Abstract
Introduction
CpG oligodeoxynucleotides (CpG ODN) play important roles in resisting inflammation and bone resorption. However, the inherent instability and rapid degradation hinder their wider application. This study aimed to evaluate whether N-acetyl-L-leucine-modified polyethyleneimine (N-Ac-L-Leu-PEI) could effectively deliver CpG ODN 2006 to RAW264.7 cells and and if it can regulate osteoclastogenesis in vitro.
Materials and Methods
Gel retardation assay was conducted to evaluate whether N- Ac-L-Leu-PEI and CpG ODN could form a stable complex. RAW264.7 cells were divided into four groups of control group, ODN group, phosphorothioate ODN group and N-Ac-L-Leu-PEI/ODN group. Fluorescence assay was conducted to evaluate the transfection rate of ODNs in different groups. Cell viability was determined by MTT assay. Cell apoptosis was determined by live-dead cell staining and flow cytometry assay. Relative expression levels of osteoclastic differentiation factors, including Nfatc, c-fos, receptor activator of nuclear factor κB (RANK), and matrix metalloproteinase 9 (MMP9), were determined by real-time PCR and Western blot.
Results
N-Ac-L-Leu-PEI and CpG ODN could form a stable complex at a mass ratio of 1:1 (w:w). MTT assay showed that the cell viability of N-Ac-L-Leu-PEI was relatively high even at a mass ratio of 8 μg/mL. The transfection rate of N-Ac-L-Leu-PEI-ODN complex was higher than 90%. The cell proliferation and apoptosis was significantly enhanced in N-Ac-L-Leu-PEI- CpG ODN group when compared to those in phosphorothioate CpG ODN. The expression levels of Nfatc, c-fos, RANK, and MMP9 were significantly decreased in N-Ac-L-Leu-PEI/ODN complex group.
Discussion
N-Ac-L-Leu-PEI could be a potential gene vehicle for the prevention of periodontitis-mediated bone resorption.
Keywords: N-acetyl-L-leucine-modified polyethyleneimine, N-Ac-L-Leu-PEI, CpG oligodeoxynucleotides, CpG ODN, proliferation, osteoclastic differentiation
Introduction
Periodontitis is a chronic infectious disease caused by a host immune response to bacteria that accumulates as dental plaque.1 Previous studies indicated that severe periodontitis, which reportedly affects 8.5% of American adults, can lead to a variety of systemic diseases such as atherosclerosis, rheumatoid arthritis, aspiration pneumonia, and even cancer.2 In addition, alveolar bone resorption caused by periodontal local innate immune response stimulated by periodontal pathogens is the main clinical symptom of periodontal disease.3 The resorption affects chewing, pronunciation, and eating, and reduces physical and mental health and quality of life.4
The clinical prevention and treatment of periodontal disease is hampered by the limited availability of drugs that can control both inflammation and bone absorption. Recent studies have shown that certain specific oligodeoxynucleotide (ODN) sequences can inhibit inflammation and reduce alveolar bone resorption.5,6 ODN can be classified into stimulatory ODN, inhibitory ODN, and inert ODN. Stimulatory ODN generally refers to CpG ODN containing a CG motif. CpG ODN can simulate the bacterial DNA to induce a protective immune response based on the recognition of the unmethylated CpG motif by toll-like receptors (TLRs).7,8 Recently, CpG ODN has been used to suppress periodontitis by downregulating innate-like B cell apoptosis9 and increasing the production of protective Th1- and Th2-cells.10 Furthermore, CpG ODN plays a special role in osteoclastogenesis.11 Thus, CpG ODN has been suggested as being potentially useful in treating bone-degenerative diseases.12
Zou13 and Krisher14 reported that CpG ODN initiated strong osteoclastic differentiation in receptor activator of nuclear factor kappa-Β ligand (RANKL) -induced cells by improving tumor necrosis factor-alpha (TNFα) and RANKLthrough TLRs, while in early osteoclast precursors, CpG ODN plays opposite roles. The collective findings indicate that CpG ODN could be an effective therapy for inflammation and osteoclastogenesis associated with periodontitis.
However, the therapeutic potential of CpG ODN is hindered by its instability and difficulty in cellular uptake.15 An effective delivery system is needed for the application of CpG ODN gene therapy. The cationic polymer polyethyleneimine (PEI) is currently the “gold standard” carrier because of its good gene loading and delivery abilities, low immunogenicity, and favorable cost.16–18 Although PEI25K was very efficient at facilitating gene transfection, cytotoxicity and hemolysis were still a big problem.19,20 Thus, Li21 grafted N-acetyl-l-leucine on the primary amino group of PEI25K by a 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide/N-hydroxysuccinimide (EDC/NHS)-mediated coupling reaction to obtain N-Ac-L-Leu-PEI. N-Ac-L-Leu-PEI significantly improved the biocompatibility and transfection efficiency, and significantly decreased protein adsorption and hemolytic activity of the PEI25K matrix. This vehicle has been successfully used to deliver the p53 gene,21 a DNAzyme22 and microRNA23,24 to trigger the pronounced suppression of tumor cells and to upregulate bone formation in vivo and in vitro.
