not in B cells, from uninfected and day 1 infected mice (Fig. 5S). However, CD86 expression was decreased in DCs in day 4 infected WT and March1−/− mice compared with those of day 1, which was consistent with elevated March1 transcripts in WT mice day 4 pi. The decline in pDCs, particularly CD86+pDCs, could contribute to the reduced CD86 level in March1−/− mice day 4 pi. The significantly higher level of CD86+DC and Mac populations in the infected March1−/− mice may activate some specific T cells to produce IL-10, IFN-γ, and other cytokines/chemokines.
IFN-γ and T Cells Play a Role in the Protection of Infected March1−/− Mice.
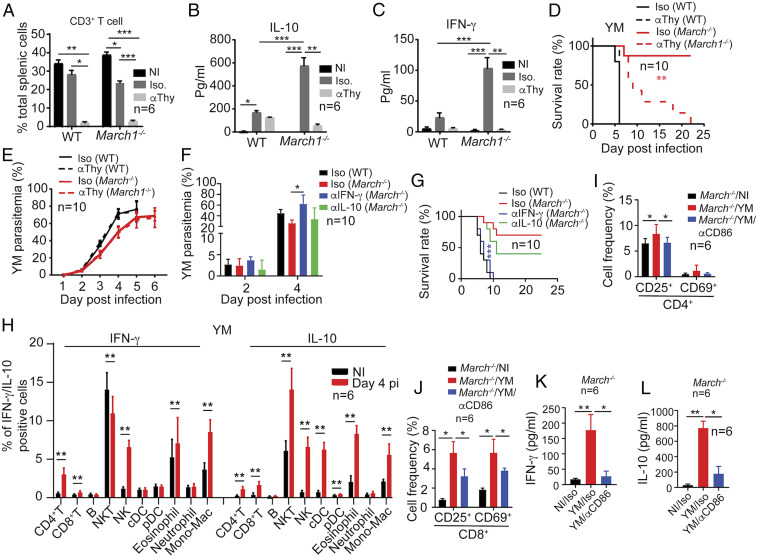
The observations of higher day 4 IFN-γ and IL-10 levels and longer host survival of infected March1−/− mice suggest that these two cytokines may play a key protective role. We therefore evaluated the effects of T cells and serum levels of IFN-γ and IL-10 on parasitemia and host survival. We injected anti-Thy1.2 antibody (Bio X Cell; BE0066; 250 μg per mouse, intravenously; i.v.) at day 1 before infection to deplete T cells and counted T cells in the spleen day 4 pi. Anti-Thy1.2 antibody treatment greatly reduced T cell counts in both WT and March1−/− mice (Fig. 6A and SI Appendix, Fig. S4). T cell depletion resulted in significant reduction in levels of both IL-10 and IFN-γ (Fig. 6 B and C) and greatly decreased the survival rates of YM-infected March1−/− mice (Fig. 6D) but did not significantly affect parasite growth in WT and March1−/− mice (Fig. 6E). These results suggest that T cells and the cytokines such as IFN-γ and/or IL-10 produced by T cells are needed to protect March1−/− mice after malaria infection. To further confirm the roles of IL-10 and IFN-γ in protection, we injected (i.v., 500 μg, four injections at day 0 to 3) anti–IL-10 (Bio X Cell; BE0049) and anti–IFN-γ (Bio X Cell; BE0055) to block these molecules in March1−/− mice infected with YM parasites. Treatment of March1−/− mice with anti–IFN-γ antibody significantly increased parasitemia and reversed the improved host survival due to the absence of March1 (Fig. 6 F and G). Mice treated with anti–IL-10 had slightly increased parasitemia and reduced survival rate compared with March1−/− mice receiving isotype control antibodies. Anti–IFN-γ treatment showed more significant impact on parasitemia and host survival than anti-Thy1.2 antibody treatment, which could be due to the presence of residual IFN-γ in mice treated with anti-Thy1.2 antibodies because IFN-γ can be produced by other cell types. We therefore stained intracellular IFN-γ and IL-10 in immune cells including T and B cells, natural killer (NK) cells, NK T cells, DCs, eosinophils, neutrophils, and monocytes/Macs and showed increased expression of IFN-γ and IL-10 in many cell types day 4 pi (Fig. 6H). STAT1 and pSTAT1 were also expressed at slightly higher levels in the spleen extracts of mice treated with anti-Thy1.2 antibodies than those treated with anti–IFN-γ antibodies (SI Appendix, Fig. S3 I and J). These results indicate the presence of a higher level of IFN-γ in the anti-Thy1.2–treated mice than those treated with anti–IFN-γ, providing a potential explanation for the more significant effect of anti–IFN-γ than anti-Thy1.2 treatment on parasitemia and host survival.
