Significance
Neonicotinoids acting on insect nicotinic acetylcholine receptors (nAChRs) are deployed for crop protection, but growing evidence for adverse effects on insect pollinators has led to restricted use of some neonicotinoids in the EU. It is therefore vital to understand the target site actions of neonicotinoids in pollinators, but to date the difficulties of heterologous expression of insect nAChRs have hampered progress. We have found that a thioredoxin (TMX3) enables robust functional expression of honeybee, bumblebee, and fruit fly nAChRs in Xenopus laevis oocytes. With this advance, we show that expressed bee nAChRs are more neonicotinoid-sensitive than those of fruit fly, and clothianidin can modulate both honeybee and bumblebee nAChRs at a concentration below that commonly observed in agricultural fields.
Keywords: neonicotinoids, nicotinic acetylcholine receptors, fruit fly, honeybee, bumblebee
Abstract
The difficulty of achieving robust functional expression of insect nicotinic acetylcholine receptors (nAChRs) has hampered our understanding of these important molecular targets of globally deployed neonicotinoid insecticides at a time when concerns have grown regarding the toxicity of this chemotype to insect pollinators. We show that thioredoxin-related transmembrane protein 3 (TMX3) is essential to enable robust expression in Xenopus laevis oocytes of honeybee (Apis mellifera) and bumblebee (Bombus terrestris) as well as fruit fly (Drosophila melanogaster) nAChR heteromers targeted by neonicotinoids and not hitherto robustly expressed. This has enabled the characterization of picomolar target site actions of neonicotinoids, findings important in understanding their toxicity.
Neonicotinoid insecticides display selective actions on insect nicotinic acetylcholine receptors (insect nAChRs) and show high plant systemic activity that enables seed treatment (1–5). Hence, for the past two decades, they have been widely used for plant protection and animal health care (3). However, adverse actions of neonicotinoids on bee behavior, colony size, and queen production have been demonstrated (6–13). Their intensive use also correlates with reduced numbers of insectivorous birds (14). Hence, on April 27, 2018, the European Union (EU) placed a ban on outdoor use of the most commonly used neonicotinoids (imidacloprid, thiamethoxam, and clothianidin). There were also calls for wider international restrictions of neonicotinoid use (15). It is therefore urgent to understand the mechanism of neonicotinoid actions and toxicity.
A major barrier to achieving this goal has been the challenge of obtaining robust, heterologous functional expression of cloned insect nAChR subunits using well-established expression vehicles such as Xenopus laevis oocytes or Drosophila melanogaster S2 cells. It is difficult to reliably achieve heterologous expression of insect nAChRs, but insect α-subunits form robust nAChRs when coexpressed with certain vertebrate non-α-subunits (16). These insect/vertebrate hybrid nAChRs have been employed to study the mode and diversity of neonicotinoid actions (5, 17, 18). However, some features of insect native nAChR interactions with neonicotinoids are not easily studied using such hybrid nAChRs. For example, studies on honeybee (Apis mellifera) cultured antennal lobe neurons (19, 20) and Kenyon cells (21) as well as on cockroach (Periplaneta americana) (22) and fruit fly (D. melanogaster) cholinergic neurons (23) showed that imidacloprid acts as a partial agonist on nAChRs. Imidacloprid blocked the desensitizing component of native nAChRs on cockroach neurons (24), acting selectively on one receptor subtype, whereas clothianidin activates two distinct receptor subtypes (25). In all cases, the subunit composition and stoichiometry of native insect nAChRs is unknown. There is an urgent need to quantify the actions of neonicotinoids, notably those restricted for crop protection use in the EU, on insect nAChRs of known subunit composition. We report that insect nAChRs of the fruit fly (D. melanogaster), the western honeybee (A. mellifera), and the buff-tailed bumblebee (Bombus terrestris) can be expressed robustly in X. laevis oocytes with the aid of cofactors, notably TMX3 (26). We show that heteromeric honeybee and bumblebee nAChRs are sensitive to picomolar imidacloprid, thiacloprid, and clothianidin, counseling caution for continued neonicotinoid use in the field.
Results and Discussion
Functional Expression of Insect nAChRs.
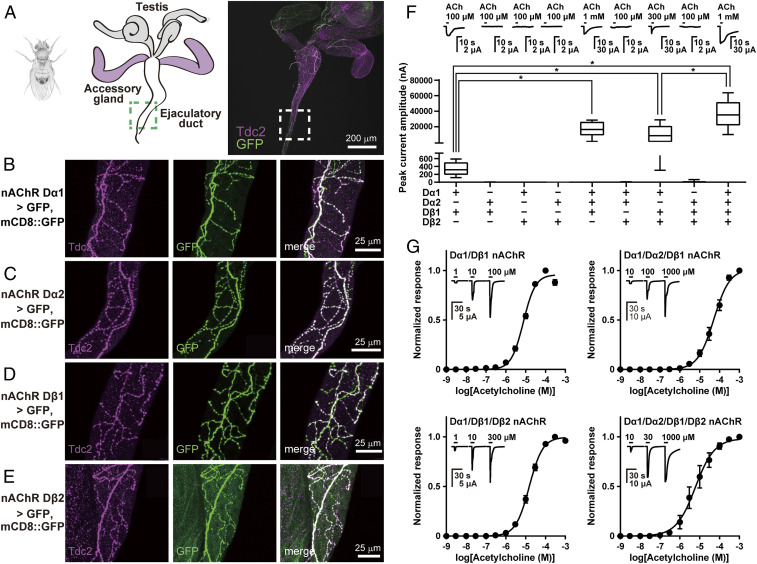
Our initial attempts at robust functional expression of insect nAChRs focused on the Dα1, Dα2, Dβ1, and Dβ2 subunits of D. melanogaster since biochemical studies point toward their coassembly (27, 28). To confirm their colocalization, we explored the expression of these nAChR subunit genes using a viral T2A peptide-mediated GAL4 transgenic knockin (29, 30) to drive the expression of a membrane-tethered GFP reporter gene. We employed the previously identified octopaminergic neurons innervating the testis ejaculatory duct (Fig. 1A) (31), since their neurites are easily identified by visualizing with membrane-tethered GFP and anti-tyrosine decarboxylase 2 (Tdc2) antibody immunostaining. We found that a GFP signal reflecting the expression of the Dα1 gene was detected in Tdc2-positive neurites (Fig. 1B). Using the same method, we showed that Dα2, Dβ1, and Dβ2 genes were also expressed in the same neurons (Fig. 1 C–E). Considering that GFP signals driven by each of four nAChR subunit genes overlapped almost perfectly with the Tdc2 signal in these neurons, these results suggest that the four D. melanogaster subunits are likely to coexist in single neurons targeting the ejaculatory duct.
Fig. 1.
