Abstract
Introduction
Characterizing flavors are widely available in e-cigarettes and motivate initiation and continued use. Flavors may enhance appeal and facilitate development of addiction to tobacco products through modulation of tobacco products’ reinforcing or aversive actions. Palatable flavors (eg, fruit) may increase appeal through primary reinforcing properties. Menthol’s cooling and anesthetic effects may increase appeal by counteracting nicotine’s aversive effects. Genetics provide a method for modeling individual differences in sensitivity to nicotine’s effects. A common polymorphism, rs16969968, encoded in the α5 nicotinic acetylcholine receptor subunit gene (CHRNA5), is a well-recognized marker for smoking risk and reduces sensitivity to nicotine aversiveness.
Methods
This pilot study tested how flavors impacted e-cigarette appeal and self-administration. In a single testing day, cigarette smokers (N = 32; 94% menthol-smokers) self-administered e-cigarettes containing e-liquids differing in nicotine level (0 mg/mL, 24 mg/mL) and flavor (unflavored, menthol, fruit-flavored) within directed and ad libitum e-cigarette paradigms. Subjective drug effects, number of puffs, rs16969968 genotype, plasma nicotine, and menthol glucuronide levels were collected.
Results
Menthol partially ameliorated nicotine aversiveness; fruit did not. In nicotine’s absence, fruit flavor increased self-reported preference and ad libitum use relative to menthol-containing or unflavored e-liquids. Individuals with high-smoking-risk rs16969968 genotype (N = 7) reported greater craving alleviation following directed administration of nicotine-containing e-liquids, showed a trend rating nicotine-containing e-liquids as less harsh, and self-administered more nicotine during ad libitum compared to individuals with low-smoking-risk genotype (N = 23).
Conclusions
While menthol countered aversiveness of nicotine-containing e-liquids, fruit flavor increased appeal of nicotine-free e-liquids. These preliminary findings suggest menthol and fruit flavor increase e-cigarettes’ appeal through distinct mechanisms.
Implications
This study provides a detailed characterization of the effects of flavors (unflavored, menthol, fruit), nicotine (0 mg/mL, 24 mg/mL) and their interactions on the subjective drug effects and ad libitum self-administration of e-cigarettes. Genetics were used to assess these effects in higher-smoking-risk (diminished sensitivity to nicotine aversiveness) and lower-risk groups. Findings could inform impact of regulation of flavors or nicotine in e-cigarettes, and their impacts on vulnerable sub-populations.
Introduction
Electronic cigarettes (e-cigarettes) are widely used, often contain flavors, and can deliver nicotine levels reaching those delivered by combustible cigarettes.1 Nicotine is addictive, and facilitates tobacco product maintenance (including e-cigarettes) and its negative health consequences.2–4 Nicotine produces aversive actions (eg, harshness, bitterness) particularly at higher doses,5,6 and rewarding actions, which have dissociable neurobiological mechanisms7 and likely represent separate factors (rather than a single continuous factor), which may each impact appeal. Sensitivity to nicotine aversiveness protects against regular tobacco use or high nicotine intake, balance between drugs’ reinforcing and aversive effects may predict regular use.6,8,9 A regulatory challenge is balancing e-cigarettes’ potential for harm-reduction versus risk.10–13 There are important clinical and regulatory implications to understanding factors, such as flavors, impacting the appeal and use of nicotine-containing e-cigarettes.
The sheer number of available e-liquid flavors presents a research challenge. However, many chemicals used in flavored e-liquids, are commonly found across a wide range of flavor categories (eg, aldehydes and other carbonyl compounds), or are common within certain flavor categories and contribute to their characteristic flavors, such as menthol (for menthol and minty e-liquids) and “fruity esters” (for fruit-flavored e-liquids).14 Investigating popular flavor categories, such as menthol and fruit,15 using e-liquids containing these common characterizing chemicals (eg, menthol, fruity esters) could identify shared and discernable mechanisms by which different categories/types of flavors impact nicotine’s appeal and use (eg, by undermining/masking aversive properties of nicotine-containing e-cigarettes, increasing rewarding properties, or both).
Flavors are motivating factors for initiation of e-cigarettes, with “sweet” and “cooling” e-cigarette flavors rated as more liked than “harsh” or “bitter” flavors.16,17 While fruit flavor increased “sweetness” and nicotine increased “throat hit” ratings, perceived sweetness but not throat hit, was associated with higher appeal (intent to use, willingness to pay, liking).18 Young adult smokers rated flavored e-liquids (including green apple) as more rewarding, were willing to work harder to receive puffs and self-administered more than unflavored e-liquid.19 Therefore, flavors that are perceived as sweet or to have cooling properties or that counteract nicotine’s aversive subjective effects may make e-cigarettes more palatable and may alter use behavior.
Flavors, like menthol, may enhance appeal of and facilitate addiction to tobacco products through modulation of tobacco products’ aversive or reinforcing actions, through central and peripheral mechanisms.20 Menthol is a common component in e-liquids, even many not labeled menthol or mint.21 Menthol has cooling, analgesic, and counterirritant properties and, even at below-characterizing levels, can decrease the harshness of nicotine and tobacco smoke,22 which could increase appeal of nicotine-containing products, while at higher levels, menthol can have its own harshness or bitterness.5 While menthol’s cooling, analgesic and counterirritant properties are due at least in part to its peripheral actions on transient receptor potential (TRP) channels,23 it may also impact nicotine’s effects through central actions.24,25
Individual differences in sensitivity to subjective nicotine effects and vulnerability to tobacco dependence are partly genetically modulated. A common single nucleotide polymorphism (SNP), rs16969968 (A/G), encodes an amino acid substitution (N>D) in CHRNA5, the gene for the α5 subunit of nicotinic acetylcholine receptor (α5 nAChR). The A-allele is more common in US European ancestry populations (frequency ~0.38) compared to African ancestry populations (frequency ~0.07).26 The A-allele of rs16969968 is associated with lower functional response to nicotinic agonists, greater risk for nicotine dependence, heaviness of smoking (eg, average cigarettes per day), and negative health outcomes from tobacco products27–29 that are likely due to reduced sensitivity to nicotine aversiveness in individuals carrying the risk allele.30 Smokers with risk allele (rs16969968 *A) rated intravenous nicotine as less aversive, but not more/less rewarding relative to smokers with no risk allele.31 However, no laboratory studies have assessed rs16969968 in humans in response to nicotine delivered by commercial tobacco products, or the impact of flavor. The ability to personalize e-cigarettes (eg, nicotine level, flavor) may enhance the likelihood of abuse by individuals who may otherwise find the nicotine content or flavor of other tobacco products not reinforcing or aversive.