Fig. 6.
Protection, T cell activation, and IFN-γ production in March1−/− mice and regulation of MARCH1 and CD86 expression after YM infection. (A) CD3+ T cells from spleens of noninfected, infected WT, or infected March1−/− mice treated with isotype immunoglobulin G control (Iso) or anti-Thy1.2 antibodies (αThy) day 4 pi were counted using flow cytometry. (B and C) Serum IL-10 and IFN-γ levels, respectively, were measured using ELISA. The experiments were repeated one time with similar results. (D and E) Host survival (D) and parasitemia (E) of mice treated with anti-Thy1.2 or isotype control antibodies. (F and G) Parasitemia and host survival curves of YM-infected March1−/− mice with or without anti–IFN-γ or anti–IL-10 antibody treatment. (H) Percentages of cells expressing intracellular IFN-γ and IL-10 in noninfected and day 4 YM-infected March1−/− mice. Cells were stained with anti–IFN-γ and anti–IL-10 and for specific cell-type markers detected using flow cytometry. (I and J) Frequencies of activated CD4+ (I) and CD8+ (J) T cell subpopulations in the spleens of YM-infected March1−/− mice with or without anti-CD86 treatment. (K and L) Serum levels of IFN-γ (K) and IL-10 (L) in YM-infected March1−/− mice treated with anti-CD86 or isotype control antibodies. Kruskal–Wallis test, median with range (A–C, E, F, and I–L): *P < 0.05, **P < 0.01, ***P < 0.001; Mann–Whitney U test, error bars represent mean and SD comparing individual pairs (H): **P < 0.01; log-rank test (D and G): **P < 0.01, ***P < 0.001. Each experiment was independently repeated.
We also treated YM-infected March1−/− mice with anti-CD86 antibody (Bio X Cell; BE0025; i.v., 500 μg, four injections on days 0 to 3) to block CD86–CD28 interaction and showed significant decreases in the frequencies of CD4+CD25+, CD4+CD69+, CD8+CD25+, and CD8+CD69+ T cells day 4 pi, suggesting impaired activation of both CD4+ and CD8+ T cells (Fig. 6 I and J). Interestingly, serum levels of IFN-γ and IL-10 were also significantly reduced after anti-CD86 treatment day 4 pi, although the levels of reduction in IFN-γ and IL-10 were not as much as those treated with anti-Thy antibody (Fig. 6 K and L). These results are consistent with previous observations of higher parasitemia in IFNifn-γ−/− and IFNAR−/− mice days 6 to 7 pi with N67 (4, 39), and significantly improved host survival after anti-CD28 antibody (200 μg) treatment to activate CD28 (28). Therefore, IFN-γ can provide protection against malaria infection, although the protection may depend on parasite–host models (40).
Discussion
This study reveals several molecular and cellular events regulated by MARCH1, including the IFN-I pathways of host innate immune response to malaria infections, in addition to the known roles in regulating CD86 and MHC-II expression (18, 38, 41–44) (summarized in SI Appendix, Fig. S5). This study also identifies many host genes that interact with different parasite genetic loci during 24 h of P. yoelii infection, providing important leads for future studies on host–parasite interactions and gene functions.
Several lines of evidence show that MARCH1 is a negative regulator of IFN-I responses: 1) March1 was clustered with several ISGs in the Ts-eQTL analysis, suggesting that it plays a role in IFN-I response. 2) In vitro expression of March1 in 293T cells suppresses IFN-I signaling pathways. 3) March1−/− mice produce significantly higher IFN-α and IFN-β than WT mice when stimulated with cGAMP and poly(I:C). 4) Deficiency of MARCH1 increases protein levels of STING, MAVS, TRAF3, and TRAF6 in uninfected mice. However, serum levels of IFN-α and/or IFN-β were significantly lower in March1−/− mice than WT mice during early malaria infection. Several factors may contribute to the observed low levels of IFN-I: 1) increased expression of genes encoding SOCS1, SOCS3, SIKE1, CACTIN, TRIM24, IL-10RA, USP18, and mir21 that are known to suppress immune/IFN-I responses (5, 45–49); 2) substantially reduced levels of key proteins of IFN-I response pathways such as STING and MAVS in infected Matrch1−/− mice compared with WT mice, possibly through MARCH1 ubiquitination of target proteins for degradation; and 3) changes in DCs, Macs, and other cell populations can also affect the levels of proteins critical for IFN-I responses.