Colocalization of nAChR subunits and their functional expression. (A) Cartoon of testis, accessory glands, and ejaculatory ducts of the fruit fly and a microscope image of these tissues. (B–E) Microscope images of ejaculatory ducts of male flies carrying UAS-GFP, UAS-mCD8::GFP with Dα1-2A-GAL4 (B), Dα2-2A-GAL4 (C), Dβ1-2A-GAL4 (D), and Dβ2-2A-GAL4 (E). (F) ACh-induced currents recorded from X. laevis oocytes expressing various D. melanogaster nAChR subunits in combination with DmRIC-3, DmUNC-50, and DmTMX3. Boxes show median and 25th to 75th percentiles of ACh response amplitudes with minimum and maximum indicated as whiskers (n = 20). *P < 0.05 (one-way ANOVA, Kruskal–Wallis test). (G) ACh concentration–response relationships. Each plot represents mean ± SEM (n = 5).
We next explored the expression of Dα1 and Dβ1 subunits selected as a minimal heteromeric subunit combination in X. laevis oocytes. Despite evidence of their colocalization, however, we found no electrophysiological evidence of functional expression, findings resembling those previously reported in experiments using D. melanogaster S2 and human embryonic kidney (HEK293) cells as expression vehicles (32). In the nematode Caenorhabditis elegans, RIC-3 and UNC-50 promote nAChR maturation (33) and nAChR trafficking (34), respectively. We therefore examined whether a simultaneous introduction of these regulators influence fruit fly nAChR expression in X. laevis oocytes. However, no successful expression of the Dα1/Dβ1 nAChR was observed, not even when we coinjected this subunit pairing, together with cRNAs encoding the nAChR subunits, cRNAs encoding the D. melanogaster orthologs of RIC-3 (DmRIC-3, CG30926) and UNC-50 (DmUNC-50, CG9773) (SI Appendix, Fig. S1). We postulated that a missing component for robust insect nAChR expression could be a thioredoxin, possibly underlying the disulfide bond formation of the Cys-loop superfamily proteins critical for subunit assembly and the coupling of ligand binding to channel gating (35, 36). We therefore coexpressed thioredoxin-related transmembrane protein 3 (DmTMX3) (CG5027) (26), a D. melanogaster ortholog of the C. elegans UNC-74 expression cofactor for levamisole-sensitive nAChRs (37), together with Dα1 and Dβ1. This resulted in robust inward current responses to 100 µM ACh (Fig. 1F and SI Appendix, Fig. S1). The peak amplitudes of the response to 100 µM ACh of the Dα1/Dβ1 nAChR expressed with either DmTMX3 and DmRIC-3, or DmTMX3 and DmUNC-50 were similar to the amplitude of the ACh responses recorded when Dα1/Dβ1 subunits were coexpressed with DmTMX3 alone. However, we observed larger ACh responses when the Dα1/Dβ1 pairing was coexpressed with all three cofactors (DmRIC-3, DmUNC-50, and DmTMX3) (SI Appendix, Fig. S1; P < 0.05 [one-way ANOVA, Kruskal–Wallis test]). Therefore, DmTMX3 plays the crucial role in robust functional expression of this D. melanogaster heteromeric nAChR in cooperation with DmRIC-3 and DmUNC-50.
We then tested the capacity of additional D. melanogaster nAChR subunits to form robust, functional receptors in the presence of DmRIC-3, DmUNC-50, and DmTMX3. Whereas individual Dα1, Dα2, Dβ1, and Dβ2 subunits as well as the Dα1/Dα2, Dβ1/Dβ2, Dα2/Dβ1, Dα2/Dβ2 pairings and the cofactors (DmRIC-3/DmUNC-50/DmTMX3) failed to form functional nAChRs (SI Appendix, Fig. S2), we observed large-amplitude currents in response to ACh in oocytes coexpressing the Dα1, Dα2, and Dβ1 subunits and those expressing Dα1, Dβ1, and Dβ2 subunits (Fig. 1F). The addition of the Dα2 subunit to the Dα1/Dβ1/Dβ2 combination resulted in the ACh response exceeding in amplitude those recorded from Dα1/Dβ1 nAChRs (Fig. 1F, P < 0.05 [one-way ANOVA, Kruskal–Wallis test]). Furthermore, coexpression of Dα1/Dβ1 with either Dα2 or Dβ2 subunit resulted in a shift of pEC50 (= −logEC50) for ACh (Fig. 1G and Table 1; P < 0.05 [one-way ANOVA, Tukey test]), suggesting that both Dα2 and Dβ2 subunits coassemble with the Dα1/Dβ1 nAChR to form robust nAChRs with features distinct from the Dα1/Dβ1 nAChR.
Table 1.
Agonist actions of acetylcholine and neonicotinoids on fruit fly, honeybee, and bumblebee nAChRs
| Acetylcholine | Imidacloprid | Thiacloprid | Clothianidin | ||||
| nAChRs | pEC50 | pEC50 | Imax | pEC50 | Imax | pEC50 | Imax |
| Fruit fly (D. melanogaster) | |||||||
| Dα1/Dβ1 | 5.12 ± 0.02a,* | 6.76 ± 0.23ab | 0.112 ± 0.009a | 7.64 ± 0.18ab | 0.065 ± 0.005a | 7.21 ± 0.12a | 0.296 ± 0.013a |
| Dα1/Dβ1(R81T) | 5.48 ± 0.04† | 6.06 ± 0.12† | 0.032 ± 0.002† | 7.01 ± 0.09† | 0.015 ± 0.001† | 5.33 ± 0.11† | 0.326 ± 0.017 |
| Dα1/Dα2/Dβ1 | 4.29 ± 0.04b | 6.39 ± 0.18a | 0.046 ± 0.004ab | 6.63 ± 0.17c | 0.020 ± 0.002bc | 5.53 ± 0.07b | 0.467 ± 0.021b |
| Dα1/Dα2/Dβ1(R81T) | 4.68 ± 0.04† | 5.76 ± 0.15 | 0.023 ± 0.002 | 6.44 ± 0.18 | 0.017 ± 0.002 | ND‡ | ND |
| Dα1/Dβ1/Dβ2 | 4.82 ± 0.02c | 6.97 ± 0.21ab | 0.244 ± 0.022c | 7.73 ± 0.19ab | 0.056 ± 0.005ab | 6.66 ± 0.07c | 0.411 ± 0.011bc |
| Dα1/Dβ1(R81T)/Dβ2 | 5.33 ± 0.03† | 5.65 ± 0.25† | 0.025 ± 0.003† | 7.29 ± 0.09 | 0.0057 ± 0.0003† | ND | ND |
| Dα1/Dα2/Dβ1/Dβ2 | 5.22 ± 0.07a | 6.92 ± 0.12ab | 0.592 ± 0.030d | 7.15 ± 0.08ac | 0.454 ± 0.019d | 6.51 ± 0.05cd | 0.821 ± 0.017d |
| Dα1/Dα2/Dβ1(R81T)/Dβ2 | 4.95 ± 0.04† | 5.69 ± 0.18† | 0.081 ± 0.006† | 7.07 ± 0.12 | 0.014 ± 0.001† | 5.49 ± 0.04† | 0.951 ± 0.022† |
| Honeybee (A. mellifera) | |||||||
| Amα1/Amα8/Amβ1 | 5.94 ± 0.04e | 7.63 ± 0.13b | 0.075 ± 0.004a | 7.94 ± 0.19ab | 0.058 ± 0.006ab | 7.96 ± 0.07e | 0.788 ± 0.020d |
| Amα1/Amα2/Amα8/Amβ1 | 5.72 ± 0.03f | 7.48 ± 0.16b | 0.070 ± 0.005a | 8.16 ± 0.13b | 0.037 ± 0.003bc | 8.09 ± 0.09e | 0.766 ± 0.024de |
| Bumblebee (B. terrestris) | |||||||
| Btα1/Btα8/Btβ1 | 5.80 ± 0.03ef | 7.60 ± 0.26b | 0.107 ± 0.010a | 7.29 ± 0.26abc | 0.094 ± 0.008a | 7.87 ± 0.06ef | 0.759 ± 0.018de |
| Btα1/Btα2/Btα8/Btβ1 | 5.66 ± 0.03f | 7.40 ± 0.20b | 0.085 ± 0.007a | 7.14 ± 0.38ac | 0.092 ± 0.011a | 7.41 ± 0.10ag | 0.680 ± 0.029e |
Data are represented as mean ± SEM (n = 5).