This preliminary study used subjective drug effect ratings during a directed e-cigarette self-administration paradigm and amount of self-administration during an ad libitum paradigm.
to assess whether menthol, compared to fruit or no flavor, counteracts aversive effects of a high dose of nicotine (24 mg/mL), and/or undermines a naturally occurring “protective” profile of aversion-sensitivity in rs16969968*G homozygotes (henceforth “protective” [GG]), vs. rs16969968*A allele carriers (henceforth “risk” (A-carrier)).
Methods
Participants
Smokers were recruited from the New Haven area using advertisements. Inclusion criteria were: aged 18–50; ≥5 cigarettes/day for the past year; urine cotinine (≥3 NicAlert; ≥100 ng/mL); not seeking smoking-cessation; lifetime e-cigarette use (ie, must have tried e-cigarettes in lifetime but no specific inclusion/exclusion for current or past regularity of use); acceptable birth control for women, able to read and write English. Subjects were excluded for history of major or current medical illnesses that the physician investigator deemed a contraindication for participation; current psychiatric diagnosis and/or treatment for Axis I disorders; current substance use disorder, other than nicotine; known allergy to propylene glycol (PG), vegetable glycerin, menthol, or green apple flavorants; pregnant or breastfeeding; inability to fulfill scheduled visits and procedures. For baseline and demographic information see Table 1. The VA Connecticut Healthcare System and Yale University Human Subjects Subcommittees approved the study. Participants were paid following participation in each visit.
Table 1.
Demographics and Baseline Measures
| Full sample (N = 32) | Protective genotype (GG; N = 23) | Risk genotype (A-Carrier; N = 7) | Statistics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Variable | N | % | N | % | N | % | df | X 2 | p | |||
| Sex | Male | 21 | 65.63 | 16 | 69.57 | 3 | 42.86 | 1 | 1.60 | .21 | |||
| Female | 11 | 34.38 | 7 | 30.43 | 4 | 57.14 | |||||||
| Race | African American | 23 | 71.88 | 19 | 82.61 | 4 | 57.14 | 2 | 3.02 | .22 | |||
| European American | 6 | 18.75 | 3 | 13.04 | 1 | 14.29 | |||||||
| Other | 3 | 9.38 | 1 | 4.35 | 2 | 28.57 | |||||||
| Ethnicity | Not Hispanic/ Not Latino | 29 | 90.63 | 22 | 95.65 | 5 | 71.43 | 1 | 2.90 | .09 | |||
| Hispanic/Latino | 3 | 9.38 | 1 | 4.35 | 2 | 28.57 | |||||||
| Highest level education | Partial high school | 2 | 6.25 | 2 | 8.70 | 0 | 0.00 | 2 | 2.00 | .37 | |||
| High School graduate / GED | 21 | 65.63 | 16 | 69.57 | 4 | 57.14 | |||||||
| Partial college training | 9 | 28.13 | 5 | 21.74 | 3 | 42.86 | |||||||
| Usual cigarette brand menthol vs. non-menthola | |||||||||||||
| Menthol-preferring | 30 | 93.75 | 22 | 95.65 | 7 | 100.00 | |||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | df | F | p | ||
| Demographics | |||||||||||||
| Age | 32 | 32.50 | 7.88 | 23 | 32.52 | 7.91 | 7 | 33.43 | 9.27 | 1, 4.4 | 0.07 | .80 | |
| Body mass index (BMI) | 32 | 32.93 | 8.88 | 23 | 32.09 | 9.45 | 7 | 33.93 | 7.91 | 1, 18.2 | 0.22 | .64 | |
| Years of Education | 26 | 12.08 | 0.80 | 17 | 12.00 | 0.94 | 7 | 12.14 | 0.38 | 1, 0.10 | 0.15 | .70 | |
| Estimated IQ (SILS) | 26 | 86.08 | 13.23 | 17 | 84.06 | 12.33 | 7 | 85.71 | 14.10 | 1, 13.59 | 0.08 | .78 | |
| Baseline smoking and e-cigarette measures | |||||||||||||
| Cigarette/nicotine dependence | FTND total score | 32 | 4.75 | 2.29 | 23 | 4.17 | 1.99 | 7 | 6.29 | 2.21 | 1, 23.9 | 5.74 | .02 |
| Heaviness of smoking | Average number of cigarettes per day | 32 | 12.19 | 5.96 | 23 | 11.09 | 5.20 | 7 | 14.29 | 7.95 | 1, 54.9 | 1.58 | .22 |
| Age of smoking onset | Age start smoking regularly (≥3x/wk) | 32 | 17.06 | 3.31 | 23 | 17.48 | 3.42 | 7 | 15.29 | 1.38 | 1, 25.8 | 2.68 | .11 |
| Smoking abstinence history | Longest lifetime smoking abstinence (mo) | 32 | 7.59 | 9.43 | 23 | 6.91 | 6.14 | 7 | 6.86 | 13.21 | 1, 0.02 | 0.00 | .99 |
| Cigarette dependence | Penn State Cigarette Dependence Scaleb | 31 | 12.58 | 9.32 | 22 | 11.14 | 4.30 | 7 | 17.29 | 18.28 | 1, 200.8 | 2.26 | .14 |
| E-cigarette dependence | Penn State E-Cigarette Dependence Scaleb | 31 | 5.19 | 3.50 | 22 | 5.36 | 3.82 | 7 | 5.14 | 2.85 | 1, 0.259 | 0.02 | .89 |
| Frequency of e-cigarette use | Days of self-reported e-cigarette use in past month | 27 | 7.19 | 9.36 | 20 | 7.85 | 10.44 | 5 | 6.80 | 5.63 | 1, 4.41 | 0.05 | .83 |
| Baseline self-reported withdrawal symptoms and smoking urges | |||||||||||||
| Withdrawal symptoms | Minnesota Nicotine Withdrawal Scale (MNWS) Total Score | 31 | 19.52 | 14.36 | 22 | 19.09 | 13.07 | 7 | 15.26 | 12.54 | 1, 78.05 | 0.47 | .50 |
| Smoking urges | Brief Questionnaire on Smoking Urges (BQSU) Factor 1 | 31 | 25.03 | 9.15 | 22 | 25.18 | 9.54 | 7 | 22.86 | 9.01 | 1, 28.70 | 0.32 | .57 |
| Brief Questionnaire on Smoking Urges (BQSU) Factor 2 | 31 | 14.65 | 7.14 | 22 | 15.14 | 7.41 | 7 | 13.00 | 6.53 | 1, 24.24 | 0.46 | .50 | |