CpG ODN 2006 is a TLR9 ligand with a backbone containing phosphodiester, but not phosphorothioate. CpG ODN 2006 selectively inhibits the growth of erythroid cells derived from human CD34 + cells.25 This study aimed to investigate whether N-Ac-L-Leu-PEI can deliver CpG ODN 2006 to RAW264.7 cells and if it can regulate osteoclastogenesis in vitro.
Materials and Methods
Materials
The primers of CpG ODN 2006, β-actin, Nfatc, c-fos, RANK, matrix metalloproteinase 9 (MMP9), and 6-carboxyfluorescein (FAM)-labeled ODN (ODN and phosphorothioate ODN) were synthesized by TaKaRa Bio (Dalian, China), and the sequences are listed in Table 1. These FAM-labeled ODNs shows green signal when excited by blue light. N-Ac-L-Leu-PEI and branched PEI25K (Sigma–Aldrich, St. Louis, USA) were provided by the School of Life Sciences, Jilin University (Jilin, China). The PrimerScript® RT reagent kit and SYBR Green Premix Ex Taq kit were purchased from TaKaRa Bio (Dalian, China). Antibodies to Nfatc, c-fos, RANK, MMP9, β-actin, and horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies were all purchased from ABclonal (Boston, MA, USA). The RAW264.7 cells (SCSP-5036) were purchased from the cell bank at the Chinese Academy of Sciences (Shanghai, China).
Table 1.
The Primer Sequences of CpG ODN 2006, β-Action, Nfatc, c-Fos, RANK and MMP9
| Name | Sequence |
|---|---|
| 2006 | 5ʹ-TCGTCGTTTTGTCGTTTTGTCGTT-3’ |
| β-actin | 5ʹ-CATCCGTAAAGACCTCTATGCCAAC-3ʹ 5ʹ-ATGGAGCCACCGATCCACA-3’ |
| Nfatc | 5ʹ-CAAGTCTCACCACAGGGCTCACTA-3ʹ 5ʹ-TCAGCCGTCCCAATGAACAG-3’ |
| c-fos | 5ʹ-ACGTGGAGCTGAAGGCAGAAC-3ʹ 5ʹ-AGCCACTGGGCCTAGATGATG-3’ |
| RANK | 5ʹ-GGCTTACCTGCCCAGTCTCATC-3ʹ 5ʹ-AAGCATCATTGACCCAATTCCAC-3’ |
| MMP9 | 5ʹ-GCCCTGGAACTCACACGACA-3ʹ 5ʹ-TTGGAAACTCACACGCCAGAAG-3’ |
Gel Retardation Assay
N-Ac-L-Leu-PEI was dissolved in double distilled water to a final concentration of 1μg/μL. Then, N-Ac-L-Leu-PEI and ODN solutions were gently mixed at different mass ratios (N-Ac-L-Leu-PEI/ODN) of 0, 0.25, 0.5, 1.0, 2.0 and 4.0 (w/w). The N-Ac-L-Leu-PEI/ODN complexes were incubated for 30 min at room temperature (approximately 25°C). The complexes were used for 1.5% agarose gel electrophoresis for 25 min at a voltage of 80V using Tris-acetate-ethylenediaminetetraacetic acid buffer. The gel was then photographed.
Fluorescence Assay
RAW264.7 cells were divided into a control (ctrl) group exposed to phosphate buffered saline (PBS), ODN group (CpG ODN 2006), phosphorothioate ODN group (phosphorothioate CpG ODN 2006), and N-Ac-L-Leu-PEI/ODN group (N-Ac-L-Leu-PEI/CpG ODN 2006). The cells were seeded in 6-well plates at a density of 5×105 cells per well and cultured at 37°C in an atmosphere of 5% CO2 for 24h. Depending on the group, PBS, FAM-ODN (1 μg/mL), FAM-phosphorothioate ODN, or N-Ac-L-Leu-PEI/FAM-ODN complex were added for an additional 6h co-culture. The cells were washed thrice and the FAM-positive cells were detected using a fluorescence microscope (IX71, Olympus, Osaka, Japan) under blue light illumination with the excitation source at 420 nm – 485 nm and emission wavelength of 515 nm.