Different letters (a−g) indicate that pEC50 and Imax data for each ligand differ between the wild-type nAChRs compared (one-way ANOVA, Tukey test, P < 0.05).
Indicates that data for the R81T mutant differ from that for the corresponding wild-type nAChR in D. melanogaster (two-way ANOVA, Bonferroni test, P < 0.05).
ND: could not be determined with accuracy because the concentration–response curve did not attain a maximum.
Neonicotinoid Actions on Fruit Fly nAChRs.
Since neonicotinoids activate native insect nAChRs (23), we investigated the agonist actions of imidacloprid, thiacloprid, and clothianidin on the D. melanogaster Dα1/Dβ1, Dα1/Dα2/Dβ1, Dα1/Dβ1/Dβ2, and Dα1/Dα2/Dβ1/Dβ2 nAChRs expressed in X. laevis oocytes. Imidacloprid, thiacloprid, and clothianidin activated all four types of recombinant nAChRs. Thiacloprid showed the highest agonist affinity in terms of pEC50, while clothianidin showed the highest agonist efficacy in terms of Imax for all receptors (Fig. 2 A–D and SI Appendix, Fig. S3 and Table 1). The subunit components had a minimal impact on the affinity of imidacloprid, whereas thiacloprid showed a lower agonist affinity for the Dα1/Dα2/Dβ1 nAChR compared to the Dα1/Dβ1 and Dα1/Dβ1/Dβ2 nAChRs (Fig. 2 A–D and Table 1; P < 0.05 [one-way ANOVA, Tukey test]). The agonist efficacy of imidacloprid and clothianidin was enhanced in the presence of the Dβ2 subunit, whereas the agonist efficacy of thiacloprid was reduced by addition of the Dα2 subunit to the Dα1/Dβ1 nAChR (Fig. 2 A–D and Table 1; P < 0.05 [one-way ANOVA, Tukey test]). All of the neonicotinoids showed highest agonist efficacy on the Dα1/Dα2/Dβ1/Dβ2 nAChRs (Fig. 2D and Table 1), suggesting that the responses to ACh and neonicotinoids of oocytes expressing more than three D. melanogaster nAChR subunits are not simply the result of a mixture of the Dα1/Dβ1 nAChR and another kind of nAChR. The concentration–response relationships of imidacloprid for the D. melanogaster Dα1/Dβ1, Dα1/Dα2/Dβ1, Dα1/Dβ1/Dβ2, and Dα1/Dα2/Dβ1/Dβ2 nAChRs resemble those observed for native nAChRs on D. melanogaster neurons (23).
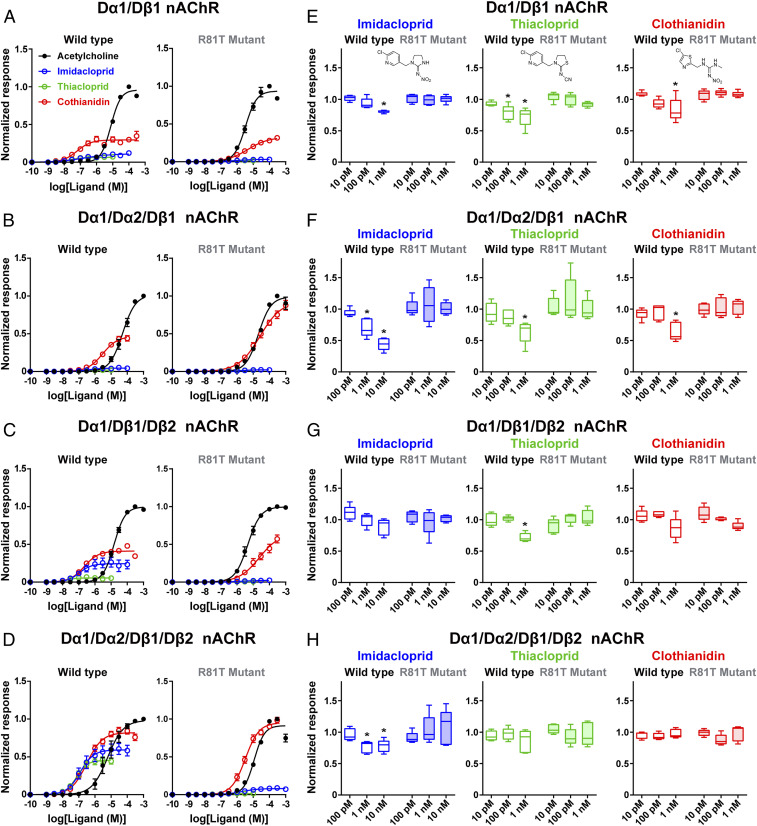
Fig. 2.
