Dear Editor,
The circadian system is key for optimal functioning by maintaining synchrony between internal circadian rhythms, behaviors, and external cues. Many clinicians are not fully aware, however, of the far-reaching implications of the circadian system for human health. Clinical attention to circadian rhythms has largely focused on sleep disturbances. The impact of the circadian system on health is, however, much broader. Clinical diagnoses are often based on single time point assessments during the day, ignoring circadian influences on physiology. Even when time is considered, using (external) clock time ignores the large interindividual differences in internal timing. Recent advances in techniques for the estimation of circadian phase will hopefully soon make it feasible to include circadian timing in clinical diagnosis and in the treatment of disease.
Although the circadian system was previously considered a single oscillator, it is now recognized as a multioscillator system. In addition to the central pacemaker in the hypothalamic suprachiasmatic nucleus (SCN), it includes peripheral oscillators in virtually every organ and cell of the body [1]. Circadian misalignment refers to abnormal timing between SCN rhythms and behavior (e.g. sleeping during the biological day), the environment (e.g. exposure to light during the biological night), or peripheral oscillators, and between different peripheral oscillators (Figure 1C).
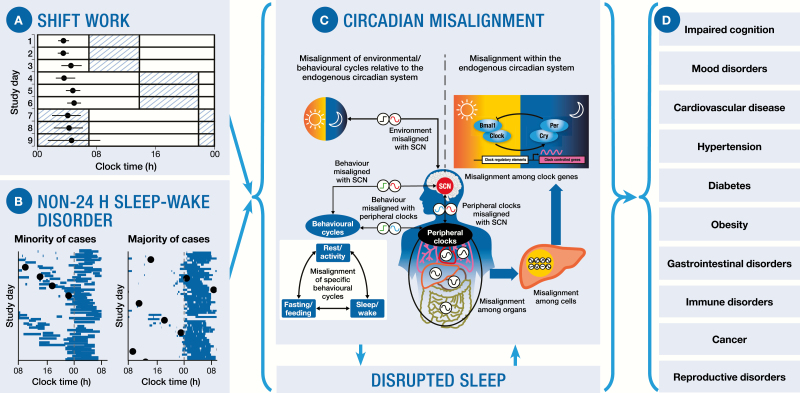
Figure 1.
Shift work and Non-24-h Sleep-Wake Disorder have significant implications for adverse clinical health outcomes. (A) Example of shiftwork: behavioral, externally driven circadian misalignment caused by the occupational requirements (adapted from [11]). Blue hatched bars represent work episodes, white bars represent free time or sleep, and filled circles (mean [SE]) represent the peak of melatonin as a marker of central circadian phase. With the change from working day shifts to night shifts, the circadian pacemaker is misaligned with the timing of work and sleep. (B) Examples of N24SWD: a chronic, intrinsically driven circadian disruption. Blue bars represent sleep episodes; filled circles represent the peak of melatonin or cortisol as markers of central circadian phase. The left panel illustrates what most clinicians consider is the typical manifestation of N24SWD, however, only a small proportion maintain entrainment between circadian and sleep cycles. The right panel illustrates that most patients are either not diagnosed or misdiagnosed (e.g. insomnia); because they do not present with a non-24-h sleep-wake profile (adapted from [12]). Circadian phase assessments are rarely conducted to confirm diagnoses. (C) The molecular mechanism generating endogenous circadian rhythms consists of intracellular transcriptional/translational positive and negative feedback loops involving a complex clock-gene regulatory network, of which only one core feedback loop is displayed here for simplicity. Shift work and N24SWD both lead to circadian misalignment and (consequential) sleep disruption. Circadian misalignment often manifests between behavioral and environmental cycles and the central pacemaker in the SCN and between behavioral and peripheral circadian oscillators found in virtually every organ and cell of the body. Internal desynchrony occurs between peripheral oscillators and the SCN, and among organs, cells and clock genes (adapted from [1]). (D) Circadian misalignment and sleep disruption have been implicated in numerous adverse health outcomes.
Recently, the Nobel Prize in Physiology or Medicine was awarded to three pioneers in circadian biology for their ground-breaking work unravelling the molecular mechanisms of the circadian clock. The Nobel Prize Committee recognized not only the value of uncovering the mechanisms that drive circadian rhythms, but also their important health implications. In modern society, circadian misalignment is common and is associated with poor health [1, 2]. Night shift work is an example of circadian misalignment caused by occupational requirements (Figure 1A). Individuals are required to work during the biological night, and sleep during the biological day, when sleep propensity and quality are low. Night work is highly prevalent, reported in about 14% of the working population. The societal impact of shift work is extensive, including high rates of chronic disease, increased healthcare costs, poor quality of life, and increased risk of—sometimes catastrophic—accidents [3].
Many disease states may be caused or exacerbated by exposure to circadian misalignment (Figure 1D), and/or the consequential recurrent and chronic sleep deficiency. Shift workers often experience impaired alertness and cognition including diminished attention, memory, and executive function [3]. Shift workers also experience psychosocial disruption and impaired emotional regulation, along with increased risk of depression.
Circadian misalignment can also have profound adverse metabolic consequences [1, 2]. Circadian misalignment and disturbed sleep are independent risk factors for developing obesity and diabetes [4]. As a component of circadian misalignment, the mistiming of food intake, i.e. during the biological night, has been shown to impair glucose tolerance, pancreatic beta cell function, and may reduce energy expenditure [5]. Extensive evidence also links shift work with numerous gastrointestinal disorders [6].