Cell Viability Assay
The viability of RAW264.7 cells treated with PEI25K and N-Ac-L-Leu-PEI as well as ODN was determined using the conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The RAW264.7 cells were seeded into wells of a 96-well plate at a density of 4×103 cells per well and incubated for 24h. In the first experiment, the cells were cultured with PEI25K (final concentration of 1 μg/mL) and different concentrations of N-Ac-L-Leu-PEI (0, 0.5, 1, 2, 4, 6, and 8 μg/mL) for 72h. In the second experiment, the cells were divided into the aforementioned four groups and cultured as described for fluorescence observation for 24, 48, and 72h. Next, 20 μL MTT solution (5 mg/mL in PBS) (Sigma-Aldrich) was added to the wells for an additional 4h incubation. The MTT solution was removed and 150 μL dimethylsulfoxide (DMSO; Sigma-Aldrich) was added to dissolve the formazan crystals that have been formed. The absorbance (A) at 490 nm was measured using a GF-M3000 microplate reader (Shandong, China) to calculate the cell viability (%) using the formula: Asample/Acontrol ×100.
Live-Dead Cell Staining
The cells of the four groups were seeded into wells of 12-well plates with 2×105 cells per well and co-cultured for 72h. The cells were washed thrice and stained using the live-dead cell staining kit (K501, Biovision, Milpitas, CA, USA) for 30 to 40 min at 37°C in the dark. The cells were washed using PBS and then observed and photographed using the fluorescence microscope (IX71, Olympus, Osaka, Japan). The green signals indicate living cells while the red signals indicate dead cells.
Flow Cytometry (FCM) Assay
RAW264.7 cells of the four groups were cultured for 72h and collected. The cells were tested for apoptosis using an Annexin V-FITC/PT kit (7sea biotech, Shanghai, China) according to the manufacturer’s instructions.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
RAW264.7 cells were induced to form osteoclasts using mouse RANKL (50 ng/mL; Peprotech, Rocky Hill, NJ, USA) for 4 days. Total RNA was isolated using a HiPure Total RNA kit (Megan, Guangzhou, China) according to the manufacturer’s instructions. The mRNAs were investigated with a PrimerScript® RT reagent kit and SYBR Green Premix Ex Taq. The PCR products were evaluated with a MxPro Mx3005P real-time PCR detection system (Agilent Technologies, Santa Clara, CA, USA). The internal control mRNA was β-actin. The cycling conditions of mRNAs were: 95°C for 30s, followed by 40 cycles at 95°C for 5s, 55°C for 30s, and 72°C for 1 min. The 2-ΔΔCt method was used to calculate the relative expression levels, and the obtained values were averaged from triplicate measurements.
Western Blot Analysis
RAW264.7 cells were treated with ODN and RANKL for 4 days and the cell proteins were harvested with RIPA lysis buffer and quantified with a bicinchoninic acid protein assay kit (Beyotime Biotech Inc., Jiangsu, China). Proteins were separated by 12% polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). These membranes were blocked with 5% bovine serum albumin in Tris-buffered saline-Tween and incubated with the following primary antibodies: Nfatc (A15339), c-fos (A0236), RANK (A13382), MMP9 (A2095), and β-actin (AC026), respectively. HRP-conjugated anti-rabbit secondary antibodies were diluted at 1:3000 and incubated at room temperature for 1h. The signals were detected using an ECL chemiluminescence kit (7Sea biotech) with a Tanon 5200 device (Tianneng, Shanghai, China).
Tartrate-Resistant Acid Phosphatase (TRAP) and Rhodamine Phalloidin Staining
RAW264.7 cells of the four groups were seeded into wells of 48-well plates with 1×104 cells per well and cultured in a growth medium containing 50 ng/mL mouse RANKL for 4.5 days. The cells were fixed by 4% paraformaldehyde for 25 min at 37°C. After being washed with PBS, the cells were stained using a TRAP kit (Sigma-Aldrich) according to the manufacturer’s instructions. The stained cells were permeabilized using 0.5% Triton X-100 for 5 min, and then stained with rhodamine phalloidin work fluid (100 nM; Solarbio, Beijing, China) for 30 min in the dark, at room temperature. The nucleus was stained by 4′, 6-diamidino-2-phenylindole (DAPI) for an additional 5 min. After washing with PBS, the plate was directly observed under fluorescence microscopy.
Statistical Analysis
All experiments were performed on at least three individuals. The results are presented as the mean ± standard deviation (SD) and were statistically analyzed with the Student’s t-test unless noted otherwise. A two-tailed P-value <0.05 was considered statistically significant. P values were indicated by *P<0.05, **P<0.01, and ***P<0.001 (similar #P), and n.s., non-significant (P>0.05). Statistical analysis was conducted using SPSS version 20.0 (IBM, Armonk, NY, USA).