Modulation by neonicotinoids of D. melanogaster nAChRs. (A–D) Concentration–response relationships of neonicotinoids for wild-type fruit fly nAChRs and their R81T mutants in which the Arg81 of the Dβ1 subunit was replaced by threonine. In the mutant nAChRs, the Dβ1 subunit possesses the R81T amino acid substitution. Each plot represents mean ± SEM (n = 5). (E–H) Neonicotinoid modulation of responses to 100 μM ACh of the wild-type and mutant fruit fly nAChRs. Boxes show median and 25th to 75th percentiles with minimum and maximum indicated as whiskers of normalized peak amplitude of response to ACh (n = 5). *P < 0.05 (one-way ANOVA, Bonferroni test).
To confirm further the contribution of the Dβ1 subunit to expressing neonicotinoid-sensitive insect nAChRs, we examined the effects of replacing the arginine 81 (R81) with threonine (T) in loop D of the D. melanogaster Dβ1 subunit on the agonist actions of the neonicotinoids, because this basic residue has been shown to interact directly with the NO2 or CN group of neonicotinoids (see Fig. 2 for chemical structures) (5, 18). The R81T mutation had no significant effect on the ACh-induced current response amplitude of nAChRs except for the Dα1/Dα2/Dβ1 receptor (SI Appendix, Fig. S4). By contrast, the mutation reduced both agonist affinity of imidacloprid and clothianidin (Fig. 2 A–D and Table 1, P < 0.05 [two-way ANOVA, Bonferroni test]). For thiacloprid, the R81T mutation reduced the efficacy more profoundly than the affinity (Fig. 2 A–D and Table 1; P < 0.05 [two-way ANOVA, Bonferroni test]). These results suggest that the effects of the R81T mutation on neonicotinoid actions are the results of changes in receptor–ligand interactions and that, in these expressed insect nAChRs, the Dβ1 subunit is a key player in neonicotinoid interactions.
In native insect neurons, imidacloprid attenuates the desensitizing component more profoundly than the nondesensitizing component of ACh responses at low concentrations (24). Hence, we investigated the effects of coapplication with the neurotransmitter of imidacloprid, thiacloprid, and clothianidin at concentrations <10 nM, below the threshold for agonist actions, on the ACh-induced response of the D. melanogaster recombinant nAChRs (Fig. 2 E–H). When imidacloprid was applied at 1 nM for 1 min followed immediately by coapplication with ACh for 2 min, it attenuated the fast desensitizing component of the ACh response of the Dα1/Dβ1, Dα1/Dα2/Dβ1, and Dα1/Dα2/Dβ1/Dβ2 nAChRs, while scarcely influencing a nondesensitizing component (SI Appendix, Fig. S5). Thiacloprid reduced the ACh response of the Dα1/Dβ1 nAChR even at 100 pM (Fig. 2E) and was more potent than imidacloprid and clothianidin in its antagonist action on the Dα1/Dβ1/Dβ2 nAChR response (Fig. 2G; P < 0.05 [one-way ANOVA, Bonferroni test]). Clothianidin tested at 1 nM blocked the ACh response of the Dα1/Dβ1 and Dα1/Dα2/Dβ1 nAChRs (Fig. 1 E and F; P < 0.05 [one-way ANOVA, Bonferroni test]), while being ineffective on the Dα1/Dβ1/Dβ2 and Dα1/Dα2/Dβ1/Dβ2 nAChRs (Fig. 2 G and H). The R81T mutation in the Dβ1 subunit attenuated the antagonist actions of the neonicotinoids on all of the D. melanogaster nAChRs (Fig. 2 E–H), confirming the role for the Dβ1 subunit in determining the antagonist activity of neonicotinoids as well as their agonist activity.
Target Site Actions of Neonicotinoids in Honeybees and Bumblebees.
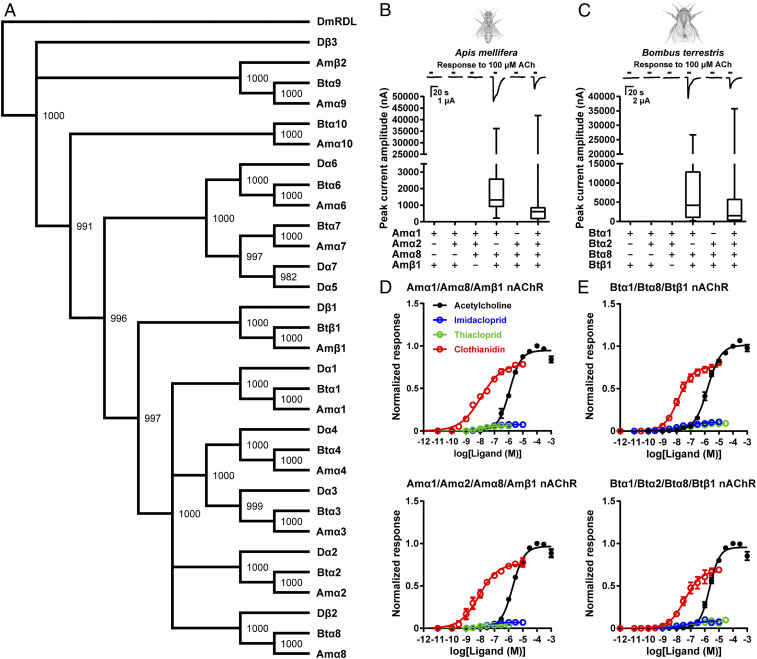
Based on the findings in the fruit fly, we attempted to address neonicotinoid actions on heteromeric nAChRs in two pollinator species, the western honeybee (A. mellifera) and the buff-tailed bumblebee (B. terrestris). We first examined the capacity of the three auxiliary proteins RIC-3, UNC-50, and TMX3 to express the nAChRs in X. laevis oocytes. In this experiment, we employed the A. mellifera α8 (Amα8) and B. terrestris α8 (Btα8) as the α8 subunits in both species show the highest amino acid sequence similarity to the fruit fly Dβ2 subunit (Fig. 3A). Coexpressing together Amα1, Amα8, and Amβ1 subunits or Amα1, Amα2, Amα8, and Amβ1 subunits with AmRIC-3, AmUNC-50, and AmTMX3 resulted in the first robust expression of honeybee nAChRs corresponding to the fruit fly Dα1/Dβ1/Dβ2 and Dα1/Dα2/Dβ1/Dβ2 nAChRs, respectively (Fig. 3B). Similarly, robust bumblebee Btα1/Btα8/Btβ1 and Btα1/Btα2/Btα8/Btβ1 nAChRs were formed in X. laevis oocytes in the presence the three equivalent bumblebee cofactors (BtRIC-3, BtUNC-50, BtTMX3) (Fig. 3C).
Fig. 3.