| N | Mean | SD | N | Mean | SD | N | Mean | SD | df | F | p | ||
| Baseline plasma nicotine and menthol indicators | |||||||||||||
| Nicotine metabolite ratio | 30 | 0.37 | 0.30 | 21 | 0.35 | 0.19 | 7 | 0.28 | 0.16 | 1, 0.02 | 0.62 | .44 | |
| Plasma cotinine (ng/mL) | 30 | 183.90 | 115.21 | 21 | 154.20 | 100.78 | 7 | 277.00 | 124.49 | 1, 79175.3 | 6.95 | .01 | |
| Plasma 3-hydroxycotinine (ng/mL) | 30 | 59.57 | 47.82 | 21 | 49.38 | 43.42 | 7 | 74.71 | 45.86 | 1, 3369.3 | 1.74 | .20 | |
| Plasma nicotine (ng/mL; low values as missing)c | 23 | 2.90 | 2.48 | 15 | 3.37 | 2.88 | 6 | 2.32 | 1.24 | 1, 4.7 | 0.72 | .41 | |
| Plasma nicotine (ng/mL; lowest values imputed) c | 30 | 2.46 | 2.31 | 21 | 2.69 | 2.65 | 7 | 2.13 | 1.24 | 1, 1.7 | 0.29 | .60 | |
| Plasma menthol glucuronide (ng/mL; low values as missing)c | 23 | 12.79 | 7.62 | 16 | 11.37 | 4.73 | 6 | 12.27 | 6.96 | 1, 3.5 | 0.12 | .73 | |
| Plasma menthol glucuronide (ng/mL; lowest values imputed) c | 30 | 10.74 | 7.64 | 21 | 9.61 | 5.21 | 7 | 11.09 | 7.08 | 1, 11.4 | 0.35 | .56 | |
aMenthol preference was based on participant’s self-reported usual brand of cigarette.
bPenn State Cigarette and E-Cigarette Dependence Scales (Foulds et al. Nicotine Tob Res. 2015;17(2):186-92.): These questionnaires were designed to measure cigarette or e-cigarette dependence using parallel items (10 items each; scores of 0–3 = not dependent, 4–8 = low dependent, 9–12 = medium dependence, 13+ = high dependence). In some cases, individuals in this sample missed up to two items. The scales are still presented as sums of the available items. When data were analyzed for only subjects with complete data on each scale, the pattern of findings remained the same and there were still no genotype differences.
cIn cases where nicotine or menthol glucuronide levels were below the level of detection of the assay, the data were analyzed two ways: first treating these datapoints as missing, then imputing the lowest detection point of the assay in for these datapoints. The pattern of findings was the same in both cases.
Procedures
After written informed consent was obtained, eligibility for study participation was determined using demographics, tobacco use, medical and psychiatric screening, biochemical (urine screens) and physiological (heart rate, blood pressure) measures. A brief e-cigarette training session introduced eligible subjects to the e-cigarette (eg, press button to activate then inhale), e-liquids (without nicotine), and “directed self-administration” puff parameters (described below).
For the Test Day, subjects were asked to refrain from smoking (10 hours) and menthol-containing products (eg, tea, gum; except cigarettes) (24 hours) or eating breakfast prior to the ~8AM session. After a light standard breakfast, an intravenous catheter was inserted into a forearm vein for blood draws. The single test day consisted of a Directed Self-Administration component, followed by lunch, then an ad libitum Self-Administration component (see Figure 1 for test day overview).
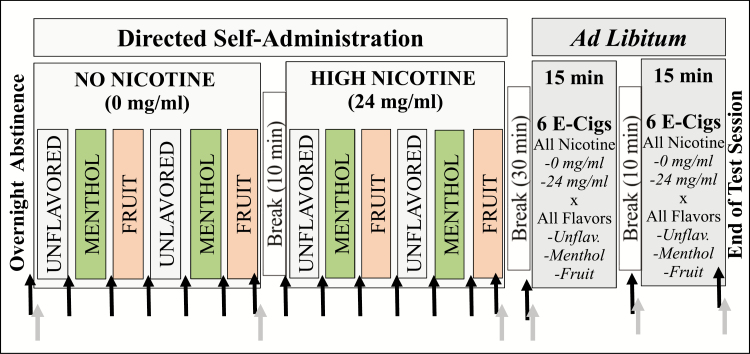
Figure 1.
Test Session Overview. The order of flavor sub-blocks was randomized across individuals (example order shown), while nicotine block order was constant for all individuals (Block 1 = 0 mg nicotine/mL e-liquid; Block 2 = 24 mg nicotine/mL e-liquid). Each flavor sub-block consisted of 3 directed puffs (4 s puffs; 9 Watts; 15 s inter-puff-interval). There was a 10 min break between the nicotine blocks, and a 5 min break between each flavor sub-block. Arrows indicate when measures were collected. Black arrows (subjective drug effects); gray arrows (blood draw timepoints to measure cotinine, 3-hydroxycotinine, nicotine, menthol glucuronide at baseline [blood draw 1] and nicotine and menthol glucuronide at all time-points [blood draw 1–6]).
“Directed Self-Administration” consisted of blocks of escalating nicotine levels (block 1 = 0 mg/mL [“0NIC”]; block 2 = 24 mg/mL [“24NIC”]) and, within each block, two sub-blocks of each flavor (unflavored, menthol, fruit [green apple]). The order of flavor sub-blocks was counterbalanced across subjects but held consistent within subjects across nicotine blocks. Breaks from puffing (10 minutes between nicotine blocks; 5 minutes between flavor sub-blocks) allowed for assessments, subject rest, and acute flavor effects to dissipate. Subjective drug effects were assessed after each sub-block. Puffing parameters, which were controlled through e-cigarette device settings (AutoMode), were three puffs of 4-second puff duration with 15-second inter-puff intervals for each flavor sub-block. Participants but not the research team were blind to e-liquid conditions. To facilitate subject recall of e-cigarette experiences during directed self-administration (to inform choices during ad libitum) the six different e-cigarettes (each containing a different nicotine*flavor e-liquid condition) were color-coded and displayed throughout the session.