Results
N-Ac-L-Leu-PEI-Mediated CpG ODN 2006 Delivery
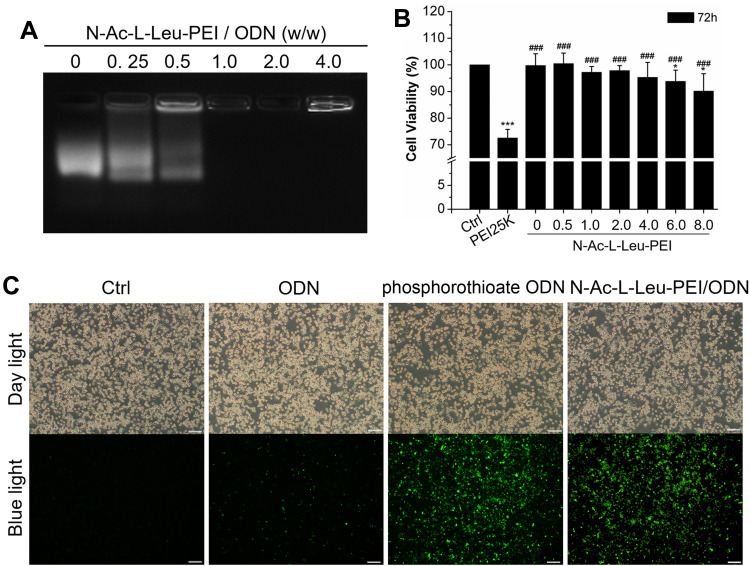
To assess whether N-Ac-L-Leu-PEI can be used for the intracellular delivery of CpG ODN 2006, a gel retardation assay was used to determine the binding capacity of N-Ac-L-Leu-PEI and CpG ODN 2006. The bands of N-Ac-L-Leu-PEI/CpG ODN 2006 complex were dispersive at mass ratios of 0, 0.25 and 0.5. The band was complete when the mass ratio reached to 1. This result indicated the migration of ODN2006 was completely retarded by N-Ac-L-Leu-PEI at the w/w ratio over 1:1 (Figure 1A).
Figure 1.
Characteristics of N-Ac-L-Leu-PEI/CpG ODN 2006 complexes. (A) The N-Ac-L-Leu-PEI/ODN complexes were prepared at different mass ratios for agarose gel retardation assays. (B) MTT assay. Data represents the mean ± SD of three independent experiments and are statistically analyzed with the Student’s t-test. *P<0.05, ***P< 0.001 vs the ctrl group; ###P <0.001 vs the PEI25K group. (C) fluorescence microscopy observation with 4:1 mass ratio. Scale bar: 100 μm.
In the MTT test, the cell viability of N-Ac-L-Leu-PEI was superior to PEI25K and exceeded 85% even at a mass ratio of 8 μg/mL for 72h (Figure 1B), while that of PEI25K was less than 75% (Figure 1B). The transfection efficiency of N-Ac-L-Leu-PEI-mediated CpG ODN 2006 delivery was estimated by fluorescence microscopy. The cell morphology in each group was observed under day light and the FAM-labeled positive signals (green) were observed under blue light. As shown in Figure 1C, there were no obvious abnormalities of the cells in each group under daylight. The green signals were almost invisible in ctrl group, were less than 10% positive in ODN group, and were more than 90% positive in both phosphorothioate ODN group and N-Ac-L-Leu-PEI/CpG ODN group, respectively. This result indicated N-Ac-L-Leu-PEI/CpG ODN 2006 could enter into RAW264.7 cells with a relatively higher efficiency. Taken together, these observations suggested that N-Ac-L-Leu-PEI/CpG ODN 2006 complex is of less cytotoxicity and satisfactory transfection efficiency, providing evidence that N-Ac-L-Leu-PEI was suitable for CpG ODN 2006 delivery in RAW264.7 cells.
Proliferation of RAW264.7s by CpG ODN 2006
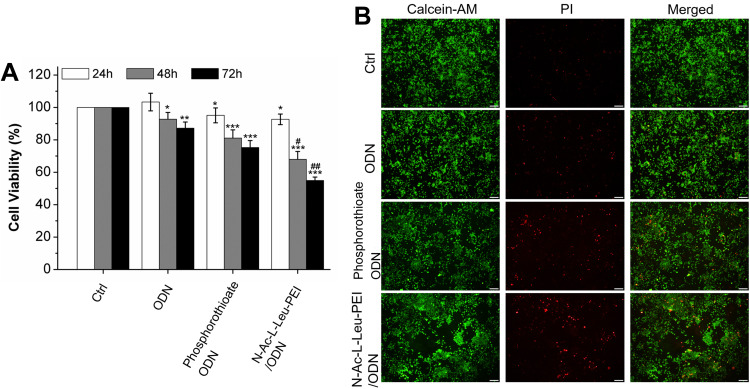
Osteoclastogenesis requires the proliferation and differentiation of osteoclast precursors. Here, we first assessed the regulatory role of CpG ODN 2006 on the proliferation of RAW264.7 cells. The MTT assay showed that CpG ODN 2006 suppressed the cell viability at 24, 48, and 72h in three groups, especially N-Ac-L-Leu-PEI/ODN, followed by phosphorothioate ODN and ODN (Figure 2A). The remarkably reduced cell viability indicated the possible involvement of cell apoptosis. This was further assessed using the live-dead cell assay. There was no significant difference on the number of live cells (green stained cells) among each group. The numbers of dead cells (red stained cells) in the ctrl, ODN, phosphorothioate ODN, and N-Ac-L-Leu-PEI/ODN groups were gradually increased (Figure 2B).