Agonist actions of neonicotinoids on honeybee and bumblebee nAChRs. (A) Relationships of D. melanogaster, A. mellifera, and B. terrestris nAChR subunit proteins. DmRDL: D. melanogaster GABAA receptor subunit RDL. Bootstrap values are shown at each node. (B and C) ACh-induced responses of the honeybee (B) and bumblebee (C) nAChRs. The boxes represent median and 25th to 75th percentiles of ACh response amplitudes with minimum and maximum shown as whiskers (honeybee, n = 20; bumblebee, n = 10). (D and E) Concentration–response relationships for neonicotinoids on the honeybee (D) and bumblebee (E) nAChRs. Each plot represents mean ± SEM (n = 5).
We evaluated agonist activity of the three neonicotinoids for the A. mellifera and B. terrestris nAChRs. Imidacloprid was a partial agonist as in native insect neurons (19–23) with similar affinity for the honeybee and the bumblebee nAChRs, while thiacloprid acted as a partial agonist with higher affinity for the honeybee Amα1/Amα2/Amα8/Amβ1 nAChRs compared to the bumblebee Btα1/Btα8/Btβ1 and Btα1/Btα2/Btα8/Btβ1 nAChRs (Fig. 3 D and E and Table 1; P < 0.05 [one-way ANOVA, Tukey test]). Of the commercial neonicotinoids, clothianidin is most widely used for crop protection and has been documented as a factor in the decline of wild bees, honeybees, and bumblebees (6, 10). Interestingly, clothianidin showed higher agonist affinity not only for the honeybee Amα1/Amα8/Amβ1 and Amα1/Amα2/Amα8/Amβ1 nAChRs but also for the bumblebee Btα1/Btα8/Btβ1 and Btα1/Btα2/Btα8/Btβ1 nAChRs than most of the fruit fly nAChRs (Fig. 2 A–D vs. Fig. 3 D and E and Table 1; P < 0.05 [one-way ANOVA, Tukey test]). Furthermore, clothianidin showed comparable affinity to thiacloprid and the highest efficacy among the neonicotinoids tested for the honeybee and bumblebee nAChRs (Fig. 3 D and E and Table 1), suggesting that both insect pollinator species possess nAChRs with features favorable for binding this insecticide.
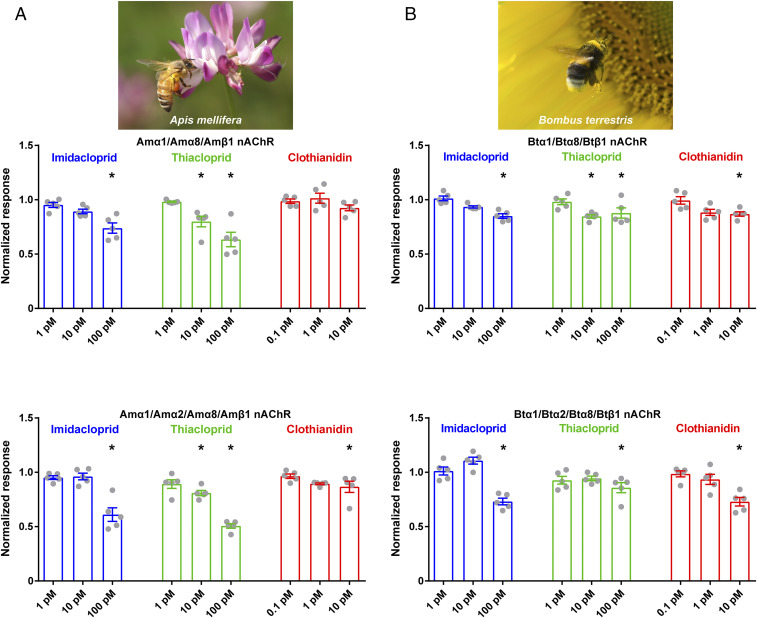
It has been shown that imidacloprid acts as a partial agonist on nAChRs expressed in honeybee neurons (19–21) and that imidacloprid and clothianidin affect the excitability of honeybee (A. mellifera) Kenyon cells at concentrations as low as 10 nM; imidacloprid reduces the peak amplitude of the ACh response of Kenyon cells with an IC50 of 295 nM (38). However, the threshold concentration for neonicotinoid modulation of honeybee and bumblebee nAChRs is not known. From an eco-toxicological perspective, evaluating the target site actions of neonicotinoids at picomolar concentrations is critical as it offers insights into sublethal effects on honeybees and bumblebees at field-relevant concentrations. We therefore examined the effects of imidacloprid, thiacloprid, and clothianidin on the ACh-induced responses of the honeybee and bumblebee nAChRs at picomolar concentrations at which they did not show agonist actions (Fig. 4 and SI Appendix, Fig. S6). Here, we show that imidacloprid and thiacloprid suppress the peak amplitude of the ACh response of Amα1/Amα8/Amβ1 and Amα1/Amα2/Amα8/Amβ1 nAChRs of honeybees as well as Btα1/Btα8/Btβ1 and Btα1/Btα2/Btα8/Btβ1 nAChRs of bumblebees at 100 pM and thiacloprid affected not only the honeybee Amα1/Amα8/Amβ1 and Amα1/Amα2/Amα8/Amβ1 nAChRs (Fig. 4A), but also bumblebee Btα1/Btα8/Btβ1 nAChR even at 10 pM (Fig. 4B; P < 0.05 [one-way ANOVA, Bonferroni test]). Thiacloprid is metabolized faster than imidacloprid in A. mellifera and B. terrestris and thus may not cause adverse effects on these insect pollinator species if used under appropriate regulation (39). In the case of clothianidin, however, no such fast metabolism has been confirmed. Clothianidin blocked the ACh response of the honeybee Amα1/Amα2/Amα8/Amβ1 as well as bumblebee Btα1/Btα8/Btβ1 and Btα1/Btα2/Btα8/Btβ1 nAChRs at 10 pM (Fig. 4 A and B; P < 0.05 [one-way ANOVA, Bonferroni test]), a concentration far below that observed in agricultural fields [1.9 ppb = 7.6 nM for nectar and 6.1 ppb = 24 nM for pollen (40)], which may indicate a risk to these insect pollinator species.
Fig. 4.
Modulation of honeybee (A. mellifera) and bumblebee (B. terrestris) nAChRs by imidacloprid, thiacloprid, and clothianidin. Neonicotinoid modulation of 100 μM ACh-induced response of the A. mellifera (A) and B. terrestris nAChRs (B) were examined according to the same method as performed for the fruit fly (D. melanogaster) nAChRs (Fig. 2). Bar graphs represent mean ± SEM, and data points are plotted in each bar graph (n = 5). *P < 0.05 (one-way ANOVA, Bonferroni test) compared with untreated control response to ACh.