In addition to finding explanations for the reduced serum IFN-I levels in the infected March1−/− mice, we also provide mechanistic insight into the mechanism of improved host survival without the protective role of an early IFN-I response reported previously (4). We showed that MARCH1 deficiency increased CD86+DC populations that may enhance T cell activation, leading to elevated day 4 serum levels of IFN-γ and longer survival of infected March1−/− mice. Consistent with this conclusion, T cell depletion significantly reduced serum IFN-γ level in WT and March1−/− mice and partially reversed the improved host survival rate due to MARCH1 deficiency. Additionally, anti–IFN-γ and anti–IL-10 treatments confirm that IFN-γ plays a major role in protecting YM-infected March1−/− mice. Indeed, blocking IFN-γ had more significant impacts on parasitemia and host survival rate than T cell depletion. Anti–IFN-γ treatment could have more completely blocked IFN-γ than T cell depletion because IFN-γ can be produced by other cell types after T cell depletion. These observations suggest that the protective role of MARCH1 deficiency is mostly mediated through IFN-γ, and both increased numbers of CD86+DCs and reduced IFN-I levels may contribute to the enhanced T cell activation and IFN-γ production in the March1−/− mice. T cell activation requires simultaneous binding of MHC molecules and costimulatory ligands such as CD80 and CD86 expressed on APCs to T cell receptors and CD28 (or CTLA4 and PD-L1) on T cells (50, 51). The increases in expression of CD86 and the number of CD86+DCs will enhance T cell activation in the March1−/− mice. Additionally, the reduced levels of IFN-I day 1 pi may also influence adaptive immunity in the March1−/− mice because Plasmodium-induced IFN-I was also shown to limit T follicular helper cell accumulation and promote Tr1 responses in mice infected with P. yoelii (3, 12). IFN-I has been shown to suppress innate immune cell function and IFN-γ production by parasite-specific CD4+ T cells (8, 11–13). Therefore, the higher IFN-γ level and better protection observed in the March1−/− mice are likely due to increases in the number of CD86+DCs and reduced IFN-I levels in early malaria infection. On the other hand, IFN-I has also been shown to be essential for DC maturation and development of CD4+T cell immunity (52). Indeed, an early (24 h) increase in IFN-I levels was found to inhibit early N67 parasitemia in WT C57BL/6 mice (4) and to play a critical role in protecting MAVS/STING-deficient mice infected with YM parasites (5). An IFN-I response initiated by MDA5 recognition of parasite RNA has also been reported to reduce P. berghei burden in the liver (6). Additionally, IFN-α was found to inhibit P. yoelii blood-stage parasites through reduced production of reticulocytes (53). Therefore, IFN-I can either enhance or inhibit host immune responses, depending on the parasite–host models and timing of its production during infection.
Analysis of cell populations has revealed additional mechanistic insight into how MARCH1 deficiency increases survival rates of malaria-infected mice. In addition to significantly increased numbers of CD86+DCs in March1−/− mice, the numbers of CD80+DC and CD80+Mac populations increased dramatically in both WT and March1−/− mice day 4 pi. CD80+DCs and CD86+DCs have been shown to bind CTLA4/PD-L1 and CD28 to regulate T cell activation differentially (50, 51, 54–56). The increased number of CD80+DCs can partially explain malaria-induced inhibition of immune response because CD80 can bind to CTLA4 and/or PD-L1 to dampen T cell response (50, 51, 54). On the other hand, the significantly increased numbers of both CD86+DCs and CD80+CD86+DCs in the infected March1−/− mice could promote CD86–CD28 interaction and T cell activation, leading to enhanced Th1-mediated response and IFN-γ production. A second interesting observation is the opposite directions in marginal zone B cell number variation, with increasing marginal zone B cells in March1−/− mice but declining in WT mice. These observations indicate better T cell help in B cell development and maturation (57) early adaptive immune responses. Since March1 deficiency can increase CD86+DC population, T cell activation, and more marginal zone B cells, inhibition of MARCH1 activity may overcome immune suppression mediated by CD80-PD-1–CTLA4 interactions through increased CD28–CD86 binding and a stronger early adaptive immune response. Further dissecting the mechanism of how MARCH1 regulates IFN-I and CD86 expression in DCs may provide an alternative for treating T cell dysfunction in cancer patients and chronic infections.