Figure 2.
Cell viability assay and live-dead cell assay. (A) MTT assay of RAW264.7 cells cultured with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 24, 48, and 72 h. Data represents the mean ± SD of three independent experiments and are statistically analyzed with the Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 vs the ctrl group at the same culture time; #P < 0.05, ##P <0.01 vs phosphorothioate ODN group at the same culture time. (B) Live-Dead cell assay of RAW264.7 cells treated with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 72 h. Green signals indicate live cells and red signals indicate dead cells. Scale bar: 100 μm.
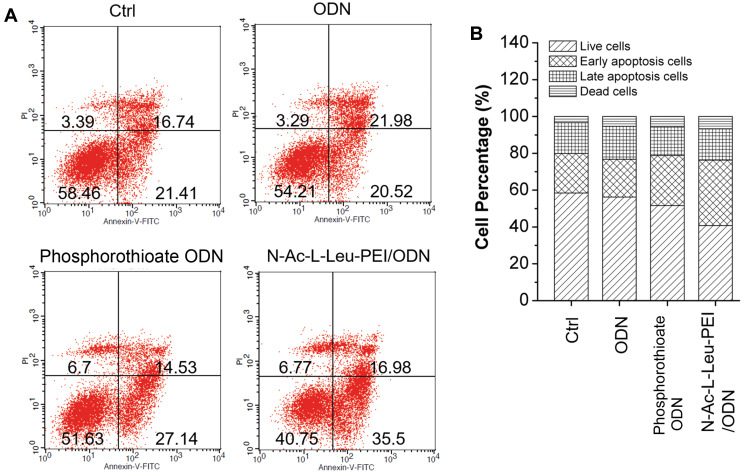
The cell apoptosis assay also indicated more apoptotic cells in the ODN and phosphorothioate ODN than ctrl, especially in the N-Ac-L-Leu-PEI/ODN group. Moreover, CpG ODN 2006 mainly triggered early cell apoptosis (Figure 3). These results indicated that CpG ODN 2006 significantly reduced the proliferation of RAW264.7 cells in part by inducing early apoptosis. N-Ac-L-Leu-PEI-mediated CpG ODN 2006 delivery produced the most significant suppression of RAW264.7 cells.
Figure 3.
Cell apoptosis assay. (A) Cell apoptosis was examined using Flow cytometry after co-culture with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 72 h. (B) Statistical analysis of the cell population.
Osteoclastic Differentiation Analysis of RAW264.7s by CpG ODN 2006
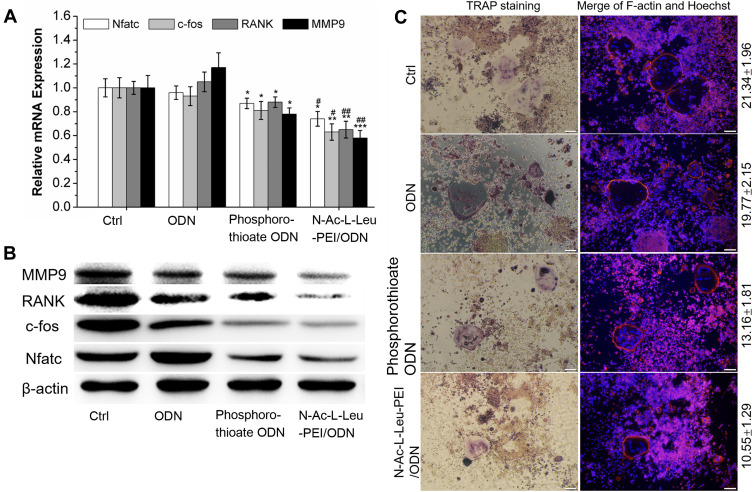
We accessed the CpG ODN 2006 regulation of osteoclastic differentiation at the gene, protein, and cell levels using RT-PCR and Western blot examinations. At both the gene and protein levels, CpG ODN 2006 significantly decreased the expression of the osteoclast differentiation factors, including Nfatc, c-fos, RANK, and MMP9 (Figure 4A and B). TRAP staining also showed that the number of osteoclasts with multiple cores, fusion, and TRAP+ were significantly decreased to 13.16 ± 1.81 and 10.55 ± 1.29 in phosphorothioate ODN and N-Ac-L-Leu-PEI/ODN group, respectively, compared to the ctrl group (21.34 ± 1.96) and ODN group (19.77 ± 2.15) (Figure 4C). It was noteworthy that the N-Ac-L-Leu-PEI/ODN inhibition of osteoclast formation was more pronounced than phosphorothioate ODN in these experiments.