In conclusion, we have succeeded in robust, functional expression in X. laevis oocytes of honeybee (A. mellifera), bumblebee (B. terrestris), and fruit fly (D. melanogaster) nAChRs using TMX3 as a key cofactor. Our data showing that neonicotinoids have a particularly high impact on the A. mellifera and B. terrestris nAChRs tested are of importance in considering the future of plant protection using neonicotinoids. Of the neonicotinoids restricted in the EU, clothianidin modulates the honeybee and bumblebee nAChRs containing the α8 subunit even at 10 pM, a concentration much lower than that to which bees are exposed in the field. As the Btα8 subunit-containing nAChRs underpin bee olfactory retrieval behavior (41), chronic exposure to sublethal doses of neonicotinoids could lead to abnormalities in synaptic timing and thereby alter this important bee behavior. Our discovery of these sublethal, picomolar actions of neonicotinoids on their targets shows precisely how cholinergic signaling by the insect neurotransmitter ACh is modified. Parasites, viral pathogens, climate change, habitat loss, and alien species can be cocontributors with neonicotinoids to the decline of bees (42, 43). It is therefore necessary to regulate neonicotinoids carefully and to consider all of the stressors in the environment.
We acknowledge that for nAChRs expressed in X. laevis oocytes, the lipid environment differs from that of insect nerve, as do pretranslation and posttranslation modifications, and codon bias differences may also exist. Also, we have only studied neonicotinoid actions on some insect nAChRs. Therefore, more work is needed on how α- and non–α-subunits are assembled to form nAChRs in particular neurons and to what extent their expression is modulated by developmental and environmental factors. Also, it is necessary to evaluate in more detail the modes of actions not only of neonicotinoids, but also of new insecticides targeting insect nAChRs when expressed not only in X. laevis oocytes, but also in other expression vehicles. Nevertheless, our discovery of the importance of cofactor TMX3 in enabling robust insect nAChR expression offers a route to functional studies on nAChRs, not only those of other beneficial insects, insect pests, and disease vectors, but also those of nontarget terrestrial and aquatic invertebrates, which have so far proved highly challenging and in many cases elusive.
Materials and Methods
Chemicals.
Imidacloprid, thiacloprid, and clothianidin were purchased from FUJIFILM Wako Pure Chemical. ACh and atropine were purchased from MilliporeSigma.
DNA Cloning.
In addition to DNAs encoding D. melanogaster Dα1 (NP_524481), Dα2 (NP_524482), Dβ1 (NP_523927), Dβ2 (NP_524483), DmRIC-3 (CAP16647), DmUNC-50 (NP_649813), and DmTMX3 (NP_648847), those encoding A. mellifera Amα1 (XP_026298411), Amα2 (NP_001011625), Amα8 (NP_001011575), Amβ1 (NP_001073028), AmRIC-3 (BCD56240), AmUNC-50 (AJE70276), and AmTMX3 (BCD56241) as well as B. terrestris Btα1 (XP_003397561), Btα2 (XP_012166790), Btα8 (XP_012163744), Btβ1 (XP_003393394), BtRIC-3 (BCD56239), BtUNC-50 (XP_012167154), and BtTMX-3 (XP_003403348) were cloned into pcDNA3.1 (+) vector (Thermo Fisher Scientific).
Functional Expression of nAChRs and Electrophysiology.
Female X. laevis were anesthetized and oocytes were obtained according to the UK Animals (Scientific Procedures) Act, 1986. Following treatment with 2 mg⋅mL−1 type IA collagenase (MilliporeSigma), in Ca2+-free standard oocyte saline (Ca2+-free, standard oocyte saline [SOS]) containing 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes) (pH 7.6), oocytes were transferred to SOS containing 100 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM Hepes (pH 7.6) for removal of the follicle cell layers (44, 45). cRNA encoding each nAChR subunit and cofactor was prepared from the cDNA construct using the mMESSAGE mMACHINE T7 Ultra Kit (Thermo Fisher Scientific). The cytoplasm of each defolliculated oocyte was injected with 50 nL of cRNA solution, where each cRNA was mixed at the same concentration (100 ng⋅μL−1). Injected oocytes were incubated in SOS supplemented with 2.5 mM sodium pyruvate, 100 units⋅mL−1 penicillin, 100 μg⋅mL−1 streptomycin, and 20 μg⋅mL−1 gentamycin and 4% horse serum (Thermo Fisher Scientific) at 16 °C for 3 to 7 d prior to recording of the responses.
Voltage-clamp electrophysiology was performed with oocytes clamped at a holding potential Eh of −100 mV. Data were analyzed using Clampfit (Molecular Devices). Oocytes were perfused at 7 to 10 mL⋅min−1 with SOS containing 0.5 μM atropine (A-SOS) to block endogenous muscarinic ACh receptor response (44–46). Stock solutions of neonicotinoids were prepared in dimethyl sulfoxide (DMSO) at 100 mM and diluted with A-SOS to prepare test solutions. DMSO in test solutions <0.1% had no effect on the nAChR responses at this concentration range. ACh was dissolved directly in A-SOS immediately before experiments. When determining concentration–response relationships for ACh and neonicotinoids, responses to 100 μM ACh were first measured by successive applications for 5 s at 3-min intervals to confirm that the responses are stable, prior to applications of these agonists for 5 s at 3-min intervals. At higher concentrations, one oocyte was used to record one response to the neonicotinoids of all of the wild type, D. melanogaster, A. mellifera, and B. terrestris nAChRs tested to prevent the effect of irreversible modulation of the wild-type nAChRs. In the cases of the D. melanogaster nAChRs, peak amplitude of the ACh- and neonicotinoid-induced response was normalized to the response to ACh at either 100 μM (Dα1/Dβ1 nAChR), 300 μM (Dα1/Dα2/Dβ1 and Dα1/Dα2/Dβ1/Dβ2 nAChRs), or 300 μM (Dα1/Dβ1/Dβ2 nAChR). In the case of the A. mellifera and B. terrestris nAChRs, the amplitude of the agonist response to was normalized to the 100 μM ACh-induced response. Experiments were repeated to confirm reproducibility (n = 5, ≥2 frogs).
The concentration–response data were fitted by nonlinear regression using Prism 6 (GraphPad Software) according to the following equation.
| [1] |
In Eq. 1, Imax is normalized maximum response, Y is normalized response, X is log [agonist concentration (molar)], and nH is the Hill coefficient.
The antagonist effects of neonicotinoids on nAChR were evaluated as follows. After successive applications of 100 μM ACh for 5 s with 3-min interval (training applications), 100 μM ACh was applied for 2 min to oocytes expressing nAChRs. Then, each neonicotinoid was applied for 1 min prior to coapplication with 100 μM ACh for 2 min. The peak amplitude of the responses to ACh in the absence (control, “C”) and presence (treated, “T”) of neonicotinoids was normalized by the mean amplitude of two training ACh responses [(TC1 + TC2)/2]. The normalized data for the control [2C/(TC1 + TC2)] and treated responses [2T/(TC1 + TC2)] were compared to evaluate the antagonist actions of the neonicotinoids (SI Appendix, Fig. S7). Experiments were repeated to confirm reproducibility of the antagonist actions of neonicotinoids (n = 5, ≥2 frogs).