The “ad libitum Self-Administration” component began following the 30-minute lunch break. Participants had free access to all six e-cigarettes (six nicotine*flavor combinations) for two 15-minute ad libitum blocks, separated by a 10-minute break. During both ad libitum blocks, participants could puff from as many or as few of the e-cigarettes as they chose (ie, all six options were available simultaneously for both blocks). During ad libitum, e-cigarettes were set to Manual Mode (ie, puff duration determined by button press duration, up to 10 seconds) to enable naturalistic puffing.
Outcome Measures
Subjective drug effects, during directed self-administration, were measured with an adapted Drug Effects Questionnaire (DEQ)and Labeled Magnitude Scale, general version (gLMS). Summary scores were created from means of conceptually-related items. The primary outcomes were five “aversive effects” summary measures: (1) “Overall Aversiveness” (mean of “feel bad effects” and “dislike” items), (2) “Harshness” (mean of “harshness in mouth,” “harshness in throat,” “harshness in chest/lungs”), (3) “Dislike sensation” (mean of “dislike sensation in mouth,” “dislike sensation in throat,” “dislike sensation in chest/lungs”), (4) “Aversive Flavor/Taste” (mean of “taste bitter,” “dislike flavor/taste”), and (5) “Other Aversive Symptoms” (mean of “headache,” “nauseous,” “urge to cough”). Ten secondary outcomes included “aversive nicotine-withdrawal-related effects” (affect, cognitive, craving for “regular” cigarette), “rewarding effects” (overall, rewarding nicotine-related, cooling, rewarding taste/flavor) and “other effects” (overall e-cigarette effect, strong sensation, other) summary measures (for details see Supplementary Materials).
“Number of puffs” was derived from built-in manufacturer-supplied firmware (MyVapors) from each e-cigarette during ad libitum.
Biochemical measures were collected to characterize the sample and validate the paradigm. DNA, extracted from peripheral blood using a commercial kit (PureGene; Gentra, Minneapolis, MN), was used to genotype CHRNA5 (rs16969968) with TaqMan method and primers (Applied Biosystems, Foster City, CA). Plasma cotinine and 3-hydroxycotinine (3HC) and nicotine, were measured employing LC/MS/MS at session baseline and used to compute the nicotine metabolite ratio (NMR:3HC/cotinine), a marker of nicotine clearance which impacts heaviness of smoking and subjective nicotine effects.32,33 Menthol glucuronide, a menthol metabolite,34,35 also assayed by LC/MS/MS in plasma, was measured at baseline and after each block (Figure 1).
E-liquids and E-cigarette Devices
E-liquids (made-to-order by Pace Engineering Concepts LLC) differed by flavor (unflavored, menthol [3.5%], fruit [green apple]) and nicotine concentration (0 mg, 24 mg nicotine/mL e-liquid) in a factorial design (ie, 6 flavor*nicotine-level combinations) (Supplementary Table 1). The nicotine level (24 mg/mL) is in the upper range of commonly commercially available levels (0–36 mg/mL for most e-liquid companies) and of levels chosen by younger e-cigarette users,36 reinforcing to adult cigarette smokers,37 yet sufficiently high to produce aversive symptoms in adults.5 Base liquids were adjusted to achieve a final propylene glycol (PG) to vegetable glycerin (VG) ratio of approximately 50/50. Since menthol crystals were dissolved with ethanol to create menthol e-liquids, equivalent ethanol levels were also added to non-menthol liquids to control for this variable.38 None of the e-liquids contained added sweeteners. E-liquids were of similar pH level. Menthol and nicotine concentrations were verified by the Yale TCORS Laboratory Core.
Six separate programmable eVic Supreme e-cigarettes (Joyetech USA (Irvine, CA)), were used with built-in MyVapors software version 1.1. Power was set to 9 Watts.
Statistical Approach
Analyses were carried out in JMP 11.0. Variables were checked for normality and transformed when necessary. Genotype groups (A-carrier, GG) were compared on baseline variables using t-tests for continuous variables and chi-square tests for categorical variables. Analyses of all outcome measures were performed using mixed effects models.
Directed Self-administration
Subjective drug effects were assessed after each nicotine*flavor directed sub-block. Separate mixed-effect models were fit for each outcome. Analyses included subjective drug effect summary score ratings from each flavor-by-nicotine sub-block as dependent variables. Nicotine level (0, 24 mg/mL), flavor (menthol, fruit (apple), unflavored) and nicotine-by-flavor were included as fixed effects of interest. Sub-block and nicotine-by-sub-block were included as fixed effects to evaluate period effects and whether those varied by nicotine (see Supplementary Materials for detailed results on sub-block and nicotine-by-sub-block). Subject and block (nested-within-subject) were included as random effects and a first-order autoregressive correlation structure of the errors (AR(1)) was used to account for correlations among repeated measures within individuals. Bonferroni corrections for multiple comparisons were applied separately for primary (pcorrected = .05/5 = .01) and secondary (pcorrected = .05/10 = .005) outcomes. Post hoc comparisons of least square means explained significant interactions and were Bonferroni-corrected within comparison type (ie, nicotine effects within flavor: p = .05/3 flavors = 0.01667; flavor effects within nicotine: p = .05/6 comparisons = 0.00833). p-values reported in the text (in their uncorrected format) all survive the relevant Bonferroni-corrected significance threshold, unless otherwise specified.
As a manipulation check, to confirm that nicotine and menthol were delivered in directed blocks and decreased following lunch break, separate mixed-effect models were fit for each outcome (plasma nicotine, plasma menthol glucuronide) and included blood draw timepoint as a fixed effect (four timepoints: prior to e-cigarette self-administration, post-directed 0NIC and 24NIC blocks, post-lunch-break). Subject and block (nested-within-subject) were included as random effects. Post hoc comparisons among blood-draw timepoints were Bonferroni-corrected.
Ad Libitum Component
The primary outcome for ad libitum was the total number of puffs on each e-cigarette (ie, each nicotine*flavor condition). The model included nicotine, flavor, and nicotine-by-flavor as fixed effects and subject as a random effect.
Secondary outcomes for ad libitum were blood levels of nicotine or menthol glucuronide. Separate models were fit for each and included blood-draw timepoint (post-lunch as ad libitum baseline, and levels at the end of each ad libitum block) and subject as a random effect. For significant effects of blood draw timepoint, post-hoc comparisons of least square means among timepoints were performed and Bonferroni-corrected.
Genetics Analyses
Analysis steps were repeated with genotype (A-carrier vs. GG) and genotype interactions with the other factors in the models (nicotine, flavor, nicotine-by-flavor) as fixed effects. To check for potential skewing by race, the key genetics findings (eg, aversiveness or craving subjective effects; self-administration of nicotine in the ad libitum component) were re-run within the African American group only (n = 23) and the pattern of findings remained consistent.