Figure 4.
Osteoclast differentiation analysis. (A) qRT-PCR detected gene expression of Nfatc, c-fos, RANK, and MMP9 in RAW264.7 cells co-culture with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 4 days. Data represents the mean ± SD of three independent experiments and are statistically analyzed with the Student’s t-test. *P<0.05, **P<0.01, ***P<0.01 vs the ctrl group; #P < 0.05, ##P <0.01 vs phosphorothioate ODN group. (B) Western blot detected protein expression of Nfatc, c-fos, RANK, and MMP9 after co-culture with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 4 days. (C) The TRAP+, F-actin ring, and multinuclear cells were examined after co-culture with PBS (ctrl), CpG ODN (ODN), phosphorothioate CpG ODN, and N-Ac-L-Leu-PEI/CpG ODN complexes (N-Ac-L-Leu-PEI/ODN, w/w= 4:1) for 4.5 days by TRAP staining, rhodamine phalloidin staining, and Hoechst staining, respectively. Data represents the mean ± SD of three independent experiments.
Discussion
CpG ODN has recently been widely used in research and preliminary clinical trials for its potential in treatment and prevention of infections and tumor formation, and as a vaccine adjuvant.26–28 The involvement of CpG ODN in osteoclastogenesis has been established.11–13,29 In this study, N-Ac-L-Leu-PEI was used to deliver CpG ODN 2006 to RAW264.7 cells, and the stability, delivery ability, and regulation of osteoclastogenesis were assessed.
CpG ODN quickly enters cells via the endocytosis pathway with the involvement of TLRs and is distributed in the cell cytoplasm.30 Phosphorothioate-modified CpG ODN was more stable than non-modified ODN against enzymatic degradation.31–33 However, modified CpG ODN can reduce the immune response and cause enlargement of the immune organs.34,35 Therefore, in recent years, macromolecule materials have been used in the delivery of CpG ODN, including graphene oxide-chitosan nanocomposites36 and N-isopropylacrylamide-modified polyethyleneimine (PEN).37 Previous studies demonstrated that the N-Ac-L-Leu-PEI/CpG ODN 2006 complex (w: w = 1: 1) was superior to microRNAs and single-stranded oligo DNAs (w: w = 2:1)22,23,38 and to plasmid DNA (w:w = 0.6–0.8:1).24 The PEN vehicle could completely retard ODN MT01 at a mass ratio of 0.5.16 Similarly, N-Ac-L-Leu-PEI leads to better cell viability than PEI25K in RAW264.7 cells.23,38 The same results were shown in our findings. This may be attributed to the declining cation composition of the complexes and non-specific protein adsorption of N-Ac-L-Leu-PEI, which triggered less cell membrane disruption.21 N-Ac-L-Leu-PEI did not result in a more efficient delivery of CpG ODN, as shown in Figure 1C that the transfection efficiency was both more than 90% in the ODNs group and the N-Ac-L-Leu-PEI/CpG ODN 2006 group. Besides, it enabled CpG ODN to play a negative role in osteoclastogenesis in vitro,23,38 which indicated the significant down-regulation of proliferation and differentiation of osteoclast precursors. The lowest cell viability and more apoptotic cells were found in the N-Ac-L-Leu-PEI/CpG ODN 2006 group in our study.
In addition, in the process of RANKL-mediated osteoclast differentiation, Nfatc, c-fos, and RANK are early differentiation markers, and MMP9 mainly affects the absorptive capacity of osteoclasts.39 TRAP is a marker enzyme of osteoclast activity.2 In this study, N-Ac-L-Leu-PEI-mediated CpG ODN 2006 delivery inhibited the release of cytokines as well as the expression of Nfatc, c-fos, Ctsk, and TRAP, which in turn reduced osteoclast formation. With regard to transfection efficiency, proliferation, and differentiation of osteoclast precursor cells, N-Ac-L-Leu-PEI-mediated CpG ODN delivery was always more prominent than the other deliveries.
Conclusion
N-Ac-L-Leu-PEI and CpG ODN could form stable complexes with relatively low cytotoxicity and high transfection efficiency. N-Ac-L-Leu-PEI-mediated CpG ODN 2006 delivery induces appreciable inhibition of the proliferation and differentiation of osteoclast precursors. The findings implicate that the N-Ac-L-Leu-PEI vehicle is a novel CpG ODN therapy strategy for the prevention and treatment of periodontitis-mediated bone resorption.