Statistical Analysis.
One-way ANOVA was used to analyze the effects of neonicotinoids on the ACh responses of the D. melanogaster, A. mellifera, and B. terrestris nAChRs expressed in X. laevis oocytes, while two-way ANOVA was employed to examine the effect of the R81T mutation on the agonist activity for each ligand. Any difference between the means compared was analyzed with P values (<0.05) using Prism 6.
Fly Culture.
D. melanogaster flies were raised on cornmeal–agar–yeast medium at 25 °C. UAS-GFP (47) was a gift from Kei Ito, University of Cologne, Cologne, Germany. UAS-mCD8::GFP (#108068), which expresses a transgene encoding a membrane-targeted GFP protein, was obtained from Kyoto Stock Center.
Transgenic Flies.
The GAL4 knockin D. melanogaster flies were generated by CRISPR/Cas9-mediated homologous recombination. For each receptor, a targeting vector was designed such that the 2A-GAL4 (29) is inserted in-frame with the last intracellular region of the protein. The targeting vector and a gRNA expression vector that cuts near the target site were coinjected into fertilized eggs maternally expressing Cas9 protein. The flanking sequences of the insertion are shown below with the site of integration indicated by a slash. The 20-bp gene-specific sequence of the gRNA is underlined: Dα1, 5′-AGAGGACTGGAAGTACGTTGCCATG/GTATTGGATCGTATGTTTCTGTGGA-3′; Dα2, 5′-TCAGGACTGGGGCTTTGTGGCCATG/GTCATGGATCGCCTATTCCTCTGGC-3′; Dβ1, 5′-CGAAGATTGGAAGTACGTGGCCATG/GTGATCGATCGCTTGCAACTATACAT-3′; and Dβ2, 5′-GGAGGACTGGAAGTTCGTATCGATG/GTGCTGGACCGCTTCTTTCTCTGGCT-3′.
Immunostaining.
The reproductive systems of male D. melanogaster were dissected in Grace’s Insect Medium, supplemented (Thermo Fisher Scientific), and fixed in 4% paraformaldehyde in Grace’s Insect medium for 30 to 60 min at room temperature (RT). The fixed samples were washed three times in PBS supplemented with 0.1% Triton X-100. After washing, the samples were transferred to blocking solution (PBS with 0.1% Triton X-100 and 2% BSA; MilliporeSigma; A3608) for 1 h at RT and incubated with a primary antibody in the blocking solution at 4 °C overnight. The primary antibodies used in this study were mouse anti-GFP monoclonal antibody (clone GFP-20; MilliporeSigma; G6539; 1:1,000) and rabbit anti-Tdc2 antibody (Abcam; ab128225; 1:2,000) (48). After washing, Fluorophore (Alexa Fluor 488 and 555)-conjugated secondary antibodies (Thermo Fisher Scientific; A11001 and A32732; 1:200) were applied in blocking solution, and the tissue was incubated for 2 h at RT. After a final wash, all samples were mounted in FluorSave reagent (MilliporeSigma). Samples were visualized on a Zeiss LSM 700 confocal microscope. Images were processed using the ImageJ package (49).
Multiple Alignments of Amino Acid Sequences of nAChRs and Tree View.
The nAChR subunit protein sequences were aligned with MAFFT v7.308 (50) (algorithm, auto; scoring matrix, BLOSUM62; Gap open penalty, 1.55; and offset value, 0.123) and then the phylogenetic tree was built with fruit fly GABAA receptor subunit DmRDL (RDL: Resistant to dieldrin; NP_523991) as the outgroup. Bootstrap analysis was conducted with 1,000 replicates. Accession numbers of insect nAChR subunits are as follows: D. melanogaster Dα1 (NP_524481), Dα2 (NP_524482), Dα3 (NP_525079), Dα4 (NP_001097669), Dα5 (NP_995708), Dα6 (NP_723494), Dα7 (NP_996514), Dβ1 (NP_523927), Dβ2 (NP_524483), Dβ3 (NP_525098); A. mellifera Amα1 (XP_026298411), Amα2 (NP_001011625), Amα3 (NP_001073029), Amα4 (NP_001091691), Amα6 (NP_001073564), Amα7 (NP_001011621), Amα8 (NP_001011575), Amα9 (NP_001091694), Amα10 (XP_392070), Amβ1 (NP_001073028), Amβ2 (NP_001091699); B. terrestris Btα1 (XP_003397561), Btα2 (XP_012166790), Btα3 (XP_012170034), Btα4 (XP_016767555), Btα6 (XP_020723485), Btα7 (XP_012167932), Btα8 (XP_012163744), Btα9 (XP_003397879), Btα10 (XP_020722434), and Btβ1 (XP_003393394).
Data Availability.
Sequence data have been deposited to DNA Data Bank of Japan (accession numbers BCD56239, BCD56240, and BCD56241). All other data are included in the manuscript and SI Appendix.
Supplementary Material
Acknowledgments
This study was supported by Japan Society for the Promotion of Science KAKENHI to M.I. (Grant 16K21507), H.T. (Grant 26250001), as well as to K.M. and R.N. (Grant 17H01472). Y.Y. was a recipient of the fellowship from the Japan Society for the Promotion of Science. We thank Kei Ito and Kyoto Stock Center (Drosophila Genetic Resource Center) at Kyoto Institute of Technology for providing us with the fruit fly strains. We also thank Yoshie Fukuta and Daiki Higuchi for assistance; Jun Nakamura and Shinji Kohara for the gift of honeybees and bumblebees, respectively; Tamara Clark for the drawings of fruit fly, honeybee, and bumblebee; and Jun Nakamura for the gift of the photographs of honeybee and bumblebee.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence data reported in this paper have been deposited to DNA Data Bank of Japan (accession nos. BCD56239, BCD56240, and BCD56241).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003667117/-/DCSupplemental.