To investigate the mechanism of how MARCH1 deficiency reduces serum IFN-I level after malaria infection, we performed genome-wide mRNA expression analyses after infection of WT and March1−/− mice with N67 and YM parasites, respectively. Upstream regulator analyses showed inhibition of many molecules in IFN (type I and type II) response pathways in March1−/− mice day 1 pi, compared with those of WT mice. The results are consistent with the reduced in vivo serum IFN-I levels day 1 pi. Importantly, our analyses identify activated upstream regulators known to inhibit IFN-I response such as TRIM24, PTGER4, SOCS1, SOCS3, BTK, UBE3C, SIKE1, CACTIN, USP18, DUSP1, and mir21 (35–37) that can contribute to the reduced serum IFN-I in infected March1−/− mice. These analyses reveal a complicated network of IFN responses regulated by MARCH1. Further investigations are necessary to dissect the precise mechanisms as to how the absence of MARCH1 activates these negative IFN-I regulators and the roles of these regulators in controlling IFN responses in malaria infections.
In summary, we show that MARCH1 plays key roles in two important antimalaria immune pathways: 1) regulating innate IFN-I response that is protective against early blood and liver stages (4–6); and 2) regulating adaptive immunity by altering immune cell populations, CD86 expression, T cell activation, and IFN-γ/IL-10 production. Manipulation of MARCH1 activity therefore may provide a promising avenue for immune therapy to reverse T cell dysfunction or exhaustion in cancer and malaria infections.
Materials and Methods
Ethics Statement.
All animal procedures in this study were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee at the National Institute of Allergy and Infectious Diseases (NIAID) (approval LMVR11E) and according to experimental protocols approved by the Animal Care and Welfare Committee at the Houston Methodist Research Institute following the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Association for Assessment and Accreditation of Laboratory Animal Care International. All mice were maintained under pathogen-free conditions.
Malaria Parasites and Mice.
Procedures for infection of mice with the parasites were as reported previously (26, 27). Briefly, parasites were thawed from frozen stocks and injected into naïve C57BL/6 mice to initiate infection. An inoculum containing 1 × 106 infected red blood cells suspended in 100 µL phosphate-buffered saline (pH 7.4) from the donor mice was injected i.v. into experimental mice (n = 3 to 6 mice per clone or strain). The N67 and YM parasites were initially obtained from MR4-BEI (https://www.beiresources.org/About/MR4.aspx) and were described previously (26). Inbred female C57BL/6 mice, aged 6 to 8 wk old, were obtained from the Charles River Laboratory, Jackson Laboratory, or NIAID/Taconic repository, NIH. March1−/− mice were obtained from Paul Roche of the National Cancer Institute (NCI), NIH, Rockville, MD, with permission from Satoshi Ishido, Showa Pharmaceutical University, Tokyo, Japan. All of the experiments were performed in accordance with NIH-approved animal study protocol LMVR11E. Parasitemia was monitored daily by microscopic examination of Giemsa-stained thin blood smears. Additional methods can be found in SI Appendix, Methods.
Data Availability.
All data are available in the figures, SI Appendix figures, and datasets. The microarray data associated with Figs. 1A and 4 A–C have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession nos. GSE114718 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE114718) and GSE119944 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119944).
Supplementary Material
Acknowledgments
We thank Dr. Paul Roche of the NCI, NIH, and Dr. Satoshi Ishido of Japan for the March1−/− mice. This work was supported by the Division of Intramural Research, NIAID, NIH, and, in part, by grants from the NCI, NIH (R01CA101795 and U54CA210181), Department of Defense Congressionally Directed Medical Research Programs, Breast Cancer Research Program (BC151081), and startup funding from the Houston Methodist Research Institute and University of Southern California (to R.-F.W.). We thank Dr. Jacques Thibodeau for discussions/suggestions and Brigit Sullivan of the NIH library for editing.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data associated with Figs. 1A and 4 A–C have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (accession nos. GSE114718 and GSE119944).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004332117/-/DCSupplemental.