Results
Of 47 subjects screened, 35 were eligible and 32 participated in test day self-administration protocol. For demographics and baseline data, see Table 1 and Supplementary Figure 1 (additional baseline e-cigarette use measures). Genotypes groups differed (A-carrier > GG) on Fagerstrom Test for Nicotine Dependence (FTND) and baseline cotinine, but not other baseline measures (Table 1).
Directed Self-administration
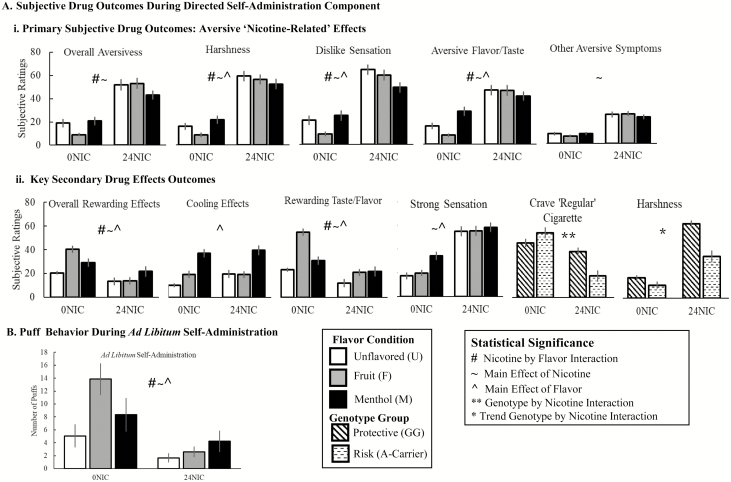
Nicotine-by-flavor interactions are described below, with significant main effects of nicotine or flavor generally noted only in absence of nicotine-by-flavor interactions (see Figure 2A for key findings and Supplementary Table 2 and Supplementary Figure 2 for full results).
Figure 2.
Key subjective drug effect and self-administration behavior outcomes.
Primary “Subjective Drug Effect” Outcomes: “Aversive Effects”
“Overall aversiveness,” “harshness,” and “dislike sensation” were rated higher for 24NIC versus 0NIC within each flavor, and higher for menthol than fruit within 0NIC (Nicotine-by-flavor “overall aversiveness”: F2, 260.1 = 4.96, p = .008; harshness: F2,247.1 = 6.14, p = .003; dislike: F2,258.7 = 7.91, p = .0005). Menthol was rated lower “dislike sensation” than unflavored in 24NIC (24NIC menthol < 24NIC unflavored; p = .0099, does not survive Bonferroni-correction threshold for flavor effects within nicotine level: pcorrected = .05/6 comparisons = 0.0083). “Aversive flavor/taste” was higher for 24NIC versus 0NIC within each flavor, and higher for menthol than fruit or unflavored in 0NIC (Nicotine-by-flavor: F2,257.8 = 8.77, p = .0002). “Other aversive symptoms” were higher for 24NIC versus 0NIC (Nicotine: F2,129.1 = 50.29, p < .0001).
Secondary “Subjective Drug Effect” Outcomes
Rewarding Effects
Fruit 0NIC was higher “overall rewarding” versus unflavored 0NIC, menthol 0NIC, or fruit 24NIC (Nicotine-by-flavor: F2,293.2 = 12.89, p < .0001). “Rewarding flavor/taste” was higher for fruit 0NIC versus unflavored or menthol 0NIC, higher for 0NIC versus 24NIC within each flavor, and within 24NIC a trend (does not survive correction) for higher menthol or fruit versus unflavored (Nicotine-by-flavor: F2,293.0 = 11.57, p < .0001). “Rewarding nicotine-related effects” showed flavor effects (unflavored < fruit < menthol) within 0NIC (Nicotine-by-flavor: F2,251.4 = 5.71, p = .004). “Cooling effect” showed a main effect of flavor (unflavored < fruit < menthol; F2, 266.0 = 30.52, p < .0001).
Nicotine-Withdrawal-Related Aversive Effects
There were no statistically significant main or interactive effects of nicotine and flavor on nicotine-withdrawal-related aversive effects.
Other effects
“Overall e-cigarette effect” was higher for 24NIC versus 0NIC within each flavor, and flavor differed within 0NIC (unflavored0NIC < fruit0NIC < menthol0NIC) (Nicotine-by-flavor: F2,269.7 = 5.60, p = .004). “Strong sensation” was higher for 24NIC versus 0NIC (nicotine: F1,181.5 = 48.97, p < .0001) and menthol versus unflavored or fruit (flavor: F2,253.9 = 14.82, p < .0001).
Genetics
Significant or trend genetics-by-nicotine or -flavor interactions are noted below (reported results did not survive Bonferroni correction, unless otherwise noted). There were no trend or significant main effects of genotype. See Figure 2 for key findings, Supplementary Table 3 for full results and Supplementary Material for more detailed description of interactions.
CHRNA5 (rs16969968) by nicotine or flavor interactions showed a pattern of effects consistent with, and extending, the “risk” effects previously reported for the rs16969968 A-allele. A trend genotype-by-nicotine interaction on “harshness” (F1,27.5 = 2.95, p = .097; Figure 2) reflected that although both genotype groups rated 24NIC as more aversive than 0NIC, “protective” (GG), versus “risk” (A-allele) rated 24NIC as harsher (24NIC A-Carrier < 24NIC GG; t = 3.23, p = .002). A genotype-by-nicotine effect on “craving for a ‘regular’ cigarette” survived Bonferroni correction (F1,27.1 = 13.07, p = .001; Figure 2); and reflected reduced self-reported craving following the 24NIC versus 0NIC within “risk” genotype (A-carrier) only (24NIC A-Carrier < 0NIC A-Carrier; t = 4.09, p = .0002). A genotype-by-nicotine interaction on aversive affect (F1,26.6 = 4.52, p = .04) reflected exacerbated aversive affect following nicotine in the “protective” group (0NIC GG < 24NIC GG; t = 1.8, p = .07). For “dislike sensation,” a genotype-by-nicotine-by-flavor interaction (F2,233.4 = 3.66, p = .03) reflected lower “dislike sensation” for 0NIC versus 24NIC for all genotype*flavor combinations, and genotype differences of flavor ratings of 0NIC e-liquids. Genotype-by-flavor interactions were observed for “rewarding flavor/taste” and trends for “aversive flavor/taste” and “aversive affect” (Supplementary Table 3).