References
- 1.Matsuda K. et al., Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 22, 573–580 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Nauen R., Ebbinghaus-Kintscher U., Elbert A., Jeschke P., Tietjen K., “Acetylcholine receptors as sites for developing neonicotinoid insecticides” in Biochemical Sites of Insecticide Action and Resistance, Ishaaya I., Ed. (Springer, 2001), pp. 77–105. [Google Scholar]
- 3.Jeschke P., Nauen R., Beck M. E., Nicotinic acetylcholine receptor agonists: A milestone for modern crop protection. Angew. Chem. Int. Ed. Engl. 52, 9464–9485 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Casida J. E., Neonicotinoids and other insect nicotinic receptor competitive modulators: Progress and prospects. Annu. Rev. Entomol. 63, 125–144 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Matsuda K., Ihara M., Sattelle D. B., Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 60, 241–255 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Rundlöf M. et al., Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Gill R. J., Ramos-Rodriguez O., Raine N. E., Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitehorn P. R., O’Connor S., Wackers F. L., Goulson D., Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Woodcock B. A. et al., Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 356, 1393–1395 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Cressey D., The bitter battle over the world’s most popular insecticides. Nature 551, 156–158 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Crall J. D. et al., Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683–686 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Wintermantel D. et al., Field-level clothianidin exposure affects bumblebees but generally not their pathogens. Nat. Commun. 9, 5446 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lämsä J., Kuusela E., Tuomi J., Juntunen S., Watts P. C., Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. Biol. Sci. 285, 20180506 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallmann C. A., Foppen R. P., van Turnhout C. A., de Kroon H., Jongejans E., Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343 (2014). [DOI] [PubMed] [Google Scholar]
- 15.Goulson D.; 232 signatories , Call to restrict neonicotinoids. Science 360, 973 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Bertrand D. et al., Physiological properties of neuronal nicotinic receptors reconstituted from the vertebrate β2 subunit and Drosophila α subunits. Eur. J. Neurosci. 6, 869–875 (1994). [DOI] [PubMed] [Google Scholar]
- 17.Ihara M., Buckingham S. D., Matsuda K., Sattelle D. B., Modes of action, resistance and toxicity of insecticides targeting nicotinic acetylcholine receptors. Curr. Med. Chem. 24, 2925–2934 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Ihara M., Matsuda K., Neonicotinoids: Molecular mechanisms of action, insights into resistance and impact on pollinators. Curr. Opin. Insect Sci. 30, 86–92 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Barbara G. S., Zube C., Rybak J., Gauthier M., Grünewald B., Acetylcholine, GABA and glutamate induce ionic currents in cultured antennal lobe neurons of the honeybee, Apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 191, 823–836 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Barbara G. S., Grünewald B., Paute S., Gauthier M., Raymond-Delpech V., Study of nicotinic acetylcholine receptors on cultured antennal lobe neurones from adult honeybee brains. Invert. Neurosci. 8, 19–29 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Déglise P., Grünewald B., Gauthier M., The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 321, 13–16 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Ihara M. et al., Actions of imidacloprid, clothianidin and related neonicotinoids on nicotinic acetylcholine receptors of American cockroach neurons and their relationships with insecticidal potency. J. Pestic. Sci. 31, 35–40 (2006). [Google Scholar]
- 23.Brown L. A., Ihara M., Buckingham S. D., Matsuda K., Sattelle D. B., Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 99, 608–615 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Salgado V. L., Saar R., Desensitizing and non-desensitizing subtypes of α-bungarotoxin-sensitive nicotinic acetylcholine receptors in cockroach neurons. J. Insect Physiol. 50, 867–879 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Thany S. H., Agonist actions of clothianidin on synaptic and extrasynaptic nicotinic acetylcholine receptors expressed on cockroach sixth abdominal ganglion. Neurotoxicology 30, 1045–1052 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Haugstetter J., Blicher T., Ellgaard L., Identification and characterization of a novel thioredoxin-related transmembrane protein of the endoplasmic reticulum. J. Biol. Chem. 280, 8371–8380 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Schloss P., Betz H., Schröder C., Gundelfinger E. D., Neuronal nicotinic acetylcholine receptors in Drosophila: Antibodies against an α-like and a non-α-subunit recognize the same high-affinity α-bungarotoxin binding complex. J. Neurochem. 57, 1556–1562 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Chamaon K., Smalla K. H., Thomas U., Gundelfinger E. D., Nicotinic acetylcholine receptors of Drosophila: Three subunits encoded by genomically linked genes can co-assemble into the same receptor complex. J. Neurochem. 80, 149–157 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Diao F., White B. H., A novel approach for directing transgene expression in Drosophila: T2A-Gal4 in-frame fusion. Genetics 190, 1139–1144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondo S. et al., Neurochemical organization of the Drosophila brain visualized by endogenously tagged neurotransmitter receptors. Cell Rep. 30, 284–297.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Rezával C., Nojima T., Neville M. C., Lin A. C., Goodwin S. F., Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 24, 725–730 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansdell S. J., Schmitt B., Betz H., Sattelle D. B., Millar N. S., Temperature-sensitive expression of Drosophila neuronal nicotinic acetylcholine receptors. J. Neurochem. 68, 1812–1819 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Halevi S. et al., The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 21, 1012–1020 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eimer S. et al., Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J. 26, 4313–4323 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unwin N., Fujiyoshi Y., Gating movement of acetylcholine receptor caught by plunge-freezing. J. Mol. Biol. 422, 617–634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemecz Á., Prevost M. S., Menny A., Corringer P. J., Emerging molecular mechanisms of signal transduction in pentameric ligand-gated ion channels. Neuron 90, 452–470 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Boulin T. et al., Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. U.S.A. 105, 18590–18595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmer M. J. et al., Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manjon C. et al., Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 28, 1137–1143.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godfray H. C. et al., A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. Biol. Sci. 281, 20140558 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis T. et al., Amelα8 subunit knockdown in the mushroom body vertical lobes impairs olfactory retrieval in the honeybee, Apis mellifera. Eur. J. Neurosci. 36, 3438–3450 (2012). [DOI] [PubMed] [Google Scholar]
- 42.Potts S. G. et al., Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Kerr J. T. et al., CLIMATE CHANGE. Climate change impacts on bumblebees converge across continents. Science 349, 177–180 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Matsuda K. et al., Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br. J. Pharmacol. 123, 518–524 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shimada S. et al., The mechanism of loop C-neonicotinoid interactions at insect nicotinic acetylcholine receptor α1 subunit predicts resistance emergence in pests. Sci. Rep. 10, 7529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lupu-Meiri M., Shapira H., Matus-Leibovitch N., Oron Y., Two types of intrinsic muscarinic responses in Xenopus oocytes. I. Differences in latencies and 45Ca efflux kinetics. Pflügers Arch. 417, 391–397 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Ito K., Awano W., Suzuki K., Hiromi Y., Yamamoto D., The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124, 761–771 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Huang J., Liu W., Qi Y. X., Luo J., Montell C., Neuromodulation of courtship drive through tyramine-responsive neurons in the Drosophila brain. Curr. Biol. 26, 2246–2256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider C. A., Rasband W. S., Eliceiri K. W., NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katoh K., Misawa K., Kuma K., Miyata T., MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data have been deposited to DNA Data Bank of Japan (accession numbers BCD56239, BCD56240, and BCD56241). All other data are included in the manuscript and SI Appendix.