References
- 1.King T., Lamb T., Interferon-γ: The Jekyll and Hyde of malaria. PLoS Pathog. 11, e1005118 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacerda-Queiroz N. et al., Mechanism of splenic cell death and host mortality in a Plasmodium yoelii malaria model. Sci. Rep. 7, 10438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mooney J. P., Wassmer S. C., Hafalla J. C., Type I interferon in malaria: A balancing act. Trends Parasitol. 33, 257–260 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Wu J. et al., Strain-specific innate immune signaling pathways determine malaria parasitemia dynamics and host mortality. Proc. Natl. Acad. Sci. U.S.A. 111, E511–E520 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X. et al., Cross-regulation of two type I interferon signaling pathways in plasmacytoid dendritic cells controls anti-malaria immunity and host mortality. Immunity 45, 1093–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liehl P. et al., Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat. Med. 20, 47–53 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigário A. M. et al., Recombinant human IFN-alpha inhibits cerebral malaria and reduces parasite burden in mice. J. Immunol. 178, 6416–6425 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Haque A. et al., Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur. J. Immunol. 41, 2688–2698 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Haque A. et al., Type I IFN signaling in CD8− DCs impairs Th1-dependent malaria immunity. J. Clin. Invest. 124, 2483–2496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S. et al., Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35, 194–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montes de Oca M. et al., Type I interferons regulate immune responses in humans with blood-stage Plasmodium falciparum infection. Cell Rep. 17, 399–412 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zander R. A. et al., Type I interferons induce T regulatory 1 responses and restrict humoral immunity during experimental malaria. PLoS Pathog. 12, e1005945 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebina I. et al., IFNAR1-signalling obstructs ICOS-mediated humoral immunity during non-lethal blood-stage Plasmodium infection. PLoS Pathog. 12, e1005999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura T., Kimura K., Yui K., Yoshida S., Reduction of conventional dendritic cells during Plasmodium infection is dependent on activation induced cell death by type I and II interferons. Exp. Parasitol. 159, 127–135 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Ishido S., Goto E., Matsuki Y., Ohmura-Hoshino M., E3 ubiquitin ligases for MHC molecules. Curr. Opin. Immunol. 21, 78–83 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Ohmura-Hoshino M. et al., A novel family of membrane-bound E3 ubiquitin ligases. J. Biochem. 140, 147–154 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Walseng E. et al., Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 107, 20465–20470 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuki Y. et al., Novel regulation of MHC class II function in B cells. EMBO J. 26, 846–854 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samji T., Hong S., Means R. E., The membrane associated RING-CH proteins: A family of E3 ligases with diverse roles through the cell. Int. Sch. Res. Notices 2014, 637295 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh J., Shin J. S., Molecular mechanism and cellular function of MHCII ubiquitination. Immunol. Rev. 266, 134–144 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ablack J. N., Metz P. J., Chang J. T., Cantor J. M., Ginsberg M. H., Ubiquitylation of CD98 limits cell proliferation and clonal expansion. J. Cell Sci. 128, 4273–4278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tze L. E. et al., CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 208, 149–165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbas T., Steimle V., Lapointe R., Ishido S., Thibodeau J., MARCH1 down-regulation in IL-10-activated B cells increases MHC class II expression. Cytokine 59, 27–30 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Mittal S. K., Cho K. J., Ishido S., Roche P. A., Interleukin 10 (IL-10)-mediated immunosuppression: March-I induction regulates antigen presentation by macrophages but not dendritic cells. J. Biol. Chem. 290, 27158–27167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y. et al., Membrane-associated RING-CH (MARCH) 1 and 2 are MARCH family members that inhibit HIV-1 infection. J. Biol. Chem. 294, 3397–3405 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J. et al., Linkage maps from multiple genetic crosses and loci linked to growth-related virulent phenotype in Plasmodium yoelii. Proc. Natl. Acad. Sci. U.S.A. 108, E374–E382 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J. et al., Genome-wide analysis of host-Plasmodium yoelii interactions reveals regulators of the type I interferon response. Cell Rep. 12, 661–672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia L. et al., Detection of host pathways universally inhibited after Plasmodium yoelii infection for immune intervention. Sci. Rep. 8, 15280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komander D., Rape M., The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Akutsu