Acknowledgments
We thank Prof. Quanshun Li, Jilin University School of Life Sciences for providing N-Ac-L-Leu-PEI. This research was supported by grants from the National Natural Science Foundation of China (No.81600879, No.81970946 and No. 51972240), Medical Support Program of the Jilin province (No.20170311032YY), Science and Technology Project of Jilin Provincial Department of Finance (No. jsz2018170-12) and Wenzhou Science and Technology Project (Y20150262).
Author Contributions
Y.Q. Shen, H.N. Wang, and W.W. Yu conceived and designed the study, and wrote the manuscript. J.F. Cao, H.Y. Li, Y. Zhen, and Z. Chen acquired and analyzed the data. H.B. Lin conducted the cell tests of the N-Ac-L-Leu-PEI/CpG ODN 2006 complex. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hegde R, Awan KH. Effects of periodontal disease on systemic health. Disease-a-Month. 2019;65(6):185–192. doi: 10.1016/j.disamonth.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramalingam S, Sundar C, Jansen JA, Alghamdi H. Chapter 1 - Alveolar bone science: structural characteristics and pathological changes In: Alghamdi H, Jansen J, editors. Dental Implants and Bone Grafts. Woodhead Publishing; 2020:1–22. [Google Scholar]
- 4.Intini G, Katsuragi Y, Kirkwood KL, Yang S. Alveolar bone loss mechanisms, potential therapeutic targets, and interventions. Adv Dent Res. 2014;26(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Q, Hu Y, Deng S, et al. Cytidine-phosphate-guanosine oligodeoxynucleotides in combination with CD40 ligand decrease periodontal inflammation and alveolar bone loss in a TLR9-independent manner. J Appl Oral Sci. 2018;26:e20170451. doi: 10.1590/1678-7757-2017-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu H, Nakagami H, Morita S, et al. New treatment of periodontal diseases by using NF-κB decoy oligodeoxynucleotides via prevention of bone resorption and promotion of wound healing. Antioxid Redox Signal. 2009;11(9):2065–2075. doi: 10.1089/ars.2008.2355 [DOI] [PubMed] [Google Scholar]
- 7.Krieg, MA. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9(7):831–835. doi: 10.1038/nm0703-831 [DOI] [PubMed] [Google Scholar]
- 8.Hemmi H, Takeuchi O, Kawai T, et al. A toll-like receptor recognizes bacterial. Nature. 2000;408(6813):740–745. doi: 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Wang Y, Lin J, et al. Lipopolysaccharides-induced suppression of innate-like B cell apoptosis is enhanced by CpG oligodeoxynucleotide and requires toll-like receptors 2 and 4. PLoS One. 2016;11(11):e0165862. doi: 10.1371/journal.pone.0165862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Hashizume T, Kurita-Ochiai T, Fujihashi K, Yamamoto M. Oral immunization with Porphyromonas gingivalis outer membrane protein and CpG oligodeoxynucleotides elicits T helper 1 and 2 cytokines for enhanced protective immunity. Mol Oral Microbiol. 2010;25(3):178–189. doi: 10.1111/j.2041-1014.2009.00560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W, Amcheslavsky A, Bar-Shavit Z. CpG oligodeoxynucleotides modulate the osteoclastogenic activity of osteoblasts via toll-like receptor 9. J Biol Chem. 2003;278(19):16732–16740. doi: 10.1074/jbc.M212473200 [DOI] [PubMed] [Google Scholar]
- 12.Amcheslavsky A, Hemmi H, Akira S, Bar-Shavit Z. Differential contribution of osteoclast- and osteoblast-lineage cells to CpG-oligodeoxynucleotide (CpG-ODN) modulation of osteoclastogenesis. J Bone Miner Res. 2005;20(9):1692–1699. doi: 10.1359/JBMR.050515 [DOI] [PubMed] [Google Scholar]
- 13.Zou W, Schwartz H, Endres S, Hartmann G, Bar-Shavit Z. CpG oligonucleotides: novel regulators of osteoclast differentiation. FASEB J. 2002;16(3):274. [DOI] [PubMed] [Google Scholar]
- 14.Krisher T, Bar-Shavit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem. 2014;115(12):2146–2154. doi: 10.1002/jcb.24891 [DOI] [PubMed] [Google Scholar]
- 15.Lambert G, Fattal E, Couvreur P. Nanoparticulate systems for the delivery of antisense oligonucleotides. Adv Drug Deliv Rev. 2001;47(1):99–112. [DOI] [PubMed] [Google Scholar]
- 16.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. [DOI] [PubMed] [Google Scholar]
- 17.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. [DOI] [PubMed] [Google Scholar]