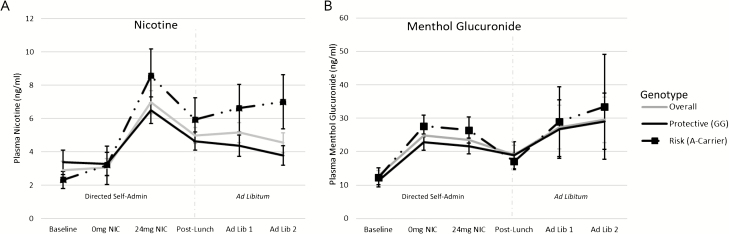
Verification of Nicotine and Menthol Delivery
Within directed blocks, plasma nicotine levels increased post-24NIC block, versus baseline or 0NIC; levels decreased post-lunch, but remained higher than baseline or post-0NIC (timepoint: F3,58.1 = 63.89, p < .0001). Plasma menthol glucuronide increased following both directed blocks (0NIC, 24NIC both contained menthol) versus baseline, and decreased post-lunch, but remained higher than baseline (timepoint: F3,63.9 = 19.98, p < .0001). There were no significant genotype differences at baseline, following directed blocks or post-lunch (which served as baseline for ad libitum) or genotype-by-timepoint effects on nicotine or menthol glucuronide within the directed component. See Supplementary Table 4 and Figure 3.
Figure 3.
Validation of Nicotine and Menthol Delivery. (A) Plasma Nicotine and (B) the menthol metabolite Menthol Glucuronide were assessed at six blood draw timepoints. In order, the timepoints were (1) Baseline (following overnight smoking abstinence and prior to e-cigarette self-administration), (2) 0 mg NIC (following 0 mg/mL nicotine Directed Self-Administration Block 1), (3) 24 mg NIC (following 24 mg/mL nicotine Directed Self-Administration Block 2), (4) Post-Lunch (30 min break from e-cigarette administration, including lunch; timepoint also served as a baseline for the Ad Libitum Component), (5 & 6) Ad Lib 1 and 2 (Ad Libitum blocks 1 and 2 [15 min each, separated by 10 min break]). The dotted gray line separates data points included in manipulation-check analyses validating nicotine and menthol delivery during directed self-administration and expected decreases following lunch (timepoints 1–4) and analyses assessing volume of nicotine and menthol self-administration during ad libitum (as a secondary outcome measure; timepoints 4–6). Means (standard error [SE]) are presented for the sample overall (gray) and split by genotype type group (black solid line = ‘Protective (GG)’, black dotted line with square markers = ‘Risk (A-Carrier)’.
Ad Libitum Self-administration
During ad libitum, subjects puffed more 0NIC fruit versus 24NIC fruit, 0NIC menthol or 0NIC unflavored (nicotine-by-flavor: F2,108.3 = 5.73, p = .004; Figure 2B; Supplementary Table 5), more 0NIC versus 24NIC (nicotine: F1,94.8 = 25.67, p < .0001), and more fruit versus unflavored or menthol (flavor: F2,131.1 = 13.23, p < .0001).
There was no significant change in plasma nicotine (F2,43.3 = 2.81, p = .07) or menthol glucuronide (F2,46.6 = 1.49, p = .24) in ad libitum (Supplemental Table 4; Figure 3).
Genetics: Ad Libitum
Carriers of the rs16969968 risk allele (A-carrier) puffed more fruit than unflavored or menthol; and the rs16969968 protective genotype (GG) puffed more fruit than unflavored while the number of menthol puffs did not significantly differ from fruit or unflavored (genotype-by-flavor: F2,116.3 = 3.44, p = .035; Supplementary Table 5).
Plasma nicotine levels were higher at the end of the ad libitum period for rs16969968 A-carriers (risk) compared to GG genotype (protective), and decreased for the GG genotype group post-ad libitum versus ad libitum baseline (post-lunch) (genotype-by-timepoint: F2,50.6 = 3.19, p = .05; Supplementary Table 4; Figure 3), indicating that A-carriers maintained their nicotine levels during ad libitum self-administration, while the GG group did not. There were no main or interactive effects of genotype on menthol glucuronide across ad libitum.
Discussion
The study had several noteworthy findings. First, nicotine delivery via e-cigarettes produced aversive effects relative to e-cigarettes without nicotine, supporting the validity of our model. Second, in the absence of nicotine, fruit flavor was rated low on aversiveness and high on rewarding effects. Third, in contrast to fruit flavor, menthol was aversive in the absence of nicotine (eg, harshness), diminished harshness of the nicotine-containing e-liquids, and had rewarding (eg, coolness) and other (eg, overall sensation) effects in both the nicotine or no-nicotine conditions. Fourth, nicotine-by-flavor interactions reflected a larger impact of nicotine aversiveness in the fruit condition relative to the menthol condition. For example, the preference for fruit (higher positive ratings, lower aversiveness ratings) observed in the no nicotine condition was largely abolished in the presence of nicotine, while the menthol conditions showed less change in response to nicotine and some indications of lower aversiveness than other flavor conditions in the presence of nicotine. Lastly, the “risk” genotype (A-carriers) of rs16969968 reported greater alleviation of negative abstinence-related symptoms (eg, “craving for regular cigarettes”), a trend towards rating the nicotine condition as less aversive, and self-administered more nicotine during the ad libitum period than the GG genotype.
Our findings suggest that menthol partially mitigated nicotine aversiveness, potentially increasing nicotine-containing e-cigarettes’ appeal, while fruit increased appeal of nicotine-free e-cigarettes with little effect on appeal of high nicotine-containing e-cigarettes. These dissociable patterns should be investigated in intermediate nicotine doses. Our results regarding menthol’s effects on nicotine aversiveness are consistent with prior work,17 for example, showing menthol (3.5%) e-cigarettes, versus non-menthol, were rated as lower in harshness or irritation in the presence of high nicotine (24 mg/mL), but higher in subjective irritation and harshness in the presence of low nicotine,5 and that youth e-cigarette users rated moderate (12 mg/mL) nicotine e-liquids as higher on “like/wanting” when combined with 3.5% menthol, versus non-menthol, but did not experience the same effects of menthol at a lower (6 mg/mL) nicotine.39
The proposed effect of rs16969968 genotype in influencing sensitivity to nicotine aversiveness was supported. Although extensive prior research has linked rs16969968*A with increased risk for heavy smoking27 and preclinical research implicated differences in nicotine aversiveness as a mechanism,30 only one prior study in humans linked this genetic variant with differences in nicotine aversiveness, and in that case nicotine was delivered intravenously.31 Therefore, the current findings are the first demonstration of differences in subjective ratings (craving alleviation; aversiveness) or self-administration behavior (blood nicotine levels following ad libitum) across CHRNA5 genetic variants in humans self-administering nicotine via any commercially available tobacco product, and first demonstration in e-cigarettes.