M., Dikic I., Bremm A., Ubiquitin chain diversity at a glance. J. Cell Sci. 129, 875–880 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Wang Q. et al., The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41, 919–933 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Burdette D. L., Vance R. E., STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 14, 19–26 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Corcoran K. et al., Ubiquitin-mediated regulation of CD86 protein expression by the ubiquitin ligase membrane-associated RING-CH-1 (MARCH1). J. Biol. Chem. 286, 37168–37180 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krämer A., Green J., Pollard J. Jr., Tugendreich S., Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30, 523–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hertzog P. J., Williams B. R., Fine tuning type I interferon responses. Cytokine Growth Factor Rev. 24, 217–225 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Tisserand J. et al., Tripartite motif 24 (Trim24/Tif1α) tumor suppressor protein is a novel negative regulator of interferon (IFN)/signal transducers and activators of transcription (STAT) signaling pathway acting through retinoic acid receptor α (Rarα) inhibition. J. Biol. Chem. 286, 33369–33379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y. et al., HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog. 9, e1003248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baravalle G. et al., Ubiquitination of CD86 is a key mechanism in regulating antigen presentation by dendritic cells. J. Immunol. 187, 2966–2973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattaradilokrat S. et al., Plasmodium genetic loci linked to host cytokine and chemokine responses. Genes Immun. 15, 145–152 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shear H. L., Srinivasan R., Nolan T., Ng C., Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J. Immunol. 143, 2038–2044 (1989). [PubMed] [Google Scholar]
- 41.Kaul S., Mittal S. K., Roche P. A., A major isoform of the E3 ubiquitin ligase March-I in antigen-presenting cells has regulatory sequences within its gene. J. Biol. Chem. 293, 4478–4485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Gassart A., De Angelis Rigotti F., Gatti E., MHC-II ubiquitination. Methods Mol. Biol. 960, 517–527 (2013). [DOI] [PubMed] [Google Scholar]
- 43.De Gassart A. et al., MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl. Acad. Sci. U.S.A. 105, 3491–3496 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walseng E. et al., Dendritic cell activation prevents MHC class II ubiquitination and promotes MHC class II survival regardless of the activation stimulus. J. Biol. Chem. 285, 41749–41754 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atzei P., Gargan S., Curran N., Moynagh P. N., Cactin targets the MHC class III protein IkappaB-like (IkappaBL) and inhibits NF-kappaB and interferon-regulatory factor signaling pathways. J. Biol. Chem. 285, 36804–36817 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang J. et al., SIKE is an IKK epsilon/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J. 24, 4018–4028 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu D. et al., SOCS3 drives proteasomal degradation of TBK1 and negatively regulates antiviral innate immunity. Mol. Cell. Biol. 35, 2400–2413 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu Y., Hayward G. S., The ubiquitin E3 ligase RAUL negatively regulates type I interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity 33, 863–877 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arimoto K. I. et al., STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat. Struct. Mol. Biol. 24, 279–289 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acuto O., Michel F., CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat. Rev. Immunol. 3, 939–951 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Butte M. J., Keir M. E., Phamduy T. B., Sharpe A. H., Freeman G. J., Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Longhi M. P. et al., Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vigário A. M. et al., Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte. Blood 97, 3966–3971 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y. et al., CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J. Immunol. 172, 2778–2784 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Manzotti C. N. et al., Integration of CD28 and CTLA-4 function results in differential responses of T cells to CD80 and CD86. Eur. J. Immunol. 36, 1413–1422 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Xiang J., Gu X., Qian S., Chen Z., Graded function of CD80 and CD86 in initiation of T-cell immune response and cardiac allograft survival. Transpl. Int. 21, 163–168 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Suan D., Sundling C., Brink R., Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol. 45, 97–102 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the figures, SI Appendix figures, and datasets. The microarray data associated with Figs. 1A and 4 A–C have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession nos. GSE114718 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE114718) and GSE119944 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119944).