- 18.Xiu KM, Yang JJ, Zhao NN, Li JS, Xu FJ. Multiarm cationic star polymers by atom transfer radical polymerization from β-cyclodextrin cores: influence of arm number and length on gene delivery. Acta Biomater. 2013;9(1):4726–4733. doi: 10.1016/j.actbio.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 19.Funhoff AM, van Nostrum CF, Lok MC, Fretz MM, Crommelin DJA, Hennink WE. Poly(3-guanidinopropyl methacrylate): A novel cationic polymer for gene delivery. Bioconjug Chem. 2004;15(6):1212–1220. doi: 10.1021/bc049864q [DOI] [PubMed] [Google Scholar]
- 20.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/S0142-9612(02)00445-3 [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Zhang L, Li Q. Induction of apoptosis in cancer cells through N-acetyl-L-leucine-modified polyethylenimine-mediated p53 gene delivery. Colloids Surf B Biointerfaces. 2015;135:S0927776515301387. [DOI] [PubMed] [Google Scholar]
- 22.Xu QH, Yuan Q, Zhang YQ, et al. Ocular metastasis in elderly male bladder cancer patients: potential risk factors. Am J Mens Health. 2020;14(2):1557988320908998. doi: 10.1177/1557988320908998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu W, Yi Z, Yang Z, Fei H, Shen Y. N-AC-L-Leu-PEI-mediated miR-34a delivery improves osteogenic differentiation under orthodontic force. Oncotarget. 2017;8(66):110460–110473. doi: 10.18632/oncotarget.22790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen Y, Liu Y, Gao H, et al. N -Acetyl- l -leucine-polyethylenimine-mediated miR-34a delivery improves osteogenesis and bone formation in vitro and in vivo. RSC Adv. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y-M, Ishii K, Hirokawa M, et al. CpG-ODN 2006 and human parvovirus B19 genome consensus sequences selectively inhibit growth and development of erythroid progenitor cells. Blood. 2010;115(22):4569–4579. doi: 10.1182/blood-2009-08-239202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nobutaka H. CpG oligodeoxynucleotide nanomedicines for the prophylaxis or treatment of cancers, infectious diseases, and allergies. Int J Nanomedicine. 2017;12:515–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014;13(2):299–312. doi: 10.1586/14760584.2014.863715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Yan Y, Fang M, et al. MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice. Int Immunopharmacol. 2012;13;4:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krisher T, Bar㏒Havit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem. 2014;115(12):2146–2154. [DOI] [PubMed] [Google Scholar]
- 30.Klinman MD. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4(4):249–259. [DOI] [PubMed] [Google Scholar]
- 31.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. Strategies for enhancing the immunostimulatory effects of CpG oligodeoxynucleotides. J Control Release. 2004;97(1):1–17. doi: 10.1016/j.jconrel.2004.02.022 [DOI] [PubMed] [Google Scholar]
- 32.Kurreck J. Antisense technologies: improvement through novel chemical modifications . Febs J. 2003;270(8):1628–1644. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal S, Zhao Q. Antisense therapeutics. Curr Opin Chem Biol. 1998;2(4):519–528. doi: 10.1016/S1367-5931(98)80129-4 [DOI] [PubMed] [Google Scholar]
- 34.Heikenwalder M, Polymenidou M, Junt T, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10(2):187–192. doi: 10.1038/nm987 [DOI] [PubMed] [Google Scholar]
- 35.Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int J Nanomed. 2012;7:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Yan T, Xu S, Feng S, Gao XD. Graphene oxide-chitosan nanocomposites for intracellular delivery of immunostimulatory CpG oligodeoxynucleotides. Mat Sci Eng C. 2017;73:144. doi: 10.1016/j.msec.2016.12.072 [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, Lin C, Hou X, et al. Enhancing the osteogenic capacity of MG63 cells through N-isopropylacrylamide-modified polyethylenimine-mediated oligodeoxynucleotide MT01 delivery. RSC Adv. 2017;7(43):27121–27127. doi: 10.1039/C6RA27182K [DOI] [Google Scholar]
- 38.Shen Y, Liu Y, Gao H, et al. N-Acetyl-l-leucine-polyethylenimine-mediated miR-34a delivery improves osteogenesis and bone formation in vitro and in vivo. RSC Adv. 2018;8(15):8080–8088. doi: 10.1039/C7RA12548H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsiao KC, Chu PY, Chang GC, Liu KJ. Elevated expression of lumican in lung cancer cells promotes bone metastasis through an autocrine regulatory mechanism. Cancers. 2020;12(1):233. doi: 10.3390/cancers12010233 [DOI] [PMC free article] [PubMed] [Google Scholar]