The validity of the paradigm was supported. This study aimed to characterize aversive subjective effects of high nicotine, while also capturing rewarding nicotine effects. The nicotine level (24 mg/mL) was sufficient to induce significant aversive effects as well as nonsignificant rewarding effects. As planned, the relative balance skewed towards aversiveness, as evidenced by higher subjective ratings and self-administration patterns. Importantly, the e-liquids used were palatable enough that subjects were willing to complete the directed self-administration component, and remained well within safe limits (no adverse events occurred). Subjective ratings and self-administration patterns were internally consistent (eg, e-liquid conditions rated as less aversive and more rewarding during directed self-administration component were self-administered at higher rates during ad libitum component), indicating internal validity. Plasma nicotine and menthol glucuronide levels rose following the directed blocks which contained nicotine or menthol, respectively, dropped slightly following the lunch break. Importantly for the genetics analyses, levels of nicotine and menthol glucuronide did not differ by genotype at any of the blood draws where the nicotine and menthol delivery were intended to remain consistent across genotype groups (ie, overnight abstinence baseline, after directed blocks, after lunch break), even though they did differ during the ad libitum component, where differences in self-administration were the key outcome. Therefore, genotype group differences on subjective ratings during the directed administration or other outcomes or nicotine levels following the ad libitum period are unlikely to be accounted for by differential levels of nicotine or menthol delivery during the directed administration or following lunch (which served as baseline for ad libitum).
This initial study had several limitations which should be addressed in future studies. First, all but two of the subjects in the study were menthol cigarette smokers. Since menthol cigarette smokers may like menthol taste/sensation more and have a different learning history with menthol (as a possible conditioned cue),40,41 differ from non-menthol smokers in subjective ratings of intravenously-delivered nicotine (ie, without accompanying smoking cues),42 menthol and non-menthol smokers could differ in their response to menthol or nicotine. Therefore, the current findings from a predominantly menthol-preferring sample may not apply equally to non-menthol smokers. Second, only one nicotine (24 mg/mL) dose was tested versus no nicotine (0 mg/mL). While this nicotine dose is considered a high level of nicotine and it successfully induced aversive effects, other nicotine-by-flavor interactions may have been detected at low or intermediate nicotine levels. Third, a fixed, escalating nicotine level (0 mg/mL followed by 24 mg/mL) minimized nicotine carryover. This approach limited order and dose effects from being fully disentangled. Fourth, the sample size (N = 32) limited power, particularly in the genetics analyses. Findings should be considered preliminary and warrant future studies with larger sample sizes that can examine confounding effects of variables such as sex, age, race, current/former/never cigarette smoker status and menthol-preferring/non-preferring smoker status.15,43–45 Lastly, the multiple outcome measures were a strength and limitation. The relatively detailed assessment of subjective response to the e-cigarette conditions enabled greater characterization of the effects and will inform future studies. To minimize the number of comparisons, items were grouped conceptually; however, with a larger sample size, data reduction could be accomplished statistically (eg, factor analysis). Corrections for multiple comparisons lowered type I error risk. To address resulting increased type II error risk, results not surviving corrections are clearly labeled in the Supplementary Tables and Materials.
One notable strength of the study was careful control of e-liquid conditions. Nicotine and menthol levels were matched and independently confirmed. The vendor confirmed that no sweeteners were added to any e-liquids. Alcohol levels were matched across e-liquids, and pH was tested and did not significantly differ across e-liquids. PG/VG ratio was matched for the final PG/VG of the total e-liquid, rather than PG/VG of the base liquid. This is an important consideration when comparing across different flavor conditions (particularly when including an unflavored condition; Supplementary Table 1), since these factors could influence nicotine absorption (eg, pH1, PG/VG46), flavor (eg, sweeteners47), subjective effects like harshness and throat hit (eg, PG/VG46,48), or other effects (eg, psychomotor effects of alcohol38). Additional strengths include confirmation of nicotine and menthol delivery, detailed characterization of subjective effects, and novel use of genetics to model different “risk” profiles.
Conclusions
This study addressed an FDA research priority regarding e-cigarette perceptions and use. Findings suggest that menthol partially mitigated nicotine aversiveness, potentially increasing nicotine-containing e-cigarettes’ appeal, while fruit increased appeal of nicotine-free e-cigarettes with little effect on appeal of high nicotine-containing e-cigarettes. These dissociable patterns should be investigated in intermediate nicotine doses. Although some e-liquid flavorants may increase harm,14 flavors (including fruit and menthol) can reduce perceived harm,16 and menthol can facilitate dependence or worsen cessation outcomes in some groups of smokers,41 but limiting flavor options may reduce e-cigarette use.49 Flavors’ effects on appeal and use of e-cigarettes, with or without nicotine, are important factors when considering regulation of e-cigarettes.
The genetics analysis addresses the FDA research priority regarding the impact of tobacco product characteristics, (eg, flavors) on initiation among vulnerable populations. The “protective” (GG) genotype found nicotine less reinforcing (rated as more “harsh,” experienced less craving alleviation from nicotine, self-administered less). This subgroup may find other aspects of tobacco products reinforcing or require more masking of nicotine aversiveness to find a product appealing. Notably, the “protective” rs16969968 genotype (GG) occurs at disproportionately higher rates in African Americans, relative to European Americans, and menthol use is more prevalent in African American smokers45 and e-cigarettes users.50 Regulators should consider that product characteristics which diminish the aversiveness of products (ie, lower nicotine levels; flavors that mask nicotine aversiveness) may disproportionately increase appeal in subgroups with more sensitivity to aversiveness (eg, “protective” [GG]). Furthermore, regulations that aim to reduce addictive potential by lowering nicotine may have less impact on subgroups that experience less reinforcement (eg, craving alleviation) from nicotine. These findings underline the importance of regulatory decision-makers considering the potential interaction of different product characteristics (eg, flavors, nicotine) and impact on vulnerable subgroups.
Funding
This research was supported by a pilot grant from the Yale Tobacco Center of Regulatory Science (Yale TCORS; P50DA036151).