The circadian system influences cardiovascular function [7, 3], including blood pressure regulation, cardiac sympathovagal balance, platelet activation, and fibrinolysis. Circadian misalignment in shift work has also been linked to autoimmune disorders and impaired immune function. Other diseases that are associated with circadian misalignment and sleep disruption may be mediated by immunological mechanisms: heart disease, metabolic syndrome, stroke, and cancers all share inflammation as a risk factor or modifier. There is also animal-based evidence of environmental circadian misalignment causing disruption to innate and adaptive immune processes, increasing susceptibility to cancer, sepsis, intestinal inflammation, and early death [8]. Shift work was classified by the World Health Organization as a probable carcinogen, and this classification was recently re-affirmed.
Circadian misalignment also occurs in common clinical conditions of reduced light perception including aging and diabetic retinopathy, and in Delayed and Advanced Sleep-Wake Phase Disorder, affecting many adolescents and older adults, respectively. Furthermore, reduced contrast between day and night light exposure, increasingly prevalent due to indoor lifestyles, widespread artificial lighting, and (blue) light-emitting electronics, weakens circadian synchronization and potentially promotes adverse health.
Another manifestation of circadian misalignment, which has received much less attention, is Non-24-h Sleep-Wake Disorder (N24SWD); typified by chronic circadian disruption due to an inability of the SCN to be synchronized by external time cues, (i.e. the light–dark cycle), and for which a pharmacological treatment has recently been FDA approved. This disorder is experienced by most totally blind individuals without light perception [9], although cases are also reported in sighted patients [10].
In N24SWD, since the endogenous circadian period (or cycle length) is typically slightly longer than 24 h, the pacemaker slowly drifts in and out of phase with the 24-h external environmental cycle. In only a minority of blind patients is this drifting of their endogenous circadian system matched by a parallel drifting of their habitual sleep–wake cycles. Most patients, however, attempt to sleep at night and remain awake during the day, to match work/school, and social schedules. In this way, what is thought to be the classical clinical presentation of N24SWD—a gradual, daily shifting of the timing of the sleep/wake cycle—is the exception (Figure 1B). Instead, in most patients while they attempt to sleep at night, the pacemaker becomes misaligned from sleep–wake and other behavioral cycles on a cyclically recurring basis, causing sleep disruption, daytime sleepiness and napping, and impaired mood. Because of the desynchrony between the sleep/wake cycle and the endogenous circadian pacemaker in the majority of cases, the condition is notoriously difficult to diagnose with diaries or wrist actigraphy and instead requires repeated assessments of the melatonin rhythm spanning weeks or months [9, 12].
Given the high prevalence of circadian misalignment in N24SWD, there is a need for future studies to better understand the pathophysiology and develop novel treatments in N24SWD, and other circadian disorders [9]. Clinical management guidelines are urgently needed to prevent, diagnose, and treat these highly prevalent circadian disorders and ultimately reduce their substantial healthcare burden.
Acknowledgments
The authors wish to thank Charles A. Czeisler for providing insights into the pathophysiology of N24SWD and circadian misalignment.
Conflict of interest statement. Drs. Sletten, Cappuccio, Davidson, Van Cauter, Rajaratnam, and Scheer received consulting fees and travel support from Vanda Pharmaceuticals to attend an Expert Perspectives Meeting on the Health Consequences of Non-24 in the Blind Population. This meeting was the basis of the current manuscript. The funders had no role in the decision to publish or in the preparation of the manuscript. Dr. Van Cauter is a consultant for Philips Respironics and the Sleep Number Bed company and receives investigator-initiated grant support from Astra-Zeneca, Merck and Shire. In 2018, she received speaker fees from Dane Garvin Ltd. Dr. Rajaratnam has been a consultant for Philips Respironics and the Alertness CRC, and an advisory board member of Sleep Health Foundation and has received funds for research paid to Monash from Teva Pharmaceuticals, Shell, Rio Tinto & Seeing Machines. Dr. Scheer received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Pfizer Pharmaceuticals, and Kellogg Company. Dr. Sletten, Dr. Cappuccio, and Dr. Davidson declare that they have no further competing interests.
References
- 1. Qian J, et al.. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27(5):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Potter GD, et al.. Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. 2016;37(6):584–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kecklund G, et al.. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. [DOI] [PubMed] [Google Scholar]
- 4. Leproult R, et al.. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mason AC, et al.. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bishehsari F, et al.. Circadian rhythms in gastrointestinal health and diseases. Gastroenterology. 2016;151(3):e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chellappa SL, et al.. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans JA, et al.. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323: doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- 9. Lockley SW, et al.. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015;386(10005):1754–1764: doi: 10.1016/S0140-6736(15)60031-9. [DOI] [PubMed] [Google Scholar]
- 10. Hayakawa T, et al.. Clinical analyses of sighted patients with non-24-hour sleep-wake syndrome: a study of 57 consecutively diagnosed cases. Sleep. 2005;28(8):945–952. [DOI] [PubMed] [Google Scholar]
- 11. Rajaratnam SM, et al.. Health in a 24-h society. Lancet. 2001;358(9286):999–1005. [DOI] [PubMed] [Google Scholar]
- 12. Uchiyama M, et al.. Non-24-hour sleep-wake rhythm disorder in sighted and blind patients. Sleep Med Clin. 2015;10(4):495–516: doi: 10.1016/j.jsmc.2015.07.006. [DOI] [PubMed] [Google Scholar]