Declaration of Interests
RG discloses consulting fees for Palo Alto Health Sciences, Knopp Biosciences, and Mathematica Policy Research, royalties from book “Statistical Methods in Psychiatry and Related Fields” published by CRC Press, and a provisional patent submission by Yale University: Chekroud, A.M., Gueorguieva, R., & Krystal, J.H. “Treatment Selection for Major Depressive Disorder” (filing date June 3, 2016, USPTO docket number Y0087.70116US00). The remaining authors have no competing interests to declare.
Supplementary Material
Acknowledgments
The authors acknowledge Stacy Minnix, Lance Barnes, Ellen Mitchell RN, and Chris Cryan for their contributions to recruitment, data collection and/or data management.
References
- 1. DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16(4):438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. USDHHS. The health consequences of smoking – 50 years of progress: A report of the Surgeon General. In: U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, ed. Atlanta, GA; 2014. [Google Scholar]
- 3. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26(6):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goldenson NI, Leventhal AM, Stone MD, McConnell RS, Barrington-Trimis JL. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171(12):1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine Tob Res. 2016;18(7):1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler CD, Kenny PJ. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76(Pt B):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Picciotto MR, Mineur YS. Molecules and circuits involved in nicotine addiction: the many faces of smoking. Neuropharmacology. 2014;76(Pt B):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sartor CE, Lessov-Schlaggar CN, Scherrer JF, et al. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav. 2010;35(8):771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103(1):69–78. [DOI] [PubMed] [Google Scholar]
- 10. Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction. 2017;112(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann N Y Acad Sci. 2015;1340(1):65–74. [DOI] [PubMed] [Google Scholar]
- 12. Bold KW, Kong G, Camenga DR, et al. Trajectories of e-cigarette and conventional cigarette use among youth. Pediatrics. 2018;141(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: A systematic review and meta-analysis. JAMA Pediatr. 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25(e1):e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonhomme MG, Holder-Hayes E, Ambrose BK, et al. Flavoured non-cigarette tobacco product use among US adults: 2013–2014. Tob Control. 2016;25(Suppl 2):ii4–ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control. 2016;25(Suppl 2):ii62–ii66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: flavor, nicotine strength, and type. PLoS One. 2018;13(3):e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, et al. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: application of a novel methodology. Drug Alcohol Depend. 2016;168:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 2016;166:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wickham RJ. How menthol alters tobacco-smoking behavior: A biological perspective. Yale J Biol Med. 2015;88(3):279–287. [PMC free article] [PubMed] [Google Scholar]
- 21. Han S, Chen H, Zhang X, Liu T, Fu Y. Levels of selected groups of compounds in refill solutions for electronic cigarettes. Nicotine Tob Res. 2016;18(5):708–714. [DOI] [PubMed] [Google Scholar]
- 22. Yerger VB, McCandless PM. Menthol sensory qualities and smoking topography: A review of tobacco industry documents. Tob Control. 2011;20(Suppl 2):ii37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Journigan VB, Zaveri NT. TRPM8 ion channel ligands for new therapeutic applications and as probes to study menthol pharmacology. Life Sci. 2013;92(8-9):425–437. [DOI] [PubMed] [Google Scholar]
- 24. Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem Senses. 2012;37(5):463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang XB, Jiang P, Gong N, et al. A-type GABA receptor as a central target of TRPM8 agonist menthol. PLoS One. 2008;3(10):e3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Auton A, Brooks LD, Durbin RM, et al. ; 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526(7571):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saccone NL, Culverhouse RC, Schwantes-An T-H, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: A meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8):e1001053. doi:10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tobacco Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M. A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology. 2015;40(12):2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37(6):1509–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P 3rd. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5(5):621–624. [DOI] [PubMed] [Google Scholar]
- 34. Jatlow P, Valentine G, Gueorguieva R, et al. Plasma menthol glucuronide as a biomarker of acute menthol inhalation. Tob Regul Sci. 2018;4(1):586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P III. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19(12):3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morean ME, Kong G, Cavallo DA, Camenga DR, Krishnan-Sarin S. Nicotine concentration of e-cigarettes used by adolescents. Drug Alcohol Depend. 2016;167:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valentine GW, Hefner K, Jatlow PI, Rosenheck RA, Gueorguieva R, Sofuoglu M. Impact of e-cigarettes on smoking and related outcomes in veteran smokers with psychiatric comorbidity. J Dual Diagn. 2018; 14(1):2–13. doi:10.1080/15504263.2017.1384877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valentine GW, Jatlow PI, Coffman M, Nadim H, Gueorguieva R, Sofuoglu M. The effects of alcohol-containing e-cigarettes on young adult smokers. Drug Alcohol Depend. 2016;159:272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krishnan-Sarin S, Green BG, Kong G, et al. Studying the interactive effects of menthol and nicotine among youth: An examination using e-cigarettes. Drug Alcohol Depend. 2017;180:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res. 2010;12(Suppl 2):S110–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeVito EE, Valentine GW, Herman AI, Jensen KP, Sofuoglu M. Effect of menthol-preferring status on response to intravenous nicotine. Tob Regul Sci. 2016;2(4):317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeVito EE, Herman AI, Waters AJ, Valentine GW, Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rath JM, Villanti AC, Williams VF, Richardson A, Pearson JL, Vallone DM. Correlates of current menthol cigarette and flavored other tobacco product use among U.S. young adults. Addict Behav. 2016;62:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lawrence D, Rose A, Fagan P, Moolchan ET, Gibson JT, Backinger CL. National patterns and correlates of mentholated cigarette use in the United States. Addiction. 2010;105(Suppl 1):13–31. [DOI] [PubMed] [Google Scholar]
- 46. Spindle TR, Talih S, Hiler MM, et al. Effects of electronic cigarette liquid solvents propylene glycol and vegetable glycerin on user nicotine delivery, heart rate, subjective effects, and puff topography. Drug Alcohol Depend. 2018;188:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miao S, Beach ES, Sommer TJ, Zimmerman JB, Jordt SE. High-intensity sweeteners in alternative tobacco products. Nicotine Tob Res. 2016;18(11):2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Q, Zhan Y, Wang L, Leischow SJ, Zeng DD. Analysis of symptoms and their potential associations with e-liquids’ components: a social media study. BMC Public Health. 2016;16:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pesko MF, Kenkel DS, Wang H, Hughes JM. The effect of potential electronic nicotine delivery system regulations on nicotine product selection. Addiction. 2016;111(4):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bowler RP, Hansel NN, Jacobson S, et al. ; for COPDGene and SPIROMICS Investigators Electronic cigarette use in US adults at risk for or with COPD: Analysis from two observational cohorts. J Gen Intern Med. 2017;32(12):1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.